Abstract
The substrates of mammalian ULK1/2 and its yeast homologue Atg1 in autophagy have remained elusive. The class III phosphatidylinositol 3-kinase component Beclin-1 has now been identified as a physiological substrate of the ULK kinases in autophagy following amino acid starvation, therefore suggesting a critical molecular link between the upstream kinases and the autophagy core machinery.
Autophagy is a specialized degradative route, which starts with the formation of autophagosomes and ends in the lysosomes, providing building blocks and energy for cellular survival1. More than 36 autophagy-related genes (Atgs) have been identified in yeast, and most of them are evolutionarily conserved2. Among these Atg proteins, there is only one serine/threonine protein kinase: Atg1 in yeast and ULK1/2 in mammals3. The kinase activity of ULK/Atg1 is required for autophagy, but its physiological substrates have, until recently, been unknown. In this issue, Guan and colleagues demonstrate that ULK phosphorylates Beclin-1 following amino acid withdrawal, and this phosphorylation step is crucial for the function of Beclin-1 in autophagy4.
Beclin-1, the mammalian homologue of yeast Atg6, serves as a core component of the class III phosphatidylinositol 3-kinase (PI(3)KC3) complex. The PI(3)KC3 complex is composed of at least six stoichiometric subunits, including VPS34 (the mammalian counterpart of yeast Vps34), p150 (counterpart of yeast Vps15), Beclin-1 (Atg6 in yeast), ATG14L (also known as Barkor; Atg14 in yeast), UVRAG (Vps38 in yeast) and Rubicon (which has no yeast counterpart)5–10. VPS34, p150 and Beclin-1 form a stable core complex, and Beclin-1 is proposed to function as a bridge between the core complex and the regulatory subunits such as ATG14L or UVRAG. Activity of PI(3)KC3 is essential for autophagy initiation, and therefore it is tightly regulated. Guan and colleagues have developed an elegant in vitro assay for VPS34 activity, in which endogenous VPS34 is immunopurified from mammalian cell lysates that have been subjected to various environmental stresses, including glucose starvation and amino acid shortage4,11. They found that VPS34 lipid kinase activity is different depending on which subunit is associated with the VPS34 complex. Following glucose or amino acid deprivation, the lipid kinase activity associated with VPS34 or Beclin-1 is reduced, suggesting that the p150–VPS34–Beclin-1 complex devoid of regulatory subunits is irrelevant to autophagy activation. In contrast, the VPS34 complex activity associated with the autophagy-specific subunit ATG14L is strongly stimulated by glucose and amino acid starvation, suggesting that only this fraction of VPS34 is responsive to stresses and the regulation of this activity is highly specific to autophagy. Separating ATG14L-dependent and ATG14L-independent VPS34 lipid kinase activity is key to successfully elucidating the regulatory mechanism for each activity.
The definition of ATG14L-dependent VPS34 activity allowed the Guan group to dissect the requirement for autophagy-specific stimulation of Vps34 activity. In two recent reports, they demonstrate that AMPK is necessary and sufficient for activation of VPS34 lipid kinase in autophagy through phosphorylation of Beclin-1 at Ser 91 and Ser 94 following glucose starvation11, and that ULK is adequate and essential for the autophagy-specific VPS34 activation following amino acid starvation through phosphorylation of Beclin-1 at a different site, Ser 14 (ref. 4). These studies clearly establish Beclin-1 as a signalling hub that responds to various signals to activate VPS34 in autophagy. This is not the first time that Beclin-1 is shown to be regulated by phosphorylation. It was recently reported that Akt phosphorylates Beclin-1 at Ser 234 and Ser 295, and that such regulation is important in the suppression of Beclin-1 in autophagy and in Akt-driven tumorigenesis12. Beclin-1 is also phosphorylated by death-associated protein kinase (DAPK), which induces its release from its inhibitory binding partner Bcl-2 (refs 13). ULK1 was shown to phosphorylate the Beclin-1-interacting protein AMBRA1, and releases it from dynein for autophagy activation14.
Guan and colleagues then moved on to a Caenorhabditis elegans model system to address the importance of ULK-mediated Beclin-1 phosphorylation in vivo4. The autophagic clearance of germline P granules (PGL granules) in somatic cells during worm embryogenesis was employed as readout15. In this system, knockout of Beclin-1 or the ULK homologue unc51 in worms resulted in failure to clear PGL granules in somatic cells. Re-expression of wild-type Beclin-1, but not a phosphorylation-deficient mutant, fully complemented the defect in PGL granule degradation. Thus, the authors concluded that unc51 and phosphorylation of Beclin-1 are required for the autophagic degradation of PGL granules in C. elegans embryogenesis.
To further strengthen the evidence that phosphorylation of Beclin-1 by ULK is required for autophagy induction, Guan and colleagues transiently expressed a phosphomimetic Beclin-1S14D mutant in FIP200−/− mouse embryonic fibroblasts (MEFs) that are defective in ULK kinase activity. The result showed that overexpression of Beclin-1S14D mutant could partially compensate for the ULK defect, suggesting that at least Beclin-1 is an important downstream substrate of ULK kinase. The incomplete rescue might be due to transient expression4. Alternatively, there might be other, as-yet undefined, targets regulated by ULK kinase in autophagy (Fig. 1).
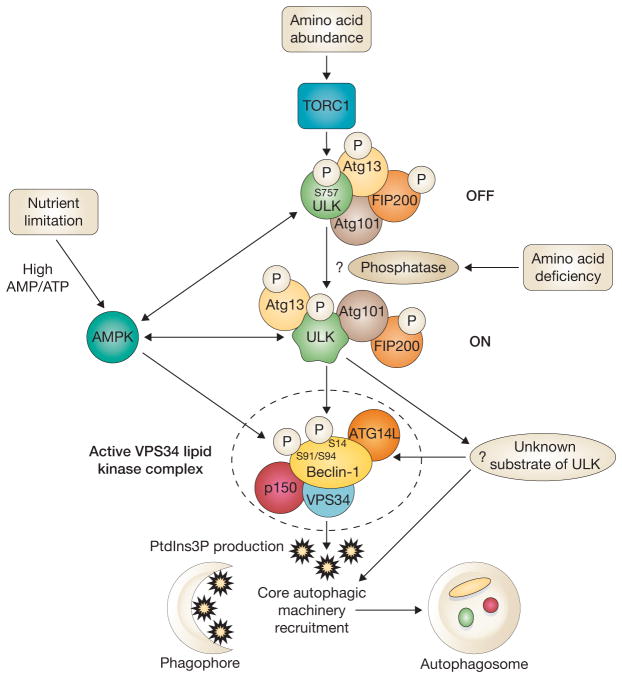
Figure 1.
ULK kinases activate the class III PI(3)K in autophagy following amino acid starvation and nutrient limitation. ULK kinases are positively regulated by AMPK following energy crisis and negatively regulated by the TORC1 kinase and/or unknown phosphatases following amino acid shortage. Guan and colleagues show that following amino acid deprivation or mTORC1 inhibition, active ULK activates VPS34 by phosphorylating ATG14L-bound Beclin-1 at Ser 14, an event that is required for PtdIns3P production and localization of the core autophagic machinery to phagophores and autophagosomes. Following nutrient limitation, AMPK activates VPS34 by phosphorylation of ATG14L-bound Beclin-1 at Ser 91 and Ser 94. It is also possible that other unknown targets of ULK (Atg1 in yeast) exist.
It is worth noting that only the ATG14L-associated VPS34 complex in this in vitro system displays a stress-inducible regulation that is consistent with VPS34 activation following autophagic stress. This is in line with the previously reported role of ATG14L as an autophagy-specific VPS34 regulator6–8. Guan and colleagues provide a plausible mechanism for the effect of ATG14L through its role in promoting the interaction between the substrate (Beclin-1) and the kinase (ULK)4. However, it is still not clear how this is achieved given that ATG14L has no reported interaction with ULK. It is likely that ATG14L binding promotes conformational changes in the pro-autophagy VPS34 complex to allow a high-affinity interaction between Beclin-1 and ULK. The authors also showed that the adaptor function of ATG14L and the phosphorylation of Beclin-1 by ULK are required for the recruitment of the VPS34 lipid kinase complex to the phagophore4. A similar situation was reported previously when ATG14L was found to promote phosphorylation of Beclin-1 by AMPK under nutrient starvation conditions11.
It seems that the in vitro system fails to distinguish between distinct protein complexes in different subcellular compartments. The p150–VPS34–Beclin-1 complex forms two mutually exclusive subcomplexes by interacting with different regulators or adaptors6–8. When binding to ATG14L, the complex is localized to phagophores and autophagosomes. Otherwise, the VPS34 complex is associated with UVRAG and Rubicon at endosomes. The ATG14L subcomplex is specific for autophagy activation, but the function of the UVRAG–Rubicon subcomplex in autophagy is still under debate. It is known to be important for endosome maturation, but is also likely to be involved in autophagosome and amphisome maturation directly or indirectly8–10. In the in vitro system employed by Guan and colleagues, both ATG14L and UVRAG displayed similar activation of VPS34 lipid kinase activity following glucose or amino acid starvation4,11. It is likely that their functions are decided not biochemically, but by their subcellular localization. This notion is supported by the observation that both ATG14L and UVRAG interact with Beclin-1 through their respective coiled-coil domains in a competitive manner5–9.
The identified ULK1 phosphorylation site in Beclin-1 is not found in its yeast homologue Atg6, which leads to the speculation that the regulation of VPS34 lipid kinase activity in response to both amino acid withdrawal and nutrient limitation could be different in yeast. There might also be other unidentified evolutionarrily conserved ULK (Atg1) substrates that are crucial for autophagy regulation in yeast. Nevertheless, the biological functions of ULK (Atg1) protein kinases in autophagy are now starting to be understood.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Mizushima N, Komatsu M. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 2.Motley AM, Nuttall JM, Hettema EH. EMBO J. 2012;31:2852–2868. doi: 10.1038/emboj.2012.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mizushima N. Curr Opin Cell Biol. 2010;22:132–139. doi: 10.1016/j.ceb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Russell RC, et al. Nat Cell Biol. 2013;15:741–750. doi: 10.1038/ncb2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang C, et al. Nat Cell Biol. 2006;8:688–699. doi: 10.1038/ncb1426. [DOI] [PubMed] [Google Scholar]
- 6.Itakura E, Kishi C, Inoue K, Mizushima N. Mol Biol Cell. 2008;19:5360–5372. doi: 10.1091/mbc.E08-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Q, et al. Proc Natl Acad Sci USA. 2008;105:19211–19216. doi: 10.1073/pnas.0810452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsunaga K, et al. Nat Cell Biol. 2009;11:385–396. doi: 10.1038/ncb1846. [DOI] [PubMed] [Google Scholar]
- 9.Zhong Y, et al. Nat Cell Biol. 2009;11:468–476. doi: 10.1038/ncb1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Q, Westphal W, Wong KN, Tan I, Zhong Q. Proc Natl Acad Sci USA. 2010;107:19338–19343. doi: 10.1073/pnas.1010554107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J, et al. Cell. 2013;152:290–303. doi: 10.1016/j.cell.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang RC, et al. Science. 2012;338:956–959. doi: 10.1126/science.1225967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zalckvar E, et al. EMBO Rep. 2009;10:285–292. doi: 10.1038/embor.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Bartolomeo S, et al. J Cell Biol. 2010;191:155–168. doi: 10.1083/jcb.201002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, et al. Cell. 2009;136:308–321. doi: 10.1016/j.cell.2008.12.022. [DOI] [PubMed] [Google Scholar]