Abstract
Background and purpose
While left atrial enlargement (LAE) increases incident stroke risk, the association with recurrent stroke is less clear. Our aim was to determine the association of LAE with recurrent stroke most likely related to embolism (cryptogenic and cardioembolic), and all ischemic stroke recurrences.
Methods
We followed 655 first ischemic stroke patients in the Northern Manhattan Stroke Study for up to 5 years. LA size from 2-D echocardiography was categorized as normal (52.7%), mild LAE (31.6%), and moderate-severe LAE (15.7%). We used Cox proportional hazard models to calculate the hazard ratios and 95% confidence intervals (HR, 95%CI) for the association of LA size and LAE with recurrent cryptogenic/cardioembolic and total recurrent ischemic stroke.
Results
LA size was available in 529 (81%) patients. Mean age at enrollment was 69±13 years; 45.8% were male, 54.0% Hispanic, and 18.5% had atrial fibrillation. Over a median of 4 years there were 65 recurrent ischemic strokes (29 were cardioembolic or cryptogenic). In multivariable models adjusted for confounders including atrial fibrillation and heart failure, moderate-severe LAE compared to normal LA size was associated with greater risk of recurrent cardioembolic/cryptogenic stroke (adjusted HR 2.83, 95% CI 1.03-7.81), but not total ischemic stroke (adjusted HR 1.06, 95% CI, 0.48-2.30). Mild LAE was not associated with recurrent stroke.
Conclusion
Moderate to severe LAE was an independent marker of recurrent cardioembolic or cryptogenic stroke in a multiethnic cohort of ischemic stroke patients. Further research is needed to determine whether anticoagulant use may reduce risk of recurrence in ischemic stroke patients with moderate to severe LAE.
Keywords: Ischemic stroke, left atrial enlargement, embolism, stroke recurrence
Introduction
Left atrial enlargement (LAE) is associated with the risk of first ischemic stroke, 1, 2 subclinical cerebrovascular disease3, paroxysmal atrial fibrillation (AF) in the general population4, 5, and detection of AF in patients with cryptogenic stroke.6-9 These associations suggest that LAE, AF, and stroke could share a common disease pathway. Several gaps in knowledge persist, however. Evidence from large population-based studies suggests that LAE is associated with incident stroke independently of diagnosed AF.1, 2 Prior studies have been limited by the lack of consideration of stroke subtypes; as a result less is known whether LAE is associated with incident cryptogenic stroke, which is thought to often arise from embolism.10, 11 Lastly, though LAE is associated with detection of AF, it is less clear if LAE increases the risk of stroke recurrence. A better understanding of the relationship between LAE and stroke risk may target prolonged monitoring strategies for AF and potentially improve secondary stroke prevention strategies. We hypothesized that LAE on echocardiography in ischemic stroke patients is associated with a higher risk of stroke recurrence, particularly of the subtypes likely related to embolism (cryptogenic and cardioembolic stroke).
Methods
Study Population
The Northern Manhattan Stroke Study (NOMASS) was designed to determine predictors of stroke recurrence and prognosis in a multi-ethnic, urban population. The methods of patient identification and enrollment of this cohort (n=655) have been described previously.12 Patients were enrolled in NOMASS if: (1) age was > 40 years; (2) they had a diagnosis of first stroke; and (3) they lived in Northern Manhattan for more than 3 months in a household with a telephone. Only patients with ischemic strokes were included in our study. Patient evaluation was conducted at Columbia University Medical Center (CUMC). The cohort for this study represents a community-based cohort of stroke patients, and therefore stroke patients were evaluated according to the practice of their local treating neurologists. While systematic screening for paroxysmal atrial fibrillation was not performed, hospitalized patients underwent EKG and cardiac telemetry. Participants who were either not hospitalized (5%) or hospitalized elsewhere were evaluated in the outpatient research clinic or by their primary physician and/or community health clinic. Patients were interviewed at 6 months and then annually for up to 5 years, and new diagnoses such as atrial fibrillation were ascertained through interviews of patients and caregivers, physicians and other providers, review of medical records and discharge reports of interval hospitalizations. Patients unable or unwilling to come to the medical center were visited by a member of the research staff, and the evaluation was conducted at home or in an alternative place of residence (e.g., nursing home). An ongoing surveillance system of admissions to CUMC was used to identify study participants who were admitted for any reason. The surveillance included other local hospitals to identify study participants who experienced recurrent stroke, MI, hospitalization, or death, and when available, their medical records were reviewed for all outcome events including death.
The study was approved by the Institutional Review Board at CUMC. All participants gave consent directly or through a surrogate when appropriate.
Echocardiographic Measurements
Transthoracic echocardiography (TTE) was performed as part of routine clinical care within three months from stroke onset. Studies were performed and measurements taken according to the guidelines of the American Society of Echocardiography13. In particular, left atrial anteroposterior diameter was measured in parasternal long-axis view at the level of the aortic valve according to a leading edge–to–leading edge convention. The measurement was replaced by qualitative assessment of LA size when an accurate measurement was not possible; the agreement between measurement and qualitative assessment in patients who had both was excellent (Cronbach alpha = 0.92) using the LA categorization that follows. Left atrial size was categorized into four groups according to left atrial diameter and gender: normal left atrial size in mm (Women: ≤ 38, Men: ≤ 40), mild LAE (Women: 39-42, Men: 41- 46), moderate LAE (Women: 43-46, Men: 47-51), and severe LAE (Women: ≥ 47, Men: ≥ 52)14. We combined moderate and severe LAE into one category due to the expected small numbers of patients with severe LAE.2 The interpretation of the echocardiographic studies was blinded to stroke recurrence.
Covariates
We adjusted for the following potential confounders in the association between LAE and stroke recurrence: baseline demographics (age, sex, and race-ethnicity), and risk factors at the time of the incident stroke evaluation (hypertension, diabetes, hyperlipidemia, smoking, AF, and congestive heart failure).
Recurrent stroke
Annual follow-up for 5 years after index stroke assessed vital status as well as interval hospitalization or illness and specifically symptoms indicative of ischemic stroke. A suspected ischemic stroke was followed up by record review to confirm whether an outcome had occurred. We also prospectively screened all discharges from CUMC to detect hospitalizations and outcomes that may not have been captured by interview. Stroke was defined as the first symptomatic occurrence of fatal and non-fatal ischemic stroke according to the World Health Organization criteria.15 Ischemic stroke subtype was classified by a consensus of two neurologists, with a third neurologist adjudicating if needed16, based on the TOAST (Trial of Org 10172 in Acute Stroke Treatment) criteria.17
Statistical Analysis
Distributions of baseline characteristics were summarized and compared by LAE categories using Chi-square tests for categorical and Kruskal-Wallis tests for continuous variables. The primary outcome was total recurrent ischemic stroke and the secondary outcome was the combined recurrent cryptogenic or cardioembolic stroke subtypes. The primary predictor was LAE categories and the secondary predictor was LA size as a continuous variable. Cox proportional hazard models were fitted to estimate hazard ratios and 95% confidence intervals (HR, 95% CI) for the association between left atrial size and the risk of recurrent stroke, unadjusted and adjusted for demographic characteristics (age, sex, race-ethnicity) and risk factors (hypertension, diabetes, hyperlipidemia, history of AF and congestive heart failure). We tested for violations of the proportionality assumption. Sensitivity analyses were performed by additionally adjusting for body mass index (BMI), systolic blood pressure, diastolic blood pressure, and stroke prevention strategies such as antihypertensive and anticoagulant medications on discharge. We tested whether the associations between LA size and recurrent stroke were modified by baseline AF with an interaction term, and further sensitivity analyses were performed by excluding those with AF at baseline. Statistical analyses were conducted using SAS software (Version 8.2, SAS Institute, Cary, NC); P<0.05 was considered statistically significant.
Results
Among 655 patients with a first ischemic stroke, LA size data was available in 529 (80.7%). Patients without echocardiography were less likely to be Hispanic (p for difference=0.05) and more likely to have cryptogenic stroke (p for difference = 0.01), but there were no other significant differences in characteristics including follow-up time and recurrent stroke incidence rate between the two groups (Table 1). The mean LA diameter was 40.6 mm (SD 6.3). Mean age at the time of stroke was 69 years; 45.8% were male, 18.2% non-Hispanic white, 25.5% non-Hispanic black, 54.0% Hispanic, and 18.5% had history of atrial fibrillation and 13.1% had congestive heart failure (Table 1). There were 279 (52.7%) with normal LA size, 167 (31.6%) with mild LAE, and 83 (15.7%) with moderate to severe LAE.
Table 1.
Baseline demographics of patients with and without echocardiogram at baseline
| Patients with echocardiogram at baseline (analytic subcohort) | Patients without echocardiogram at baseline (excluded from analysis) | p-value* | |
|---|---|---|---|
| Number of participants (n)* | 529 | 126 | |
| Demographics: | |||
| Age, mean ±SD, (in years) | 69.1 ± 12.6 | 71.9 ± 12.5 | 0.07 |
| Male, N (%) | 242 (45.8) | 50 (39.7) | 0.22 |
| Race- Ethnicity | |||
| Non-Hispanic White, N (%) | 96 (18.1) | 28 (22.2) | ref |
| Non-Hispanic Black, N (%) | 135 (25.5) | 46 (36.5) | 0.57 |
| Hispanic, N (%) | 286 (54.1) | 50 (39.7) | 0.05 |
| Risk factors and medical history (%): | |||
| Hypertension | 442 (83.6) | 104 (82.5) | 0.78 |
| Diabetes mellitus | 237 (44.8) | 58 (46.0) | 0.80 |
| Congestive heart failure | 96 (18.2) | 30 (23.8) | 0.15 |
| Atrial fibrillation | 97 (17.8) | 25 (19.8) | 0.59 |
| Current smoking | 108 (20.5) | 24 (19.1) | 0.71 |
| History of hypercholesterolemia | 265 (50.1) | 64 (50.8) | 0.89 |
| Stroke etiologic subtypes, N (%) | |||
| Atherosclerotic | 88 (16.6) | 12 (9.5) | 0.05 |
| Lacunar | 129 (24.4) | 23 (18.3) | 0.14 |
| Cardioembolic | 105 (19.7) | 22 (17.5) | 0.54 |
| Cryptogenic | 193 (36.5) | 63 (50.0) | 0.01 |
| Recurrent ischemic stroke, (N) % | 65 (12.3) | 16 (12.7) | 0.9 |
| Follow-up time median (inter quartile range), (in year) | 4.0 (1.3 – 5.0) | 3.0 (0.9 – 5.0) | 0.18 |
Kruskal-Wallis test for continuous variables and chi-square with 1DF test for categorical variables were used.
Over a median of 4 years (interquartile range 1.3-5.0 years) of follow-up, recurrent stroke occurred in 80 patients (15%), of which 65 were confirmed to be ischemic in origin and were included in the primary analysis; 13 were cardioembolic (16.3%), 16 cryptogenic (20.0%), 12 large artery atherosclerosis (15.0%), and 15 lacunar (18.8%). None of the patients with cryptogenic stroke at baseline and recurrent stroke were found to have AF during annual follow-up. Baseline demographics based on left atrial size category are listed in table 2.
Table 2.
Baseline demographics based on the left atrial size categories
| Overall (n=529) | Normal n=279 (52.7%) | Mild LAE n=167 (31.6%) | Moderate-Severe LAE N=83 (15.7%) | P-value* | |
|---|---|---|---|---|---|
| Age (Mean ± SD) | 69 ± 13 | 68 ± 12 | 69 ± 13 | 75 ± 13 | < 0.001 |
| Sex (% men) | 45.8 | 39.8 | 54.5 | 48.2 | 0.0094 |
| Race-Ethnicity | |||||
| White (%) | 18.2 | 12.9 | 22.8 | 26.5 | ref |
| Black (%) | 25.5 | 28.3 | 24.0 | 19.3 | 0.0045 |
| Hispanic (%) | 54.1 | 57.4 | 49.1 | 53.0 | 0.0073 |
| Diabetes (%) | 44.8 | 41.6 | 52.7 | 39.8 | 0.0443 |
| Hypertension (%) | 83.6 | 82.4 | 82.6 | 89.2 | 0.3243 |
| Congestive heart failure (%) | 13.1 | 7.9 | 16.2 | 24.4 | 0.0002 |
| Hypercholesterolemia (%) | 50.1 | 51.3 | 54.5 | 37.4 | 0.0328 |
| Body Mass Index (median) | 25.9 | 25.6 | 26.5 | 26.2 | 0.06 |
| Smoking status | |||||
| Never | 46.0 | 43.8 | 42.7 | 59.8 | ref |
| Past | 33.3 | 31.9 | 37.8 | 29.3 | 0.1471 |
| Current | 20.7 | 24.3 | 19.5 | 11.0 | 0.0151 |
Kruskal-Wallis test for continuous variables and chi-square with 2 DF test for categorical variables were used.
Association with total recurrent ischemic stroke
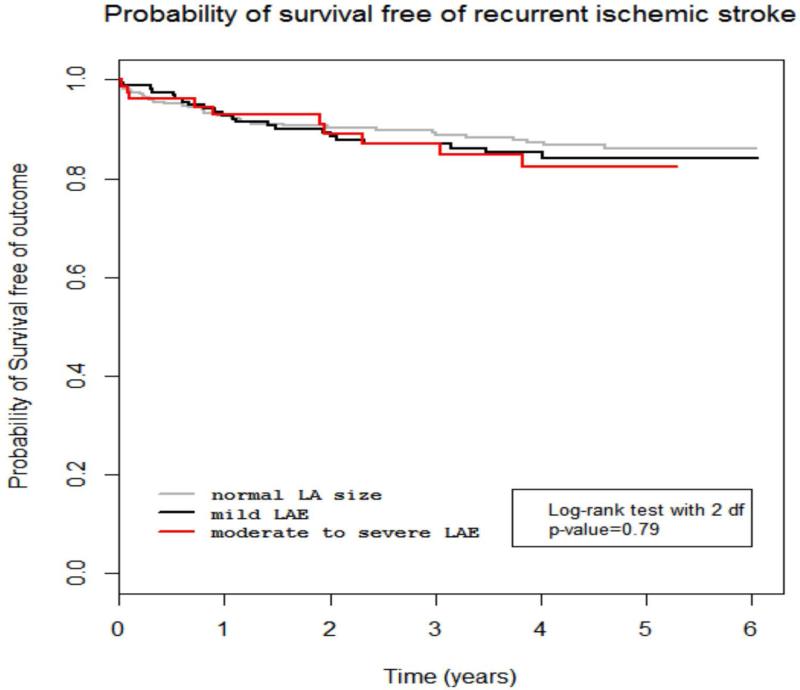
In univariate and adjusted models LA diameter was not associated with the risk of total recurrent ischemic stroke (Table 3). There was also no association between moderate to severe LAE and total recurrent ischemic stroke (unadjusted HR 1.24, 95% CI 0.61-2.52; adjusted HR 1.06, 95% CI, 0.48-2.30). Kaplan-Meier survival analysis is displayed in Figure 1. Furthermore, there was no interaction between LAE and atrial fibrillation (p=0.97), suggesting that the effect of LAE on recurrent ischemic stroke was not modified by atrial fibrillation. The results remained unchanged after excluding those with atrial fibrillation (data not shown).
Table 3.
Association of left atrial size and recurrent ischemic stroke and recurrent cryptogenic or cardioembolic stroke
| Left atrial size | Model 1, Unadjusted, Hazard ratio (95% confidence interval) | Model 2* Hazard ratio (95% confidence interval) | Model 3** Hazard ratio (95% confidence interval) | |
|---|---|---|---|---|
| Recurrent ischemic stroke of any subtype | Normal Left atrial size | reference | reference | reference |
| Mild left atrial enlargement | 1.15 (0.67-1.97) | 1.10 (0.63-1.90) | 1.06 (0.60-1.87) | |
| Moderate to severe left atrial enlargement | 1.24 (0.61-2.52) | 1.06 (0.51-2.19) | 1.06 (0.48-2.30) | |
| Left atrial size (per SD+ increase) | 1.09 (0.84-1.41) | 1.03 (0.79-1.34) | 1.03 (0.76-1.38) | |
| Recurrent cardioembolic or cryptogenic stroke | Normal left atrial size | reference | reference | reference |
| Mild left atrial enlargement | 1.56 (0.64-3.84) | 1.53 (0.61-3.80) | 1.32 (0.51-3.39) | |
| Moderate to severe left atrial enlargement | 4.35 (1.81-10.48) | 3.83 (1.54-9.54) | 2.83 (1.03-7.81) | |
| Left atrial size (per SD+ increase) | 1.76 (1.25-2.48) | 1.73 (1.21-2.49) | 1.55 (1.01-2.37) |
SD: Standard Deviation
Adjusted for age, sex, and race-ethnicity;
Adjusted for age, sex, race-ethnicity, hypertension, diabetes, hypercholesterolemia, smoking, atrial fibrillation, and congestive heart failure
Figure 1.
Probability of survival free of total recurrent ischemic stroke for normal left atrial size, mild left atrial enlargement, and moderate to severe left atrial enlargement.
Association with combined recurrent cardioembolic or cryptogenic stroke subtype
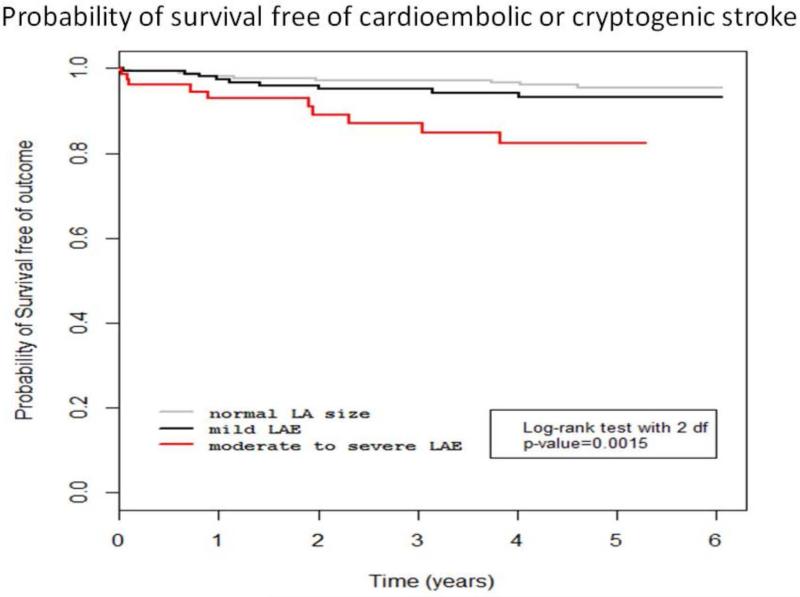
In an unadjusted model, compared to those with normal LA size, those with moderate to severe LAE had greater risk of recurrent combined cardioembolic or cryptogenic stroke (unadjusted HR 4.35, 95% CI 1.81-10.48). After adjusting for baseline demographics and risk factors, including AF and congestive heart failure, the associations persisted (adjusted HR 2.83, 95% CI 1.03 to 7.81) (Table 3). Mild LAE was not associated with combined recurrent cryptogenic or cardioembolic stroke subtypes (adjusted HR 1.32, 95% CI 0.51 to 3.39). The Kaplan-Meier survival analysis is displayed in Figure 2.
Figure 2.
Probability of survival free of recurrent cryptogenic/cardioembolic stroke for normal left atrial size, mild left atrial enlargement, and moderate to severe left atrial enlargement.
LA diameter as a continuous variable was also associated with greater risk of combined recurrent cardioembolic or cryptogenic stroke (unadjusted HR 1.76 per SD change in LA diameter, 95% CI 1.25-2.48; adjusted HR 1.55 per SD change in LA diameter, 95%CI 1.01-2.37).
There was no interaction between LAE and AF (p=0.31), suggesting that the effect of LAE on combined recurrent cryptogenic or cardioembolic stroke subtypes was not modified by atrial fibrillation. After excluding patients with a history of AF in sensitivity analyses, those with moderate to severe LAE (vs. normal LA size) had a trend toward greater risk of recurrent cardioembolic or cryptogenic stroke (adjusted HR 2.77, 95%CI 0.74-10.35), and the association of continuous LA size with recurrent cardioembolic or cryptogenic stroke remained significant (adjusted HR 1.85, 95%CI 1.03-3.33). None of the patients with moderate to severe atrial enlargement and non-cardioembolic stroke at baseline were found to have AF at the time of the second stroke.
Sensitivity Analyses
Sensitivity analyses were performed adjusting for body mass index, systolic blood pressure, diastolic blood pressure, and antihypertensive medications, and we found similar results (supplementary table I). In addition, in a sensitivity analysis adjusting for anticoagulant use on discharge, the association between moderate to severe LAE and recurrent cryptogenic/cardioembolic stroke remained significant (adjusted HR 3.44, 95%CI 1.14-10.31) (supplemental table I).
Discussion
In a multiethnic population, we found that those with moderate to severe LAE identified on echocardiography performed for clinical purposes was associated with greater risk of recurrent cryptogenic or cardioembolic stroke than those with normal LA size. This association persisted even after adjusting for baseline demographics and established stroke risk factors, including the two most likely potential confounders, history of AF and congestive heart failure. In sensitivity analyses excluding the 97 participants with atrial fibrillation at baseline, we found a similar effect size, though the association did not reach statistical significance, probably because of reduced power.
We focused our analysis on the combined outcome of recurrent cryptogenic or cardioembolic stroke because of the expectation that these two subtypes may share a common embolic mechanism. It is increasingly recognized that among patients with unexplained superficial hemispheric infarcts and no significant arterial stenosis or definite cardiac source, lower risk or unrecognized cardiac sources of embolism are likely explanations for the stroke.10, 11, 18
The explanation of the association of LAE with recurrent cryptogenic/cardioembolic stroke could be due to several reasons. The mechanism of stroke recurrence in patients with moderate to severe LAE could be at least partially explained by concomitant AF. In our study, however, the association between recurrent stroke risk and left atrial dilatation remained even after adjusting for history of atrial fibrillation. We acknowledge, however, that our measures of atrial fibrillation are imperfect, and subsequent AF after stroke could also explain our outcome. For example, recent research suggests that prolonged AF monitoring can increase the detection of paroxysmal atrial fibrillation19-22, but it was not routinely performed in our cohort, so we may have underestimated the prevalence of atrial fibrillation. However, none of the patients who had cryptogenic stroke at baseline and subsequent recurrent stroke were found to have atrial fibrillation during follow up. Another proposed mechanism is that an increase in left atrial volume produces reduced flow velocity in the left atrial appendage,23 therefore contributing to stasis and predisposing to clot formation. This is supported by transesophageal echocardiographic data suggesting an association between left atrial dilatation and spontaneous echocardiographic contrast, and embolic events1. However, transesophageal echocardiography was not routinely performed in our study cohort to confirm this hypothesis. Another potential explanation is that because LAE is a manifestation of organ damage secondary to chronic hypertension, the increased stroke risk is instead a reflection of the well-established association of hypertension with recurrent stroke. Within our study, however, the association between LAE and stroke risk was independent of hypertension, arguing against this being an explanation for our findings. We cannot exclude residual confounding as an explanation, however.
Although atrial fibrillation is the most robust marker of left atrial dysfunction associated with ischemic stroke, other markers of left atrial dysfunction such as elevated N-terminal pro-Brain Natriuretic Peptide (NT pro-BNP), p-wave terminal force in lead V1 (PTFV1) of a 12-lead electrocardiogram 24, 25, and paroxysmal supraventricular tachycardia (PSVT),26 have been associated with increased stroke risk independent of atrial fibrillation. Post-hoc analysis of the Warfarin-Aspirin Recurrent Stroke Study (WARSS) study, moreover, showed that treatment with warfarin was superior to aspirin in reducing risk of stroke and death among the 5% of stroke patients, none of whom had atrial fibrillation at onset, with the most highly elevated NT pro-BNP levels 27, suggesting that biomarkers of atrial dysfunction may have therapeutic implications. Similarly, left atrial enlargement may serve as one of the markers of atrial cardiopathy that is also associated with increased risk of embolic strokes.24 These results may thus spur future research to determine whether anticoagulant use reduces risk of recurrence in ischemic stroke patients with moderate to severe LAE.
Limitations and Strengths
Our study has several limitations. First, we were missing LA size values on 19% of patients; however, baseline characteristics as well as follow-up time and incidence rate of recurrent stroke were more or less similar between the groups with and without LA size available, which suggests that no selection bias was introduced. Second, we did not routinely monitor our cohort for AF on follow up visits and thus paroxysmal atrial fibrillation may have been under-detected. Third, the categorization of left atrial size measurements was based on clinical readings and not standardized measurements from a core lab, however the simple linear measurement of LA diameter used has good reproducibility and generalizability14 in the echocardiographic evaluation of stroke patients whose left atrial size measurements is also based on clinical readings. In addition, our study lacked data on LA volume, which is a more accurate way to assess LA size, and is more strongly associated with cardiovascular events. Having found an effect for LA diameter, however, it is likely that such effects would have been even stronger had we used LA volume.
Strengths of our study include the fact that it is a prospective multi-ethnic study with a relatively long follow-up, making the results robust and generalizable. Furthermore, we collected data on a wide range of potentially confounding risk factors, allowing us to estimate the independent effect of LAE. Our study also included data about stroke subtype using TOAST criteria and studied the association between left atrial size and recurrent stroke subtype.
Conclusion
Moderate to severe LAE is an independent marker of recurrent stroke of embolic subtypes in patients with ischemic stroke. Future studies are needed to improve stroke secondary prevention strategies in patients with moderate to severe LAE to reduce their risk of embolic stroke.
Supplementary Material
Footnotes
Disclosures:
Dr. Yaghi received finding from NINDS StrokeNet. Dr. Elkind Dr. Mitchell S. V. Elkind receives compensation for providing consultative services for Biotelemetry/Cardionet, BMS-Pfizer Partnership, Biogen IDEC, Boehringer-Ingelheim, Daiichi-Sankyo, and Janssen Pharmaceuticals; serves on the National, Founders Affiliate, and New York City chapter boards of the American Heart Association/American Stroke Association; and receives royalties from UpToDate for a chapter related to cryptogenic stroke. Dr. Willey received funding from the NIH and is a consultant for heartware incorporated. Dr. Homma is a consultant for St. Judes Medical, BMS/Pfizer, and Daiichi Sankyo. Dr. Sacco received funding from NINDS.
References
- 1.Benjamin EJ, D'Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The framingham heart study. Circulation. 1995;92:835–841. doi: 10.1161/01.cir.92.4.835. [DOI] [PubMed] [Google Scholar]
- 2.Di Tullio MR, Sacco RL, Sciacca RR, Homma S. Left atrial size and the risk of ischemic stroke in an ethnically mixed population. Stroke; a journal of cerebral circulation. 1999;30:2019–2024. doi: 10.1161/01.str.30.10.2019. [DOI] [PubMed] [Google Scholar]
- 3.Russo C, Jin Z, Liu R, Iwata S, Tugcu A, Yoshita M, et al. La volumes and reservoir function are associated with subclinical cerebrovascular disease: The cabl (cardiovascular abnormalities and brain lesions) study. JACC. Cardiovascular imaging. 2013;6:313–323. doi: 10.1016/j.jcmg.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wozakowska-Kaplon B. Changes in left atrial size in patients with persistent atrial fibrillation: A prospective echocardiographic study with a 5-year follow-up period. International journal of cardiology. 2005;101:47–52. doi: 10.1016/j.ijcard.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Mattioli AV, Sansoni S, Lucchi GR, Mattioli G. Serial evaluation of left atrial dimension after cardioversion for atrial fibrillation and relation to atrial function. The American journal of cardiology. 2000;85:832–836. doi: 10.1016/s0002-9149(99)00876-0. [DOI] [PubMed] [Google Scholar]
- 6.Fujii S, Shibazaki K, Kimura K, Sakai K, Aoki J. A simple score for predicting paroxysmal atrial fibrillation in acute ischemic stroke. Journal of the neurological sciences. 2013;328:83–86. doi: 10.1016/j.jns.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 7.Shin HY, Jeong IH, Kang CK, Shin DJ, Park HM, Park KH, et al. Relation between left atrial enlargement and stroke subtypes in acute ischemic stroke patients. Journal of cerebrovascular and endovascular neurosurgery. 2013;15:131–136. doi: 10.7461/jcen.2013.15.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stahrenberg R, Edelmann F, Haase B, Lahno R, Seegers J, Weber-Kruger M, et al. Transthoracic echocardiography to rule out paroxysmal atrial fibrillation as a cause of stroke or transient ischemic attack. Stroke; a journal of cerebral circulation. 2011;42:3643–3645. doi: 10.1161/STROKEAHA.111.632836. [DOI] [PubMed] [Google Scholar]
- 9.Haft JI, Teichholz LE. Echocardiographic and clinical risk factors for atrial fibrillation in hypertensive patients with ischemic stroke. The American journal of cardiology. 2008;102:1348–1351. doi: 10.1016/j.amjcard.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Sacco RL, Ellenberg JH, Mohr JP, Tatemichi TK, Hier DB, Price TR, et al. Infarcts of undetermined cause: The nincds stroke data bank. Annals of neurology. 1989;25:382–390. doi: 10.1002/ana.410250410. [DOI] [PubMed] [Google Scholar]
- 11.Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O'Donnell MJ, et al. Embolic strokes of undetermined source: The case for a new clinical construct. Lancet neurology. 2014;13:429–438. doi: 10.1016/S1474-4422(13)70310-7. [DOI] [PubMed] [Google Scholar]
- 12.Elkind MS, Cheng J, Rundek T, Boden-Albala B, Sacco RL. Leukocyte count predicts outcome after ischemic stroke: The northern manhattan stroke study. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2004;13:220–227. doi: 10.1016/j.jstrokecerebrovasdis.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Picard MH, Adams D, Bierig SM, Dent JM, Douglas PS, Gillam LD, et al. American society of echocardiography recommendations for quality echocardiography laboratory operations. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2011;24:1–10. doi: 10.1016/j.echo.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification. European journal of echocardiography : the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Organisation WH. Proposal for the multinational monitoring of trends and determinants in cardiovascular disease ( monica project) 1983 WHO/MNC/82.1 Rev 1. [Google Scholar]
- 16.Gan R, Sacco RL, Kargman DE, Roberts JK, Boden-Albala B, Gu Q. Testing the validity of the lacunar hypothesis: The northern manhattan stroke study experience. Neurology. 1997;48:1204–1211. doi: 10.1212/wnl.48.5.1204. [DOI] [PubMed] [Google Scholar]
- 17.Adams HP, Jr., Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke; a journal of cerebral circulation. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 18.Ntaios G, Papavasileiou V, Milionis H, Makaritsis K, Manios E, Spengos K, et al. Embolic strokes of undetermined source in the athens stroke registry: A descriptive analysis. Stroke; a journal of cerebral circulation. 2015;46:176–181. doi: 10.1161/STROKEAHA.114.007240. [DOI] [PubMed] [Google Scholar]
- 19.Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J, et al. Atrial fibrillation in patients with cryptogenic stroke. The New England journal of medicine. 2014;370:2467–2477. doi: 10.1056/NEJMoa1311376. [DOI] [PubMed] [Google Scholar]
- 20.Bhatt A, Majid A, Razak A, Kassab M, Hussain S, Safdar A. Predictors of occult paroxysmal atrial fibrillation in cryptogenic strokes detected by long-term noninvasive cardiac monitoring. Stroke research and treatment. 2011;2011:172074. doi: 10.4061/2011/172074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rizos T, Guntner J, Jenetzky E, Marquardt L, Reichardt C, Becker R, et al. Continuous stroke unit electrocardiographic monitoring versus 24-hour holter electrocardiography for detection of paroxysmal atrial fibrillation after stroke. Stroke; a journal of cerebral circulation. 2012;43:2689–2694. doi: 10.1161/STROKEAHA.112.654954. [DOI] [PubMed] [Google Scholar]
- 22.Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, et al. Cryptogenic stroke and underlying atrial fibrillation. The New England journal of medicine. 2014;370:2478–2486. doi: 10.1056/NEJMoa1313600. [DOI] [PubMed] [Google Scholar]
- 23.Tabata T, Oki T, Fukuda N, Iuchi A, Manabe K, Kageji Y, et al. Influence of left atrial pressure on left atrial appendage flow velocity patterns in patients in sinus rhythm. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 1996;9:857–864. doi: 10.1016/s0894-7317(96)90478-2. [DOI] [PubMed] [Google Scholar]
- 24.Kamel H, Soliman EZ, Heckbert SR, Kronmal RA, Longstreth WT, Jr., Nazarian S, et al. P-wave morphology and the risk of incident ischemic stroke in the multi-ethnic study of atherosclerosis. Stroke; a journal of cerebral circulation. 2014;45:2786–2788. doi: 10.1161/STROKEAHA.114.006364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohsaka S, Sciacca RR, Sugioka K, Sacco RL, Homma S, Di Tullio MR. Electrocardiographic left atrial abnormalities and risk of ischemic stroke. Stroke; a journal of cerebral circulation. 2005;36:2481–2483. doi: 10.1161/01.STR.0000185682.09981.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamel H, Elkind MS, Bhave PD, Navi BB, Okin PM, Iadecola C, et al. Paroxysmal supraventricular tachycardia and the risk of ischemic stroke. Stroke; a journal of cerebral circulation. 2013;44:1550–1554. doi: 10.1161/STROKEAHA.113.001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longstreth WT, Jr., Kronmal RA, Thompson JL, Christenson RH, Levine SR, Gross R, et al. Amino terminal pro-b-type natriuretic peptide, secondary stroke prevention, and choice of antithrombotic therapy. Stroke; a journal of cerebral circulation. 2013;44:714–719. doi: 10.1161/STROKEAHA.112.675942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.