Abstract
Background and Purpose
Recent symptoms stand as a major determinant of stroke risk in carotid stenosis patients, likely reflective of atherosclerotic plaque destabilization. In view of emerging links between vascular and adipose biology, we hypothesized that human perivascular adipose characteristics associate with carotid disease symptom status.
Methods
Clinical history, carotid plaques, blood, and subcutaneous and perivascular adipose tissues were prospectively collected from patients undergoing carotid endarterectomy (CEA). Nine adipose associated biologic mediators were assayed and compared in patients with symptomatic (n=15) versus asymptomatic (n=19) disease. Bonferroni correction was performed for multiple testing (α/9=0.006).
Results
Symptomatic patients had 1.9-fold higher perivascular adiponectin levels (p=0.005). Other circulating, subcutaneous, and perivascular biomarkers, as well as microscopic plaque characteristics, did not differ between symptomatic and asymptomatic patients.
Conclusions
Symptomatic and asymptomatic CEA patients display a tissue-specific difference in perivascular adipose adiponectin. This difference, which was not seen in plasma or subcutaneous compartments, supports a potential local paracrine relationship to vascular disease processes which may relate to stroke mechanisms.
Keywords: Carotid artery stenosis, Perivascular adipose tissue, Adipokine, Atherosclerosis
Introduction
Symptomatic status in carotid stenosis confers a 26% two-year stroke risk without surgical intervention.1 Plaque destabilization is thought to account for these differences when degrees of stenosis are comparable; however underlying mechanisms are not well-understood.2
Perivascular adipose tissue is increasingly recognized for its active role in cell-cell signaling, modulation of smooth muscle function, remodeling, and inflammation.3 In mice, transplantation of visceral adipose tissue to the carotid leads to impaired endothelial function and atherogenesis.4 Circulating plasma adipokine levels correlate with carotid intima-media thickness (CIMT) and symptom status in humans.5-6
Theorizing complex signaling interplay among subcutaneous/perivascular adipose tissues and the adjacent carotid, we investigated our hypothesis that specific adipose-related biomarkers would uniquely link to clinical features of CEA patients.
Methods
Study Participants and Data Collection
Patients undergoing CEA for symptomatic or asymptomatic carotid stenosis7 at a single institution in 2013 provided written informed consent for prospective collection of demographic, clinical, and duplex ultrasonography data under a Partners Human Research Committee IRB-approved protocol. All patients underwent conventional CEA via a longitudinal arteriotomy.
Sample Procurement and Protein Assay
At the time of surgery, peripheral blood, subcutaneous, and perivascular tissues were harvested. After protein isolation as previously described,8 adiponectin, interleukin (IL)-1β, IL-6, IL-8, leptin, monocyte chemoattractant protein (MCP)-1, plasminogen activator inhibitor (PAI)-1, resistin, and tumor necrosis factor (TNF) were quantified via Luminex multiplex assay (Supplemental methods).
Histology
Random plaque sections were prepared by conventional histologic methods and stained with hematoxylin/eosin, Masson's trichrome, and immunohistochemical anti-CD68 staining (Ventana Medical Systems, Inc., Tucson, AZ). Fibrous cap, necrotic core, angiogenesis, macrophage content, plaque hemorrhage and calcification were semi-quantitatively assessed by a blinded vascular pathologist.
Statistical Analysis
Categorical variables were compared using Fisher's exact testing. Continuous data were analyzed using Wilcoxon rank sum or the Student's t-test based on normality of distribution. Bonferroni correction was used for multiple comparisons. All statistical analyses were conducted using SAS software, v9.3 (SAS Institute, Inc., Cary, NC.)
Results
Nineteen patients with asymptomatic carotid stenosis and 15 patients with symptomatic disease were enrolled. Both groups were similar in terms of baseline characteristics including prior TIA/stroke unrelated to the current carotid lesion, antiplatelet therapy, statin use, and carotid artery peak systolic velocity. CEA represented primary intervention in all patients, none of whom had had prior cervical radiation therapy. Symptomatic patients displayed a variety of clinical manifestations (Table 1). BMI was similar between groups. Spearman correlation revealed a trend toward a negative correlation between perivascular adiponectin and BMI (r = -0.38, p = 0.03) which was not significant after Bonferroni correction, as well as a significant positive correlation between leptin and BMI (r = 0.59, p = 0.0003).
Table 1.
Baseline patient characteristics.
| Asymptomatic (n=19) | Symptomatic (n=15) | P-value | |
|---|---|---|---|
| Mean age (SD) | 71(6.64) | 68(8.92) | 0.34* |
| Female (%) | 8(42) | 5(33) | 0.73 |
| Race | |||
| White (%) | 18(95) | 15(100) | >0.99 |
| BMI (SD) | 28.7(3.8) | 28.8(4.9) | 0.97* |
| Comorbidities | |||
| Remote TIA/stroke (%) | 2(11) | 2(13) | >0.99 |
| Peripheral arterial disease (%) | 7(37) | 2(13) | 0.24 |
| Prior myocardial infarction (%) | 2(11) | 2(13) | >0.99 |
| Prior coronary intervention (%) | 5(26) | 4(27) | >0.99 |
| Heart failure (%) | 3(16) | 1(7) | 0.61 |
| Hypertension (%) | 18(95) | 15(100) | >0.99 |
| Hyperlipidemia (%) | 18(95) | 12(80) | 0.29 |
| Diabetes mellitus (%) | 4(21) | 3(20) | >0.99 |
| Renal insufficiency, Cr≥2 (%) | 1(5) | 0(0) | >0.99 |
| Smoking status | |||
| Never (%) | 5(26) | 5(33) | >0.99 |
| Current (%) | 4(21) | 2(13) | 0.67 |
| Preoperative medications | |||
| Aspirin (%) | 17(89) | 14(93) | >0.99 |
| Clopidogrel (%) | 2(11) | 2(13) | >0.99 |
| Warfarin (%) | 2(11) | 1(7) | >0.99 |
| Low molecular weight heparin (%) | 1(5) | 1(7) | >0.99 |
| Statin (%) | 18(95) | 15(100) | >0.99 |
| Beta blocker (%) | 14(74) | 12(80) | >0.99 |
| ACE-inhibitor (%) | 9(47) | 8(53) | >0.99 |
| Preoperative internal carotid stenosis | |||
| Mean PSV, cm/s (SD) | 393(114) | 402(139) | 0.84* |
| Degree of internal carotid stenosis | 0.37 | ||
| <49% (%) | 0 | 0 | |
| 50-69% (%) | 2(11) | 4(27) | |
| 70-99% (%) | 17(89) | 11(73) | |
| Carotid disease symptomatology | |||
| Stroke (%) | -- | 2(13) | -- |
| Hemispheric TIA (%) | -- | 5(33) | -- |
| Amaurosis fugax (%) | -- | 8(53) | -- |
| Days from symptoms to CEA (IQR) | -- | 30(7-106) | -- |
SD, standard deviation; TIA, transient ischemic attack; Cr, creatinine; ACE, angiotensin-converting enzyme; PSV, peak systolic velocity; IQR, interquartile range
P-value obtained by Student's t-test due to normal distribution. Others obtained by non-parametric testing.
Comparison of symptomatic versus asymptomatic patients revealed that the former had 1.9-fold higher perivascular adiponectin (p=0.005) (Figure 1; Supplemental Table I). This association was robust, remaining statistically significant after Bonferroni adjustment (α/9=0.006). Symptomatic patients demonstrated trends toward: (1) plasma IL-1β decrease (2.7-fold, p=0.008), (2) subcutaneous adiponectin elevation (1.5-fold, p=0.04) and PAI-1 decrease (2-fold, p=0.01), and (3) perivascular IL-1β elevation (1.4-fold, p=0.015) (Supplemental Tables I-III).
Figure 1.
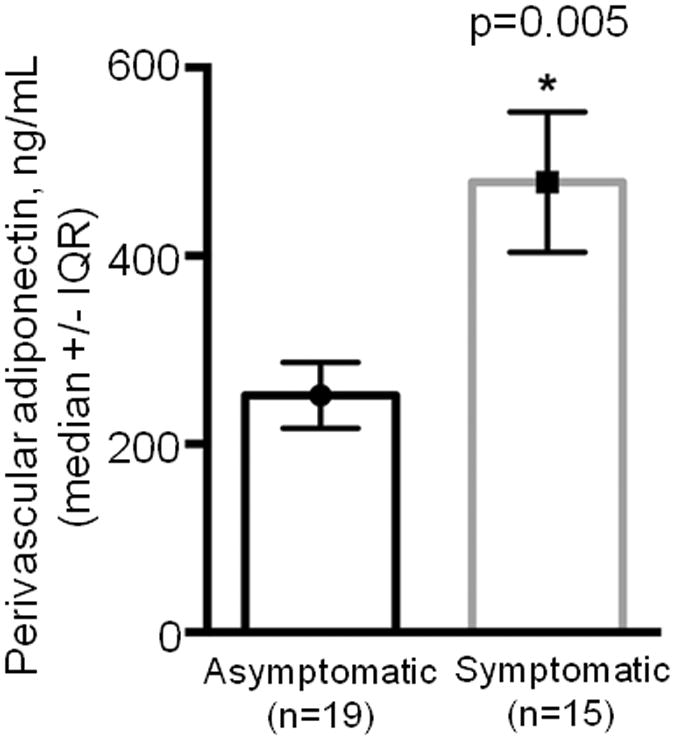
Adiponectin levels in perivascular tissue of patients with carotid stenosis. IQR, interquartile range
Endarterectomy specimen pathologic scoring did not differ between symptomatic and asymptomatic patients (Figure 2; Supplemental Table IV). There were no significant differences between perivascular mediator levels and histological characteristics.
Figure 2.
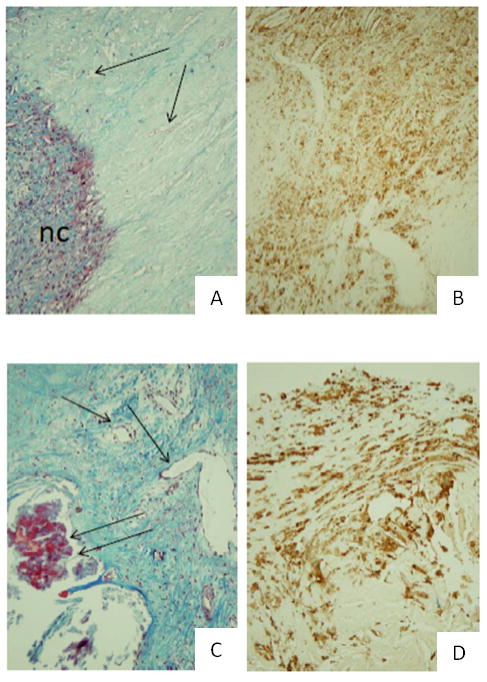
Representative histology of endarterectomy specimens from asymptomatic (A-B) and symptomatic (C-D) patients, stained with Masson trichrome (A,C) and CD68 (B,D). All 200×. nc, necrotic core; double arrows, calcification; single arrow, neovascularization
Discussion
Here we find that perivascular adiponectin expression differs based on symptom status in patients undergoing CEA. This differential expression profile was only present in the perivascular compartment—symptomatic patients had similar circulating adiponectin levels when compared to their asymptomatic counterparts, but had significantly higher adiponectin expression in tissue contiguous to the carotid artery. There were several other adipose-related biomarker trends between these small cohorts, further supporting potential signaling pathways between the vasculature and these tissues.
Little data exist on perivascular adiponectin in humans. In a prior report we did note decreased perivascular adiponectin compared to the subcutaneous compartment in major leg amputation specimens.9 Decreased plasma adiponectin levels have been found to be associated with increased coronary artery atherogenesis, plaque vulnerability, and CIMT.10-12 Here a seemingly paradoxical relationship was found between local perivascular adiponectin (traditionally viewed as a protective vascular mediator) and symptom status (a clinical marker of destabilized plaque).1-2 This may suggest disparate roles for local versus systemic adiponectin, or undefined effects of adiponectin.
In this cohort, pathologic characteristics of carotid plaques were similar between symptomatic and asymptomatic patients. The observation that perivascular expression of adiponectin was significantly associated with symptom status while plaque histology was not supports that the former association has a larger effect size. Note that carotid plaques are complex, and the microscopic analyses may suffer from sampling errors. Alternatively, local adiponectin expression and symptom development may be linked via plaque destabilization-independent mechanisms.
Limitations are acknowledged. In total we obtained single perivascular adipose samples from 34 patients. Assuming a type I error rate α equal to 0.05 and type II error rate β equal to 0.10 (90% power), our study is powered to detect effect sizes of 0.6; subtle differences in protein levels can be missed, and the interesting trends noted in the other mediators may be biologically noteworthy. Type I error (e.g. in the association seen between symptom status and perivascular adiponectin) was addressed by Bonferroni correction. Nearly all of our patients were Caucasian, which may limit the generalizability of our results. The duration of plaque-stabilizing medication use and compliance in each group were not clear from our source data. The current piece examines beyond the vessel wall to the surrounding adipose, thus detailed plaque morphology (complete plaque sectioning with search for intraplaque hemorrhage) was not the focus of the study since knowledge relating plaque characteristics and symptoms is widely accepted. We do include some basic plaque morphology data because it may relate to the adipose phenotype. Most importantly, precise mechanisms cannot be derived from this observational data, though the novel associations discovered should spur such important studies.
Conclusions
Symptomatic CEA patients exhibit two-fold higher local, perivascular adipose adiponectin levels. This novel link suggests previously unrecognized relationships among the neurologic, vascular, and adipose organs, and may lead to strategies to improve patient selection and other measures for stroke risk reduction.
Supplementary Material
Acknowledgments
Funding sources: This research was generously supported by NIH 5T32CA009535-26, 5T35HL110843, American Heart Association 12GRNT9510001/12GRNT1207025, Swiss National Science Foundation P1LAP3_158895, the Lea Carpenter du Pont Vascular Surgery Fund, and the Carl and Ruth Shapiro Family Foundation.
Disclosures: This investigator-initiated work represents a joint research venture between Brigham and Women's Hospital and Novartis Institutes, who provided a portion of the research expenses. Dr. C. Keith Ozaki is also supported by the American Heart Association Grant-in-Aid.
References
- 1.North American Symptomatic Carotid Endarterectomy Trial Collaborators (NASCET) Beneficial effect of CEA in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 2.Golledge J, Greenhalgh RM, Davies AH. The symptomatic carotid plaque. Stroke. 2000;31:774–781. doi: 10.1161/01.str.31.3.774. [DOI] [PubMed] [Google Scholar]
- 3.Britton KA, Fox CS. Perivascular adipose tissue and vascular disease. Clin Lipidol. 2011;6:79–91. doi: 10.2217/clp.10.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohman MK, Luo W, Wang H, Guo C, Abdallah W, Russo HM, et al. Perivascular visceral adipose tissue induces atherosclerosis in ApoE-/- mice. Atheroscl. 2011;219:33–9. doi: 10.1016/j.atherosclerosis.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin HJ, Park S, Yoon SJ, Choi DS, Cho DK, Kim JS, et al. Association between serum resistin and CIMT in hypertension patients. International Journal of Cardiology. 2008;125:79–84. doi: 10.1016/j.ijcard.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 6.Rubio-Guerra AF, Cabrera-Miranda LJ, Vargas-Robles H, Maceda-Serrano A, Lozano-Nuevo JJ, Escalante-Acosta BA. Correlation between levels of circulating adipokines and adiponectin/resistin index with CIMT in hypertensive type 2 diabetic patients. Cardiology. 2013;125:150–3. doi: 10.1159/000348651. [DOI] [PubMed] [Google Scholar]
- 7.Ricotta JJ, AbuRahma A, Ascher E, Eskandari M, Faries P, Lal BK. Updated SVS guidelines for management of extracranial carotid disease. JVS. 2011;54:e1–31. doi: 10.1016/j.jvs.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 8.Mauro CR, Nguyen BT, Yu P, Tao M, Gao I, Seidman MA, et al. Inflammatory “adiposopathy” in major amputation patients. Ann Vasc Surg. 2013;27:346–52. doi: 10.1016/j.avsg.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mauro CR, Ilonzo G, Nguyen BT, Yu P, Tao M, Gao I, et al. Attenuated adiposopathy in perivascular adipose tissue compared with subcutaneous human adipose tissue. Am J Surg. 2013;206:241–4. doi: 10.1016/j.amjsurg.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–6. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 11.Sawada T, Shite J, Shinke T, Otake H, Tanino Y, Ogasawara D, et al. Low plasma adiponectin levels are associated with presence of thin-cap fibroatheroma in men with stable coronary artery disease. Int J Cardiol. 2010;142:250–6. doi: 10.1016/j.ijcard.2008.12.216. [DOI] [PubMed] [Google Scholar]
- 12.Gardener H, Sjoberg C, Crisby M, Goldberg M, Mendez A, Wright CB, et al. Adiponectin and CIMT in the Northern Manhattan Study. Stroke. 2012;43:1123–5. doi: 10.1161/STROKEAHA.111.641761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


