Abstract
Background
Stellate ganglion nerve activity (SGNA) is important in cardiac arrhythmogenesis. However, direct recording of SGNA requires access to the thoracic cavity. Skin of upper thorax is innervated by sympathetic nerve fibers originating from the stellate ganglia (SG) and is easily accessible.
Objective
To test the hypothesis that thoracic skin nerve activity (SKNA) can be used to estimate SGNA.
Methods
We recorded SGNA and SKNAs using surface electrocardiogram leads in 5 anesthetized and 4 ambulatory dogs. Apamin injected into the right SG abruptly increased both right SGNA and SKNA in 5 anesthetized dogs. We integrated nerve activities and averaged heart rate in each one-min window over 10 min. We implanted a radiotransmitter to record left SGNA in 4 ambulatory dogs, including two normal dogs, one dog with myocardial infarction and one dog with intermittent rapid atrial pacing. After 2 weeks of recovery, we simultaneously recorded the SKNA and left SGNA continuously for 30 min when the dogs were ambulatory.
Results
There was a positive correlation (average r=0.877, 95% confidence interval (CI) 0.732 to 1.000, p<0.05 for each dog) between integrated SKNA (iSKNA) and SGNA (iSGNA) and between iSKNA and heart rate (average r=0.837, 95% CI 0.752 to 0.923, p<0.05). Similar to that found in the anesthetized dogs, there was a positive correlation (average r=0.746, 95% CI 0.527 to 0.964, p<0.05) between iSKNA and iSGNA and between iSKNA and heart rate (average r=0.706, 95% CI 0.484 to 0.927, p<0.05).
Conclusions
SKNAs can be used to estimate SGNA in dogs.
Keywords: autonomic nervous system, sympathetic nerve, stellate ganglion, cardiac arrhythmia
Introduction
Sympathetic tone is important in cardiac arrhythmogenesis.1 Previous studies have shown that directly recorded stellate ganglion nerve activity (SGNA) immediately precedes heart rate acceleration and spontaneous cardiac arrhythmias in ambulatory dogs,1 suggesting that SGNA may be useful in cardiac arrhythmia prediction and risk stratification. Because direct recording from the stellate ganglion requires thoracotomy, it is difficult to record SGNA from humans. The skin of the canine upper thorax is extensively innervated by sympathetic nerves from the stellate ganglion.2 We3 recently reported that subcutaneous nerve activity (SCNA) recorded by bipolar subcutaneous electrodes in the thorax correlated well with the SGNA and heart rate in ambulatory dogs. Therefore, SCNA can be used to estimate the sympathetic tone. However, a skin incision is still needed for implanting the subcutaneous electrodes. For clinical applications for cardiovascular risk stratification, it is highly desirable to develop a completely non-invasive method for direct skin sympathetic nerve activity recording. We hypothesize that it is possible to record skin nerve activity (SKNA) from the surface of the skin in dogs, and that the SKNA recorded from the upper chest wall can be used to estimate the SGNA. The purpose of the present study was to develop a method for SKNA recording and to compare the SKNA with SGNA and with heart rate. The results were used to test the hypothesis that SKNA can be used to estimate SGNA in both anesthetized and ambulatory dogs.
Methods
The animal protocol was approved by the Institutional Animal Care and Use Committee of the Indiana University School of Medicine and the Methodist Research Institute, Indianapolis, IN, and conformed to the Guide for Care and Use of Laboratory Animals. A total of 9 dogs were studied.
Protocol 1: Right SGNA and SKNA in anesthetized dogs
Five dogs (A, B, C, D, E) were used in this protocol. The first two dogs were also used for SCNA recording and those results were included in a previous report.3 The dogs were intubated and underwent isoflurane general anesthesia. Thoracotomy was performed through the right 3rd intercostal space and the hair on the thoracic skin was removed. A pair of bipolar electrodes was inserted under the fascia of the right stellate ganglion. Another pair of bipolar electrodes with the interelectrode distance of 4 cm was inserted into the subcutaneous tissues of the right 3rd intercostal space for SCNA recording. Electrocardiogram (ECG) patches (Tyco/Healthcare Kendall, Medi-Trace 100, Hampshire, U.K.) were secured on the skin using adhesive tapes for surface ECG and SKNA recording. Two pairs of those ECG patch electrodes were taped on the skin to record ECG Leads I and II, along with SKNA. Lead I was recorded between electrodes at the level of the 2nd rib with an interelectrode distance of 22 cm (Figure 1A). Lead II was recorded between electrodes on right second rib and the left lower abdomen, with an interelectrode distance of 48 cm (Figure 1A). An additional patch was secured to the right lower abdomen to serve as ground.
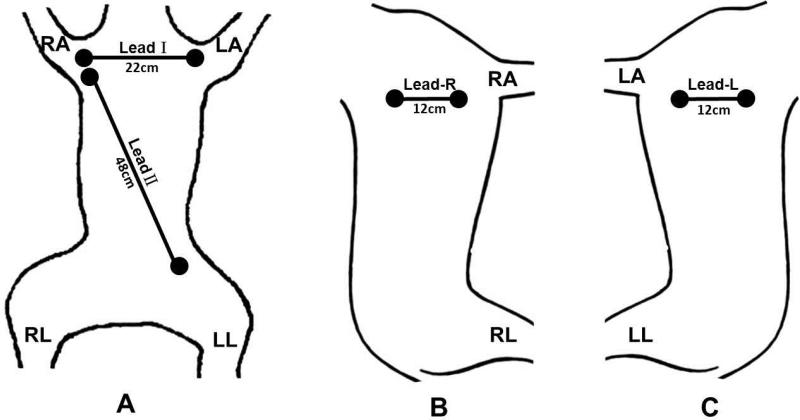
Figure 1.
Schematics of electrode location on the surface of the skin showing dogs in supine (A), left decubitus (B) and right decubitus (C) positions. In A, the two electrodes for Lead I recording were placed on the second rib and were 22 cm apart (11 cm from the midline). The Lead II electrodes were separated by 48 cm. B shows the location of the right bipolar electrode pair for recording SKNA-R. C shows the location of the left bipolar electrode pair for recording SKNA-L.
To explore whether or not other locations on the chest wall can also be used for SKNA recording, we placed one pair of bipolar electrodes each at the level of the right and left 3rd rib in dogs B and C, respectively, to form bipolar electrodes with 12 cm interelectrode distance (Figures 1B and 1C). In dogs D and E, we moved these bipolar electrodes downwards to the lower 1/3rd of the chest to determine if SKNA from the lower chest can also be used for recording sympathetic nerve activity. These electrodes were connected to a World Precision Instrument Iso-Damm-8 amplifier (Sarasota, Florida), with a noise level of < ±2.5 μV and a recording bandwidth set at 10 Hz-3 KHz. The signals were digitized by Digidata 1400a using AxoScope software (Sunnyvale, Calif) at 10,000 times per second per channel. After all surgical procedures were performed, the anesthetic agents were switched from isoflurane to alpha- chloralose (up to 100 mg/kg) and morphine. We then injected 1 ml apamin (concentration 0.2 ng/μL) directly into the right stellate ganglion. Apamin, a neurotoxin, is a specific blocker of the small conductance calcium activated K (SK) channel.4 Inhibition of the SK channel is known to facilitate neuronal discharges.3, 5 Data were acquired for 10 min after apamin injection.
Protocol 2: Left SGNA and SKNA in ambulatory dogs
Four dogs (F, G, H, I) were used in this protocol. All four dogs were chronically instrumented for different research protocols. The non-invasive recording made in this study does not affect the results of those research protocols, but helps reduce the use of animals. The dogs underwent left thoracotomy through the third intercostal space. A DSI (Data Sciences International, St. Paul, MN) D70EEE radiotransmitter was implanted to record the left SGNA (LSGNA) and the subcutaneous ECG according to methods reported elsewhere.3 Dogs F and G were normal dogs and did not undergo other procedures. Dog H had undergone left thoracotomy and left circumflex coronary artery ligation to create myocardial infarction. These dogs were allowed to recover for 2 weeks after the initial surgery before they were studied. Dog I had undergone a modified Secura implantable cardioverter-defibrillator (Medtronic, Minneapolis, MN) implantation for intermittent rapid left atrial pacing in an attempt to induce paroxysmal AF according to methods reported elsewhere.6 That dog was allowed to recover for 2 weeks from surgery, and was then paced intermittently for 8 weeks. However, it was in sinus rhythm when used in the present study. At the time of the study, all wounds had healed and the dogs were ambulatory. After clipping the hair on the chest, we placed 4 ECG patches on the skin to record surface ECG Leads I and II according to the methods described in Protocol 1. An additional two pairs of bipolar electrodes were placed on the upper 1/3rd of the left and right thorax for bipolar ECG recordings. Soft, non-adhesive elastic bands were wrapped around the chest to help secure the ECG patches in place. The locations of surface ECG patch electrodes were the same as shown in Figure 1. These skin electrodes were connected to the same equipment as described in Protocol 1. A DSI RLA3000 Mini “wand” Receiver was used to transmit the signals from the implanted DSI D70EEE radiotransmitter to the same Digidata 1400a equipment. The data from skin and the D70EEE were digitized simultaneously at 10,000 times per s per channel (same as in Protocol 1). Continuous recordings were made for 30 min while the dog was awake and lying or standing in the dog run. Hand clapping and speaking among the investigators were used to encourage sympathetic nerve discharges.
Data Analysis
We analyzed recordings using custom written DSIView software. The same ECG signals were used for both SKNA and ECG analyses. We performed quantitative analyses by integrating SGNA (iSGNA), SKNA (iSKNA), SCNA (iSCNA) min by min. In Protocol 1, the signals of SKNAI were low-pass filtered at 100 Hz to display the surface ECG for heart rate analyses. The signals of SKNA-I, SCNA and that of right SGNA (RSGNA) were either high-pass filtered (at 150 Hz, 300 Hz, 500Hz or 700 Hz) or wavelet filtered7 to display nerve activities. The wavelet transform7 is a time–frequency analysis and signal coding tool favored for the interrogation of complex non-stationary signals. In our application, we used discrete wavelet filtering based on a standard wavelet (Daubechies wavelet, db06) to remove the ECG, and kept the remnant aperiodic, non-stationary components to represent the nerve activities. In addition, fast Fourier transform (FFT) integration analyses were used to analyze the correlation between SKNA-I and RSGNA with the integrated magnitude spectrum between 200 Hz – 1000 Hz from a 2-s recording. In Protocol 2, the signals of SKNA-I, SCNA and LSGNA were high-pass filtered at 150 Hz or wavelet filtered to display the nerve activity. The same signals of SKNA-I was also low-pass filtered at 100 Hz to display ECG for heart rate analyses. The data were reported as mean and 95% confidence interval (CI). Pearson correlation coefficients were calculated between heart rate, iSGNA, iSCNA and iSKNA. A p value of ≤0.05 was considered statistically significant.
Results
Protocol 1: Correlation between iSGNA and iSKNA after apamin injection
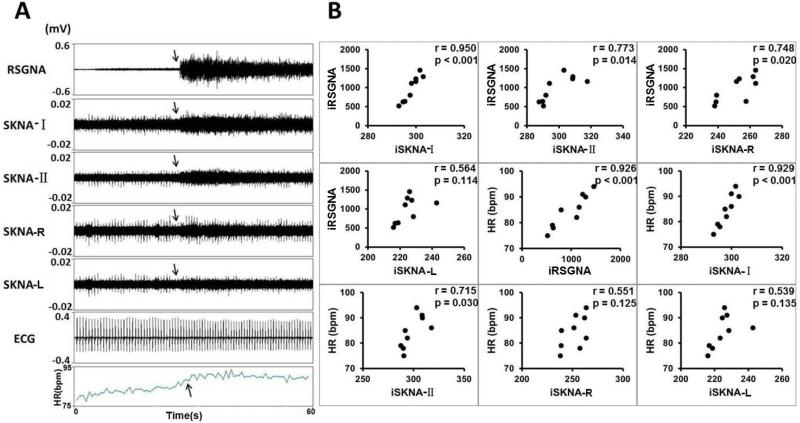
Apamin injection induced robust activity of SKNA and SGNA in all dogs studied. Figure 2A shows a typical recording from Dog D. Apamin-induced SGNA, SKNA and heart rate acceleration. Figure 2B shows the relationship among integrated right SGNA (iRSGNA), integrated SKNA recorded by ECG Lead I (iSKNA-I), ECG Lead II (iSKNA-II), right chest (iSKNA-R), left chest (iSKNA-L) and heart rate of the same dog. All of them strongly correlated with each other.
Figure 2.
Apamin increases nerve activities and heart rate (Protocol 1). A shows the actual recording made in Dog D after apamin injection, showing elevated heart rate (HR), along with the elevation of right stellate ganglion nerve activity (RSGNA), skin nerve activity from Lead-I (SKNA-I), skin nerve activity from Lead II (SKNA-II), skin nerve activity from right chest bipolar lead (SKNA-R) and left chest bipolar lead (SKNA-L). The ECG was from the same recording obtained from SKNA-I, but low pass filtered at 100 Hz. B shows correlation between integrated nerve activities recorded from different locations, and between integrated nerve activities and heart rate. Each filled circle represents the average of nerve activity and heart rate over a onemin window. A positive correlation was found in each comparison, but 3 of them (iRSGNA vs iSKNA-L, HR vs iSKNA-R and HR vs iSKNA-L) were statistically insignificant. The strongest correlation was found between iRSGNA and iSKNA- I, followed by the correlation between HR with iRSGNA and between HR and iSKNA-I.
Protocol 2: Monitoring of spontaneous SKNA, SGNA and heart rate in Ambulatory Dogs
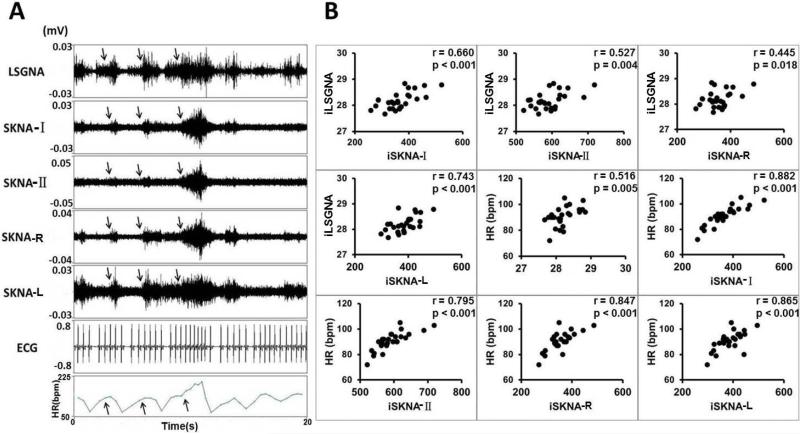
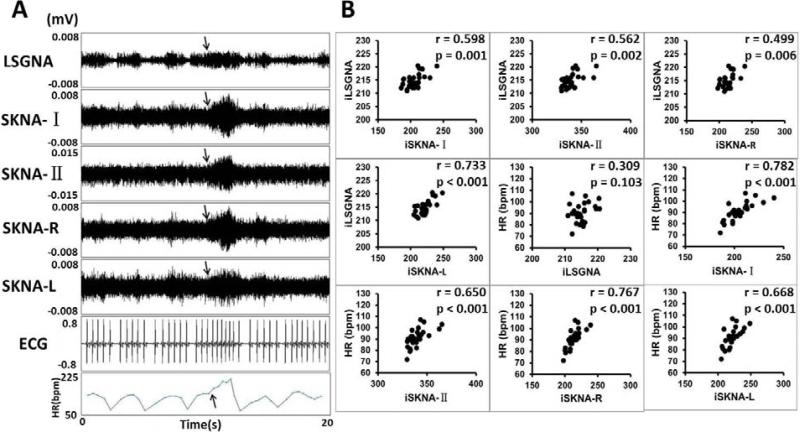
Simultaneous recording of SGNA and SKNAs was successful in all dogs studied. There were electrical signals resembling nerve activities on the surface of skin. During the recording period, the sound created by the investigators (speaking and clapping of the hands) or barking of other dogs in the same room often caused abrupt activation of the SGNA. Figure 3A shows a typical recording from dog I. The SGNA was associated with elevated heart rate and the SKNA at all locations. The arrow shows that largest heart rate acceleration associated with largest LSGNA and SKNA recordings. Figure 3B shows significantly positive correlations among integrated left SGNA (iLSGNA) and iSKNA-I, iSKNA-II, iSKNA-R, iSKNA-L and heart rate.
Figure 3.
SKNA in ambulatory dogs (Protocol 2). The recording came from Dog I. The LSGNA was recorded by DSI D70EEE radiotransmitter, while the remaining nerve activities and ECG were recorded by the Iso-Damm-8 amplifier. All data were then digitized simultaneously by Digidata 1400a. A shows increased LSGNA activity was associated with increased SKNA and heart rate (arrows). B shows positive correlations among the nerve activities and the heart rate. The p values of all correlations were < 0.05. The best correlation was that between iLSGNA and iSKNA-L.
Table 1 shows the correlation coefficients and the p values of all dogs studied. As shown in this table, there is consistently a strong positive correlation (mean 0.877) found between iSGNA and iSKNA-I in all dogs of Protocol 1. A good correlation (mean 0.746) was found between iSGNA and iSKNA-I in Protocol 2. Both right and left integrated SGNA (iRSGNA and iLSGNA) correlated well with the ipsilateral integrated SKNA (iSKNA-R and iSKNA-L) respectively. All other correlations were positive and mostly statistically significant.
Table 1.
Correlations among nerve activities measured at different sites and the heart rate
| Protocol 1. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dog# | iRSGNA vs. iSKNA-I | iRSGNA vs. iSKNA-II | iRSGNA vs. iSKNA-R | iRSGNA vs. iSKNA-L | HR vs. iRSGNA | HR vs. iSKNA-I | HR vs. iSKNA-II | HR vs. iSKNA-R | HR vs. iSKNA-L |
| A | r = 0.948 | 0.933 | NA | NA | 0.834 | 0.886 | 0.859 | NA | NA |
| p <0.001 | <0.001 | 0.005 | 0.001 | 0.003 | |||||
| B | r = 0.749 | 0.729 | 0.864 | 0.715 | 0.875 | 0.823 | 0.907 | 0.725 | 0.586 |
| p =0.020 | 0.026 | 0.003 | 0.031 | 0.002 | 0.006 | 0.001 | 0.027 | 0.097 | |
| C | r = 0.985 | 0.881 | 0.951 | 0.885 | 0.867 | 0.783 | 0.933 | 0.600 | 0.583 |
| p <0.001 | 0.002 | <0.001 | 0.002 | 0.002 | 0.013 | <0.001 | 0.088 | 0.099 | |
| D | r = 0.950 | 0.773 | 0.748 | 0.564 | 0.926 | 0.929 | 0.715 | 0.551 | 0.539 |
| p <0.001 | 0.014 | 0.020 | 0.114 | <0.001 | <0.001 | 0.030 | 0.125 | 0.135 | |
| E | r = 0.751 | 0.603 | 0.802 | 0.772 | 0.881 | 0.766 | 0.548 | 0.453 | 0.507 |
| p =0.020 | 0.086 | 0.009 | 0.015 | 0.002 | 0.016 | 0.126 | 0.221 | 0.163 | |
| Mean | 0.877 | 0.784 | 0.841 | 0.734 | 0.877 | 0.837 | 0.792 | 0.582 | 0.554 |
| 95%CI | (0.732, 1.000) | (0.622, 0.945) | (0.703, 0.980) | (0.521, 0.947) | (0.836, 0.918) | (0.752, 0.923) | (0.593, 0.992) | (0.402, 0.762) | (0.494, 0.614) |
| Protocol 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dog# | iLSGNA vs. iSKNA-I | iLSGNA vs. iSKNA-II | iLSGNA vs. iSKNA-R | iLSGNA vs. iSKNA-L | HR vs. iLSGNA | HR vs. iSKNA-I | HR vs. iSKNA-II | HR vs. iSKNA-R | HR vs. iSKNA-L |
| F | r = 0.820 | 0.607 | 0.728 | 0.788 | 0.647 | 0.801 | 0.537 | 0.809 | 0.769 |
| p <0.001 | 0.002 | <0.001 | <0.001 | 0.001 | <0.001 | 0.008 | <0.001 | <0.001 | |
| G | r = 0.803 | 0.604 | 0.510 | 0.463 | 0.591 | 0.499 | 0.482 | 0.510 | 0.463 |
| p <0.001 | 0.013 | 0.018 | 0.035 | 0.016 | 0.049 | 0.059 | 0.018 | 0.035 | |
| H | r = 0.540 | 0.546 | 0.339 | 0.506 | 0.421 | 0.759 | 0.789 | 0.644 | 0.352 |
| p =0.025 | 0.023 | 0.123 | 0.016 | 0.093 | <0.001 | <0.001 | 0.001 | 0.108 | |
| I | r = 0.819 | 0.772 | 0.760 | 0.912 | 0.574 | 0.763 | 0.776 | 0.749 | 0.627 |
| p <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Mean | 0.746 | 0.632 | 0.584 | 0.667 | 0.558 | 0.706 | 0.646 | 0.678 | 0.553 |
| 95%CI | (0.527, 0.964) | (0.477, 0.787) | (0.270, 0.899) | (0.321, 1.000) | (0.404, 0.712) | (0.484, 0.927) | (0.393, 0.899) | (0.469, 0.887) | (0.261, 0.844) |
HR, heart rate; NA, not available.
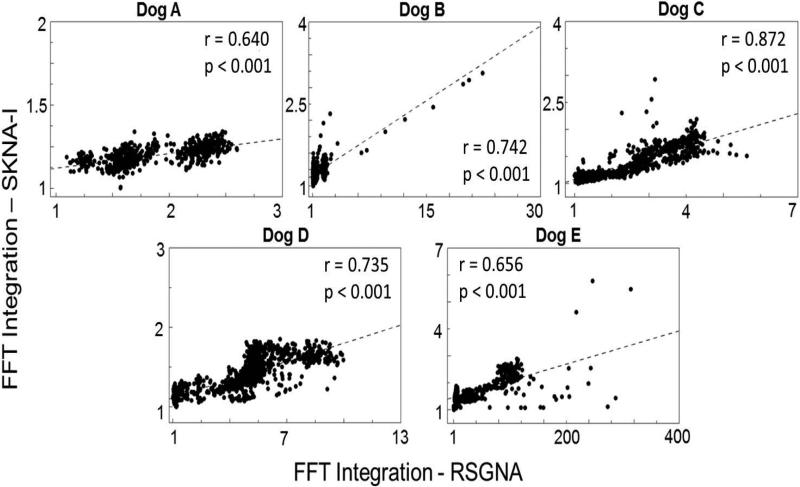
Correlation between iSGNA and iSKNA using FFT integration analyses
As shown in Figure 4, changes in the frequency content from the RSGNA recordings correlates to changes in the frequency content from the SKNA-I recordings in all dogs of Protocol 1. The correlation coefficients (mean 0.729, 95% CI 0.615 to 0.843) were significant (p<0.05) for all dogs studied.
Figure 4.
Correlation between integrated SKNA and integrated right SGNA (RSGNA) using FFT integration analyses (Protocol 1). Each dot represents the integrated magnitude spectrum between 200 Hz – 1000 Hz from the resulting FFT calculated from a 2-s recording.
Wavelet filtering and different frequencies of high-pass filter settings
We applied wavelet filtering to the same data shown in Figure 3. As shown in Figure 5A, wavelet filtering successfully removed the ECG artifacts but preserved the patterns of nerve activities. Figure 5B shows significant and positive correlations among filtered nerve activities and heart rate. Table 2 shows the correlation between iSKNA-I and iRSGNA, iSCNA or heart rate at different high pass filter settings. There were significant correlations between iSKNA-I and iRSGNA, between iSKNA and iSCNA and between iSKNA and heart rate at different high-pass filter settings. Table 3 shows the same correlations after the signals were filtered by the wavelet filter. The correlations were significant and positive for all dogs studied.
Figure 5.
SKNA with wavelet filter in ambulatory dog I (same raw data as that used in Figure 3). Panel A shows the nerve activities after wavelet filtering. Note the elimination of the surface ECG artifacts by wavelet filtering. Panel B shows the correlations among nerve activities and heart rate (HR). Significant and positive correlations are present.
Table 2.
Correlations with iSKNA-I using different high-pass filter settings in Protocol 1
| iRSGNA | iRSGNA | iRSGNA | iRSGNA | iSCNA | iSCNA | iSCNA | iSCNA | HR | HR | HR | HR | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dog# | 700HZ | 500HZ | 300HZ | 150HZ | 700HZ | 500HZ | 300HZ | 150HZ | 700HZ | 500HZ | 300HZ | 150HZ |
| A | r = 0.948 | 0.842 | 0.875 | 0.673 | 0.842 | 0.985 | 0.927 | 0.825 | 0.886 | 0.742 | 0.825 | 0.894 |
| p <0.001 | 0.002 | 0.001 | 0.033 | 0.002 | <0.001 | <0.001 | 0.003 | 0.001 | 0.014 | 0.003 | <0.001 | |
| B | r = 0.749 | 0.875 | 0.659 | 0.800 | 0.735 | 0.869 | 0.918 | 0.916 | 0.823 | 0.744 | 0.639 | 0.672 |
| p =0.020 | 0.002 | 0.038 | 0.010 | 0.024 | 0.002 | <0.001 | 0.001 | 0.006 | 0.022 | 0.046 | 0.047 | |
| C | r = 0.985 | 0.992 | 0.948 | 0.676 | 0.717 | 0.838 | 0.977 | 0.850 | 0.783 | 0.670 | 0.774 | 0.756 |
| p <0.001 | <0.001 | <0.001 | 0.046 | 0.030 | 0.005 | <0.001 | 0.004 | 0.013 | 0.048 | 0.009 | 0.018 | |
| D | r = 0.950 | 0.928 | 0.960 | 0.933 | 0.683 | 0.684 | 0.767 | 0.709 | 0.929 | 0.904 | 0.931 | 0.917 |
| p <0.001 | <0.001 | <0.001 | <0.001 | 0.042 | 0.042 | 0.016 | 0.032 | <0.001 | 0.001 | <0.001 | 0.001 | |
| E | r = 0.751 | 0.948 | 0.862 | 0.898 | 0.711 | 0.750 | 0.759 | 0.733 | 0.766 | 0.716 | 0.749 | 0.673 |
| p =0.020 | <0.001 | 0.003 | 0.001 | 0.032 | 0.020 | 0.018 | 0.025 | 0.016 | 0.030 | 0.020 | 0.047 | |
| Mean | 0.877 | 0.917 | 0.861 | 0.796 | 0.738 | 0.825 | 0.870 | 0.807 | 0.837 | 0.755 | 0.784 | 0.782 |
| 95%CI | (0.732, 1.000) | (0.843, 0.990) | (0.711, 1.000) | (0.646, 0.946) | (0.662, 0.814) | (0.682, 0.968) | (0.746, 0.994) | (0.701, 0.913) | (0.752, 0.923) | (0.645, 0.865) | (0.651, 0.916) | (0.636, 0.929) |
HR, heart rate
Table 3.
Correlations with iSKNA-I using wavelet filter.
| Protocol 1 | Protocol 2 | ||||||
|---|---|---|---|---|---|---|---|
| Dog# | iRSGNA | iSCNA | HR | Dog# | iLSGNA | iSCNA | HR |
| A | r = 0.637 | 0.855 | 0.692 | F | r = 0.609 | 0.505 | 0.693 |
| p =0.047 | 0.002 | 0.027 | p <0.001 | 0.014 | <0.001 | ||
| B | r = 0.703 | 0.697 | 0.784 | G | r = 0.569 | 0.581 | 0.516 |
| p =0.030 | 0.037 | 0.012 | p = 0.021 | 0.003 | 0.041 | ||
| C | r = 0.733 | 0.700 | 0.785 | H | r = 0.455 | 0.868 | 0.732 |
| p =0.025 | 0.036 | 0.012 | p = 0.034 | <0.001 | <0.001 | ||
| D | r = 0.817 | 0.697 | 0.850 | I | r = 0.598 | 0.744 | 0.782 |
| p =0.007 | 0.037 | 0.004 | p = 0.001 | <0.001 | <0.001 | ||
| E | r = 0.909 | 0.783 | 0.706 | ||||
| p =0.001 | 0.013 | 0.034 | |||||
| Mean | 0.760 | 0.746 | 0.763 | 0.558 | 0.675 | 0.681 | |
| 95%CI | (0.629, 0.891) | (0.658, 0.835) | (0.683, 0.844) | 0.445, 0.670) | (0.415, 0.934) | (0.497, 0.865) | |
CI, confidence interval; HR, heart rate
Discussion
The main novel finding is that it is feasible to record sympathetic nerve activities from the surface of the skin. This method is completely non-invasive, thus can facilitate the clinical studies of cardiac sympathetic tone in humans. We also found that among the nerve activities registered from all recording leads, the SKNA-I most accurately estimated the SGNA.
Correlation between SKNA and SGNA
Stellate (cervicothoracic) ganglion is a sympathetic ganglion formed by the fusion of the inferior cervical ganglion and the first thoracic ganglion, and is known to be an important source of cardiac sympathetic innervation.8 Extensive clinical studies have shown that the left stellate ganglion is important in cardiac arrhythmogenesis, and that left stellate ganglion ablation is antiarrhythmic in patients with cardiac arrhythmias.9-12 Our previous studies have documented a direct relationship between SGNA and cardiac arrhythmias in ambulatory dogs.6, 13-15 In addition to being major sources of cardiac sympathetic innervation, stellate ganglia also gives rise to sympathetic nerves that innervate blood vessels and sweat glands in skin.2 We3 have recently showed that it is feasible to record SCNA from ambulatory dogs continuously over long periods of time. We extended the latter observations in the present study by documenting the feasibility of directly recording sympathetic nerve activities from the surface of the skin, and that SKNA correlated well with SGNA and SCNA. Because SCNA can be used to predict susceptibility to ventricular tachycardia and fibrillation in a canine model of ventricular arrhythmia and sudden cardiac death,16 it is possible that SKNA can also be used for that arrhythmia prediction and risk stratification.
Methods of signal filtering
The electrical signals on the skin can come from multiple different sources, including the heart, the skeletal muscle, the subcutaneous nerves and the motion artifacts. Therefore, the high pass filter setting may play an important role in determining the sensitivity and specificity of the SKNA recordings. A major source of electrical signal on the skin is ECG, which has frequencies below 150 Hz. That frequency was used as the standard low pass filter setting for ECG recordings.3 A second important source of electrical signal is the skeletal muscle activity. McAuley et al17 reviewed and summarized approximately 30 published reports on electromyogram (EMG) frequency distribution during muscle vibration and tremor in distal limb of healthy subjects. They found that the frequency peaks are always < 100 Hz. Most of the muscle activities are associated with much lower frequency (between 10 and 50 Hz). Komi and Tesch18 studied the EMG of 11 male physical education students using equipment that has a frequency range of 10 to 10,000 Hz. They found that EMG of non-fatigued muscle peaked at < 100 Hz, with small amount of the activities reaching nearly 400 Hz. The fatigued muscles generate much narrower frequency spectrum and does not typically reach 200 Hz. The vibration of muscle spindles can reach 200 Hz.19 A high pass filtering of >150 Hz eliminates most of the ECG and EMG signals, thus increases the specificity of SKNA. However, it also eliminates some real nerve signals that have frequencies below 150 Hz.20 Further increases of the high pass filter setting will further increase the specificity but in the meantime reduce the sensitivity of SKNA recordings. In the present study, we tested multiple different high pass filter settings including that of 700 Hz, which is typically used for microneurography studies.21 We found significant correlations between SKNA and SGNA at all high pass filter settings. These findings suggest that 150 Hz high pass filter setting may have sufficient sensitivity and specificity in recording SKNA. An alternative method of filtering the signal is to use the wavelet filter. We showed that using this alternative filtering method, significant correlations are maintained among nerve activities and heart rate in all dogs studied.
SKNA-I may be the best surrogate for SGNA
In addition to filter setting, the location of the recording electrodes may be important in detecting the SKNA. We implanted four pairs of bipolar electrodes (leads I, II, right and left) on the surface of the skin to record SKNAs. All dogs (9/9) showed a significant correlation between iSKNA-I and iRSGNA or iLSGNA. Eight (8/9) dogs showed a strong correlation between iSKNA-II and iRSGNA or iLSGNA. Seven (7/8) dogs showed a good correlation between iSKNA-R or iSKNA-L and iRSGNA or iLSGNA. All dogs (9/9) showed a significant correlation between iSKNA-I and heart rate. Seven dogs (7/9) showed a strong correlation between iSKNA-II and heart rate. Five (5/8) dogs showed a good correlation between iSKNA-R and heart rate and three (3/8) dogs showed a good correlation between iSKNA-L and heart rate. These data suggest that SKNA-I may be the best recording lead to estimate the SGNA and the cardiac sympathetic tone. A good correlation between iSGNA and ipsilateral iSKNA is compatible with the finding that the skin sympathetic innervation came from ipsilateral stellate ganglion.22 However, because the left and right stellate ganglia in dogs usually fires simultaneously,23 it is not surprising to see that the integrated SKNA recorded from any location on the chest correlated positively with both right and left SGNA.
Clinical Implications
Our results support the feasibility of simultaneous recording the ECG and SKNA from the skin surface using the same ECG patch electrodes. The same signals are low-pass filtered for ECG signals and high-pass or wavelet filtered for SKNA signals. These techniques may be useful in non-invasive clinical investigations of sympathetic tone in humans.
Limitations
The correlations between SGNA and SKNA for all combinations appear to be stronger in Protocol 1 than Protocol 2. A possible explanation is that the equipment used to record SGNA in Protocol 1 had much higher frequency bandwidth than D70EEE transmitter used in Protocol 2. The D70EEE radiotransmitters are adequate in recording the large nerve discharges associated with the abrupt onset of sinus tachycardia or other atrial tachyarrhythmias, but often miss the smaller changes of nerve discharges associated with transient shortening of RR interval.15 The weaker correlation is likely the result of insufficient frequency content of the D70EEE, which reduced the sensitivity in detecting nerve signals.
Clinical Perspectives.
Sympathetic nerve activity is a critical modulator of cardiovascular function. Studies in ambulatory animals showed that stellate ganglion nerve activity (SGNA) is important in cardiac arrhythmogenesis. In this manuscript, we disclose a novel method for direct measurement of sympathetic nerve activity by recording skin nerve activity (SKNA) using standard ECG electrodes. The SKNA and ECG can be recorded simultaneously from the skin surface using the same ECG patch electrodes. SKNA recorded from the Lead I ECG and from other bipolar surface ECG leads can be used to estimate the SGNA and cardiac sympathetic tone. Direct recording of SGNA requires access to the thoracic cavity. Therefore, it is difficult to directly record SGNA in human subjects. In our recent study, we have found that subcutaneous nerve activity (SCNA) can be used to estimate the sympathetic tone and SGNA. However, a skin incision or puncture is still needed for implanting the subcutaneous electrodes. The methods of SKNA recording are non-invasive, thus can be easily translated to humans. Before the discoveries are translated into clinical practice, the equipment used to record SKNA should be improved. A portable and convenient device is needed for long-term and continuous SKNA recording. In addition, clinical trials should be performed to verify the relationship between SKNA and cardiac arrhythmogenesis in humans. The results of the latter studies will help determine how much information SKNA adds beyond the heart rate (or heart rate variability) in estimating the sympathetic tone.
Acknowledgments
We thank Nicole Courtney, Jessica Warfel and Christopher Corr for their assistance. We thank Ju Mei, MD, for his support of Drs Jiang and Yuan.
Sources of Funding
The study is funded by the NIH grants R01 HL71140, P01 HL78931, R21 HL106554, R41HL12474, a Medtronic-Zipes Endowment and the Indiana University Health-Indiana University School of Medicine Strategic Research Initiative.
Abbreviations
- CI
confidence interval
- DSI
Data Sciences International
- ECG
electrocardiogram
- EMG
electromyogram
- FFT
fast Fourier transform
- HR
heart rate
- SCNA
subcutaneous nerve activity
- SG
stellate ganglia
- SGNA
stellate ganglion nerve activity
- SK
small conductance calcium activated K
- SKNA
skin nerve activity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting galley proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Peng-Sheng Chen and Shien-Fong Lin have equity interest in Arrhythmotech, LLC. These observations were used in part to support a patent application filed by the Indiana University.
Conclusions: It is feasible to record sympathetic nerve activities from the surface of skin. SKNAs can be used to estimate SGNA in dogs.
References
- 1.Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res. 2014;114:1500–1515. doi: 10.1161/CIRCRESAHA.114.303772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taniguchi T, Morimoto M, Taniguchi Y, Takasaki M, Totoki T. Cutaneous distribution of sympathetic postganglionic fibers from stellate ganglion: A retrograde axonal tracing study using wheat germ agglutinin conjugated with horseradish peroxidase. J Anesth. 1994;8:441–449. doi: 10.1007/BF02514624. [DOI] [PubMed] [Google Scholar]
- 3.Robinson EA, Rhee KS, Doytchinova A, et al. Estimating Sympathetic Tone by Recording Subcutaneous Nerve Activity in Ambulatory Dogs. J Cardiovasc Electrophysiol. 2014 doi: 10.1111/jce.12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adelman JP, Maylie J, Sah P. Small-Conductance Ca(2+)-Activated K(+) Channels: Form and Function. Annu Rev Physiol. 2012;74:245–269. doi: 10.1146/annurev-physiol-020911-153336. [DOI] [PubMed] [Google Scholar]
- 5.Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nat Rev Neurosci. 2003;4:573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- 6.Tan AY, Zhou S, Ogawa M, Song J, Chu M, Li H, Fishbein MC, Lin SF, Chen LS, Chen PS. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008;118:916–925. doi: 10.1161/CIRCULATIONAHA.108.776203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Addison PS. Wavelet transforms and the ECG: a review. Physiol Meas. 2005;26:R155–199. doi: 10.1088/0967-3334/26/5/R01. [DOI] [PubMed] [Google Scholar]
- 8.Armour JA. Functional anatomy of intrathoracic neurons innervating the atria and ventricles. Heart Rhythm. 2010;7:994–996. doi: 10.1016/j.hrthm.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Moss AJ, McDonald J. Unilateral cervicothoracic sympathetic ganglionectomy for the treatment of long QT interval syndrome. N. Engl. J. Med. 1971;285:903–904. doi: 10.1056/NEJM197110142851607. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz PJ, Priori SG, Cerrone M, et al. Left cardiac sympathetic denervation in the management of high-risk patients affected by the long-QT syndrome. Circulation. 2004;109:1826–1833. doi: 10.1161/01.CIR.0000125523.14403.1E. [DOI] [PubMed] [Google Scholar]
- 11.Wilde AA, Bhuiyan ZA, Crotti L, Facchini M, De Ferrari GM, Paul T, Ferrandi C, Koolbergen DR, Odero A, Schwartz PJ. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N.Engl.J.Med. 2008;358:2024–2029. doi: 10.1056/NEJMoa0708006. [DOI] [PubMed] [Google Scholar]
- 12.Vaseghi M, Rn Msn Np JG, Kanaan C, Ajijola OA, Marmureanu A, Mahajan A, Shivkumar K. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: Intermediate and long-term follow-up. Heart Rhythm. 2014;11:360–366. doi: 10.1016/j.hrthm.2013.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogawa M, Zhou S, Tan AY, Song J, Gholmieh G, Fishbein MCLH, Siegel RJ, Karagueuzian HS, Chen LS, Lin SF, Chen PS. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. J Am Coll Cardiol. 2007;50:335–343. doi: 10.1016/j.jacc.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 14.Zhou S, Jung BC, Tan AY, Trang VQ, Gholmieh G, Han SW, Lin SF, Fishbein MC, Chen PS, Chen LS. Spontaneous stellate ganglion nerve activity and ventricular arrhythmia in a canine model of sudden death. Heart Rhythm. 2008;5:131–139. doi: 10.1016/j.hrthm.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Choi E-K, Shen MJ, Han S, Kim D, Hwang S, Sayfo S, Piccirillo G, Frick K, Fishbein MC, Hwang C, Lin S-F, Chen P-S. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation. 2010;121:2615–2623. doi: 10.1161/CIRCULATIONAHA.109.919829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doytchinova A, Patel J, Zhou S, Chen LS, Lin H, Shen C, Everett IV TH, Lin S-F, Chen P-S. Subcutaneous Nerve Activity and Spontaneous Ventricular Arrhythmias in Ambulatory Dogs. Heart Rhythm. doi: 10.1016/j.hrthm.2014.11.007. http://www.sciencedirect.com/science/article/pii/S154752711401265X. [DOI] [PMC free article] [PubMed]
- 17.McAuley JH, Rothwell JC, Marsden CD. Frequency peaks of tremor, muscle vibration and electromyographic activity at 10 Hz, 20 Hz and 40 Hz during human finger muscle contraction may reflect rhythmicities of central neural firing. Exp Brain Res. 1997;114:525–541. doi: 10.1007/pl00005662. [DOI] [PubMed] [Google Scholar]
- 18.Komi PV, Tesch P. EMG frequency spectrum, muscle structure, and fatigue during dynamic contractions in man. Eur J Appl Physiol Occup Physiol. 1979;42:41–50. doi: 10.1007/BF00421103. [DOI] [PubMed] [Google Scholar]
- 19.Roll JP, Vedel JP, Ribot E. Alteration of proprioceptive messages induced by tendon vibration in man: a microneurographic study. Exp Brain Res. 1989;76:213–222. doi: 10.1007/BF00253639. [DOI] [PubMed] [Google Scholar]
- 20.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. 2010;90:513–557. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- 21.Victor RG, Leimbach WN, Jr., Seals DR, Wallin BG, Mark AL. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension. 1987;9:429–436. doi: 10.1161/01.hyp.9.5.429. [DOI] [PubMed] [Google Scholar]
- 22.Baron R, Janig W, With H. Sympathetic and afferent neurones projecting into forelimb and trunk nerves and the anatomical organization of the thoracic sympathetic outflow of the rat. J Auton Nerv Syst. 1995;53:205–214. doi: 10.1016/0165-1838(94)00171-f. [DOI] [PubMed] [Google Scholar]
- 23.Jung BC, Dave AS, Tan AY, Gholmieh G, Zhou S, wang DC, Akingba G, Fishbein GA, Montemagno C, Lin SF, Chen LS, Chen PS. Circadian variations of stellate ganglion nerve activity in ambulatory dogs. Heart Rhythm. 2006;3:78–85. doi: 10.1016/j.hrthm.2005.09.016. [DOI] [PubMed] [Google Scholar]