Abstract
Background & Aims
High-definition anorectal manometry (HDAM-3D) provides a topographic and 3-dimensional profile of anorectal pressure. We assessed anorectal sensori-motor function in healthy adults, the reproducibility of measurements made with HDAM-3D, and the accuracy of data analysis by its software.
Methods
Anal sphincter pressures and rectal sensory thresholds were measured in 78 healthy subjects via placement of a 10 mm rigid probe, with 256 circumferentially arrayed pressure sensors, and a balloon in the rectum. The bearing down maneuver was assessed in a subset of 18 subjects. We compared data analyzed by experts with findings from automated software analysis. Measurements made in a subset of 16 subjects, 2 weeks apart, were compared to determine reproducibility.
Results
Resting, squeezing, and sustained squeezing pressures were significantly higher in men than women (P<.05); other parameters were similar. Desire and urgency to defecate were similar between men and women, but the maximal tolerable volume was significantly lower in women (P<.05). Older women (>50 y) had significantly lower resting (P<.01) and sustained squeeze pressures (P<.04). Dyssynergic patterns of defecation were observed in 12/18 subjects (67%) who attempted to defecate without the 60 cc rectal balloon distension and in 6/18 subjects (33%) with the 60 cc rectal balloon distension. Rest–retest values correlated, (r = 0.81) as did conclusions made by experts vs software analyses of data (r = 0.99).
Conclusions
Based on HDAM-3D measurements in healthy adults, anal sphincter pressures are higher in men than women, but sensory and other parameters are similar; older subjects have weaker sphincters. Many people were found to have dyssynergic patterns of defecation, which could be related to the probe or other technical issues, so this technique may not be suitable for assessing defecation patterns. Measurements made by HDAM-3D are reproducible and data can be accurately analyzed by its software.
Keywords: Anorectal disorder, balloon expulsion, neuromuscular, rectal motor function
Introduction
Anorectal disorders are common and affect 10–20% of the population (1, 2). Previous studies have shown that physiological and morphological testing of anorectal function provides important and useful pathophysiological information (3, 4), and that it may impact patient management (3).
Currently, several techniques are available for the assessment of anorectal function (5, 6) including anorectal manometry and balloon expulsion test. Anorectal manometry comprises of a series of physiological measurements (3, 5, 7, 8). There is limited information regarding normal anorectal function using water perfused and solid state manometry systems in healthy adults (9–11). Although minimal standards for performing these tests have been recommended by the American and European Motility Societies (7, 8,12), these have not been widely adopted, largely due to technical limitations, costs and lack of standardized and user friendly equipment.
Recently, newer technologies have become available for the assessment of anorectal neuromuscular function that have incorporated a greater number of circumferentially arrayed, and closely spaced sensors. This configuration allows interpolation of manometric recordings into topographical plots and facilitates display of high resolution pressure images (13, 14). A further refinement has been the introduction of 3-dimensional high definition anorectal manometry (HDAM-3D) that comprises of a rigid probe with 256 circumferentially arrayed pressure sensors that provides 3-D reconstruction of pressure profiles. Recent studies with HDAM-3D have provided novel information regarding the sensorimotor response, the recto-anal inhibitory reflex, and recto-anal contractile response (15, 16) and anal/vaginal functional morphology (17).
Recently, normative manometric data for women, using high resolution anorectal manometry (2-D) have been published (14), but there is no information on HDAM-3D. Furthermore, HRM and HDM use different rectal balloons and sensor configurations.
Our aims were: i) to perform comprehensive evaluation of anal sphincter function, rectal motor function and rectal sensory thresholds using HDAM-3D technology in age and gender-matched healthy adults, and ii) to assess the reproducibility of anorectal function and various measurements performed with this technology, and iii) to compare the accuracy and levels of agreement between the measurements provided by the commercial software and those assessed by an expert.
Materials and Methods
Subjects
All subjects filled out standard bowel symptom questionnaires (18). Enrolled subjects had no symptoms of constipation or incontinence, were not taking any medications other than oral contraceptive pill and multivitamins, and had no history of GI surgery other than appendectomy. All subjects had a normal physical examination. The study was approved by the Institutional Review Boards of the University of Iowa, USA, and University of Veracruz, Mexico, and all participants gave written informed consent.
Study protocol
After an overnight fast, subjects attended the motility lab. No routine bowel preparation was used. HDAM-3D was performed with the subject lying in the left lateral position. The HDM probe (Given Imaging, Yoqneam, Israel) is 6.4 cm in length and has an outer diameter of 10.75 mm. It has 256 pressure sensors that are arranged in 16 rows, and each row has 16 circumferentially oriented sensors (Figure 1). Each sensor is 4 mm long and 2 mm wide. The rigid probe has a central lumen for inflation and a Luer-lock at one end through which a balloon is attached. The balloon is composed of non-latex clear thermoplastic elastomer, 3.3-cm long, with a capacity of up to 400 cc. The probe is attached to an amplifier and recorder system, and the manometric and topographic images are displayed on a computer monitor using specialized software (Motility Acquisition AR System v.2.2, Given Imaging, Yoqneam, Israel). The HDM system operates at a frequency response of > 20 Hz, a scan rate of 10 Hz, and an output resolution of 0.1 mmHg. The probe is calibrated immediately before the procedure by placing it into a calibration chamber, where it is zeroed to atmospheric pressure and set to a range of pressures up to 300 mmHg. The sensor calibration residual is ± 2 mmHg in the 0 to 100 mm Hg range, and 2% of reading in the 100 to 300 mmHg range. A digital rectal examination was performed before placement of the probe and a saline enema was given if stool was detected. The lubricated probe was inserted such that a panel of pressure sensors were located across the anal canal. Because the probe has circumferential pressure sensors, it was oriented such that the posterior portion corresponded to the dorsal aspect of the subject. This facilitated standardization of the vector measurements in the rectum and anal canal. The probe was held in place manually during the entire study by an operator. After a 10-min run-in period, subjects were first instructed to squeeze the anus as tight as possible and for at least 30 s and the maneuver was repeated. Next, the subjects were given a small party balloon.
Figure 1.
HDAM-3D probe depicting the array of circumferential pressure sensors and a balloon
They were instructed to inflate the balloon with their mouth by blowing air as hard as possible and for as long as possible on two separate occasions. Thereafter, a cohort of subjects were asked to perform the push or bearing down maneuver as if to defecate, on two separate occasions, both without and with 60 cc of air introduced into the rectal ballon. The rectal sensation, the rectoanal reflexes and rectal compliance were evaluated simultaneously by sequentially inflating the rectal balloon with a hand-held syringe in a stepwise, graded fashion using intermittent balloon distention technique (9, 12). Rectoanal inhibitory reflex (RAIR) was assessed qualitatively as present or absent. The subjects were given a sensation chart and asked to describe their sensations (first sensation, constant sensation, desire to defecate, and urgency to defecate). The rectal balloon was distended with air using 10 cc increments until the subject reported a first sensation, and thereafter with 30 cc increments until the maximal tolerable volume or 320 cc was reached. Each distention was held for at least 30 seconds and after deflation, a rest period of 2 minutes was allowed before the balloon was re-inflated to the next volume.
The threshold volume at which the subject reported a first sensation, constant sensation, desire to defecate, urge to defecate, and the maximum tolerable volume were recorded (12). Subsequently, the manometry probe was removed.
Measurements and Data Analysis
Anal Sphincter Pressure
The measurements were performed with the help of a computer software (Manoview Analysis®, Given Imaging, Yoqneam, Israel). The software provided maximal and mean rectal and anal pressures for the resting and squeeze frames and the maximal rectal pressure and residual anal pressure for the bearing down maneuver. The presence of a dyssynergic manometric pattern was noted and measurements performed as described previously (7,19).
Reproducibility
In order to assess the reproducibility of manometric and sensory measurements, and to assess the intra-subject variability, the test was repeated in 16 healthy subjects, approximately two weeks apart, using an identical protocol.
Comparative analysis of measurements obtained by the software and an expert
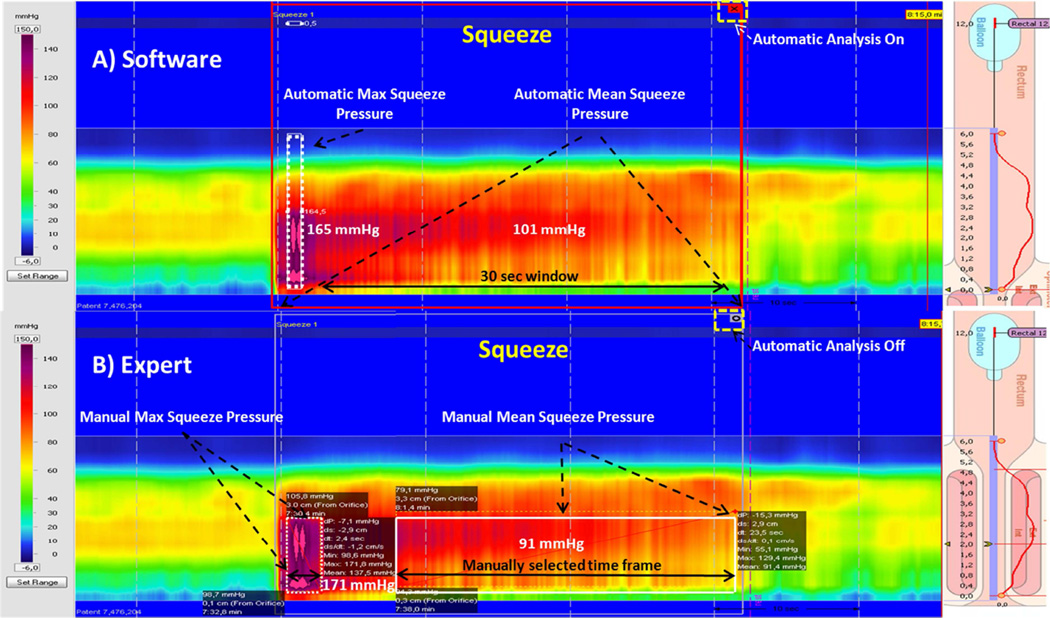
In order to assess the accuracy of measurements provided by the commercial software, we evaluated the anal and rectal topographic pressure changes during squeeze, when blowing into a party balloon and during bearing down maneuvers in a randomly selected cohort of 28 subjects. The data were analyzed by an expert investigator (SR, >25 yrs experience). The expert analyses were performed with the help of both the topographic plots and the conventional pressure plots, without activating the automated analysis ie. the small icon at the top right hand corner of the resting pressure profile (Fig 2 The correlations were performed by a third investigator (JMR).
Figure 2.
Illustration of a pressure topographic profile obtained with HDAM-3D, and depiction of the measurements as obtained by the software (top panel) and the expert (lower panel). The profile shows resting and squeeze topographic images from a single subject; the lollipops shown on the right (cartoon of anorectum) define the upper and lower borders of the anal high pressure zone and the electronic sleeve (e-Sleeve). The top panel shows software analysis of maximum squeeze pressure. Expert anaylses were performed manually as shown in the lower panel, and this included measurements for maximum and sustained squeeze pressures. To measure the anal or rectal pressures manually, the expert always used; (a) smart mouse tool and (b) did not switch on the automated analysis i.e. did not click the small icon on the top right hand corner of the box depicting that the auto analyses was off.
Effect of aging and parity on manometric data
Manometric data obtained from subjects who were less than 50 years old were compared with those who were older than 51 years and also analyzed by gender. Manometric data from nulliparous women were compared with those who had 1 or >2 vaginal deliveries.
Statistical analysis
The manometric data are expressed and summarized as mean and 95% CI. The Student t test was used to compare the gender differences between the various anorectal parameters. Wilcoxon signed rank test was used to assess the differences for the test-retest reproducibility data. Spearman (Rho) correlation test and the Bland-Altman method was used to assess the agreement for reproducibility of test data. Pearson’s correlation coefficient was used to assess the agreement between measurements performed by the expert and the software program. Categorical analyses were performed using x2 test. Finally, the Mann-Whitney U test was used to assess the effect of age on manometric data. Statistical analyses were performed using Graphpad Prism v 5.0 (San Diego, CA) and p< 0.05 was considered statistically significant.
Results
Subject Demographics
A total of 78 healthy subjects, 36 men, mean age 37 years (range 18–82) and 42 women, mean age 40 years (range 18–71) were enrolled from two tertiary care centers; 27 were recruited from Iowa, USA and 51 from Veracruz, Mexico. The subjects were matched according to their age and gender as follows: 18–50 years (n=60, F:M, 32:28) and 51 years (n= 18, F:M, 10:8). Among female subjects, 19 were nulliparous and 23 had a history of previous vaginal delivery (11 had 1; 4 had 2 and 8 subjects had 3 or more deliveries). All subjects tolerated the procedure without any adverse events.
Anal Sphincter pressures
The maximal resting sphincter pressure, maximal squeeze sphincter pressure and sustained squeeze pressure were all significantly lower in women (p<0.05) compared to men. Although some other parameters were also lower in women, they were not significantly different (Table 1). There were no differences in pressure profiles between the two recruiting sites. The maximal resting pressure in nulliparous women was 92 mm Hg (85.6–98.5, 95% CI), in those who had 1 vaginal delivery it was 82.3 mm Hg (79.1–85.6 95% CI), and in those with 2 or more vaginal deliveries it was 77.8 mm Hg (65.3–90.2, 95% CI), and these differences were not statistically different (p= 0.18). Similar and statistically insignificant differences were observed for the maximal squeeze pressure (p=0.06), rectal pressure during straining (p=0.09) and anal residual pressure during bearing-down (p=0.66).
Table 1.
Anorectal sensori-motor function in healthy adults and differences between genders
| All (n=78) | Female (n=42) | Male(n=36) | |
|---|---|---|---|
| Length of anal sphincter (mean, cms) | 4.1 (4–4.3) | 4 (3.8–4.2) | 4.3 (4.1–4.5) |
| Maximum anal resting pressure (mean, mmHg) | 83 (78–87) | 76 (71–81) | 90 (83–96)* |
| Maximum squeeze pressure (mean, mmHg) | 233 (218–249) | 205 (186–224) | 266 (245–287)* |
| Sustained squeeze pressure (mean, mmHg) | 124 (105–194) | 104 (82–127) | 151 (120–182)* |
| % Increase in anal sphincter pressure during squeeze | 63 (58–58) | 61 (53–69) | 66 (68–73) |
| Squeeze duration (mean, s) | 29 (28–30) | 28 (27–30) | 30 (28–30) |
| Rectal resting pressure (mean, mmHg) | 11 (8–14) | 10 (6–13) | 15 (13–16) |
| Rectal squeeze pressure (mean, mmHg) | 16 (11–22) | 14 (7–21) | 19 (7–31) |
| Rectal pressure during party balloon inflation (mmHg) | 57 (51–62) | 55 (48–61) | 60 (52–67) |
| Anal pressure during party balloon inflation (mean, mmHg) | 132 (122–141) | 132 (117–147) | 131 (120–141) |
| Rectal pressure bearing down (mean, mmHg) | 41 (37–45) | 39 (34–45) | 43 (35–51) |
| Anal residual pressure during bear down (mean, mmHg) | 38(31–44) | 36 (28–43) | 40 (28–52) |
| Defecation index | 1.1 (0.4–1.5) | 1.1 (0.5–1.7) | 1.1 (0.3–1.9) |
| Defecation index when bearing down with 60 cc | 1.9 (1.5–2.3) | 1.4 (1.1–2.7) | 1.7 (1.2–2.2) |
| First sensation | 20 (18–22) | 24 (21–26) | 22 (20–25) |
| Desire to defecate | 89 (81–96) | 88 (79–96) | 94 (82–103)* |
| Urgency to defecate | 146 (137–155) | 139 (130–147) | 163 (140–167)* |
| Maximal Tolerable Volume | 192 (182–202) | 193 (182–204) | 206 (192–222)* |
Mean (95% CI),
p<0.05
Rectal Sensation
The thresholds for first sensory perception, desire to defecate and urgency to defecate were lower in women compared to men, but they were not significantly different (p>0.05). However, the maximal tolerable volume was significantly lower in women when compared to men (p<0.05) (Table 1).
Rectoanal Reflexes
All subjects showed a normal rectoanal inhibitory reflex. The minimal mean rectal volume that induced anal relaxation was 16.1± 1.4 cc, and this volume was not different between men and women, 11.67 ± 1.1 vs 16.6 ± 3.2 cc (p=0.2).
Bearing Down maneuver
A dyssynergic pattern of defecation was seen in 12/18 (67%) subjects (F11:M7) during attempted defecation when lying in bed. Additionally, when bearing down on the bed with a 60 cc balloon inflated in the rectum, a dyssynergic pattern was seen in 6/18 (33%) subjects (F4:M2). This suggests that a dyssynergic pattern is more likely to be prevalent without balloon inflation when compared to bearing down with a 60 cc balloon inflated in the rectum (p=0.02).
Reproducibility
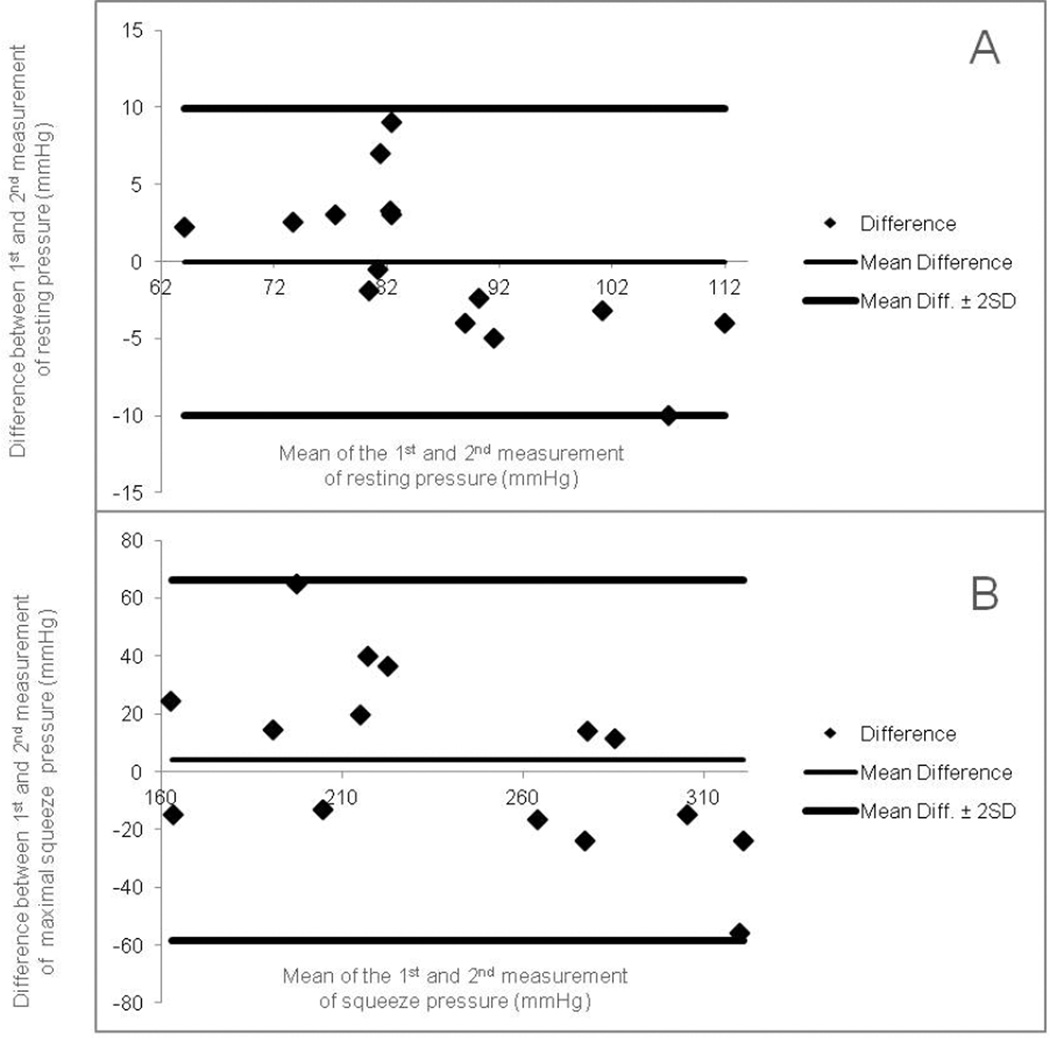
Test-retest assessment was performed in 16 healthy subjects (F10:M6, mean age= 33 y, range 21–62) at least two weeks apart. The anorectal pressure and sensory parameters were similar between test 1 and test 2, and there were no differences (p>0.05) except for the anal residual pressure when bearing down with 60 cc balloon, and for the first and constant sensations. Correlation coefficients between both tests were excellent to good (r >0.8). Bland-Altman plots for resting and maximal squeeze pressures are shown in Figure 3.
Figure 3.
This shows reproducibility of manometric data as assessed by Bland-Altman plots; for (A) resting sphincter pressures and (B) maximal squeeze pressures. Lines are plotted indicating the limits of agreement (0 ± 1.96 S.D.).
Expert versus Software agreement
For this purpose, data from 28 randomly selected subjects were analyzed in a blinded fashion. The results obtained from the expert’s measurements were compared with those obtained from the software, and are summarized in table 2. There were no significant differences between the data measured by the expert and that performed with the aid of software. Correlations for these data were good to excellent, with r values ranging from 0.81 to 0.99.
Table 2.
Manometric data as assessed by an expert and with the aid of the software program and their correlations.
| Data Analysis | Data Analysis | C orrelations | |||
|---|---|---|---|---|---|
| By Expert | By Software | p | r2 | p | |
| Resting pressure (mmHg) | 75 (67–82) | 78 (71–85) | 0.5 | 0.99 | 0.0001 |
| Maximum squeeze (mmHg) | 219 (192–247) | 217 (188–247) | 0.9 | 0.99 | 0.0001 |
| Rectal pressure during party balloon inflation (mmHg) | 52 (43–61) | 50 (38–63) | 0.8 | 0.97 | 0.0001 |
| Anal pressure during party balloon inflation (mmHg) | 139 (122–156) | 134 (118–151) | 0.7 | 0.99 | 0.0001 |
| Rectal pressure bearing-down (mmHg) | 49 (38–59) | 49 (38–59) | 0.9 | 0.93 | 0.0001 |
| Anal pressure bearing-down (mmHg) | 57 (45–70) | 61 (48–74) | 0.5 | 0.98 | 0.0001 |
| Defecation index | 1.1 (0.7–1.5) | 0.7(0.5–0.9) | 0.08 | 0.97 | 0.0001 |
| Rectal pressure bearing-down with 60cc balloon (mmHg) | 75 (65–89) | 77 (65–88) | 0.9 | 0.93 | 0.0001 |
| Anal pressure bearing-down with 60cc balloon (mmHg) | 48 (36–61) | 55 (43–66) | 0.4 | 0.86 | 0.0001 |
| Defecation index with 60cc balloon | 1.9 (1.5–2.3) | 1.1(0.7–1.5) | 0.4 | 0.81 | 0.0001 |
p value, Student t-test; mean (95% CI), Confidence Interval
Effect of age related changes on anorectal physiology
When analyzed separately by gender, the resting sphincter pressure and sustained squeeze pressure were higher in younger women (p<0.05) compared to older women. Rest of the parameters were similar between the younger and older groups. Intrarectal pressures during straining were higher in older subjects when compared to younger subjects, and this parameter was significantly different only in women (p<0.05). There were no significant differences for any of the parameters in men (Table 3).
Table 3.
Effects of aging on anorectal function
| Male | Female | |||
|---|---|---|---|---|
| (mmHg) | < 50 yr | > 50 yr | < 50 yr | > 50 yr |
| Maximum resting pressure | 93 (86–100) | 85 (69–101) | 81 (77–86)* | 65 (50–79) |
| Maximum squeeze pressure | 270 (247–294) | 252 (185–319) | 213 (191–235) | 173 (133–213) |
| Sustained squeeze pressure | 149 (120–177) | 156 (35–277) | 116 (89–142)* | 70 (41–99) |
| Rectal pressure during bearing down | 38 (32–45) | 56 (32–80) | 35 (30–40) | 52 (40–64)* |
| Anal residual pressure during bearing down | 37 (26–47) | 66 (23–110) | 37 (27–46) | 40 (21–59) |
mean, (95% CI)
p < 0.05 < 50 yr vs > 50 yr
Discussion
In this study, we conducted a comprehensive assessment of anorectal sensori-motor function using a novel high definition 3-D anorectal manometry system in a large cohort of healthy adults. Our study provides normative data from a carefully selected healthy western population who were matched for age and gender. Although some differences were seen in the anal and rectal pressure profiles, the sensory data, particularly the maximal tolerable volume was significantly different between men and women. This finding not only underscores a need for normative data but also emphasizes the importance of gender difference that should be considered when interpreting findings from patients with anorectal disorders.
The study protocol and measurements were performed as recommended by the American and European societies of Neurogastroenterology and Motility (12). The anal sphincter pressures (resting, maximal squeeze and sustained squeeze) were significantly lower in women, and is consistent with the gender differences that have been reported previously using solid state anorectal probe in healthy subjects (9). Similarly, the sensory data showed some differences between men and women, unlike the data reported previously with solid state anorectal manometry (9). The differences in sensory parameters may be due to the differences in the rectal balloons and their stiffness, as well as the rectal wall compliance. Previously, a commercially available latex balloon was used with the solid state manometry probe whereas with the HDAM-3D system, the manufacturer recommends a non-latex balloon, (Manoshield-3D®, Given Imaging, Yoqneam, Israel) that is less elastic.
In women, although we observed that increasing parity was associated with a trend towards lower anal sphincter pressures, there was no significant difference, possibly due to a Type II error.
There are some limitations of the HDAM-3D system. Unlike conventional anorectal manometry probes that are typically 4–6 mm in diameter and are flexible, the HDAM probe is approximately twice the diameter, and rigid and does not conform to the anorectal angle. Furthermore, the probe has to be hand-held, and this may introduce artifacts especially if it is not held in the neutral position during maneuvers such as squeeze and bearing down. These factors along with the greater number of pressure sensors and the higher resolution of this system may have accounted for the higher squeeze sphincter pressures and larger anal high pressure zone observed with HDAM when compared to those obtained with solid state manometry (9) or high resolution manometry (13,14). The differences observed in normative data using different technologies underscores the need to be cognizant of these findings when interpreting data for either clinical assessments or for research studies. Physiological studies have shown that the external anal sphincter principally operates at short sarcomere length (22), i.e. if the sphincter is stretched for example by a larger diameter and rigid probe, then the force of its contraction is likely to be higher and this may partly explain the higher squeeze pressures found with HDAM-3D. Also, with this technology it is important to continuously monitor and be aware of probe movement especially after maneuvers such as squeeze, cough or bearing down, and adjust the probe accordingly.
Dyssynergic pattern has been described in otherwise healthy subjects (9). The prevalence of this pattern in healthy subjects is 22% when bearing-down in the lying position on a bed and only 4% when bearing-down with a 60 cc balloon inflated in rectum, in the sitting position and on a commode (9, 23). In this study, when bearing down in the lying position on a bed, we found that 67% of a cohort of healthy subjects showed dyssynergic pattern without and 33% with a 60 cc balloon inflated in the rectum. Because the probe had to be held in place by an operator, it was not possible to perform a bearing down maneuver on the commode with this technology. Thus, the incidence of dyssynergia was higher with this technology, and a similar higher incidence has been reported with the high resolution manometry system in healthy women (14). These observations could be due to the increased sensitivity of these newer systems or alternatively, this could be due to the unphysiological position (lying on bed) when the maneuver is performed or probe movement causing an artifact, despite efforts to hold it in place. Because of the high rate of dyssynergia seen in the initial cohort of healthy subjects, we decided not to perform this maneuver in subsequent subjects. Based on these observations we feel that HDAM-3D may not be ideally suited for the manometric assessment of dyssynergic defecation, and that the test may yield a higher rate of false positive result. However, the presence of a normal relaxation pattern during attempted defecation most likely will exclude dyssynergia.
Although minimum standards for the assessment of anorectal function have been recommended (12), there is a lack of standardized approach for performing anorectal manometry amongst various motility laboratories, in part due to different technologies and equipment. By using standardized technology (probe, software, study protocol), and tools for performing measurements, it is possible to provide uniform and more accurate interpretation of anorectal testing. This may lead to improved diagnosis and treatment of anorectal disorders (23, 24). However, there is a learning curve, both for the interpretation and use of commercial software. When the data obtained from the software program were compared with that assessed by an expert, we found fairly good to excellent correlations for most of the anorectal parameters. This provides an independent validation of the accuracy of software measurements. However, the computer software does not provide data regarding certain parameters such as the sustained squeeze pressure, defecation index, dyssynergic pattern or rectal compliance, and currently these parameters have to be assessed manually. Overall, the software appears to be useful and reliable.
The manometric data showed good to excellent reproducibility for most of the parameters with very little intrasubject variability when the test was repeated two weeks apart. This suggested that the HDAM 3-D assessment provided reliable and reproducible data. This information can be important when evaluating temporal changes, for example, assessment of an intervention (eg, effect of drugs) or disease progression. Another unique advantage of the HDAM-3D system, unlike 2-D HRM, is that it provides information regarding axial and radial asymmetry of the anal sphincter (15–17), but this aspect was not assessed in this study.
In conclusion, HDAM 3-D provides reliable and reproducible topographic assessment of anorectal motor and sensory function, with better spatial and temporal resolution and visual images. Our study provides normative data for most of the commonly measured anorectal sensory and motor parameters. Future studies should address the clinical utility of this novel technology, particulary the benefits of higher resolution in the assessment of fecal incontinence, dyssynergic defecation, and other pelvic floor disorders.
Supplementary Material
Acknowledgements
We would like to thank Given Imaging (Sierra Scientific Instruments) for loaning the equipment for this study. Dr. SSC Rao was supported by NIH grant No. 2R01 KD57100-05A2. We thank Kasaya Tantiphlachiva, Michelle Nguyen and Kalyani Meduri for technical assistance with these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None
Portions of this paper were presented at Digestive Disease Week 2012, San Diego and published as an abstract; Gastroenterology 2012 Vol. 142, Issue 5, Supplement 1, Page S-826 and Gastroenterology 2012 Vol. 142, Issue 5, Supplement 1, Pages S-905-S-906.
Guarantor: Satish SC Rao, study concept and design, subject recruitment, study supervision, data analysis and interpretation, manuscript preparation, critical revision.
Contributing Authors:
Enrique Coss-Adame: data acquisition, data analysis, manuscript writing and preparation.
Jessica Valestin: Subject recruitment, data acquisition
Amyra Ali-Azamar: subject recruitment, data acquisition
Jose M Remes-Troche: Study concept and design, subjects recruitment, supervision of data analysis, manuscript revision.
References
- 1.Drossman DA, Zhiming L, Andruzzi E, et al. US householder survey of functional gastrointestinal disorders: Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–1580. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 2.Talley NJ, O’Keefe EA, Zinsmeister AR, et al. Prevalence of gastrointestinal symptoms in the elderly: A population based study. Gastroenterology. 1992;102:895–901. doi: 10.1016/0016-5085(92)90175-x. [DOI] [PubMed] [Google Scholar]
- 3.Rao SSC, Patel RS. How useful are manometric tests of anorectal function in the management of defecation disorders? Am J Gastroenterol. 1997;92:469–475. [PubMed] [Google Scholar]
- 4.Rao SS, Singh S. Clinical utility of colonic and anorectal manometry in chronic constipation. J Clin Gastroenterol. 2010;44:597–609. doi: 10.1097/MCG.0b013e3181e88532. [DOI] [PubMed] [Google Scholar]
- 5.Rao SS, Ozturk R, Laine L. Clinical utility of diagnostic tests for constipation in adults: a systematic review. Am J Gastroenterol. 2005;100:1605–1615. doi: 10.1111/j.1572-0241.2005.41845.x. [DOI] [PubMed] [Google Scholar]
- 6.Azpiroz F, Enck P, Whitehead WE. Anorectal functional testing. Review of a collective experience. Am J Gastroenterol. 2002;97:232–240. doi: 10.1111/j.1572-0241.2002.05450.x. [DOI] [PubMed] [Google Scholar]
- 7.Rao SS, Meduri K. What is necessary to diagnose constipation? Best Pract Res Clin Gastroenterol. 2011;25:127–140. doi: 10.1016/j.bpg.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wald A. Colonic and anorectal motility testing in clinical practice. Am J Gastroenterol. 1994;89:2109–2115. [PubMed] [Google Scholar]
- 9.Rao SSC, Hatfield R, Soffer E, Rao S, Beaty J, Conklin JL. Manometric tests of anorectal function in healthy adults. Am J Gastroenterol. 1999;94:773–783. doi: 10.1111/j.1572-0241.1999.00950.x. [DOI] [PubMed] [Google Scholar]
- 10.Diamant ND, Kamm MA, Wald A, Whitehead WE. AGA technical review on anorectal testing techniques. Gastroenterology. 1999;116:735–760. doi: 10.1016/s0016-5085(99)70195-2. [DOI] [PubMed] [Google Scholar]
- 11.Rao SSC, Diamant N, Enck P, et al. Current methods of performing anorectal manometry (ARM) – an inter-center comparison. Gastroenterology. 1999;116:G4633. [Google Scholar]
- 12.Rao SSC, Azpiroz F, Diamant ND, et al. Minimal standards of anorectal manometry. Neurogastroenterol Motil. 2002;14:553–559. doi: 10.1046/j.1365-2982.2002.00352.x. [DOI] [PubMed] [Google Scholar]
- 13.Jones MP, Post J, Crowell MD. High-resolution manometry in the evaluation of anorectal disorders: a simultaneous comparison with water-perfused manometry. Am J Gastroenterol. 2007;102(4):850–855. doi: 10.1111/j.1572-0241.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 14.Noelting J, Ratuapli SK, Bharucha AE, et al. Normal values for high-resolution anorectal manometry in healthy women: effects of age and significance of rectoanal gradient. Am J Gastroenterol. 2012;107:1530–1536. doi: 10.1038/ajg.2012.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheeney G, Remes-Troche JM, Attaluri A, Rao SS. Investigation of anal motor characteristics of the sensorimotor response (SMR) using 3-D anorectal pressure topography. Am J Physiol Gastrointest Liver Physiol. 2011;300:G236–G240. doi: 10.1152/ajpgi.00348.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheeney G, Nguyen M, Valestin J, Rao SS. Topographic and manometric characterization of the recto-anal inhibitory reflex. Neurogastroenterol Motil. 2012;24:e147–e154. doi: 10.1111/j.1365-2982.2011.01857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raizada V, Bhargava V, Karsten A, Mittal RK. Functional morphology of anal sphincter complex unveiled by high definition anal manometery and three dimensional ultrasound imaging. Neurogastroenterol Motil. 2011;23:1013–1914. doi: 10.1111/j.1365-2982.2011.01782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talley NJ, Phillips SF, Wiltgen CM, et al. Assessment of functional gastrointestinal disease: the bowel disease questionnaire. Mayo Clin Proc. 1990;65:1456–1479. doi: 10.1016/s0025-6196(12)62169-7. [DOI] [PubMed] [Google Scholar]
- 21.Rao SS, Mudipalli RS, Stessman M, Zimmerman B. Investigation of the utility of colorectal function tests and Rome II criteria in dyssynergic defecation (Anismus) Neurogastroenterol Motil. 2004;16:589–596. doi: 10.1111/j.1365-2982.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 22.Mittal RK, Sheean G, Padda BS, et al. The external anal sphincter operates at short sarcomere length in humans. Neurogastroenterol Motil. 2011:643–e258. doi: 10.1111/j.1365-2982.2011.01700.x. [DOI] [PubMed] [Google Scholar]
- 23.Rao SS, Kavlock R, Rao S. Effect of body position and stool characteristics on defecation in humans. Am J Gastroenterol. 2006;101:2790–2796. doi: 10.1111/j.1572-0241.2006.00827.x. [DOI] [PubMed] [Google Scholar]
- 24.Bharucha AE, Rao SS. An update on anorectal disorders for gastroenterologist. Gastroenterology. 2014;146:37–45. doi: 10.1053/j.gastro.2013.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.