Abstract
This study investigated the reduplicated, or repetitive vocalizations of hearing infants and infants with profound hearing loss with and without cochlear implants using a new measure of repetition in order to address questions not only about the effects of cochlear implantation on repetitive babbling, but also the reason that repetitive vocalizations occur at all and why they emerge around 7 to 8 months of age in hearing infants. Participants included 16 infants with profound hearing loss and 27 hearing infants who participated at a mean age of 9.9 months and/or a mean age of 17.7 months. Mean age at cochlear implantation for infants with profound hearing loss was 12.9 months and mean duration of implant use was 4.2 months. Before cochlear implantation, the data show repetitive vocalizations were rare. However, four months after cochlear implant activation, infants with hearing loss produced both repetitive vocalizations, and repetitions per vocalization, at levels commensurate with hearing peers. The results support the hypothesis that repetition emerges as a means of vocal exploration during the time that hearing infants (and infants with cochlear implants) form auditory-motor representations and neural connections between cortical areas active in syllable production and syllable perception, during the transition from non-linguistic to linguistic vocalization.
Keywords: cochlear implant, consonant-vowel repetition, reduplicated babbling, infant vocalization, auditory feedback, hearing loss
Hearing infants1 regularly produce characteristic strings of repetitive syllables (e.g., [dadada]) during a peak period in the second half of the first year (Fagan, 2009; Locke, 1993; Mitchell & Kent, 1990; van der Stelt & Koopmans-van Beinum, 1986). These well-noted vocal behaviors emerge around 7 to 8 months of age on average and are widely recognized by parents and non-parents alike. Despite their wide recognition, the reason these syllable strings emerge at all remains puzzling after decades of investigation and varied hypotheses regarding their role in the context of infant development (Kent, 1984). Ideas about their emergence and function have included that they have little to no relationship to later language (Lenneberg, 1967), that repetitive vocalizations and limb movements occur as part of a general period of cyclic organization (Kent, 1984; Thelen, 1981), and that they contribute to motor coordination and control (Ejiri & Masataka, 2001). Little research has directly examined repetitive babbling in the context of infants’ general interest in generating auditory feedback from their own vocalizations or the developmental timing of repetitive babbling in the context of neurocognitive connections that form around the same time.
Infants who receive cochlear implants at an early age present opportunities to evaluate repetition in the context of access to auditory feedback, before and after cochlear implantation. Further, because strings of syllable repetition are uncommon in spoken English words (Crystal, 2003) and infants with profound hearing loss do not hear spoken input before cochlear implantation (Northern & Downs, 2002), vocalizations that occur soon after cochlear implantation can in part address the relative influence of auditory feedback and linguistic input on vocal behavior immediately following cochlear implantation. Thus, investigating repetitive vocalizations in infants with profound hearing loss before and after cochlear implantation will potentially address infants’ motivation to generate auditory feedback as the reason repetitive vocalizations occur at all, and the timing of their emergence in relation to the formation of cognitive representations as the reason that they occur when they do.
Repetitive Babbling in Hearing Infants
Although, canonical babbling is the term often used for single, well-formed consonant-vowel (CV) syllables (Oller, 2000) without regard to repetition, reduplicated (or repetitive) babbling is the term specifically used to describe vocalizations produced with two or more consecutive CV repetitions (e.g., [dadada]). Reduplicated babbling, a notable milestone in infant development, typically emerges around 7 or 8 months of age (Fagan, 2009; Mitchell & Kent, 1990; van der Stelt & Koopmans-van Beinum, 1986). Studies of both canonical and reduplicated vocalizations have typically focused on age of onset, using a proportional measure for canonical syllable onset and documented observations of repetitive vocalization for reduplicated babble onset.
However, a relatively new measure of repetition, the discrete number of repetitions per reduplicated vocalization, was first examined in a longitudinal study of hearing infants (Fagan, 2009). This measure showed that repetitions per vocalization rose abruptly, peaked at 9.5 months, and unexpectedly declined within two to three months of emergence. Repetitions per vocalization declined with the emergence of word production and, in fact, were infrequent in early words as well. Just 18% of 13-month-old infants’ first words contained two reduplicated syllables (e.g., [dada]; Winitz & Irwin, 1958; see also Vihman, 1996). Thus, although non-word strings of CV repetition peaked before the end of the first year, they continued to occur in smaller numbers.
Substantially increased motor control and flexibility enables infants to produce CV repetitions in the second half of the first year (Kent, 1981; Stark, 1978); however, infants did not continue to produce long strings of repetition in substantial numbers at older ages when they had even greater motor control. Thus, the reason that infants repeated CV syllables during the early interval and not at later ages cannot be explained by motor competence alone.
Repetitive Babbling: Effects of Hearing Loss
As with studies of hearing infants, studies of vocal development and hearing loss have typically focused on age of canonical syllable onset using proportional criteria (Eilers & Oller, 1994; Kent, Osberger, Netsell, & Hustedde, 1987; Oller & Eilers, 1988; Stoel-Gammon, 1988). Infants with hearing loss consistently produced fewer and later-emerging canonical syllables compared to hearing infants, despite otherwise typical motor development (Ertmer, Young, & Nathani, 2007; Kent et al., 1987; Oller & Eilers, 1988; Stoel-Gammon & Otomo, 1986; von Hapsburg & Davis, 2006). Both canonical syllable ratios and age of onset were closely related to degree of hearing loss, with infants with profound hearing loss showing comparatively greater delays than infants with mild to moderate hearing loss (Stoel-Gammon & Otomo, 1986; von Hapsburg & Davis, 2006).
Compared to hearing infants, reduplicated or repetitive babbling also emerges much later, if at all, in infants with hearing loss (Kent et al. 1987; Koopmans-van Beinum, Clement, & van den Dikkenberg-Pot, 2001; Oller & Eilers, 1988; Stoel-Gammon & Otomo, 1986). For example, one infant with profound hearing loss produced no repetitive CV vocalizations before 20 months (Kent et al., 1987) and another stopped producing reduplicated syllables with the onset of hearing loss at 8 months (Stoel-Gammon & Otomo, 1986). Thus, it is clear that production both of single well-formed syllables and multi-syllable repetition depend to some degree on audition for their development. In fact, when infants with hearing loss did produce repetitive syllables, it was attributed to residual hearing or benefit from amplification (Koopmans-van Beinum et al., 2001; Oller & Eilers, 1988).
For children with profound hearing loss, these documented patterns of delay have changed with the availability and use of cochlear implants. Even with relatively late cochlear implantation, at 4 ½ years of age, children showed general improvement in speech development after cochlear implantation (Ertmer et al., 2007; Tomblin, Peng, Spencer, & Lu, 2008). In some cases, for infants who were 11 to 29 months old at time of cochlear implantation, repetitive babbling was reported to emerge 5 to 7 months later (Kishon-Rabin, Taitelbaum-Swead, Ezrati-Vinacour, & Hildesheimer, 2005). However, emergence was determined only by a questionnaire administered to parents 2–16 months after cochlear implantation, when most infants were more than 2 years old.
Schauwers, Gillis, Daemers, De Beukelaer, and Govaerts (2004) determined babbling both in terms of canonical syllable ratios (Oller & Eilers, 1988) and the production of vocalizations with multiple articulatory movements—repetitive and non-repetitive. Production criteria for the latter measure required two qualifying utterances per session across three consecutive observation sessions. Infants in the study, who received cochlear implants between 6 and 18 months, met the criteria for both measures several months after cochlear implantation. Using similar criteria, infants who received cochlear implants at 9.5 months produced two instances of repetition 2–3 months after cochlear implant activation (Colletti et al., 2005). In these studies of hearing loss, however, mean number of CV repetitions per vocalization was not investigated. Measuring repetitions per vocalization in infants with hearing loss will allow quantitative measures of change in repetition, distinct both from non-repetitive canonical syllable ratios and criterion measures of predetermined numbers of repetitive utterances per session(s).
Cortical Connections and Benefits of Repetition
Relatively recent studies of infant brain activation patterns found that neural connections between cortical areas active in syllable production and syllable perception were formed during the prime period of vocal repetition in hearing infants, from 6 to 12 months (Imada, Zhang, Cheour, Taulu, Ahonen, & Kuhl, 2006). For example, cortical auditory and motor areas were activated when 7-month-old infants listened to native and non-native speech syllables (Kuhl, Ramirez, Bosseler, Lotus Lin, & Imada, 2014). Although infants had no previous exposure to non-native input, hearing infants often produce non-native sounds in early vocalizations (Locke, 1993). Presumably, the auditory-motor pathways documented in hearing infants (Kuhl et al., 2014; Imada et al., 2006) would not form during the same period in infants with profound hearing loss. However, if infants with cochlear implants explore and repeat syllables in the same way that hearing infants do, their vocalizations may contribute to the formation of such connections.
Self-generated vocalizations and repetitions provide infants with redundant, temporally synchronous, sensory feedback linking motor actions with auditory perceptual feedback. Arguably, this redundant feedback generated by infants themselves, facilitates stable representations of sensorimotor experience (Bruner, 1964; Lickliter and Bahrick, 2004; Thelen, 1981) that can be called up and flexibly combined to form later words and sentences. Rivière (2014) described these stable patterns as automatic responses linked to memories of past actions. The A-not-B developmental error is one example of an automatic response arising from past sensorimotor experience. However, motor actions that were widely variable and poorly controlled (e.g., early reaching; Diedrich, Thelen, Smith, & Corbetta, 2000), as early vocalizations are known to be, did not build strong motor memories (Clearfield, Diedrich, Smith, & Thelen, 2006).
Together, the striking regularity in the timing of repetitive vocalizations in hearing infants, neurocognitive events that occur during the same period (Imada et al., 2006), and emerging evidence of repetitive vocalizations after cochlear implantation must be considered in attempts to explain the role of CV repetition in infant development. This study will test the proposal that repetitive vocalizations, which differ from typical adult utterances (and from earlier infant vocalizations), are produced by infants to generate auditory feedback, feedback likely to contribute to the connections between cortical areas activated by syllable perception and production that form during the prime period of vocal repetition in hearing infants (Kuhl et al., 2014; Imada et al., 2006). If repetitive vocalizations are motivated by infants’ interest in auditory exploration, number of repetitions per vocalization should be uncommon before cochlear implantation and should increase following cochlear implantation. Evidence that infants with hearing loss produce repetitive vocalizations following access to hearing would support their potential usefulness in forming sensorimotor representations.
The purpose of this study is to test this hypothesis, in part, by using a new measure of vocal repetition—not previously used in infants with hearing loss—to study the effect of access to auditory feedback on vocal repetition. This measure, the specific number of sequential CV repetitions produced within each vocalization (Fagan, 2009) will be used to investigate the number of repetitions infants produce before and shortly after cochlear implantation in comparison to hearing infants. This study will also extend previous research by addressing questions regarding the timing, motivation, and role of self-generated CV repetition in infant development.
Method
Participants
Participants were 16 infants with profound hearing loss and 27 hearing infants. Infants with profound hearing loss (13 boys, 3 girls) were identified during newborn hearing screenings and participated in auditory intervention programs as part of the accepted protocol for cochlear implant candidacy (Heman-Ackah, Roland, Haynes, & Waltzman, 2012). They had no known developmental diagnoses other than hearing loss. Hearing infants (11 boys, 16 girls) passed newborn hearing tests and had no known developmental concerns.
For infants with hearing loss, mean age at cochlear implant surgery (11 unilateral, 2 simultaneous bilateral) was 12.9 months (SD = 2.3, range = 8–16 months); mean age at implant activation was 14.0 months (SD = 2.2, range = 10–17 months). All infants scored within the average range on the Motor Skills domain of the Vineland Adaptive Behavior Scales, Second Edition (M SS = 101.0, SD = 8.3; Sparrow, Cicchetti, & Balla, 2005). Participants in this study also contributed to a separate report on vocalization frequency alone (Fagan, 2014).
All infants participated at one of two time points, Time 1 (n = 26) or Time 2 (n = 31), with a subset of these infants (n = 14) contributing data to both time points. Independent samples t-tests were used to ensure no infant contributed more than one data point to each analysis. Mean infant age at Time 1 was 9.9 months (SD = 1.3); mean age at Time 2 was 17.7 months (SD = 2.9). Time 1 was scheduled to take place shortly before cochlear implantation for infants with hearing loss (Pre-CI); Time 2 took place 4.2 months (SD = 2.6) after cochlear implant activation (Post-CI). Age at Time 1 represents a central age in many studies of vocal development and Time 2 was scheduled to observe changes in behavior closely following cochlear implantation. Hearing infants (i.e., H1 and H2) were scheduled during the same age range as infants with hearing loss.
All infants were born to hearing parents who communicated with their infants primarily with spoken English. Mean years of education for mothers of infants with hearing loss was 14.3 years (SD = 2.9), and for mothers of hearing infants, 14.8 years (SD = 2.8). Four infants were biracial, 6 were African American, and 33 were Caucasian.
Procedure
Infants, seated on the lap of a caregiver, were video-recorded playing with two sets of 12 objects (e.g., rattle, car, ball) presented to infants one at a time for 30 seconds each (i.e., 12 minutes total). Following object presentation, parents engaged with infants for two additional minutes of unstructured interaction.
Infants wore a vest containing a small microphone and transmitter with wireless connection to a camera-mounted receiver. Audible infant vocalizations containing speech-like consonant (C) and/or vowel (V) sounds (e.g., [a, ba, baba]) were identified from the 14-minute videotapes by trained coders unfamiliar with the purpose of the study. Vocalizations were separated by an audible breath or brief silence, confirmed using acoustic analysis software. All other vocal behaviors (i.e., cry, laugh, growl, effortful grunt, raspberry, squeal, vegetative, and rare tongue clicks) were excluded from analysis for this study. Number of infant vocalizations and other vocal behaviors were previously reported elsewhere (Fagan, 2014).
For this study, speech-like vocalizations were first categorized generally as vowel only (V), consonant only (C), or CV vocalizations. Next, V vocalizations were further classified as fully resonant vowels (FV) or less mature quasi-resonant vowel (QV) vocalizations, and CV vocalizations were classified as marginal (loosely formed) syllables (MS), or as well-formed canonical syllables (CS), using descriptive vocal resonance criteria described by Oller (2000). A standard ratio for marking canonical syllable production (i.e., canonical syllables/total utterances ≥0.20; Oller & Eilers, 1988) was calculated for each infant.
Finally, CV non-word vocalizations containing two or more consecutive, perceptually equivalent CV repetitions were analyzed in terms of number of sequential CV units per vocalization (e.g., [dada] = 2). Word productions were uncommon in all but the H2 group; Pre-CI infants produced no words, H1 = 1, Post-CI = 8, and H2 = 386 words. Words containing CV repetition were also infrequent and excluded from analysis of repetition; only 10.6% of words contained CV repetition, consistent with previous research (Winitz & Irwin, 1958). Because Pre-CI infants produced fewer vocalizations than hearing infants at Time 1, statistical analyses were conducted using both behavior counts and proportions. The pattern of results did not differ; therefore, results based on behavior counts are presented.
To assess reliability, 13 randomly selected videotaped records (23%) were independently coded by a second trained coder. The intra-class correlation coefficient (ICC) for number of vocalizations identified was .97. Point by point percent agreement for vocalization type (i.e., V, C, CV) was 89.5%, and resonance type (i.e., QV, FV, MS, CS), 89.6%. Coders verified number of CV repetitions per vocalization in all files by mutual agreement (i.e., 100% agreement).
Results
General Vocalization Type
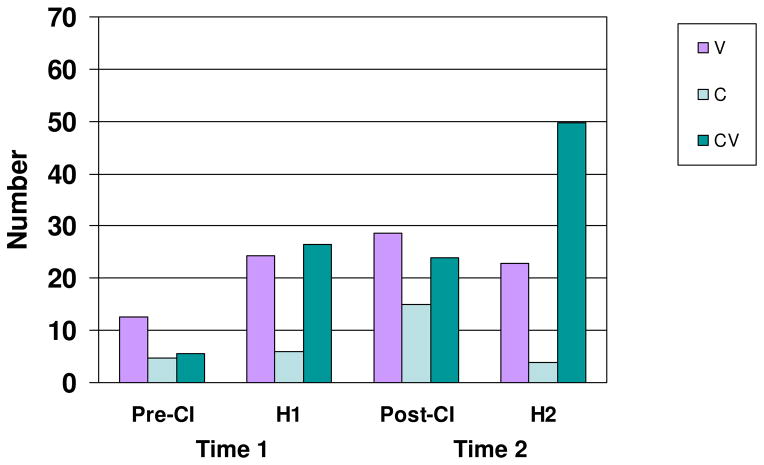
All vocalization types, categorized as V, C, and CV, were examined first. Paired-samples t-tests indicated, at Time 1, Pre-CI infants produced significantly more V vocalizations (M = 12.6) than CV vocalizations (M = 5.6), t(10) = 2.38, p < .05, d = 0.65; whereas, hearing infants (H1) produced V and CV vocalizations in similar numbers (M = 24.4 and 26.5, respectively), t(14) = 0.36, p = .72. H1 infants produced almost five times more CV vocalizations than Pre-CI infants, a significant difference, independent samples t(24) = 2.73, p < .01, d = 1.16. C vocalizations were comparatively infrequent. Figure 1 presents mean numbers of each vocalization type by group.
Figure 1.
Mean number of vowel (V), consonant (C), and consonant-vowel (CV) vocalizations by Group and Time. Pre-CI = before cochlear implantation; H1 = hearing infants at Time 1; Post-CI = after cochlear implantation; H2 = hearing infants at Time 2.
As shown in Figure 1, Post-CI production patterns resembled the production patterns of younger hearing infants (H1) in that CV and V vocalizations were produced in roughly equivalent numbers (M = 28.6 and 24.0, respectively), t(12) = 1.16, p = .26. In fact, Post-CI infants did not differ significantly from H1 infants in numbers of CV, t(26) = 0.28, p = .77; V, t(26) = 0.42, p = .67; or C vocalizations, t(26) = 1.01, p = .32.
Although Post-CI infants produced four times more CV vocalizations than Pre-CI infants, they still produced significantly fewer CV vocalizations than their H2 hearing peers, t(29) = 2.39, p < .05, d = 0.90. H2 infants produced more than twice as many CV as V vocalizations (49.7 vs. 22.8) at Time 2, the only group to produce CV vocalizations in significantly greater numbers than V vocalizations, t(17) = 2.99, p < .01, d = 1.01.
In summary, three patterns are notable in Figure 1: Pre-CI infants were unique in that they were the only group that produced primarily V vocalizations; Post-CI infants resembled younger hearing infants in their vocalization patterns; and even after cochlear implantation, infants with hearing loss produced significantly fewer CV vocalizations than hearing peers (H2).
Resonance Characteristics
Next, V and CV productions were examined for descriptive vocal resonance characteristics. Figure 2 presents mean numbers for each resonance type by group. As shown in Figure 2, the vowels predominant in early Pre-CI vocalizations (see Figure 1) were primarily less well-developed quasi-vowels (QV), and the rare CV syllables produced by Pre-CI infants were primarily marginal syllables (MS). None of the Pre-CI infants met the standard ratio (≥ 0.20) for marking the canonical syllable stage of vocal development (Oller & Eilers, 1988). However, 53% (n = 8) of H1 infants did. These data were consistent with previous research in showing canonical syllable production (i.e., individual canonical syllables) was rare in infants with hearing loss before cochlear implantation. For Post-CI (and also H1) infants, by contrast, canonical syllables were proportionally more than five times greater than before cochlear implantation (M = 0.22 vs. 0.04). At Time 2, 69% (n = 9) of Post-CI infants and 89% (n = 16) of H2 infants met or exceeded the standard canonical stage production ratio (≥ 0.20).
Figure 2.
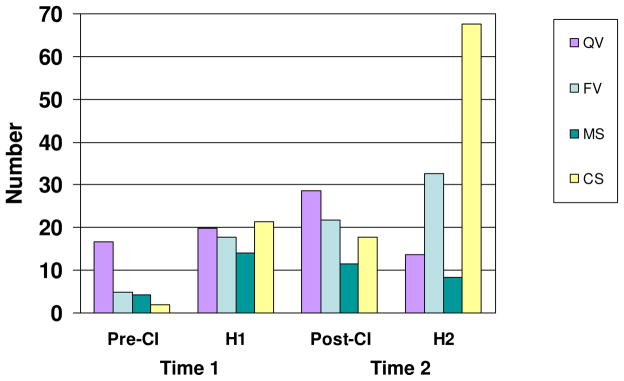
Mean number of quasi-vowel (QV), fully resonant vowel (FV), marginal syllable (MS), and canonical syllable (CS) vocal resonant types by Group and Time. Pre-CI = before cochlear implantation; H1 = hearing infants at Time 1; Post-CI = after cochlear implantation; H2 = hearing infants at Time 2.
Number of Repetitive CV Vocalizations
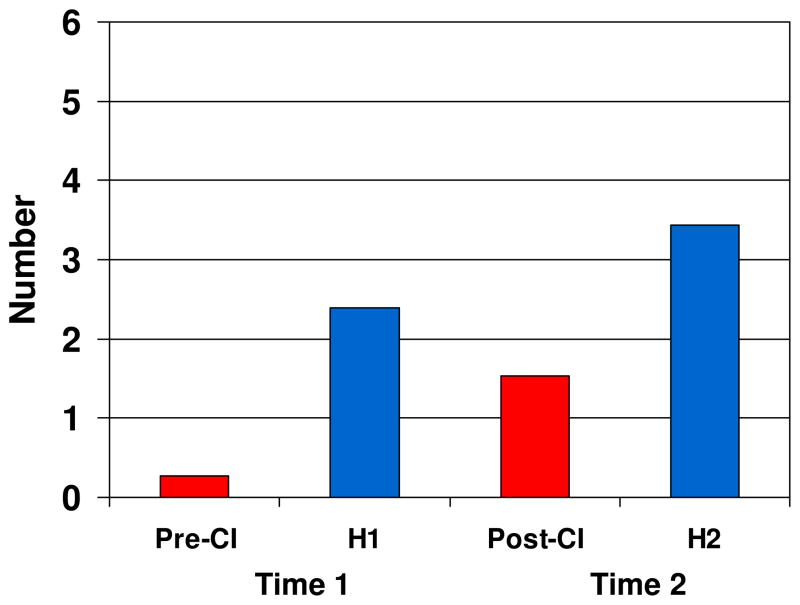
The occurrence of non-word vocalizations containing sequential CV repetitions (of any number) was examined next. At Time 1, compared to H1 infants, Pre-CI infants produced significantly fewer vocalizations containing CV repetition, t(24) = 2.18, p < .05, d = 0.94. However, at Time 2, although Post-CI infants produced fewer vocalizations with CV repetition than H2 infants did (M = 1.5, SD = 1.9 vs. M = 3.4, SD = 4.4), the difference was not statistically significant, t(29) = 1.44, p = .12, d = 0.55. Figure 3 shows mean number of vocalizations that contained CV repetition by group.
Figure 3.
Mean number of non-word vocalizations that contained consonant-vowel (CV) repetition by Group and Time. Pre-CI = before cochlear implantation; H1 = hearing infants at Time 1; Post-CI = after cochlear implantation; H2 = hearing infants at Time 2.
Individually, only two infants in the Pre-CI group (18%) produced any non-word vocalizations (n = 1; n = 2) containing CV repetition at Time 1, compared to nine (60%) H1 infants. At Time 2, however, seven (54%) Post-CI infants and 13 (72%) H2 infants produced vocalizations containing repetition. For all infants who produced repetitive CV vocalizations, the number of instances ranged from 1 to 21. The three Post-CI infants who had used their implants for the shortest amount of time (only 5–6 weeks) all produced CV repetitions. In summary, within four months of cochlear implantation, the number of infants with profound hearing loss who produced repetitive vocalizations more than tripled, and the number of repetitive vocalizations they produced increased more than five fold (Pre-CI M = 0.27, Post-CI M = 1.9), to levels statistically equivalent to H2 infants.
Number of CV Repetitions per Vocalization
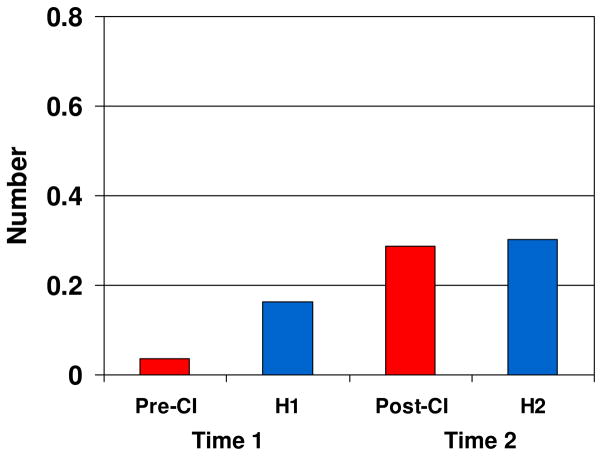
Finally, specific numbers of sequential syllable repetition per CV vocalization were examined. At Time 1, Pre-CI infants produced significantly fewer CV repetitions per vocalization (M = 0.037, SD = .08) than H1 infants did (M = .163, SD = .19), t(23) = 1.94, p < .05, d = 0.86. However, at Time 2, the difference in repetitions per CV vocalization was no longer significant, t(29) = .09, p = .92, d = 0.03. Figure 4 shows mean number of repetitions per non-word CV vocalization by group. Although p values were not corrected for multiple comparisons, significant results were supported by moderate to large effect sizes.
Figure 4.
Mean number of consonant-vowel (CV) repetitions per non-word CV vocalization by Group and Time. Pre-CI = before cochlear implantation; H1 = hearing infants at Time 1; Post-CI = after cochlear implantation; H2 = hearing infants at Time 2.
In summary, both measures of repetition—number of repetitive vocalizations, and repetitions per vocalization—increased to levels consistent with hearing peers within four months of cochlear implantation. This increase in both CV repetition measures was especially noteworthy given that Post-CI infants produced fewer CV vocalizations overall than H2 infants.
Of further note, total repetitive CV syllables across non-word vocalizations was significantly positively correlated with overall number of vocalizations, r = .45, p < .001, an indication that infants who vocalized frequently also tended to produce strings of CV repetition, underscoring the association between auditory access, vocalization, and repetition. Number of vocalizations containing repetition was not significantly correlated with chronological age (r = .10, p = .45), age at implant activation (r = −.13, p = .66), or months of cochlear implant use (r = −.29, p = .33) for infants in this study.
Discussion
This study investigated a widely recognized vocal behavior in infant development—vocalizations characterized by strings of repetitive syllables—by extending use of a new measure of CV repetition (Fagan, 2009) to infants with profound hearing loss before and after cochlear implantation. This measure, the specific number of sequential CV repetitions produced within each CV vocalization, was used to investigate effects of auditory feedback on CV repetition and address questions regarding the emergence and timing of reduplicated babbling in infant development.
Results indicated 9.9-month-old infants with profound hearing loss and average motor competence rarely if ever produced repetitive CV vocalizations before cochlear implantation. In fact, for 82% of the infants with profound hearing loss, repetition of CV syllables in reduplicated strings was non-existent before cochlear implantation. For the two remaining Pre-CI infants, CV repetition was rare; one infant produced a single utterance with syllable repetition, the other only two. However, the number of infants who produced repetitive vocalizations, the number of repetitive vocalizations they produced, and the number of CV repetitions per vocalization all increased significantly within four months of cochlear implant activation. Remarkably, only 5 to 6 weeks post-implant activation, infants who had used their implants for the shortest period of time produced strings of CV repetition. Infants with cochlear implants demonstrated the motivation to explore vocal repetition, as hearing infants did, even though they did not gain access to auditory feedback until 14 months of age.
In this study, vocal resonance results (non-repetitive) were consistent with earlier studies of hearing loss (in infants without cochlear implants) in showing infants with profound hearing loss rarely produced well-formed syllables (e.g., Ertmer et al., 2007), despite average motor competence. After cochlear implantation, vocal resonance results showing increased CV vocalizations and canonical syllables, extended previous research to younger infants with earlier cochlear implantation. Even after cochlear implantation, however, infants with hearing loss produced significantly fewer CV vocalizations than hearing peers (H2). The fact that they nevertheless produced similar numbers of repetitive vocalizations and similar numbers of CV repetition per vocalization, highlighted their interest in reduplicated babbling.
Recent research has also shown the sheer number of vocalizations produced by infants with cochlear implants tripled within 45 days of cochlear implant activation, further indication of infants’ interest in self-generated auditory feedback (Fagan, 2014)—both repetitive and non-repetitive. Moreover, number of repetitive syllables in non-word vocalizations in this study was significantly correlated with overall number of vocalizations, underscoring the role of auditory access in motivating both vocalization and repetition.
The Role of Repetition
Together, the rapidity with which repetitive vocalizations emerged following access to auditory feedback, the uncommon nature of syllable repetition in spoken English (Crystal, 2003), and largely inaccessible spoken input before cochlear implantation (Northern & Downs, 2002), support the argument that infants’ interest in generating repetitive auditory feedback motivated reduplicated babbling. Arguably, syllable repetition is an effective way for infants who can hear their own vocalizations to form auditory-motor representations of speech sounds and syllables (Bruner, 1964; Fogassi & Ferrari, 2007; Lickliter & Bahrick, 2004; Piaget, 1952). For hearing infants, reduplicated babbling emerges at a time in development when infants have the motor competence to produce stable controlled repetitions, the motivation to explore their own agency in generating sound (Palmer, 1989), and the apparent capacity to form neural connections evidenced by auditory-motor activation patterns (Imada et al., 2006; Kuhl et al., 2014). For infants with profound hearing loss, repetitive vocalizations emerged with access to cochlear implants, in infants apparently no less motivated than hearing infants to explore their own agency in generating syllable repetition.
Several relevant studies have provided indirect evidence supporting a role for repetitive CV vocalizations in facilitating motor exploration and cognitive representations. As an example, redundancy in recorded syllable sequences facilitated 6.5-month-old infants’ ability to recognize target syllables (Goodsitt, Morse, Ver Hoeve, & Cowan, 1984). Infants were more successful at recognizing a target syllable (e.g., [ba]) when it was presented with contrasting but redundant syllable pairs ([ko ba ko]) than when presented with mixed syllable pairs ([ko ba ti]). Although infants recognized target syllables under both conditions, the reduced complexity provided by redundant syllables facilitated infants cognitive processing of syllable strings.
Further, infants who did not yet regularly produce alveolar stops (i.e., [t]) at 10–12 months of age preferred listening to words that contained the alveolar stop than to words that did not (DePaolis, Vihman, & Nakai, 2013). However, infants who produced [t] frequently, instead preferred listening to words that contained a consonant they did not yet produce (i.e., [s]). Thus, infants beyond the typical age of reduplicated babble onset must have formed cognitive representations of their own regular motor production patterns and compared their representations to consonants they did not yet produce (DePaolis et al., 2013; DePaolis, Vihman, & Keren-Portnoy, 2011; Majorano, Vihman, & DePaolis, 2014). Infants’ selective listening showed they represented their own sensorimotor experience in an accessible way and that this sensorimotor experience influenced their subsequent behavior.
Experience-guided behavior has been documented in the manual modality as well. For example, both 9- and 11-, but not 7-month-old infants, who had manipulated an object subsequently looked longer at two adults manipulating the same object than a different object (Hauf, Aschersleben, & Prinz, 2007). Thus, as in studies of speech production described above, infants’ own perceptual motor experience influenced their attention to the behavior of others (DePaolis et al., 2013; see also Fogassi & Ferrari, 2007). It is noteworthy that 6- and 7-month-old infants (infants at the youngest ages in studies of reduplicated babbling and brain activation), apparently did not yet represent their motor experience in the same way that 9- and 11-month-old infants did (Hauf et al., 2007; Majorano et al., 2014) or, if they had, they did not seem to access the representations in the same way.
Other research has demonstrated the importance of self-generated action experience and perceptual-motor representations for early social-cognitive development (Daum, Prinz, & Aschersleben, 2011; Gerson & Woodward, 2014). Motor experience and the mirror neuron system are thought to have a role in enabling individuals to understand the actions of others and, potentially, to represent elements of speech (Fogassi & Ferrari, 2007; Kohler et al., 2002; Marshall, Young, & Meltzoff, 2011; Rizzolatti & Arbib, 1998; Vihman, 2002). Together these studies highlight the functional importance of infant experience in forming cognitive representations and accessing them to process the actions of others and shape their own future actions. These accessible sensorimotor plans explain in part why infants regularly produce their first words using consonants from established motor routines (Vihman, 1993), rather than with the consonants that are most frequent in mothers’ speech to infants (DePaolis et al., 2011; Majorano et al., 2014).
Rather than diminishing the role of linguistic input in infant development, this research raises awareness regarding infants’ role in development. Learning is dependent on infants’ interest in the environment and their motivation to obtain feedback not only from others, but also from their own exploratory activities. Infants’ motivation to explore sound surely contributes to the striking regularities observed in the emergence of repetitive babbling and may also help to ensure a degree of resilience when environmental input is less reliable (e.g., Kaplan, Bachorowski, Smoski, & Hudenko, 2002; Rea, Bonvillian, & Richards, 1988).
One limitation of the present study is that infants were observed at only one time point following cochlear implantation. However, measuring CV repetition using repetitions per vocalization documented the emergence of syllable repetition in a single session following cochlear implantation. Additional longitudinal research is needed to investigate infant vocalizations and vocalization reinforcement, as repetition may be reinforced by adults when it does occur, in infants with cochlear implants and in hearing infants.
In summary, although single vowels and CV syllables form the bulk of infant productions, the marked and regular occurrence of reduplicated syllables, even in comparatively small numbers, suggests they are interesting to infants, highly motivated and functional in infant development. Production of these controlled, repetitive vocalizations may contribute to the formation and strengthening of cortical connections active in speech perception and speech production—connections not yet evident only a month before the typical onset of reduplicated babbling in hearing infants. The relatively new and growing body of research on infants with cochlear implants has contributed to questions regarding the emergence and timing of reduplicated babbling by showing that production of repetitive syllable strings, almost nonexistent before cochlear implantation, is reliant on and almost immediately responsive to the auditory feedback infants discover in generating vocal repetition.
Highlights.
Infants with hearing loss produce fewer repetitive vocalizations than hearing peers
Four months after cochlear implantation, repetitions match those of hearing infants
Cochlear implants quickly influenced changes in repetition
Footnotes
Individuals with normal hearing thresholds.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bruner JS. The course of cognitive growth. American Psychologist. 1964;19:1–15. [Google Scholar]
- Clearfield MW, Diedrich FJ, Smith LB, Thelen E. Young infants reach correctly in A-not-B tasks: On the development of stability and perseveration. Infant Behavior & Development. 2006;29:435–444. doi: 10.1016/j.infbeh.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Colletti V, Carner M, Miorelli V, Guida M, Colletti L, Fiorino FG. Cochlear implantation at under 12 months: Report on 10 patients. Laryngoscope. 2005;115:445–449. doi: 10.1097/01.mlg.0000157838.61497.e7. [DOI] [PubMed] [Google Scholar]
- Crystal D. The Cambridge Encyclopedia of the English Language. 2. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- Daum MM, Prinz W, Aschersleben G. Perception and production of object-related grasping in 6-month-olds. Journal of Experimental Child Psychology. 2011;108:810–818. doi: 10.1016/j.jecp.2010.10.003. [DOI] [PubMed] [Google Scholar]
- DePaolis RA, Vihman MM, Keren-Portnoy T. Do production patterns influence the processing of speech in prelinguistic infants? Infant Behavior & Development. 2011;34:590–601. doi: 10.1016/j.infbeh.2011.06.005. [DOI] [PubMed] [Google Scholar]
- DePaolis RA, Vihman MM, Nakai S. The influence of babbling patterns on the processing of speech. Infant Behavior & Development. 2013;36:642–649. doi: 10.1016/j.infbeh.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Diedrich FJ, Thelen E, Smith LB, Corbetta D. Motor memory is a factor in infant perseverative errors. Developmental Science. 2000;3:479–494. [Google Scholar]
- Eilers RE, Oller DK. Infant vocalizations and the early diagnosis of severe hearing impairment. Journal of Pediatrics. 1994;124:199–203. doi: 10.1016/s0022-3476(94)70303-5. [DOI] [PubMed] [Google Scholar]
- Ejiri K, Masataka N. Co-occurrence of preverbal vocal behavior and motor action in early infancy. Developmental Science. 2001;4:40–48. doi: 10.4992/jjpsy.69.433. [DOI] [PubMed] [Google Scholar]
- Ertmer DJ, Young NM, Nathani S. Profiles of vocal development in young cochlear implant recipients. Journal of Speech, Language, and Hearing Research. 2007;50:393–407. doi: 10.1044/1092-4388(2007/028). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan MK. Mean length of utterance before words and grammar: Longitudinal trends and developmental implications of infant vocalizations. Journal of Child Language. 2009;36:495–527. doi: 10.1017/S0305000908009070. [DOI] [PubMed] [Google Scholar]
- Fagan MK. Frequency of vocalization before and after cochlear implantation: Dynamic effect of auditory feedback on infant behavior. Journal of Experimental Child Psychology. 2014;126:328–338. doi: 10.1016/j.jecp.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF. Mirror neurons and the evolution of embodied language. Current Directions in Psychological Science. 2007;16:136–141. [Google Scholar]
- Gerson SA, Woodward AL. Learning from their own actions: The unique effect of producing actions on infants’ action understanding. Child Development. 2014;85:264–277. doi: 10.1111/cdev.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodsitt JV, Morse PA, Ver Hoeve JN, Cowan N. Infant speech recognition in multisyllabic contexts. Child Development. 1984;55:903–910. [PubMed] [Google Scholar]
- Hauf P, Aschersleben G, Prinz W. Baby do—baby see! How action production influences action perception in infants. Cognitive Development. 2007;22:16–32. [Google Scholar]
- Heman-Ackah SE, Roland JT, Jr, Haynes DS, Waltzman SB. Pediatric cochlear implantation: Candidacy evaluation, medical and surgical considerations, and expanding criteria. Otolaryngologic Clinics of North America. 2012;45:41–67. doi: 10.1016/j.otc.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Imada T, Zhang Y, Cheour M, Taulu S, Ahonen A, Kuhl PK. Infant speech perception activates Broca’s area: A developmental magnetoencephalography study. NeuroReport. 2006;17:957–962. doi: 10.1097/01.wnr.0000223387.51704.89. [DOI] [PubMed] [Google Scholar]
- Kaplan PS, Bachorowski J, Smoski MJ, Hudenko WJ. Infants of depressed mothers, although competent learners, fail to learn in response to their own mothers’ infant-directed speech. Psychological Science. 2002;13:268–271. doi: 10.1111/1467-9280.00449. [DOI] [PubMed] [Google Scholar]
- Kent RD. Articulatory-acoustic perspectives on speech development. In: Stark RE, editor. Language behavior in infancy and early childhood. New York: Elsevier; 1981. pp. 105–126. [Google Scholar]
- Kent RD. Psychobiology of speech development: Coemergence of language and a movement system. American Journal of Physiology. 1984;246:R888–R894. doi: 10.1152/ajpregu.1984.246.6.R888. [DOI] [PubMed] [Google Scholar]
- Kent RD, Osberger MJ, Netsell R, Hustedde CG. Phonetic development in identical twins differing in auditory function. Journal of Speech and Hearing Disorders. 1987;52:64–75. doi: 10.1044/jshd.5201.64. [DOI] [PubMed] [Google Scholar]
- Kishon-Rabin L, Taitelbaum-Swead R, Ezrati-Vinacour R, Hildesheimer M. Prelexical vocalization in normal hearing and hearing-impaired infants before and after cochlear implantation and its relation to early auditory skills. Ear & Hearing. 2005;26:17S–29S. doi: 10.1097/00003446-200508001-00004. [DOI] [PubMed] [Google Scholar]
- Kohler E, Keysers C, Umilta MA, Fogassi L, Gallese V, Rizzolatti G. Hearing sounds, understanding actions: Action representation in mirror neurons. Science. 2002;297:846–848. doi: 10.1126/science.1070311. [DOI] [PubMed] [Google Scholar]
- Koopmans-van Beinum FJ, Clement CJ, van den Dikkenberg-Pot I. Babbling and the lack of auditory speech perception: A matter of coordination? Developmental Science. 2001;4:61–70. [Google Scholar]
- Kuhl PK, Ramirez RR, Bosseler A, Lotus Lin JF, Imada T. Infants’ brain responses to speech suggest analysis by synthesis. Proceedings of the National Academy of Sciences. 2014;111:11238–11245. doi: 10.1073/pnas.1410963111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenneberg EH. Biological foundations of language. New York: John Wiley & Sons; 1967. [Google Scholar]
- Lickliter R, Bahrick LE. Perceptual development and the origins of multisensory responsiveness. In: Calvert G, Spence C, Stein BE, editors. Handbook of multisensory processes. Cambridge, MA: MIT Press; 2004. pp. 643–654. [Google Scholar]
- Locke JL. The child’s path to spoken language. Cambridge, MA: Harvard University Press; 1993. [Google Scholar]
- Majorano M, Vihman MM, DePaolis RA. The relationship between infants’ production experience and their processing of speech. Language Learning and Development. 2014;10:179–204. [Google Scholar]
- Marshall PJ, Young T, Meltzoff AN. Neural correlates of action observation and execution in 14-month-old infants: An event-related EEG desynchronization study. Developmental Science. 2011;14:474–480. doi: 10.1111/j.1467-7687.2010.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PR, Kent RD. Phonetic variation in multisyllable babbling. Journal of Child Language. 1990;17:247–265. doi: 10.1017/s0305000900013751. [DOI] [PubMed] [Google Scholar]
- Northern JL, Downs MP. Hearing in Children. Philadelphia: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- Oller DK. The emergence of the speech capacity. Mahwah, NJ: Lawrence Erlbaum; 2000. [Google Scholar]
- Oller DK, Eilers RE. The role of audition in infant babbling. Child Development. 1988;59:441–449. [PubMed] [Google Scholar]
- Palmer CF. The discriminating nature of infants’ exploratory actions. Developmental Psychology. 1989;25:885–893. [Google Scholar]
- Piaget J. In: The origins of intelligence in children. Cook M, translator. New York: International Universities Press; 1952. [Google Scholar]
- Rea CA, Bonvillian JD, Richards HC. Mother-infant interactive behaviors: Impact of maternal deafness. American Annals of the Deaf. 1988;133:317–324. doi: 10.1353/aad.2012.0645. [DOI] [PubMed] [Google Scholar]
- Rivière J. Embodiment in children’s choice: Linking bodily constraints with decisional dynamics. Current Directions in Psychological Science. 2014;23:408–413. [Google Scholar]
- Rizzolatti G, Arbib MA. Language within our grasp. Trends in Neuroscience. 1998;21:188–194. doi: 10.1016/s0166-2236(98)01260-0. [DOI] [PubMed] [Google Scholar]
- Schauwers K, Gillis S, Daemers K, De Beukelaer C, Govaerts PJ. Cochlear implantation between 5 and 20 months of age: The onset of babbling and the audiologic outcome. Otology & Neurotology. 2004;25:263–270. doi: 10.1097/00129492-200405000-00011. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales. 2. Circle Pines, MN: American Guidance Service; 2005. [Google Scholar]
- Stark RE. Features of infant sounds: The emergence of cooing. Journal of Child Language. 1978;5:379–390. doi: 10.1017/s0305000900002051. [DOI] [PubMed] [Google Scholar]
- Stoel-Gammon C. Prelinguistic vocalizations of hearing-impaired and normally hearing subjects: A comparison of consonantal inventories. Journal of Speech and Hearing Disorders. 1988;53:302–315. doi: 10.1044/jshd.5303.302. [DOI] [PubMed] [Google Scholar]
- Stoel-Gammon C, Otomo K. Babbling development of hearing-impaired and normally hearing subjects. Journal of Speech and Hearing Disorders. 1986;51:33–41. doi: 10.1044/jshd.5101.33. [DOI] [PubMed] [Google Scholar]
- Thelen E. Rhythmical behavior in infancy: An ethological perspective. Developmental Psychology. 1981;17:237–257. [Google Scholar]
- Tomblin JB, Peng S, Spencer LJ, Lu N. Long-term trajectories of the development of speech sound production in pediatric cochlear implant recipients. Journal of Speech, Language, and Hearing Research. 2008;51:1353–1368. doi: 10.1044/1092-4388(2008/07-0083). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Stelt JM, Koopmans-van Beinum FJ. The onset of babbling related to gross motor development. In: Lindblom B, Zetterstrom R, editors. Precursors of early speech. New York: Stockton Press; 1986. pp. 163–173. [Google Scholar]
- Vihman MM. Vocal motor schemes, variation and the production-perception link. Journal of Phonetics. 1993;21:163–169. [Google Scholar]
- Vihman MM. Phonological development: The origins of language in the child. Oxford, England: Blackwell; 1996. [Google Scholar]
- Vihman MM. The role of mirror neurons in the ontogeny of speech. In: Stamenov M, Gallese V, editors. Mirror neurons and the evolution of brain and language. Amsterdam: John Benjamins; 2002. pp. 305–314. [Google Scholar]
- von Hapsburg D, Davis BL. Auditory sensitivity and the prelinguistic vocalizations of early-amplified infants. Journal of Speech, Language, and Hearing Research. 2006;49:809–822. doi: 10.1044/1092-4388(2006/057). [DOI] [PubMed] [Google Scholar]
- Winitz H, Irwin OC. Syllabic and phonetic structure of infants’ early words. Journal of Speech and Hearing Research. 1958;1:250–256. doi: 10.1044/jshr.0103.250. [DOI] [PubMed] [Google Scholar]