Abstract
Background and Purpose
This study examines the role of thrombin’s protease-activated receptors (PAR)-1,-4 in mediating cyclooxygenase (COX)-2 and mammalian target of rapamycin (mTOR) following germinal matrix hemorrhage (GMH).
Methods
GMH was induced by intraparenchymal infusion of bacterial collagenase into the right ganglionic eminence of P7 rat pups. Animals were treated with either PAR-1, -4, COX-2, or mTOR inhibitors by 1 hour, and up to five days.
Results
We found increased thrombin activity 6–24 hrs after GMH, and PAR-1, -4, inhibition normalized COX-2 and mTOR by 72 hrs. Early treatment with NS398 or rapamycin substantially improved long-term outcomes in juvenile animals.
Conclusions
Suppressing early PAR signal transduction, and postnatal NS398 or rapamycin treatment, may help reduce GMH severity in susceptible preterm infants.
Keywords: Stroke, experimental; Neonatal rats; Germinal Matrix Hemorrhage; Hydrocephalus; Neurological dysfunction
Introduction
Germinal matrix hemorrhage (GMH) is the leading cause of mortality and morbidity from prematurity, since this brain region is selectively vulnerable to spontaneous bleeding within the first 72 hours of preterm life.1 Cerebroventricular expansion contributes to long-term injury through mechanical compression of surrounding brain tissues.2 Neurological outcomes include hydrocephalus, mental retardation, and cerebral palsy.1, 3 Current neonatal intensive care treatments are ineffective at preventing GMH, and neurosurgical shunts are prone to devastating complications.4
Importantly, the blood constituent thrombin has been identified as a causative factor in hydrocephalus formation.5 Thrombin activates a subfamily of G protein-coupled receptors, named proteinase-activated-receptors (PAR; specifically -1 and -4),6 leading to phosphorylation and activation of mTOR7 and increased COX-2 expression.8
Therefore, we hypothesized that modulation of the thrombin- (PAR)-1,-4 – (COX)-2/ mTOR pathway could be a promising strategy to improve outcomes after GMH.
Materials and Methods
Animal Surgeries
All studies, protocols and procedures were approved by the Loma Linda University IACUC. One hundred and fifty-seven P7 rat pups (comparable to human 30–32 gestational weeks9; 14–19 g; Harlan Laboratories, Indianapolis, IN) were randomly subjected to either GMH or sham operations. GMH was induced by a stereotactically guided infusion (using bacterial collagenase, denatured collagenase, blood, or thrombin) to mimic preterm right-sided ganglionic eminence bleeds.9 Details are in the Data Supplement.
Tissue Processing and Analysis
Rats were euthanized at 72 hrs (for western blot), at various time points between 6 hrs and 21 days (for thrombin assay), and after 28 days (for neuropathological analysis) post-GMH, and analyzed by blinded experts.9–11 Details are in the Data Supplement.
Animal Treatments and Experimental Groups
For the 72 hrs (short-term; n=49) western blot study: GMH animals received i.p. co-injections of PAR-1 (SCH79797) and PAR-4 (P4pal-10) antagonists (1, 3, 7, 10, or 15 mg/kg given 1, 24, and 48 hrs post-GMH). For thrombin assay time-course study (n=49, n=7/per time point); GMH animals were euthanized at 0hr, 6 hrs, 1 d, 5 d, 7 d, 10 d, and 21 d after GMH. For the 28 day (long-term; n=26) study: COX-2 (NS398) or mTOR (rapamycin) treatment consisted of six i.p. injections (1, 6, 24, 36, 48, and 60 hrs after GMH). Inhibitors were prepared using distilled water containing five percent Dimethyl Sulfoxide (DMSO) solvent. Controls received the vehicle (5% DMSO). All drugs were purchased from Sigma-Aldrich (St. Louis, MO).10–11
Assessment of Neurological Deficits
Cognitive (T-maze, Water-maze) and sensorimotor (Rotarod, Foot Fault) testing from 21 to 28 days post-GMH was performed by experienced blinded investigators as described.9–11 Details in the Data Supplement.
Statistical Analysis
P values of less than 0.05 were considered statistically significant. Neurobehavioral data was analyzed using one-way ANOVA on ranks with Student–Newman–Keuls post-hoc test. All other data was analyzed by one-way ANOVA with Tukey post-hoc test. Data are expressed as mean±SEM.
Results
Hemorrhage progression and hydrocephalus formation following GMH were shown in Supplemental Figure I, and changes in body weight after GMH was shown in Supplemental Figure II.
GMH activated Thrombin
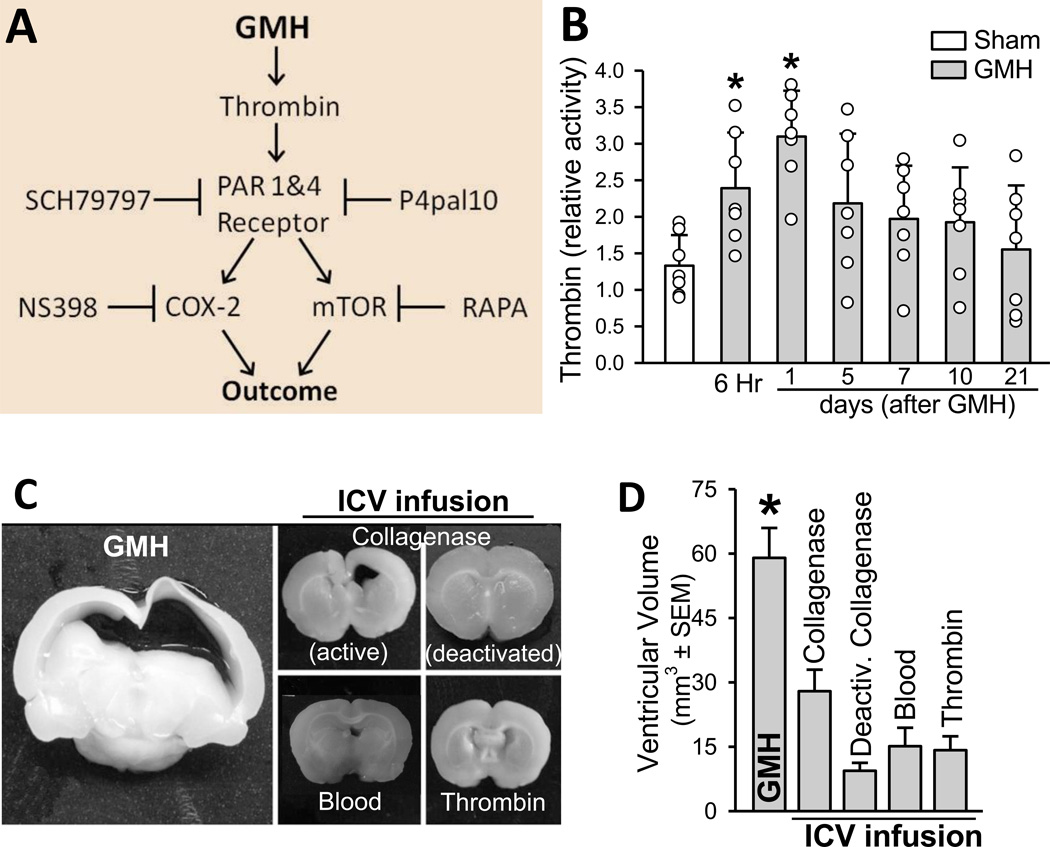
Thrombin activity increased at 6 and 24 hrs following collagenase infusion compared to sham (P < 0.05), and then normalized over 5 to 21 days (Figure 1B).
Figure 1.
A. Proposed mechanism. B. Time-course of thrombin activity (n=7/group; * P < 0.05 compared with sham). C. Representative 2mm coronal brain section pictographs (28 days after infusion). D. Quantification of ventricular volume (n=6/group; * P < 0.05 compared with vehicle). ICV indicates intracerebroventricular; RAPA, rapamycin.
Molecular Mediators of Post-hemorrhagic Hydrocephalus
Post-hemorrhagic hydrocephalus at 28 days was greatest in the group receiving direct intraparenchymal infusion of collagenase into the ganglionic eminence (P < 0.05; Figures 1C, 1D) compared to intracerebroventricular injections of collagenase, heat-deactivated collagenase, donor blood, or thrombin.
Western Blots
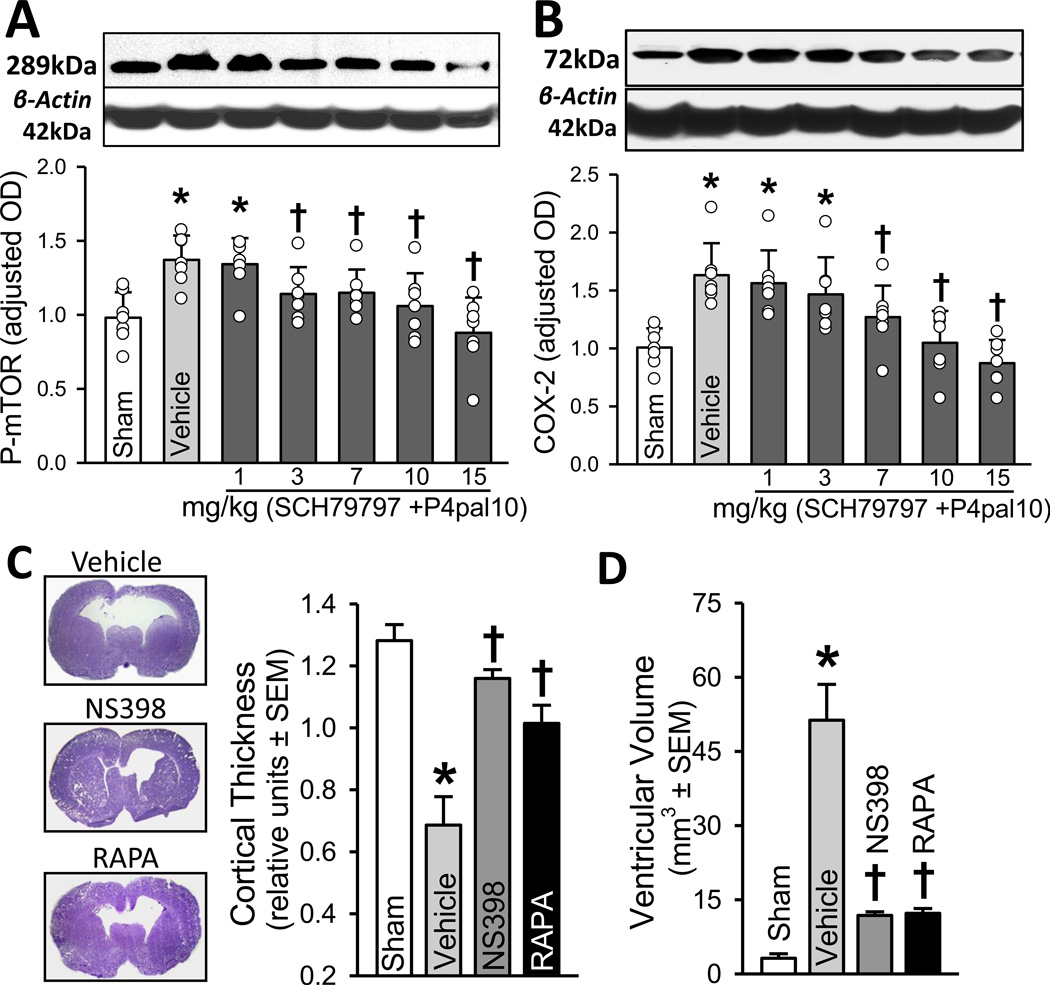
Early protease-activated receptor-1 and -4 signal inhibition reduced mTOR phosphorylation and COX-2 expression (P < 0.05; Figures 2A, 2B) in a dose responsive fashion at 72 hrs post-GMH induction.
Figure 2.
A, B. Western blot analyses of p-mTOR (left) and COX-2 expression (right) at 72 hrs after GMH (n=7/group). C. Representative Nissl stained brain micrograph sections (left), quantification of cortical thickness (right) and D. ventricular volume at 28 days after GMH. * P < 0.05 compared with sham; † P < 0.05 compared with vehicle.
Early Signal Inhibition Improved Long-term Outcome
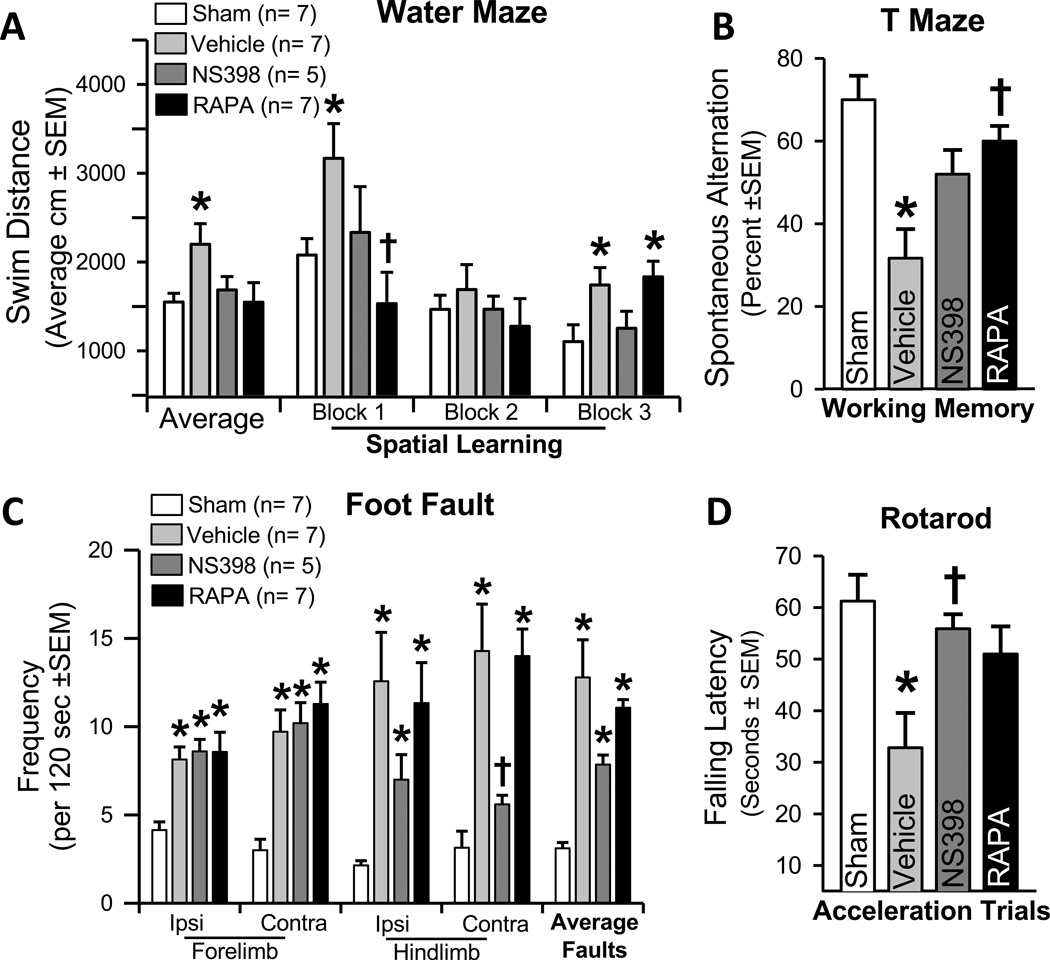
Treatments using either COX-2 or mTOR inhibitors significantly ameliorated long-term cortical thickness, ventricular volume, and neurodeficits (P < 0.05; Figures 2C, 2D, 3A-D) compared to vehicle treated animals at 28 days post-GMH.
Figure 3.
Neurological assessments at 21–28 days after GMH using A. Morris water maze, B. T-maze, C. Foot fault, and D. Rotarod test (n=5–7/group). * P < 0.05 compared with sham; † P < 0.05 compared with vehicle.
Discussion
This study investigated the effectiveness of modulating thrombin- PAR-1 and PAR-4 in reversing COX-2 and p-mTOR upregulation, as well as the effect of direct COX-2 and p-mTOR inhibition upon post-hemorrhagic hydrocephalus, and neurological deficits. Prior studies hypothesized the mechanism of hydrocephalus involved increased production of infiltrating extracellular-matrix (ECM) proteins throughout the cerebroventricular system, leading to the disruption of CSF outflow.1–2, 9, 11–13 Our results suggest that thrombin-induced PAR-1,-4 stimulation up-regulates detrimental signaling: exacerbating inflammatory (i.e. COX-2 mediated) and proliferative responses (i.e. p-mTOR mediated) that are potentially upstream of ECM protein dysregulation.1, 5, 7–9, 11, 14 Multiple parallels (especially thrombin)1, 2, 7–11 exist between our study, and the pathophysiology of adult intracerebral hemorrhage.4, 5, 12–14 Thus in extension: our findings may have a much broader therapeutic implication in terms of further adult stroke mechanistic study (for detailed comparisons, however—please reference15, these fall outside the scope of this paper).
To address the question of molecular mediators of GMH, our first aim demonstrated that intraparenchymal infusion of collagenase generated the majority of hydrocephalus. This is likely the sum contribution of blood products14 (e.g. red blood cell lysis, inflammation) and thrombin. In fact, thrombin demonstrated greatest activity in the acute phase, between 6–24 hrs post-ictus, with tendency to remain elevated up to 10 days, and normalized by 21 days.
We next hypothesized that thrombin binds to PAR-1, -4 receptors, and consequently up-regulates COX-2 and p-mTOR. Since thrombin is most active in the acute phase, we examined levels at 72 hrs post-ictus, and determined COX-2 and p-mTOR were significantly greater in vehicle treated animals compared to sham. Furthermore, inhibiting PAR-1, -4 using SCH79797 (PAR-1 antagonist) and p4pal10 (PAR-4 antagonist) significantly normalized COX-2 and p-mTOR levels at 72 hrs.
Then, we asked if directly inhibiting COX-2 or p-mTOR following GMH could circumvent long-term post-hemorrhagic ventricular dilation, cortical cell loss, as well as improve sensorimotor and neurocognitive outcomes. Our findings demonstrated vehicle treated animals had significantly worsened outcomes compared to shams, and treating with either NS398 (COX-2 inhibitor) or rapamycin (mTOR inhibitor) significantly improved brain neuropathology and neurological ability. Thus, by attenuating early inflammatory (i.e. COX-2), and proliferative (i.e. p-mTOR) signaling pathways, we improved long-term outcome in the juvenile animals.
In summary, this study is the first to show thrombin-PAR-1, -4 signal inhibition normalizing early COX-2 and p-mTOR expression levels, and this in turn, improving long-term neurological outcomes after GMH.
Supplementary Material
Acknowledgments
Sources of Funding
This research was supported by the National Institutes of Health grant RO1 NS078755 to Dr Zhang.
Footnotes
Disclosures: None
References
- 1.Ballabh P. Intraventricular hemorrhage in premature infants: Mechanism of disease. Pediatr Res. 2010;67:1–8. doi: 10.1203/PDR.0b013e3181c1b176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aquilina K, Chakkarapani E, Love S, Thoresen M. Neonatal rat model of intraventricular haemorrhage and post-haemorrhagic ventricular dilatation with long-term survival into adulthood. Neuropathol Appl Neurobiol. 2011;37:156–165. doi: 10.1111/j.1365-2990.2010.01118.x. [DOI] [PubMed] [Google Scholar]
- 3.Heron M, Sutton PD, Xu J, Ventura SJ, Strobino DM, Guyer B. Annual summary of vital statistics: 2007. Pediatrics. 2010;125:4–15. doi: 10.1542/peds.2009-2416. [DOI] [PubMed] [Google Scholar]
- 4.Whitelaw A. Intraventricular haemorrhage and posthaemorrhagic hydrocephalus: Pathogenesis, prevention and future interventions. Semin Neonatol. 2001;6:135–146. doi: 10.1053/siny.2001.0047. [DOI] [PubMed] [Google Scholar]
- 5.Gao F, Liu F, Chen Z, Hua Y, Keep RF, Xi G. Hydrocephalus after intraventricular hemorrhage: The role of thrombin. J Cereb Blood Flow Metab. 2014;34:489–494. doi: 10.1038/jcbfm.2013.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kataoka H, Hamilton JR, McKemy DD, Camerer E, Zheng YW, Cheng A, et al. Coughlin SR. Protease-activated receptors 1 and 4 mediate thrombin signaling in endothelial cells. Blood. 2003;102:3224–3231. doi: 10.1182/blood-2003-04-1130. [DOI] [PubMed] [Google Scholar]
- 7.Jiang X, Zhu S, Panetti TS, Bromberg ME. Formation of tissue factor-factor viia-factor xa complex induces activation of the mtor pathway which regulates migration of human breast cancer cells. Thromb Haemost. 2008;100:127–133. doi: 10.1160/TH07-12-0722. [DOI] [PubMed] [Google Scholar]
- 8.Lo HM, Chen CL, Tsai YJ, Wu PH, Wu WB. Thrombin induces cyclooxygenase-2 expression and prostaglandin e2 release via par1 activation and erk1/2- and p38 mapk-dependent pathway in murine macrophages. J Cell Biochem. 2009;108:1143–1152. doi: 10.1002/jcb.22341. [DOI] [PubMed] [Google Scholar]
- 9.Lekic T, Manaenko A, Rolland W, Krafft PR, Peters R, Hartman RE, et al. Rodent neonatal germinal matrix hemorrhage mimics the human brain injury, neurological consequences, and post-hemorrhagic hydrocephalus. Experimental neurology. 2012;236:69–78. doi: 10.1016/j.expneurol.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leitzke AS, Rolland WB, Krafft PR, Lekic T, Klebe D, Flores JJ, et al. Isoflurane post-treatment ameliorates gmh-induced brain injury in neonatal rats. Stroke. 2013;44:3587–3590. doi: 10.1161/STROKEAHA.113.001988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manaenko A, Lekic T, Barnhart M, Hartman R, Zhang JH. Inhibition of transforming growth factor-beta attenuates brain injury and neurological deficits in a rat model of germinal matrix hemorrhage. Stroke. 2014;45:828–834. doi: 10.1161/STROKEAHA.113.003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strahle J, Garton HL, Maher C, Muraszko K, Keep R, Xi G. Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Translational Stroke Research. 2012;3:25–38. doi: 10.1007/s12975-012-0182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crews L, Wyss-Coray T, Masliah E. Insights into the pathogenesis of hydrocephalus from transgenic and experimental animal models. Brain Pathol. 2004;14:312–316. doi: 10.1111/j.1750-3639.2004.tb00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao C, Du H, Hua Y, Keep RF, Strahle J, Xi G. Role of red blood cell lysis and iron in hydrocephalus after intraventricular hemorrhage. J Cereb Blood Flow Metab. 2014;34:1070–1075. doi: 10.1038/jcbfm.2014.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keep RF, Zhou N, Xiang J, Andjelkovic AV, Hua Y, Xi G. Vascular disruption and blood-brain barrier dysfunction in intracerebral hemorrhage. Fluids Barriers CNS. 2014;11:18. doi: 10.1186/2045-8118-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.