Abstract
Background and Purpose
Cerebral Amyloid Angiopathy (CAA) is a common cause of recurrent intracerebral hemorrhage (ICH) in the elderly. Previous studies have shown that CAA induces inflammation and expression of matrix metalloproteinase-2 and -9 (gelatinases) in amyloid-laden vessels. Here, we inhibited both using minocycline in CAA mouse models to determine if spontaneous ICH could be reduced.
Methods
Tg2576 (n=16) and 5×FAD/ApoE4 knock-in mice (n=16), aged to 17 and 12 months, respectively, were treated with minocycline (50 mg/kg, i.p.) or saline every other day for two months. Brains were extracted and stained with X-34 (to quantify amyloid), Perl’s blue (to quantify hemorrhage), and immunostained to examined Aβ load, gliosis (GFAP, Iba-1), and vascular markers of blood-brain-barrier integrity (ZO-1 and collagen IV). Brain extracts were used to quantify mRNA for a variety of inflammatory genes.
Results
Minocycline treatment significantly reduced hemorrhage frequency in the brains of Tg2576 and 5×FAD/ApoE4 mice relative to the saline-treated mice, without affecting CAA load. Gliosis (GFAP and Iba-1 immunostaining), gelatinase activity, and expression of a variety of inflammatory genes (MMP-9, Nox4, CD45, S-100b, Iba-1) were also significantly reduced. Higher levels of microvascular tight junction and basal lamina proteins were found in the brains of minocycline-treated Tg2576 mice relative to saline-treated controls.
Conclusions
Minocycline reduced gliosis, inflammatory gene expression, gelatinase activity, and spontaneous hemorrhage in two different mouse models of CAA, supporting the importance of MMP-related and inflammatory pathways in ICH pathogenesis. As an FDA-approved drug, minocycline might be considered for clinical trials to test efficacy in preventing CAA-related ICH.
Keywords: APOE, Cerebral amyloid angiopathy, gliosis, Intracerebral hemorrhage, matrix metalloproteinase, minocycline
Introduction
Although ischemic stroke is the most common stroke subtype, intracerebral hemorrhage (ICH) results in greater morbidity and mortality1. The most common cause of ICH in the elderly is cerebral amyloid angiopathy (CAA)2, a disorder caused by the deposition of β-amyloid peptide (Aβ) in small cerebral vessels—most prominently, penetrating arterioles of the cortex3,4. While CAA is commonly found in the brains of AD patients, it is also present in the elderly without AD5. ICH is a recurrent complication of CAA6; however, the pathogenesis of CAA-induced ICH has not been fully established. A better understanding of the underlying pathophysiology of CAA-induced ICH may result in the identification of potential targets for intervention. Further, the frequent recurrence of ICH in patients with CAA provides opportunity for prevention in this high-risk population.
The recognition that several mouse models of Alzheimer’s disease (AD), including Tg2576 and APP23, also develop CAA and microvascular hemorrhage7, 8 has provided animal models to study CAA-induced ICH. These mice develop age-dependent accumulations of amyloid plaques and amyloid angiopathy, and at a later stage of pathogenesis spontaneously develop hemorrhage9, 10. In addition to providing a model to study the cellular and molecular pathogenesis of CAA-related ICH, these mice provide a preclinical model to test the efficacy of interventions to prevent recurrent ICH.
Earlier studies demonstrated that exogenous Aβ could induce expression and activity of matrix metalloproteinase-9 (MMP-9) and MMP-2, gelatinases implicated in vascular remodeling and hemorrhage, in cerebral endothelial cells (CECs) and vascular smooth muscle cells, in vitro11,12,13. Isolated rat microvessels exposed to exogenous Aβ demonstrated increased gelatinase expression and decreased endothelial tight junction proteins, claudin-1 and -5, suggesting a mechanism for blood-brain-barrier (BBB) breakdown14. Moreover, gelatinase expression was found to be increased in amyloid-laden vessel and especially prominent in vessels with evidence of prior hemorrhage in Tg2576 mice15. Pharmacological inhibition of MMP reduced not only CAA-associated gelatinase activity but microvascular oxidative stress in Tg2576 mice16. In another microvascular CAA model lacking spontaneous ICH (Tg-SwDI mice), minocycline reduced microglial activation and improved behavioral deficits17. These studies raised the possibility that enhanced gelatinase activity in CAA vessels might play a role in CAA-related ICH. In this study, we examined the efficacy of minocycline, an FDA-approved antibiotic known to inhibit gelatinase activity and inflammation, in reducing spontaneous hemorrhage in two different mouse models of CAA.
Methods
Animals
Tg2576 (a.k.a. APPsw) 18 mice of both genders, expressing human APP695 with Swedish mutations at positions 670/671 under control of the prion promoter, were used in this study. 5×FAD/apoE4-knockin mice were produced by crossing 5×FAD transgenic mice (Tg7031 line)19 containing five familial AD mutations (APP K670N/M671L + I716V + V717I and PS1 M146L + L286V) with apoE4-targeted replacement mice (endogenous murine Apoe gene was replaced with the APOE4 gene)20. All experimental protocols were approved by the Animal Studies Committee at Washington University, and all studies were conducted in accordance with the US Public Health Service’s Policy on Humane Care and Use of Laboratory Animals.
Minocycline Treatment
Mice were treated minocycline (50 mg/kg, i.p., Sigma-Aldrich, St. Louis, Mo., USA) or vehicle (saline) every other day for 8 weeks. Age- and sex-matched Tg2576 mice (17 ± 2 months old) were treated with minocycline. Eight mice were used in each group (5 females and 3 males). In addition, male 5×FAD/ApoE4 mice (12 ± 1 months old) were randomly assigned to treatment with minocycline (n=8) or saline (n=8). A power analysis was performed based on hemorrhage frequency (6.2 hemorrhages per hemisphere) and standard deviation (2.77) in 12 m Tg2576 mice 21 (and unpublished data). A sample of size of 8 mice per group was found to provide at least 85% statistical power at a significance level of 5% to detect a 50% reduction in the mean number of hemorrhages due to the treatment. Total body weight was measured weekly and injection volumes were modified accordingly. Mice were sacrifice 6 h after the last injection.
Brain extraction and preparation
Mice were deeply anesthetized with isoflurane and transcardially perfused with 0.01M PBS. The brains were removed, and hemispheres were separated. Left hemispheres were immediately dissected, snap-frozen on dry ice, and stored at −80°C for biochemical analysis. Right hemispheres were fixed in 4% paraformaldehyde for 24 h and transferred to 30% sucrose in 0.1M PB. Coronal sections, 50 μm thick, were made with a sliding microtome and stored in 0.1M PB, 30% sucrose, and 30% ethylene glycol at −20°C until further analysis.
Histology
Right hemispheres were sectioned and stained for hemorrhage using Perl’s Blue. Hemorrhages were counted by an assessor who was blinded to treatment assignments; and graded based on size (grade 1: 1-3 blue puncta; grade 2: 4-10 puncta; grade 3: >10 puncta)7 in 25-30 brain sections (50μm thick) from each mouse, spaced 300μm apart. To quantify CAA and amyloid plaque load, 4 regularly-spaced brain sections from rostral anterior commissure to caudal hippocampus from each mouse was stained with Congo Red or X-34. Area stained with the amyloid dye were quantified and expressed as mean fraction of section area from each mouse22. Groups were compared using Student’s t-test or Mann-Whitney Rank Sum Test.
Immunohistochemistry
For Aβ immunohistochemistry, brain sections were incubated with mouse anti-Aβ antibody (HJ3.4, 1:1000)23 overnight at 4°C. Vectastain ABC (1:400) followed by 0.025% 3, 3-diaminobenzidine tetrachloride in 0.25% NiCl2 and 0.05% H2O2 was used to develop staining. Immunofluorescent double labeling was performed as follows: a mixture of primary antibodies was incubated with the brain sections overnight at 4°C. Fluorescently labeled secondary antibodies were then incubated at room temperature for 1 hr. Primary antibody pairs: mouse anti-GFAP monoclonal antibody (1:1000; sigma, St Louis, MO, USA) and rabbit anti-Iba1 antibody (1:1000; Wako Pure Chemicals Industries, Tokyo, Japan); rabbit anti-ZO-1antibody (1:250, Invitrogen, Camarillo, CA, USA) and biotin-conjugated tomato lectin (1:400; Vector Laboratories, Inc., Burlingame, CA, USA); mouse anti-collagen IV antibody (1:200, Sigma) and tomato lectin. Secondary antibodies: Cy3-conjugated donkey anti-rabbit IgG antibody (1:800; Jackson ImmunoResearch); AlexaFluoro 488-conjugated donkey anti-mouse IgG antibody; AlexaFluoro 488-conjugated streptavidin (1:100; Molecular Probes).
Aβ ELISA
To measure Aβ, dissected cortices were homogenized in PBS and then in 5M guanidine in TBS, pH 8.0. After each homogenization step, samples were centrifuged at 12,000 rpm for 20 min, and supernantants were collected for sandwich ELISA23. Aβx–40 and Aβx–42 were assessed using mouse monoclonal capture antibodies HJ2 (anti-Aβ35–40) and HJ7.4 (anti-Aβ37–42)24, respectively, and a biotinylated central domain antibody, HJ5.1 (anti-Aβ13–28) wsa used as the detecting antibody followed by streptavidin-poly-HRP-40 (Fitzgerald Industries). All ELISAs were developed using Super Slow ELISA 3, 3′,5,5′-tetramethylbenzidine (Sigma), and absorbance was read on a Bio-Tek Epoch plate reader (Winooski, VT) at 650 nm. Standard curves were generated from synthetic human Aβ1–40 or Aβ1–42 peptides (American Peptide, Sunnyvale, CA, USA).
RT-PCR
Messenger RNA was extracted from the left hemisphere and reverse transcribed with the cDNA Reverse Transcription kit. qPCR was performed using the ABI 7500 in the default thermal cycling mode with Power SYBR. Mouse β-actin was used as a normalization reference. Relative mRNA levels were calculated using the comparative Ct method, and expressed as a percentage of control. Please see http://stroke.ahajournals.org for table I primer sequences used in RT-PCR.
Gelatin-substrate zymography
Some of the left cortices were homogenized in 500μl of lysis buffer and supernatant was collected after centrifugation. The brain supernatant was incubated with 50μl of gelatin-Sepharose 4B (Pharmacia Biotech, Uppsala, Sweden) for 1 h with constant shaking. After centrifugation, the pellet was then incubated for 30 min with 50μl of elution buffer consisting of lysis buffer plus 10% dimethylsulfoxide (DMSO). Each 4μl of eluted sample from the left cortex was mixed with 25μl of 4×nonreducing sample buffer loaded onto 7.5% SDS-PAGE containing 0.1% gelatin, and electrophoresed at 180V. The gel was incubated for 20h at 37°C in buffer for 20 min and was stained for 1 h with 0.1% Coomassie Blue 25.
Statistical Analysis
All data were expressed as mean ± SD. Differences between groups were determined using two-tailed Student’s t test for two-group comparisons, and one-way ANOVA followed by post hoc Tukey’s test for comparison among multiple groups. Differences were deemed statistically significant at *p < 0.05 and **p < 0.01.
Results
Minocycline reduced hemorrhage frequency in Tg2576 mice
Mice tolerated minocycline treatment without adverse effects: average weights before, during, and after treatment did not differ between groups (Supplemental Fig IA). One mouse died after 4 weeks of minocycline treatment, and was subsequently replaced by another age- and sex-matched mouse.
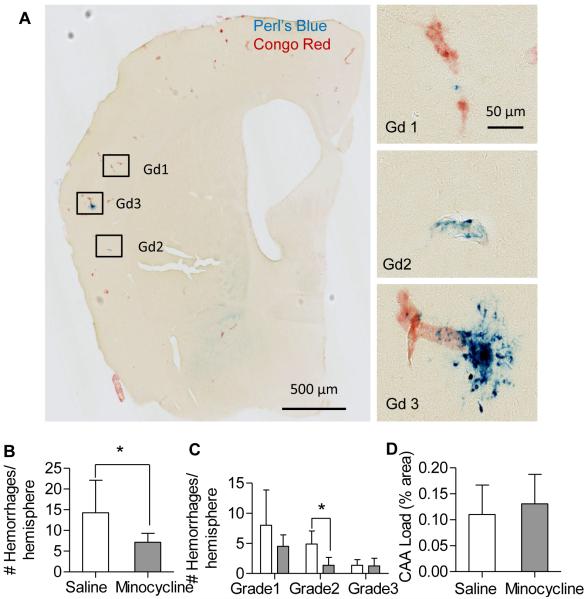
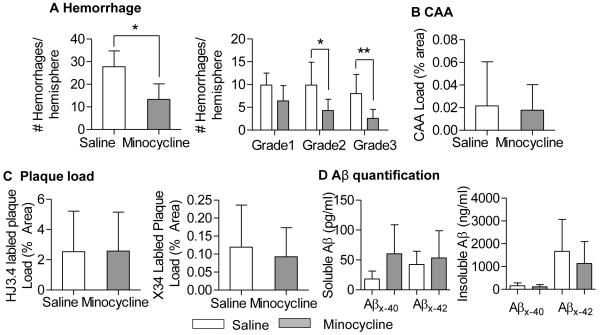
After two months of treatment with minocycline (50 mg /kg, ip, q.o.d.) or saline, brains sections were stained with Congo Red to assess amyloid load and Perl’s Blue to quantify hemorrhage frequency. The Tg2576 mice treated with minocycline had almost half the number of hemorrhages compared to those treated with saline (Fig 1B). Hemorrhages were graded by size, based on the methods of Jucker (Fig 1A)7. Grade 1 hemorrhages were most frequent, regardless of treatment. Though minocycline treatment had fewer microhemorrages for all grades, only grade 2 hemorrhages had statistically significantly fewer microhemorrhages in the minocycline-treated group compared to saline treatment (Fig 1C). Five of the eight mice were females, and when compared separately, showed a trend toward fewer microhemorrhages (12 vs. 7.4 hemorrhages/hemisphere, p = 0.059).
Figure 1.
Chronic minocycline treatment reduced hemorrhage frequency without altering CAA load in Tg2576 mice. A. Representative photomicrographs of Tg2576 brain sections stained with Perl’s Blue and Congo Red, to demonstrate microhemorrhage size (grade 1, 1-3 puncta; grade 2, 3-10 puncta; grade 3, >10 puncta). Bar=50μm. B. The number of hemorrhages was significantly lower in the minocycline-treated mice compared to controls (n=8 mice/group; 30 brain slices per animal). C. Comparison of hemorrhage frequency by grade. D, CAA, stained with Congo Red, was quantified and expressed as percent area (4 equally spaced brain sections from each animal). No significant difference was found between treatment groups. Values are mean ± SD. *p< 0.05.
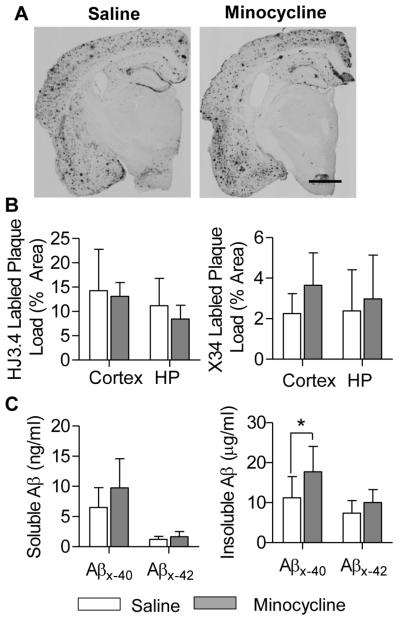
CAA load was quantified by measuring the cross-sectional area of Congo Red stained vessels and expressed as % area of the total section. There was no significant difference in CAA load between minocycline-treated vs. saline-treated mice (Fig 1D). In addition, minocycline treatment appeared to have little or no effect on amyloid plaque load. Plaque load, determined using anti-Aβ antibody (HJ3.4) and amyloid dye (X-34), was unchanged after chronic minocycline treatment (Fig 2B). These findings were corroborated by brain tissue Aβ levels measured by ELISA. Soluble Aβ was unchanged by minocycline treatment; however, insoluble Aβx-40was modestly increased by minocycline (Fig 2C). These findings are consistent with previous studies which have found variable effects of minocycline on amyloid accumulation in a variety of APP transgenic mouse models17,26-28.
Figure 2.
Minocycline had little effect on amyloid plaque load in Tg2576 mice. Plaques stained with HJ3.4 immunohistochemistry (A, bar=1mm) or X-34 were quantified (n=8 mice/group), and showed no significant difference between treatment groups (B). PBS-soluble and insoluble Aβ were serially extracted from cortex and hippocampus, and quantified with ELISA (C). No difference in Aβx-40 and Aβx-42 levels were found between groups, except in PBS-insoluble Aβx-40 levels (*p=0.045). Values are mean ± SD.
Minocycline reduced gliosis and gelatinase activity
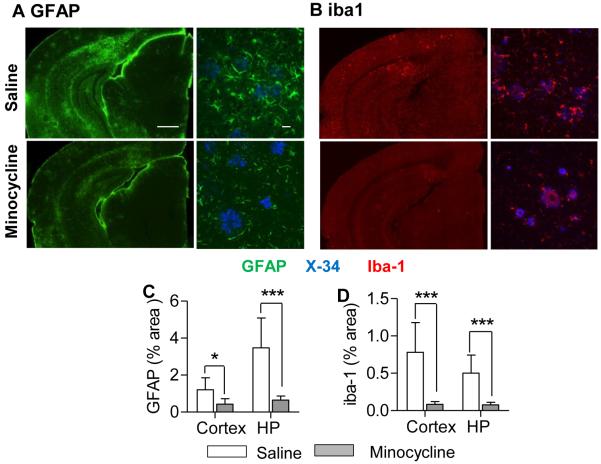
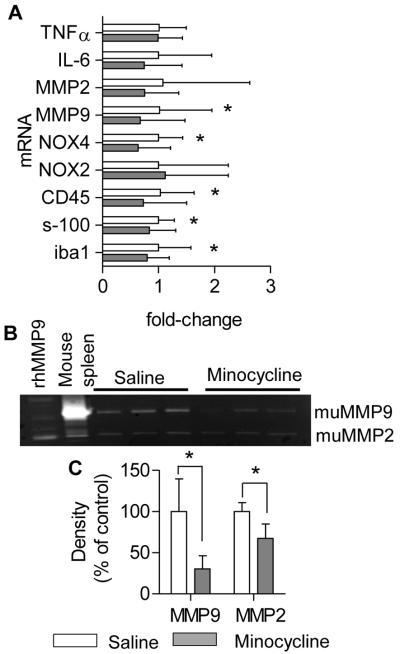
A large body of literature demonstrates that chronic administration of minocycline inhibits inflammation and gliosis in numerous different models of CNS disease (for review, see 29). In Tg2576 mice, minocycline treatment markedly decreased astrocytosis (GFAP immunohistochemistry) and microgliosis (Iba-1 immunohistochemistry), in both cortex and hippocampus (Fig 3A-D). Real-time qPCR confirmed the reduction in gliosis with reduced expression of Iba-1, CD-45, and S-100b mRNA (Fig 4A). In addition, several other genes associated with vascular inflammation were reduced by chronic minocycline treatment, including NOX4 and MMP-9. NOX4 expression has been reported to be selectively increased in other APP transgenic models30. To confirm that decreased MMP mRNA resulted in a reduced MMP activity, we performed gelatin zymography on brain extracts from minocycline-treated mice. Minocycline significantly reduced both MMP-9 and MMP-2 activity compared to saline-treated controls (Fig 4B, C).
Figure 3.
Minocycline reduced gliosis in Tg2576 mice. Representative brain sections from Tg2576 mice show dramatic decreases in GFAP (A) and Iba-1 (B) immunostaining following chronic treatment with minocycline. Bars; left panel=500μm, right panel=20μm. GFAP and Iba-1 immunostaining was quantified, normalized to plaque load, and expressed as % area; minocycline-treated mice showed significantly reduced astrocytosis (C) and microgliosis (D) compared to saline-treated mice. Values are mean ± SD.; n = 8 mice/group. *p < 0.01.
Figure 4.
Minocycline decreased expression of vascular inflammatory genes in Tg2576 mice. A. QPCR was performed on cortical extracts using primers indicated. mRNA for MMP9, NOX4, CD45, s-100, and Iba1 were significantly reduced in minocycline-treated mice compared to control (n = 8 mice/group). Gelatin zymography (B) revealed that minocycline treatment reduced both MMP-2 and MMP-9 activities in the brains of Tg2576 mice compared to controls (n=3 mice/group) (C). Values are mean ± SD.; *p< 0.05.
Minocycline enhances expression of BBB proteins
Previous studies have demonstrated that vascular MMP-9 expression can lead to degradation of basement membrane proteins (including collagen IV) and endothelial tight junction proteins (ZO-1, occluding, and claudin 5), leading to compromise of the BBB31. To determine if minocycline reduces degradation of proteins involved in neurovascular integrity, we examined the expression of the tight junction protein, ZO-1, and the basement membrane protein collagen IV (ColIV). Focusing on vascular expression, we co-stained all sections with biotin-conjugated tomato lectin to visualize capillaries and small arterioles (Fig 5A, B). We quantified expression of these antigens (ZO-1 and Col IV) within vessels stained with lectin (normalized to the lectin-positive vascular footprint), and expressed as % capillary area. Minocycline treatment increased the expression of vascular ZO-1 and Col IV by more than 2-fold (Fig 5C, D). Staining of vessels with tomato lectin was unchanged by minocycline treatment (data not shown), indicating that minocycline increased expression of ZO-1 and Col IV without altering vessel density.
Figure 5.
Minocycline increased vascular ZO-1 and Col IV immunostaining in Tg2576 mice. Confocal microscopy of ZO-1 (A), Col IV (B) and lectin-positive microvessels (A-B, bar=50μm) in the cortex of Tg2576 mice treated with saline or minocycline. Minocycline-treated mice showed greater microvascular ZO-1 (C) and Col IV (D) immunostaining compared to saline-treated mice. Values are mean ± SD.; n = 8 mice/group. *p< 0.01.
Minocycline reduces microhemorrhage in 5×FAD ApoE4 mice
Preclinical stroke studies over the past three decades have demonstrated the importance of replication using different animal models (STAIR criteria)32, 33. To confirm the efficacy of minocycline in reducing spontaneous hemorrhage, we repeated the minocycline study using a novel mouse model of CAA-related spontaneous hemorrhage. 5×FAD mice (line 7031)19 with targeted replacement of mouse Apolipoprotein E with human Apolipoprotein E ε4 (5×FAD/ApoE4) develop amyloid plaques and CAA by 5-6 months of age, and hemorrhages appear spontaneously beginning at 7 months of age (unpublished observation). Minocycline treatment (50 mg /kg, ip, qOD) was initiated at 12 months of age (when significant CAA and hemorrhages were already present) and treated for 2 months. Brain sections were stained with Perl’s Blue to quantify hemorrhage frequency. Minocycline treatment reduced the number of hemorrhages by greater than 50%. Both grade 2 and grade 3 hemorrhages were significantly reduced by minocycline (Fig 6A). Similar to the study in Tg2576 mice, 2 months of minocycline treatment did not alter CAA or plaque load (Fig 6 B-D), nor did it affect body weight over this period of time (Supplemental Fig IB).
Figure 6.
Chronic minocycline treatment reduced hemorrhage frequency in 5×FAD/ApoE4 mice. A. 5×FAD/ApoE4 mice treated with minocycline (n=8) for 8 weeks showed fewer microhemorrhages compared to mice treated with saline (n=8). CAA load (B, Congo Red-stained vessels), and plaque burden (C, HJ3.4 immunostained and X-34 stained plaques) were similar between groups. D. Soluble and insoluble Aβx-40 and Aβx-42 (quantified with ELISA) showed no differences between groups. Values are mean ± SD. *p< 0.05, **p<0.01.
Discussion
CAA is the leading cause of ICH in the elderly and recurrence is common; yet, there are no treatments for the prevention of CAA-related ICH. In the present study, we examined the efficacy of chronic minocycline treatment on hemorrhage frequency in two different mouse models of CAA. We found that minocycline reduced hemorrhage frequency in both CAA models without affecting amyloid load. Minocycline also inhibited MMP-2 and −9 activity, reduced gliosis and expression of select neuroinflammatory genes in brains of Tg2576 mice. These changes occurred in parallel with increases in vascular tight junction and basal lamina proteins, suggesting that minocycline preserved structural integrity of cerebral vessels, leading to reduction in hemorrhage frequency. In both models, treatment was initiated during advanced stages of disease, when microhemorrhage was already present. Therefore, the reduced frequency of microhemorrhage likely reflects a reduction in the accumulation of microhemorrhage during the 2-month treatment period.
Minocycline, a semi-synthetic second generation tetracycline with FDA-approval for the treatment of acne vulgaris, has a large literature supporting its neuroprotective properties34. The tetracyclines were first described to have anti-inflammatory, anti-apoptotic, and anti-gelatinase activities limiting angiogenesis and tumor metastasis35-38. Minocycline became the tetracycline of choice for central nervous system (CNS) delivery, owing to its lipophilic nature allowing it to pass through the blood-brain barrier39. Indeed, minocycline has emerged as the most effective neuroprotectant among the tetracyclines, demonstrating activities in experimental models of cerebral ischemia40, 41, traumatic brain injury42, and several neurodegenerative diseases such as Parkinson’s disease43, 44, Huntington’s disease45, 46; and amyotrophic lateral sclerosis47. In AD mouse models, minocycline has been shown to improve behavioral performance, but have little effect on amyloid plaque load17,26,28. Consistent with these earlier reports, our findings also demonstrate little effect on plaque accumulation.
Double-blind randomized clinical trials examining the efficacy of minocycline in a variety of neurodegenerative disease, including Parkinson disease48, 49, Amyotropic lateral sclerosis (ALS)50, 51, and Huntington’s Disease52, 53 have not demonstrated definitive efficacy in attenuating disease progression. In these clinical trials, the precise target of minocycline’s mechanism of action was not identified, and therefore target engagement was not confirmed. In all trials except for one, safety after chronic administration of minocycline in these aged populations was clearly demonstrated. In the phase III trial in ALS patients, neurological deterioration occurred more rapidly in the minocycline group compared to the placebo group51; however, a higher dose of minocycline was used in this trial compared to all others.
The dose of minocycline used in the current study (50 mg/kg, ip) produces peak plasma concentrations extrapolated to be equivalent to that following 1000 mg, po in humans 54, according to published pharmacokinetic studies in rodents55. Given every other day, this is approximately 2.5 times the usual daily dose of minocycline for treatment of bacterial infections. Minocycline is a known sclerosing agent and has been reported to induce pleuritis in rodents when injected intrapleurally56. In our study, we administered minocycline via intraperitoneal injection every other day to minimize its sclerosing effects. Although one mouse in the minocycline-treated group died after 1 month of dosing; the remaining animals appeared healthy (maintaining constant body weight) without overt evidence of peritonitis.
Our studies demonstrate that minocycline inhibits gliosis, certain vascular inflammatory mediators, mediators of oxidative stress, and gelatinases, consistent with findings in other studies. All of these mediators have been implicated in CAA-related pathology. Chronic minocycline treatment of transgenic mice expressing the vasculotropic Dutch/Iowa (E693Q/D694N) mutant APP did not alter CAA load but reduced microgliosis and inflammation in the brains of these mice. Moreover, cognitive deficits were improved with minocycline treatment17. Of note, this model of microvascular CAA does not result in spontaneous hemorrhage. In another study, CAA-induced free radical formation was inhibited by chronic treatment with minocycline and other more specific MMP-9 inhibitors in Tg2576 mice16. While these inhibitors did not have a direct antioxidant effect, it is hypothesized that minocycline’s antioxidant effect was indirectly mediated via its MMP-9 inhibitory activity. Therefore, the effect of minocycline in attenuating CAA-related pathology has been confirmed in several animal models and by several independent laboratories.
Our findings are the first to demonstrate that chronic minocycline treatment reduced spontaneous hemorrhage in an established CAA model (Tg2576 mice). In addition, we have confirmed these findings in another newly developed CAA mouse model, the 5×FAD/ApoE4 mouse (Fig 6). This mouse model is highly relevant to human disease, because ApoE4 genotype has been independently associated with severe CAA, CAA-related vasculopathy, and intracerebral hemorrhage57, 58. Even in the absence of CAA pathology, recent studies suggest that the ApoE4 genotype alone may induce neurovascular injury with resultant BBB leak. A recent study utilizing targeted replacement of human ApoE genes in mice demonstrated that ApoE4 specifically induced a pro-inflammatory pathway in pericytes resulting in the induction of MMP-9, degradation of BBB proteins (ZO-1 and collagen IV), and BBB leak31. In parallel with its MMP-9 and -2 inhibitory activity, we have found that minocycline preserved elevated expression of the tight junction protein, ZO-1, and the basement membrane protein, collagen IV, in brain microvessels (identified with tomato lectin, Fig 5). Both of these proteins are known substrates of the gelatinases59, 60.
A limitation of this study, and other minocycline treatment studies, is the absence of a definitive target that is responsible for its mechanism of action. Circumstantial evidence from CAA animal models suggests that the gelatinases may be the relevant target. MMP-9 is expressed in CAA vessels, especially those with evidence of prior hemorrhage15 Inhibition of MMP’s with non-specific inhibitors (such as minocycline) reduced spontaneous hemorrhage (see above) and neurobehavioral outcomes17 Further, more specific MMP-9 inhibitors reduced other downstream consequences of CAA, including CAA-induced oxidative stress16.
Conclusion
The current study demonstrates the efficacy of minocycline in reducing spontaneous hemorrhage in two different mouse models of CAA, during advanced stages of disease. Minocycline reduced gliosis, inhibited the expression of inflammatory mediators and gelatinase activity, resulting in the preserved expression of the BBB proteins, ZO-1 and collagen IV. Because minocycline is already FDA-approved and known to be safe in elderly populations, we propose that minocycline be considered as a candidate treatment in clinical trials for the prevention of ICH in CAA patients.
Supplementary Material
Acknowledgements
The Tg2576 (a.k.a. APPsw) mouse was a generous gift from Dr. K. Ashe (University of Minnesota, Minneapolis, MN). The 5×FAD mouse (line 7031) was a gift from Dr. Robert Vassar, Northwestern University, Chicago, IL. The ApoE4 mouse was a gift from Dr. Patrick Sullivan, Duke University, Durham, NC.
Sources of Funding This work was supported by NIH R01 NS084028, R21 NS082529, the Argo E. & Edna Edwards Landau Fund, and the Lillian Straus Fund (to J-M.L.), a grant from the Cure Alzheimer’s fund, the JPB Foundation and NIH R01 NS090934 (to D.M.H.).
Footnotes
Authorship Experiments were conceived and designed by P.Y., S.M.G., D.M.H., and J.-M.L. Experiments were performed by P.Y., A.Z., Q. X., A. K., E.G, and R.P. Data were analyzed by P.Y., A.Z., and J.-M.L. The manuscript was written by P.Y., A.Z., and J.-M.L. with revisions from all others.
Potential Conflicts of Interest D.M.H. is a co-founder of C2N Diagnostics LLC, is on the scientific advisory boards of AstraZeneca, Genentech, and C2N Diagnostics, and consults for for Eli Lilly. Washington University receives grants from the Tau Consortium, Cure Alzheimer’s Fund, the JPB Foundation, Eli Lilly, Janssen, and C2N Diagnostics.
References
- 1.Rincon F, Mayer SA. The epidemiology of intracerebral hemorrhage in the united states from 1979 to 2008. Neurocrit Care. 2013;19:95–102. doi: 10.1007/s12028-012-9793-y. [DOI] [PubMed] [Google Scholar]
- 2.Tanskanen M, Makela M, Myllykangas L, Rastas S, Sulkava R, Paetau A. Intracerebral hemorrhage in the oldest old: A population-based study (vantaa 85+) Front Neurol. doi: 10.3389/fneur.2012.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenberg SM. Cerebral amyloid angiopathy and vessel dysfunction. Cerebrovasc Dis. 2002;13(Suppl 2):42–47. doi: 10.1159/000049149. [DOI] [PubMed] [Google Scholar]
- 4.Vinters HV, Roos RA. Introduction to the amyloid symposium. Brain Pathol. 1996;6:109. doi: 10.1111/j.1750-3639.1996.tb00792.x. 10.1111/j.1750-3639.1996.tb00792.x. [DOI] [PubMed] [Google Scholar]
- 5.Viswanathan A, Greenberg SM. Cerebral amyloid angiopathy in the elderly. Annals of neurology. 2011;70:871–880. doi: 10.1002/ana.22516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller JH, Wardlaw JM, Lammie GA. Intracerebral haemorrhage and cerebral amyloid angiopathy: Ct features with pathological correlation. Clin Radiol. 1999;54:422–429. doi: 10.1016/s0009-9260(99)90825-5. [DOI] [PubMed] [Google Scholar]
- 7.Winkler DT, Bondolfi L, Herzig MC, Jann L, Calhoun ME, Wiederhold KH, et al. Spontaneous hemorrhagic stroke in a mouse model of cerebral amyloid angiopathy. J Neurosci. 2001;21:1619–1627. doi: 10.1523/JNEUROSCI.21-05-01619.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fryer JD, Simmons K, Parsadanian M, Bales KR, Paul SM, Sullivan PM, et al. Human apolipoprotein e4 alters the amyloid-beta 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:2803–2810. doi: 10.1523/JNEUROSCI.5170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fryer JD, Taylor JW, DeMattos RB, Bales KR, Paul SM, Parsadanian M, et al. Apolipoprotein e markedly facilitates age-dependent cerebral amyloid angiopathy and spontaneous hemorrhage in amyloid precursor protein transgenic mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:7889–7896. doi: 10.1523/JNEUROSCI.23-21-07889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herzig MC, Winkler DT, Burgermeister P, Pfeifer M, Kohler E, Schmidt SD, et al. Abeta is targeted to the vasculature in a mouse model of hereditary cerebral hemorrhage with amyloidosis. Nat Neurosci. 2004;7:954–960. doi: 10.1038/nn1302. [DOI] [PubMed] [Google Scholar]
- 11.Deb S, Gottschall PE. Increased production of matrix metalloproteinases in enriched astrocyte and mixed hippocampal cultures treated with beta-amyloid peptides. Journal of neurochemistry. 1996;66:1641–1647. doi: 10.1046/j.1471-4159.1996.66041641.x. [DOI] [PubMed] [Google Scholar]
- 12.Backstrom JR, Lim GP, Cullen MJ, Tokes ZA. Matrix metalloproteinase-9 (mmp-9) is synthesized in neurons of the human hippocampus and is capable of degrading the amyloid-beta peptide (1-40) J Neurosci. 1996;16:7910–7919. doi: 10.1523/JNEUROSCI.16-24-07910.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung SS, Zhang W, Van Nostrand WE. Pathogenic a beta induces the expression and activation of matrix metalloproteinase-2 in human cerebrovascular smooth muscle cells. J Neurochem. 2003;85:1208–1215. doi: 10.1046/j.1471-4159.2003.01745.x. [DOI] [PubMed] [Google Scholar]
- 14.Hartz AM, Bauer B, Soldner EL, Wolf A, Boy S, Backhaus R, et al. Amyloid-beta contributes to blood-brain barrier leakage in transgenic human amyloid precursor protein mice and in humans with cerebral amyloid angiopathy. Stroke. 2012;43:514–523. doi: 10.1161/STROKEAHA.111.627562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JM, Yin KJ, Hsin I, Chen S, Fryer JD, Holtzman DM, et al. Matrix metalloproteinase-9 and spontaneous hemorrhage in an animal model of cerebral amyloid angiopathy. Ann Neurol. 2003;54:379–382. doi: 10.1002/ana.10671. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Alloza M, Prada C, Lattarulo C, Fine S, Borrelli LA, Betensky R, Greenberg SM, et al. Matrix metalloproteinase inhibition reduces oxidative stress associated with cerebral amyloid angiopathy in vivo in transgenic mice. Journal of neurochemistry. 2009;109:1636–1647. doi: 10.1111/j.1471-4159.2009.06096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan R, Xu F, Previti ML, Davis J, Grande AM, Robinson JK, et al. Minocycline reduces microglial activation and improves behavioral deficits in a transgenic model of cerebral microvascular amyloid. J Neurosci. 2007;27:3057–3063. doi: 10.1523/JNEUROSCI.4371-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, et al. Correlative memory deficits, abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 19.Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan PM, Mace BE, Maeda N, Schmechel DE. Marked regional differences of brain human apolipoprotein e expression in targeted replacement mice. Neuroscience. 2004;124:725–733. doi: 10.1016/j.neuroscience.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Fryer JD, Taylor JW, DeMattos RB, Bales KR, Paul SM, Parsadanian M, et al. Apolipoprotein e markedly facilitates age-dependent cerebral amyloid angiopathy and spontaneous hemorrhage in amyloid precursor protein transgenic mice. J Neurosci. 2003;23:7889–7896. doi: 10.1523/JNEUROSCI.23-21-07889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraft AW, Hu X, Yoon H, Yan P, Xiao Q, Wang Y, et al. Attenuating astrocyte activation accelerates plaque pathogenesis in app/ps1 mice. Faseb J. 2013;27:187–198. doi: 10.1096/fj.12-208660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J, Castellano JM, Jiang H, Basak JM, Parsadanian M, Pham V, et al. Overexpression of low-density lipoprotein receptor in the brain markedly inhibits amyloid deposition and increases extracellular a beta clearance. Neuron. 2009;64:632–644. doi: 10.1016/j.neuron.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, et al. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat Neurosci. 2011;14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan P, Hu X, Song H, Yin K, Bateman RJ, Cirrito JR, et al. Matrix metalloproteinase-9 degrades amyloid-beta fibrils in vitro and compact plaques in situ. The Journal of biological chemistry. 2006;281:24566–24574. doi: 10.1074/jbc.M602440200. [DOI] [PubMed] [Google Scholar]
- 26.Seabrook TJ, Jiang L, Maier M, Lemere CA. Minocycline affects microglia activation, abeta deposition, and behavior in app-tg mice. Glia. 2006;53:776–782. doi: 10.1002/glia.20338. [DOI] [PubMed] [Google Scholar]
- 27.Parachikova A, Vasilevko V, Cribbs DH, LaFerla FM, Green KN. Reductions in amyloid-beta-derived neuroinflammation, with minocycline, restore cognition but do not significantly affect tau hyperphosphorylation. J Alzheimers Dis. 2010;21:527–542. doi: 10.3233/JAD-2010-100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biscaro B, Lindvall O, Tesco G, Ekdahl CT, Nitsch RM. Inhibition of microglial activation protects hippocampal neurogenesis and improves cognitive deficits in a transgenic mouse model for alzheimer’s disease. Neurodegener Dis. 2012;9:187–198. doi: 10.1159/000330363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elewa HF, Hilali H, Hess DC, Machado LS, Fagan SC. Minocycline for short-term neuroprotection. Pharmacotherapy. 2006;26:515–521. doi: 10.1592/phco.26.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruce-Keller AJ, Gupta S, Knight AG, Beckett TL, McMullen JM, Davis PR, et al. Cognitive impairment in humanized appxps1 mice is linked to abeta(1-42) and nox activation. Neurobiol Dis. 2011;44:317–326. doi: 10.1016/j.nbd.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, et al. Apolipoprotein e controls cerebrovascular integrity via cyclophilin a. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- 33.Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garrido-Mesa N, Zarzuelo A, Galvez J. Minocycline: Far beyond an antibiotic. Br J Pharmacol. 2013;169:337–352. doi: 10.1111/bph.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Golub LM, Ramamurthy NS, McNamara TF, Greenwald RA, Rifkin BR. Tetracyclines inhibit connective tissue breakdown: New therapeutic implications for an old family of drugs. Crit Rev Oral Biol Med. 1991;2:297–321. doi: 10.1177/10454411910020030201. [DOI] [PubMed] [Google Scholar]
- 36.Golub L, Greenwald R, Ramamurthy N, Zucker S, Ramsammy L, McNamara T. Tetracyclines (tcs) inhibit matrix metalloproteinases (mmps): In vivo effects in arthritic and diabetic rats and new in vitro studies. Matrix Suppl. 1992;1:315–316. [PubMed] [Google Scholar]
- 37.Greenwald R, Golub LM. Tetracyclines in arthritis. J Rheumatol. 1993;20:1990. [PubMed] [Google Scholar]
- 38.Sapadin AN, Fleischmajer R. Tetracyclines: Nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258–265. doi: 10.1016/j.jaad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Brogden RN, Speight TM, Avery GS. Minocycline: A review of its antibacterial and pharmacokinetic properties and therapeutic use. Drugs. 1975;9:251–291. doi: 10.2165/00003495-197509040-00005. [DOI] [PubMed] [Google Scholar]
- 40.Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A. 1998;95:15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koistinaho M, Malm TM, Kettunen MI, Goldsteins G, Starckx S, Kauppinen RA, et al. Minocycline protects against permanent cerebral ischemia in wild type but not in matrix metalloprotease-9-deficient mice. J Cereb Blood Flow Metab. 2005;25:460–467. doi: 10.1038/sj.jcbfm.9600040. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez Mejia RO, Ona VO, Li M, Friedlander RM. Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery. 2001;48:1393–1399. doi: 10.1097/00006123-200106000-00051. [DOI] [PubMed] [Google Scholar]
- 43.Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the mptp model of parkinson’s disease. Proc Natl Acad Sci U S A. 2001;98:14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas M, Le WD. Minocycline: Neuroprotective mechanisms in parkinson’s disease. Curr Pharm Des. 2004;10:679–686. doi: 10.2174/1381612043453162. [DOI] [PubMed] [Google Scholar]
- 45.Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of huntington disease. Nat Med. 2000;6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- 46.Thomas M, Ashizawa T, Jankovic J. Minocycline in huntington’s disease: A pilot study. Mov Disord. 2004;19:692–695. doi: 10.1002/mds.20018. [DOI] [PubMed] [Google Scholar]
- 47.Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]
- 48.A randomized, double-blind, futility clinical trial of creatine and minocycline in early parkinson disease. Neurology. 2006;66:664–671. doi: 10.1212/01.wnl.0000201252.57661.e1. [DOI] [PubMed] [Google Scholar]
- 49.Dodel R, Spottke A, Gerhard A, Reuss A, Reinecker S, Schimke N, et al. Minocycline 1-year therapy in multiple-system-atrophy: Effect on clinical symptoms and [(11)c] (r)-pk11195 pet (memsa-trial) Mov Disord. 2010;25:97–107. doi: 10.1002/mds.22732. [DOI] [PubMed] [Google Scholar]
- 50.Gordon PH, Moore DH, Gelinas DF, Qualls C, Meister ME, Werner J, et al. Placebo-controlled phase i/ii studies of minocycline in amyotrophic lateral sclerosis. Neurology. 2004;62:1845–1847. doi: 10.1212/01.wnl.0000125321.92112.7e. [DOI] [PubMed] [Google Scholar]
- 51.Gordon PH, Moore DH, Miller RG, Florence JM, Verheijde JL, Doorish C, et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: A phase iii randomised trial. Lancet Neurol. 2007;6:1045–1053. doi: 10.1016/S1474-4422(07)70270-3. [DOI] [PubMed] [Google Scholar]
- 52.Bonelli RM, Hodl AK, Hofmann P, Kapfhammer HP. Neuroprotection in huntington’s disease: A 2-year study on minocycline. Int Clin Psychopharmacol. 2004;19:337–342. doi: 10.1097/00004850-200411000-00004. [DOI] [PubMed] [Google Scholar]
- 53.A futility study of minocycline in huntington’s disease. Mov Disord. 2010;25:2219–2224. doi: 10.1002/mds.23236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saivin S, Houin G. Clinical pharmacokinetics of doxycycline and minocycline. Clin Pharmacokinet. 1988;15:355–366. doi: 10.2165/00003088-198815060-00001. [DOI] [PubMed] [Google Scholar]
- 55.Fagan SC, Edwards DJ, Borlongan CV, Xu L, Arora A, Feuerstein G, et al. Optimal delivery of minocycline to the brain: Implication for human studies of acute neuroprotection. Exp Neurol. 2004;186:248–251. doi: 10.1016/j.expneurol.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 56.Light RW, Wang NS, Sassoon CS, Gruer SE, Vargas FS. Comparison of the effectiveness of tetracycline and minocycline as pleural sclerosing agents in rabbits. Chest. 1994;106:577–582. doi: 10.1378/chest.106.2.577. [DOI] [PubMed] [Google Scholar]
- 57.Greenberg SM, Rebeck GW, Vonsattel JP, Gomez-Isla T, Hyman BT. Apolipoprotein e epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann Neurol. 1995;38:254–259. doi: 10.1002/ana.410380219. [DOI] [PubMed] [Google Scholar]
- 58.Rannikmae K, Kalaria RN, Greenberg SM, Chui HC, Schmitt FA, Samarasekera N, et al. Apoe associations with severe caa-associated vasculopathic changes: Collaborative meta-analysis. J Neurol Neurosurg Psychiatry. 2014;85:300–305. doi: 10.1136/jnnp-2013-306485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zlokovic BV. Neurovascular pathways to neurodegeneration in alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Candelario-Jalil E, Thompson J, Taheri S, Grossetete M, Adair JC, Edmonds E, et al. Matrix metalloproteinases are associated with increased blood-brain barrier opening in vascular cognitive impairment. Stroke. 2011;42:1345–1350. doi: 10.1161/STROKEAHA.110.600825. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.