Abstract
Purpose
Elements in the endocytic process that are determinants of the activities of antifolates delivered by folate-receptor alpha (FRα) were explored.
Methods
Antifolate growth inhibition was assessed with a 1- or 5-day exposure in reduced folate carrier-null HeLa cell lines that express a high level of FRα in the presence or absence of the proton-coupled folate transporter (PCFT). pH-dependent rates of dissociation from FRα were also determined.
Results
With a 1-day drug exposure which is relevant to the pulse clinical administration of these drugs, FRα expression enhanced raltitrexed activity and modestly enhanced ZD9331 activity, but did not significantly augment the activity of pemetrexed or lomotrexol. With a 5-day drug exposure, FRα-mediated growth inhibition was increased for raltitrexed and ZD9331 and emerged for lomotrexol. While the FRα-augmented activity of lomotrexol and raltitrexed did not require PCFT, augmentation of ZD9331 activity required the co-expression of PCFT with both 1- and 5-day exposures. In contrast, there was no augmentation of pemetrexed activity by FRα under any condition. The activities of these agents correlated with their rate of dissociation from the receptor at acidic pH: raltitrexed > ZD9331 > lomotrexol > pemetrexed consistent with insufficient pemetrexed release from FRα for export from the endosomes.
Conclusions
FRα is unlikely to contribute to the pharmacological activity of antifolates, such as pemetrexed, that bind tightly to, and dissociate slowly from, the receptor particularly when the exposure time is brief. While PCFT was required for FRα-mediated ZD9931 activity, the activities of the other antifolates was independent of PCFT.
Keywords: Folate receptor, Endocytosis, Antifolates, Pemetrexed, PCFT
Introduction
Transport of the water-soluble B9 folate vitamins across the intestinal epithelium and into cells requires folate-specific transporters. The proton-coupled folate transporter (PCFT-SLC46A1) mediates the intestinal absorption of folates, and the reduced folate carrier (RFC-SLC19A1) mediates transport into systemic tissues [1-4]. Both are facilitative carriers ubiquitously expressed in malignant cells [1, 2, 4]. Folate receptors (FRs) are expressed on epithelial membranes (i.e., apical membranes of the proximal renal tubule and choroid plexus and basolateral membrane of retinal pigment epithelial cells) and in epithelial and hematological cancers [5-7]. Since RFC appears to be the primary route by which antifolates express their limiting toxicities, antifolates that have a very low affinity for RFC are being developed specifically designed for FR-mediated and/or PCFT-mediated transport. An example of the former is the cyclopenta[g]quinazoline-based thymidylate synthase inhibitor, BGC 945 [8]. The pyrrolo[2,3-d] pyrimidime-based glycinamide ribonucleotide formyltransferase (GARFT) inhibitors are agents with a high affinity for both FRα and PCFT [2, 9-11]. The cytotoxic activity of FR-targeted antifolates requires their internalization within endosomes by FR-mediated endocytosis, dissociation from FR upon acidification of the endosomes followed by export from endosomes to reach their intracellular targets. Neither the mechanism(s) of export nor the relationship between dissociation from the receptor and export has been fully clarified.
There is evidence that PCFT is one route of export of folates from endosomes. (1) Subjects with the autosomal recessive disorders, hereditary folate malabsorption (OMIM-229050), and cerebral folate deficiency (OMIM-613068) have markedly impaired transport of folates across the choroid plexus into the cerebrospinal fluid. The former is caused by loss-of-function mutations in the PCFT gene, while the latter is caused by loss-of-function mutations in the FRα gene [12-17]. This genetic confirmation that both transporters are required to sustain transport across this epithelium, neither alone is sufficient, is consistent with a requirement for PCFT for FR function, presumably export of folates from the endosome. (2) PCFT augments FRα-mediated 5-formyltetrahydrofolate (5-CHO-THF) transport into the cytosol [18]. However, a low level of FRα-mediated 5-CHO-THF transport into the cytosol is present in the absence of PCFT, and a new class of FR-targeted GARFT inhibitors are active even in the absence of PCFT consistent with the presence of another mechanism of endosomal export [9-11, 18].
Another class of drugs designed for transport mediated solely by FRs are conjugates in which folic acid is linked through a cleavable sulfhydryl bond to a cytotoxic molecule [19]. An example is EC145 (vintafolide), a folic acid-desacetylvinblastine monohydrazide conjugate in clinical trials [20, 21]. Following endocytosis of this agent, the cytotoxic moiety is released from the conjugate when the sulfhydryl bond is reduced following which the desacetylvinblastine moiety, which is lipid soluble, diffuses out of the endosome into the cytosol. According to this strategy, folic acid need not dissociate from the receptor nor is a specific endosomal export mechanism for the cytotoxic component required. A previous study demonstrated that EC0905, an analog of EC145 [22], is highly active in an RFC- and PCFT-null HeLa cell line with modestly increased FRα expression consistent with an intact endocytic mechanism. In contrast, these cells are highly resistant to pemetrexed consistent with a failure of endocytosed drug to be exported from the endosome and/or released from the receptor [23].
The objectives of the current study were to better understand the elements of the endocytic process that are the determinants of FRα delivery of antifolates, in particular pemetrexed, into tumor cells and the role, if any, of PCFT as a contributor to this route of transport. A focus was to quantify the interaction of antifolates with FRα, in particular, the relative rates of dissociation from the receptor as a function of pH. Two HeLa cell lines which express very high levels of FRα, but do not express RFC, in the presence or absence of PCFT, were utilized in these studies in order to discriminate among binding and transport phenomena intrinsic to endocytic route.
Materials and methods
Cell lines and culture conditions
Cells utilized for these studies included: R5 cells (derived from wild-type HeLa cells with a genomic deletion of RFC but intact PCFT), R5-FR12G (a clonal derivative of R5-cells transfected to a high level of FRα expression), and R1-11-FR2 (a PCFT-null R5 clonal derivative transfected to a high-level FRα expression). The origins of these cells have been described in detail previously [18, 24]. All cells were grown in folate-free RPMI 1640 medium supplemented with 10 % dialyzed fetal bovine serum, 100 units/mL penicillin, 100 μg/mL streptomycin, and 25 nM (6R,S)5-CHO-THF. Hygromycin (0.3 mg/mL) was included in the growth medium to maintain the R1-11-FR2 and R5-FR12G cells.
Chemicals
[3′,5′,7,9-3H]folic acid, [3′,5′,7, 9-3H(N)](6S)5-CHO-THF, and generally labeled [3H]pemetrexed, were purchased from Moravek Biochemicals (Brea, CA). Unlabeled folates/antifolates were obtained from commercial sources: pemetrexed from LC laboratories, Woburn, MA; (6R,S) and (6S)5-CHO-THF from Schircks Laboratories, Jona, Switzerland; folic acid from Sigma, St. Louis, MO; ZD9331 and raltitrexed from AstraZeneca, Cheshire, UK; lomotrexol from the Eli Lilly company, Indianapolis, IN; trimetrexate from Warner Lambert, Ann Arbor, MI; and EC0905 from Endocyte, West Lafayette, IN.
Growth inhibition by cytotoxic drugs
Cells were detached with 0.5 mM EDTA in PBS and seeded into 96-well plates (0.1 mL) at a density of 1000 cells/well for HeLa and 2000 cells/well for the other cell lines. On the second day, 0.1 mL of growth medium containing a range of drug concentrations in the presence or absence of folic acid was added. For the 1-day drug exposure, the medium containing drug was removed after 24 h. The cells were then washed rapidly once with 0.1 mL folate-free RPMI medium following which 0.2 mL of this medium containing 25 nM (6R,S)5-CHO-THF was added, and the cells were grown for four more days. Otherwise cells were grown continuously in their respective media for 5 days (5-day exposure). Cell growth was analyzed by sulforhodamine B staining.
Buffers
The following buffers were used: HBS (20 mM HEPES, 5 mM dextrose, 140 mM NaCl, 5 mM KCl, 2 mM MgCl2) with pH adjusted to 7.4 and 7.0. MBS (20 mM MES, 5 mM dextrose, 140 mM NaCl, 5 mM KCl, 2 mM MgCl2) with pH adjusted to 6.5, 6.0, 5.5, and 5.0. Acid buffer (10 mM sodium acetate, 150 mM NaCl, adjusted to pH 3.5 with acetic acid) was used to dissociate folates/antifolates from FRα.
Folic acid surface binding and its inhibition by other antifolates
Cells grown in 12-well plates to near confluence were washed consecutively with 2 mL ice-cold HBS (pH 7.4), acidic buffer (pH 3.5), and HBS (pH 7.4), each for 5 min. Cells were then incubated with 0.2, 0.5, or 1.0 μM[3H]folic acid alone, or with a mixture of 0.5 μM [3H]folic acid and unlabeled folates or antifolates, in ice-cold HBS (pH 7.4) for 20 min. After three washes with ice-cold HBS (pH 7.4, 2 mL, 5 min), surface-bound [3H]folic acid was released by incubation with 0.5 mL of ice-cold acid buffer (pH 3.5) for 5 min. Tritium in the acid buffer was quantified on a liquid scintillation spectrometer and normalized to protein determined with the BCA Protein Assay (Pierce, Rockford, IL) after cells were dissolved in 0.5 mL of 0.2 M NaOH.
pH-dependent antifolate/folate dissociation from FRα
Three different assays were utilized: (1) Direct measurement of radiolabeled substrates at 0 °C—cells in 12-well plates were washed (2 mL, 5 min) consecutively with ice-cold HBS (pH 7.4), acid buffer (pH 3.5), and HBS (pH 7.4) to remove unlabeled 5-CHO-THF from the medium bound to FRα. The cells were then incubated with 1 mL of 0.5 μM [3H]folic acid, [3H](6S)5-CHO-THF, or [3H]pemetrexed in HBS (pH 7.4) for 20 min on ice to saturate surface FRα. The unbound tritiated compounds were removed by washing with ice-cold HBS (pH 7.4) three times (2 mL, 1 min). The cells were then incubated with 2 mL of buffers at a pH of from 5.0 to 7.4 for 5 min on ice following which tritium that remained bound to FRα was released with a 5-min incubation with 0.5 mL acid buffer (pH 3.5) and quantified as described above. The greater the quantity of tritium retrieved, the slower the rate of dissociation from FRα. (2) Indirect measurement of non-radiolabeled substrates at 0 °C—cells in 12-well plates were washed (2 mL, 5 min) consecutively with ice-cold HBS (pH 7.4), acid buffer (pH 3.5), and HBS (pH 7.4) to remove 5-CHO-THF from the medium bound to FRα at the cell surface. The cells were then incubated for 20 min with 1 mL ice-cold HBS (pH 7.4) containing 2 μM of non-labeled ZD9331, raltitrexed, lomotrexol, or pemetrexed to saturate FRα. Following this, the cells were washed once with 2 mL ice-cold HBS (pH 7.4) for 1 min and exposed to 2 mL of ice-cold buffers at different pH’s for 5 min to release the FRα-bound anti-folate. After a 1-min wash with 2 mL HBS (pH 7.4), cells were incubated for 1 min with 0.5 μM [3H](6S)5-CHO-THF in HBS (1 mL, pH 7.4) to reload the receptors. After three 1-min washes with 2 mL ice-cold HBS (pH 7.4), the tritium was released from the cell surface with a 5-min incubation with 0.5 mL acidic buffer (pH 3.5) and quantified. In this experimental design, the greater the amount of tritium released, the more rapid the rate of dissociation of antifolate from the receptor. As a control, cells were incubated with HBS (pH 7.4) in the absence of the antifolates. (3) Indirect measurement of non-radiolabeled substrates at 37°. The procedure was the same as (2) except that the dissociation step was performed with a 10-min incubation at 37° in pre-warmed buffers (1 mL, pH 5.0–7.4) containing 2 μM of antifolate.
Results
Characterization of cell lines employed in these studies
The HeLa cell line and its three variants, developed in this laboratory, were used in the current study to assess the contribution of FRα-mediated transport to antifolate activities and the role of PCFT in this process. These were: (1) HeLa wild-type cells (RFC+PCFT+); (2) R5 cells (RFC−PCFT+); (3) R5-FR12G cells (RFC−PCFT+FR+++++); and (4) R1-11-FR2 cells (RFC−PCFT−FR+++++). Hence, only wild-type HeLa cells express RFC. Cells were grown in folate-free medium supplemented with 25 nM (6R,S)5-CHO-THF. Folic acid was not utilized as a folate growth source to allow determination of FRα-mediated antifolate growth inhibition. R1-11 (RFC−PCFT−FR−) cells do not grow at this concentration of (6R,S)5-CHO-THF and, thus, were not included in the current study [25, 26].
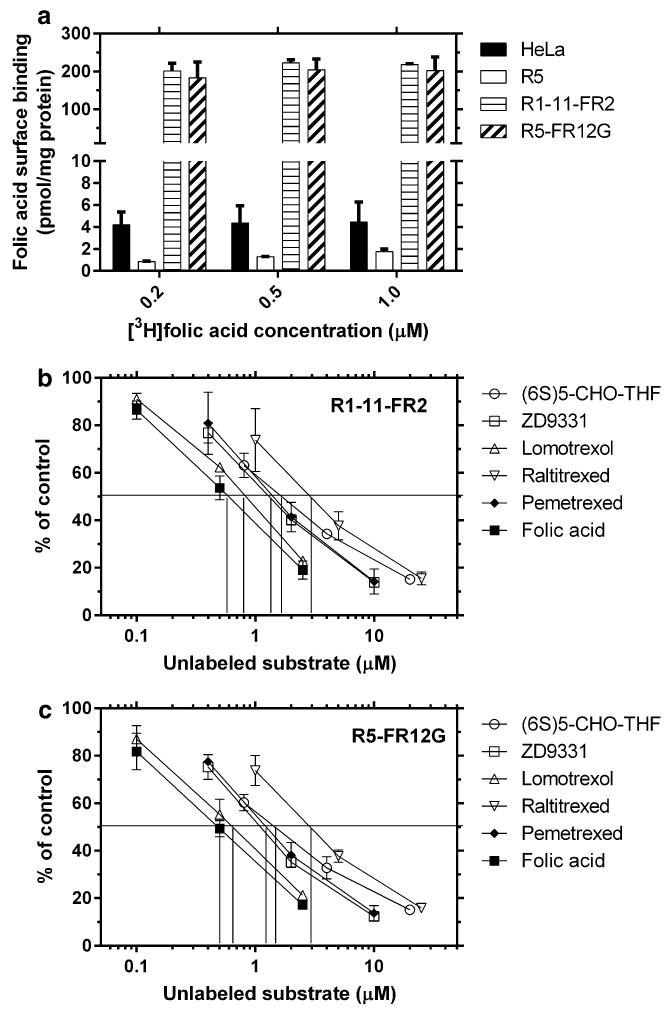
FRα surface expression was determined using [3H]folic acid concentrations (0.2, 0.5, and 1 μM) sufficiently high to ensure that the folic acid content in the buffer was far in excess of that required to saturate surface FRα sites (Fig. 1a). The FRα level in HeLa cells was greater than in R5 cells. Levels in R1-11-FR2 and R5-FR12G cells were markedly increased, identical, and constant over the concentration range, consistent with saturation of the receptors. This FRα level in R1-11-FR2 and R5-FR12G cells was ~3 times greater than that of KB cells [7].
Fig. 1.
Folic acid surface binding capacity in the cell lines studied and the effect of unlabeled folates/antifolates on binding of tritiated folic acid to FRα in R1-11-FR2 and R5-FR12G cells. a Cells were incubated with 200, 500, and 1000 nM [3H]folic acid for 20 min on ice and then washed with neutral buffer. Tritium on the cells surface was released by acidic buffer and quantified. b, c Competitive binding with [3H]folic acid in R1-11-FR2 (b) and R5-FR12G cells (c). Three different concentrations of each unlabeled folate or antifolate were employed during incubation with 500 nM [3H] folic acid following which tritium released from the cell surface was measured. Data in all panels are the mean ± SEM from three independent experiments
Competitive binding of antifolates to FRα in FR1-11-FR2 and R5-FR12G cells
Four antifolates (ZD9331, lomotrexol, raltitrexed, and pemetrexed) were studied; all have been reported to have excellent, though variable, affinities for FRα [27, 28]. As indicated in Fig. 1b, c, the pattern and extent to which these antifolates competed with [3H]folic acid for binding to FRα were nearly identical in the R1-11-FR2 and R5-FR12G cells. The potency of inhibition, reflective of affinity of antifolates for FRα, was in following order: lomotrexol > ZD9331, pemetrexed > raltitrexed. The competitive potency of lomotrexol for FRα was comparable to that of folic acid; the potency of (6S)-5-CHO-THF, the folate source in the growth medium, was between that of pemetrexed and raltitrexed.
Growth inhibition by trimetrexate—an indication of the cell folate pool
Growth inhibition by trimetrexate was assessed in the four cell lines with a 5-day drug exposure. This antifolate is highly sensitive to the intracellular folate level which generally affects the activity of antifolates. Among the four antifolates used in the current study, lomotrexol is the most sensitive to cell folate levels, followed by pemetrexed and raltitrexed. ZD9331 is the least sensitive to intracellular folates since its structure precludes formation of polyglutamate derivatives, a process suppressed by intracellular folates [29, 30]. Trimetrexate is a relatively weak dihydrofolate reductase inhibitor that enters cells by passive diffusion and does not form polyglutamate derivatives. Increased levels of intracellular folates enhance competition with trimetrexate for binding to dihydrofolate reductase and decrease the activity of this agent [29]. As indicated in Table 1, trimetrexate activity was markedly increased in R5 versus wild-type HeLa cells consistent with a contraction in the folate pool that accompanies the loss of RFC, as previously reported. This contributes to the preservation of pemetrexed activity (by augmenting its polyglutamation) in these cells [31, 32]. Trimetrexate activity was markedly decreased (35-fold) in R5-FR12G versus R5 cells, consistent with a marked FRα-mediated increase in the cellular folate pool that appeared to be, in part, PCFT dependent since trimetrexate activity was tenfold weaker in the R5-FR12G as compared to the R1-11 FR2 cells. Trimetrexate activity was also determined when 200 nM folic acid was added to the growth media during the assay, a common approach to block FR-mediated uptake. Addition of 200 nM folic acid did not alter trimetrexate activity in HeLa and R5 cells. On the other hand, folic acid at this concentration markedly (~eightfold) increased the activities of trimetrexate in both FRα-expressing cells, consistent with its potent inhibition of FRα-mediated (6S)5-CHO-THF transport and consequent folate depletion.
Table 1.
IC50 (μM) for EC0905 and antifolates in HeLa, R5, R1-11-FR2, and R5-FR12 cells
| HeLa |
R5 |
R5-FR12G |
R1-11-FR2 |
|||||
|---|---|---|---|---|---|---|---|---|
| Control | +Folic acid | Control | +Folic acid | Control | +Folic acid | Control | +Folic acid | |
| One-day exposure | ||||||||
| EC0905 | 0.78 ± 0.26 | 0.93 ± 0.06 | 0.62 ± 0.14 | 0.65 ± 0.15 | 0.00013 ± 0.00002 | 0.23 ± 0.07 | 0.00014 ± 0.00004 | 0.17 ± 0.03 |
| ZD9331 | >20 | >20 | >20 | >20 | 0.90 ± 0.15 | 6.0 ± 1.1 | >20 | >20 |
| Raltitrexed | 0.012 ± 0.002 | 0.010 ± 0.001 | 0.30 ± 0.06 | 0.40 ± 0.06 | 0.057 ± 0.012 | 3.3 ± 0.7 | 0.0057 ± 0.0003 | 2.8 ± 0.6 |
| Pemetrexed | 0.17 ± 0.03 | 0.17 ± 0.03 | 0.10 ± 0.03 | 0.10 ± 0.03 | 3.3 ± 1.3 | 7.3 ± 1.3 | 6.7 ± 0.3 | 11 ± 1 |
| Lomotrexol | 0.28 ± 0.06 | 0.33 ± 0.06 | 0.43 ± 0.04 | 0.57 ± 0.07 | 7.0 ± 1.5 | 8.3 ± 1.0 | 2.2 ± 0.3 | 9.7 ± 1.5 |
| Five-day exposure | ||||||||
| Trimetrexate 0.12 ± 0.02 | 0.12 ± 0.02 | 0.012 ± 0.003 | 0.014 ± 0.002 | 0.42 ± 0.04 | 0.049 ± 0.006 | 0.042 ± 0.007 | 0.0052 ± 0.0006 | |
| EC0905 | 0.25 ± 0.03 | 0.28 ± 0.02 | 0.12 ± 0.02 | 0.15 ± 0.03 | 0.00020 ± 0.00003 | 0.096 ± 0.003 | 0.00020 ± 0.00003 | 0.11 ± 0.01 |
| ZD9331 | 0.28 ± 0.06 | 0.30 ± 0.06 | 0.87 ± 0.10 | 1.1 ± 0.1 | 0.0037 ± 0.001 | 1.7 ± 0.3 | 9.7 ± 1.0 | 12 ± 1 |
| Raltitrexed | 0.0057 ± 0.0003 | 0.0063 ± 0.0003 | 0.067 ± 0.003 | 0.15 ± 0.01 | 0.0083 ± 0.0003 | 0.70 ± 0.12 | 0.0024 ± 0.0001 | 1.7 ± 0.1 |
| Pemetrexed | 0.070 ± 0.006 | 0.076 ± 0.012 | 0.033 ± 0.008 | 0.043 ± 0.007 | 0.083 ± 0.020 | 0.21 ± 0.05 | 1.1 ± 0.2 | 4.3 ± 0.3 |
| Lomotrexol | 0.076 ± 0.03 | 0.13 ± 0.01 | 0.093 ± 0.007 | 0.40 ± 0.06 | 0.014 ± 0.001 | 1.43 ± 0.19 | 0.0097 ± 0.0017 | 7.0 ± 0.6 |
Cells were seeded in 96-well plates for 1 day before exposure to drugs over a spectrum of concentrations in the presence or absence of folic acid. For 1-day exposure, cells were washed once with folate-free medium after 24 h and grown in folate-free medium containing 25 nM (6R,S)5-CHO-THF for additional 4 days. For 5-day exposure, cells were grown in medium containing drugs continuously for 5 days. The folic acid concentration was 1 μM for EC0905 and 200 nM when added with the other drugs. IC50 is the concentration of drug required to reduce cell growth by 50 %. Data are the mean ± SEM from three independent experiments
Cell growth inhibition after a 1-day exposure to EC0905 or antifolates
Growth inhibition by EC0905 (a folic acid-desoxyvinblastine conjugate), ZD9331, raltitrexed, pemetrexed, and lomotrexol after a 1-day exposure was assessed, and their IC50 values are presented in Table 1. The EC0905 IC50s in HeLa and R5 cells were in the 0.6–0.9 μM range and were barely affected by the presence of folic acid. Hence, the level of FRα expression in these cell lines was insufficient to augment the delivery and activity of this agent. The EC0905 IC50 was less than 1 nM in R5-FR12G and R1-11-FR2 cells and the addition of folic acid completely abolished its activity. Hence, FRα-mediated endocytosis was intact in both R5-FR12G and R1-11-FR2 cells allowing assessment of FRα-dependent activities of the antifolates.
The IC50 for ZD9331 in HeLa and R5 cells was >20 μM but was decreased by an order of magnitude to 0.9 μM in R5-FR12G cells; the addition of 200 nM folic acid increased the IC50 by >6.7-fold. In contrast, the IC50 was >20 μM for ZD9331 in R1-11-FR2 cells and was unaffected by folic acid. Hence, ZD9331 growth inhibition mediated by FRα, albeit weak, required the co-expression of PCFT.
Raltitrexed was much more active in HeLa cells than in R5 cells that express only PCFT, consistent with its high affinity for RFC and very low affinity for PCFT at neutral pH [31]. Raltitrexed activity was increased in R5-FR12G cells relative to R5, but its activity was tenfold greater in the R1-11-FR2 cells that lack PCFT. In both cases, there was a marked fall to a comparable level of activity in the presence of 200 nM folic acid. Hence, there was a modest increase in raltitrexed activity in the cells that express PCFT and FRα but, paradoxically, its activity was much greater when PCFT was absent.
Pemetrexed had comparable activities in HeLa and R5 cells; its activity was sustained in the latter cells due in part to its high affinity for PCFT, as reported previously, and the concurrent contraction of the folate pools which augments its polyglutamation as indicated by the high sensitivity of R5 cells to trimetrexate [24, 31]. Pemetrexed activity was substantially lower in both cell lines that overexpress FRα with only a small decrease in activity with the addition of folic acid. A similar pattern was observed for lomotrexol. Hence, pemetrexed and lomotrexol activities were low in cells with high expression of FRα irrespective of the co-expression of PCFT.
Cell growth inhibition after a 5-day exposure to EC0905 or antifolates
A 5-day exposure markedly prolongs the duration over which drug can enter the cells and synthesize the polyglutamate derivatives of pemetrexed, raltitrexed, and lomotrexol. The EC0905 IC50s for the R5-FR12G and R1-11-FR2 were essentially the same as obtained with the 1-day exposure, indicating that the short exposure was sufficient to achieve the full activity of the conjugate in these cells at this level of FRα expression. However, the activities of all the anti-folates were substantially increased with the longer duration of exposure.
For ZD9331, the IC50s were decreased in all the cell lines as compared to the 1-day exposure. However, there was a marked decrease in the IC50 in R5-FR12G cells to 1.4 % that of HeLa and R5 cells, and this activity was essentially abolished by the presence of folic acid increasing the IC50 by a factor of 500. In contrast, ZD9331 was not active in R1-11-FR2 cells, similar to what was observed with the 1-day exposure. Hence, PCFT was absolutely required for FRα-mediated ZD9331 growth inhibition regardless of the interval of drug exposure. The pattern of raltitrexed activity with a 5-day exposure was similar to that of the 1-day exposure in all cell lines except that raltitrexed was more active with the longer exposure; activity was markedly suppressed by folic acid despite the contraction of folate pools that occurs under these conditions. Raltitrexed activity was modestly increased in the R1-11-FR2 cells in the absence of PCFT. Hence, FRα-mediated growth inhibition by raltitrexed did not require co-expression of PCFT. The increased activity in the absence of PCFT correlated with the contraction of the folate pools in these cells as reflected by the increased trimetrexate activity, as indicated above.
Pemetrexed growth inhibition was modestly increased in all cell lines as compared to the 1-day exposure; however, there was no impact at all of PCFT in cells that express high levels of FRα and there was only a small decrease in activity upon the addition of folic acid. Hence, the data suggest that FRα did not contribute to the activity of this drug in the presence or absence of PCFT. In contrast, growth inhibition by lomotrexol was markedly increased in both R1-11-FR2 and R5-FR12G cells, as compared to the 1-day exposure, and there was a substantial decrease in activity with the addition of folic acid. However, the presence of PCFT in R5-FR12G cells did not augment the activity of this agent as compared to PCFT-null R1-11-FR2 cells.
The pH-dependent dissociation of folates and antifolate substrates from FRα
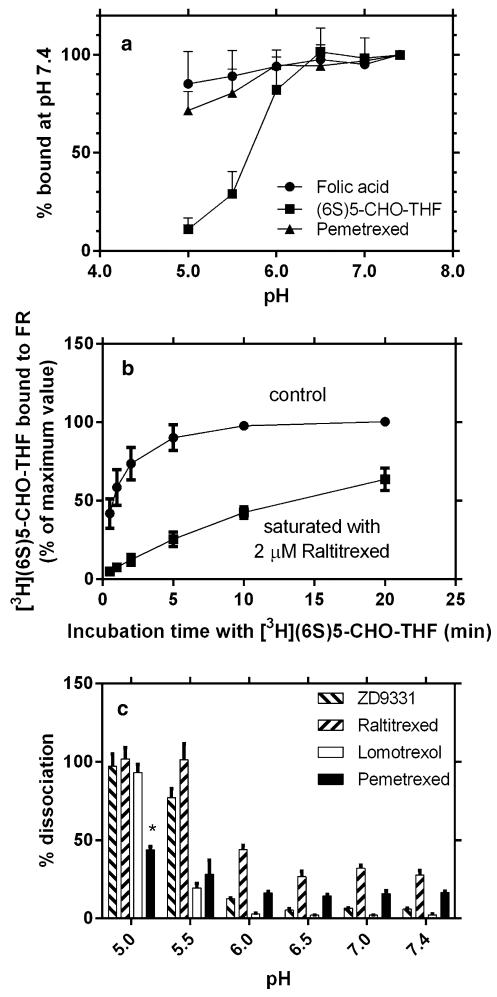
In FRα-mediated endocytosis, the endosomal pH must be decreased for the dissociation of substrate from receptor to occur before the folate can be exported into the cytosol. Folic acid is bound so tightly to FRα that it dissociates from the receptor very slowly; because of this it has been considered to be a very poor substrate for FRα-mediated transport [5]. Dissociation was assessed for tritiated folic acid, (6S)5-CHO-THF, and pemetrexed at 0 °C. Surface FRα was first saturated with the tritiated compounds; the cells were then washed with 0 °C buffer and incubated with ice-cold folate-free buffers at different pHs, and tritium remaining bound to the cell surface was quantified. As indicated in Fig. 2a, most folic acid and pemetrexed remained bound to the cells even when the pH was decreased to 5.0. The slight decrease in bound pemetrexed at low pH was not significant (P = 0.20 and 0.48 at pH 5.0 and pH 5.5, respectively) as compared to pH 7.4. In contrast, (6S)5-CHO-THF dissociation started at pH 6 and only 30 and 10 % remained bound to the cells at pH 5.5 and 5, respectively.
Fig. 2.
pH-dependent dissociation of folate/antifolate substrates from the surface of R1-11-FR2 cells at 0 °C. a Dissociation of tritiated substrates from FRα as a function of pH at 0 °C. Cells were incubated with 0.5 μM [3H]folic acid, [3H](6S)5-CHO-THF, or [3H] pemetrexed for 20 min to saturate FRα on the cell surface. The cells were then washed with 0 °C neutral buffer and incubated with ice-cold folate-free buffers at a spectrum of pHs for 5 min, following which tritium that remained bound to the cell surface was released by acid buffer and quantified. b Time course of dissociation of raltitrexed at 0 °C at pH 7.4. Cells were incubated in buffer alone (control) or with 2 μM raltitrexed to saturate FRα at the cell surface. After a rapid wash with pH 7.4 buffer, cells were exposed to 0.5 μM [3H] (6S)5-CHO-THF and tritium bound to the cell surface was assessed as a function of time. c The pH dependence of dissociation from FRα was assessed using a strategy similar to the one described in panel b. In this case, a 1-min incubation with 0.5 μM [3H](6S)5-CHO-THF was employed. One hundred percent indicates the control cells not exposed to unlabeled folate substrates. *Pemetrexed dissociation at pH 5.0 was significantly higher than dissociation at pH ≥ 6.0, but not significantly different from dissociation at pH 5.5. Data in panel a are the mean SEM from four independent experiments, while values in panels b and ± c are the mean ± SEM from three independent experiments
Since raltitrexed, ZD9331, and lomotrexol in their tritiated forms were not available, an indirect measurement of binding to FRα at 0 °C was utilized. FRα at the cell surface was first saturated with these unlabeled antifolates; the cells were washed with 0 °C buffer and then incubated with ice-cold buffers at different pHs to allow dissociation. The cells were then washed with buffer at neutral pH, and the unoccupied receptors were quantified with [3H](6S)5-CHO-THF. The time course of dissociation of raltitrexed at pH 7.4 is illustrated in Fig. 2b. It can be seen that a brief incubation (<2 min) with [3H](6S)5-CHO-THF detected a major portion of unoccupied receptors in the control cells, but only ~10 % of the bound raltitrexed was replaced. Even a 20-min incubation with [3H](6S)5-CHO-THF resulted in dissociation of only 60 % of the bound raltitrexed, indicating that the dissociation process under these conditions (pH 7.4, 0 °C) was slow.
Figure 2c compares the pH-dependent dissociation of ZD9331, raltitrexed, lomotrexol, and pemetrexed at 0 °C using the indirect measurement. As the pH was decreased, the extent of dissociation for all the antifolates was relatively unchanged between pH 7.4 and 6.0. However, at pH 5.5, raltitrexed dissociation was complete and ZD9331 was 75 % dissociated. At pH 5.0, ZD9331 dissociation was complete. Lomotrexol dissociation was detected at pH 5.5, but complete dissociation required pH 5.0. In contrast, pemetrexed dissociation at pH 5.5 was not different from the dissociation at higher pHs (P > 0.05). Even at pH 5.0, only 45 % of the pemetrexed had dissociated from the cell surface. The high background dissociation at neutral pH for raltitrexed, and to a lesser extent pemetrexed, was due to the wash procedure and the replacement of unlabeled substrates with [3H](6S)-5-CHO-THF.
Using a similar approach, the percentage of anti-folate substrates dissociated from FRα at the cell surface was assessed after surface receptors were first saturated with 2 μM folates/antifolates at 0 °C. The cells were then exposed to a spectrum of buffers at different pHs, this time at 37 °C for 10 min, each of which contained 2 μM of the same substrate allowing dissociation to approach an equilibrium between the bound and free antifolates. After a rapid wash, cells were incubated with [3H](6S)5-CHO-THF for 1 min on ice to assess the amount of unoccupied receptors. As indicated in Fig. 3, folic acid did not dissociate from receptors on the cell surface, at all, even at pH 5.0 under these conditions. There was marked dissociation of raltitrexed below pH 6.0 reaching ~80 % at pH 5.0. Dissociation of ZD9331 began at pH 5.5 and reached 50 % at pH 5.0. Lomotrexol did not dissociate until the pH reached 5.5. Pemetrexed dissociation from the receptors was minimal even at pH 5.0 under these conditions.
Fig. 3.
pH-dependent dissociation of folate substrates in the presence of 2 μM of the same substrate at 37 °C. FRα on the surface of R1-11-FR2 cells was first saturated with 2 μM substrate at 0 °C. The cells were then exposed for 10 min to buffers at 37 °C each of which contained 2 μM of the same substrate at different pHs. After a brief wash at 0 °C, cells were incubated with 0.5 μM [3H](6S)5-CHO-THF for 1 min at 0 °C to assess the amount of unoccupied receptors on the cell surface. The control (100 %) represents cells that were incubated with buffer only (in the absence of substrate) at 0 °C and exposed to neutral buffer only (in the absence of substrate) at 37 °C before incubation with [3H](6S)5-CHO-THF. Data are the mean ± SEM from three independent experiments
Discussion
The objectives of this study were to better understand the elements of the endocytic process that are the determinants of FRα-mediated delivery of antifolates into tumor cells and the role that PCFT might play in this process. The pair of cell lines, R1-11-FR2 and R5-FR12G, offered unique models for this investigation. (1) Both lack RFC and have high, comparable expression of FRα. (2) R5-FR12G expresses PCFT, but R1-11-FR2 does not. (3) FRα-mediated endocytosis was functional in both cell lines since both were highly sensitive to EC0905. The data indicate that (1) the requirement for PCFT in FRα-mediated antifolate growth inhibition in these cell lines is substrate-specific, (2) the rate of dissociation of antifolate from the receptor at acidic pH is a critical determinant of the activity of these agents, and (3) the impact of FRα expression on antifolate activities is modulated by concurrent alterations in the levels of cellular folates that suppress the polyglutamation of antifolates within cells.
FRα-mediated antifolate activities were very limited when the interval of drug exposure was 1 day except for raltitrexed. Interestingly, raltitrexed has the lowest affinity for FRα at neutral pH evaluated (Fig. 1b, c) and the highest propensity to dissociate from FRα at neutral or lower pHs, among the four antifolates studied (Figs. 2c, 3). Since this short-term exposure better simulates the conditions in which these drugs are given as pulse infusions followed by their rapid renal and hepatic clearance, these data indicate that antifolates with very high affinity for FRα should be poor substrates for delivery by this transporter in the clinical setting. This is due to a low level of dissociation of anti-folate from the receptor in the endosomes and thus a slow delivery of drug into the cytosol. Hence, what is required is for the antifolate to have high enough affinity to assure sufficient binding to FRα after administration of the drug but not so high an affinity that dissociation from FRα at endosome pH is too slow to result in antitumor activity. Another element in FRα-mediated antifolate activity is receptor-mediated uptake of 5-methyltetrahydrofolate (the physiological folate) that increases the level of intracellular folates and reduces the efficacy of antifolates that require polyglutamation for their activity [29, 33].
The 5-day exposure is much less clinically relevant but provides additional mechanistic information. Under these conditions, FRα-mediated raltitrexed activity was further enhanced and lomotrexol activity emerged—both activities were PCFT independent. In contrast, even with a 1-day exposure, the activity of ZD9331 was augmented by FRα and this was markedly increased at 5 days; however, this only occurred in cells that expressed PCFT and was blocked by folic acid. Hence, while PCFT was absolutely required for FRα-mediated ZD9331 activity, this was not the case for the other antifolates, observations that suggest another mechanism by which these antifolates exit endosomes in these cells. This is also relevant for a new generation of GARFT inhibitors with high affinity for FRα and very low affinity for RFC that are active in tumor cells that lack PCFT [9-11]. The best explanation for these findings is that ZD9331 export from endosomes is PCFT dependent and export of the other antifolates is not, at least in these cells.
At the very low concentration of folic acid employed in these studies (200 nM) to block FRα function, neither RFC- or PCFT-mediated transport of (6S)5-CHO-THF is suppressed. The sole impact of the added folic acid reflected inhibition of FRα-mediated endocytosis. Hence, the marked inhibition of raltitrexed activity by the addition of folic acid confirmed the critical role of receptor-mediated endocytosis as a determinant of the activity of this agent. This was also observed for the longer exposure to lomotrexol. The greater activities mediated by FRα for the two agents in the absence of PCFT also correlated with a decrease in the folate pool as reflected by the increased sensitivity to trimetrexate. Under both conditions, a decrease in activity in the presence of folic acid occurred despite a marked decrease in intracellular folates, indicating that increased polyglutamylation due to the reduced intracellular folate level was insufficient to compensate the loss of FRα-mediated drug transport.
Pemetrexed is standard-of-care in the treatment of advanced mesothelioma and non-squamous non-small cell lung cancer [34-36]. FRα is expressed in these tumors [37-39]; however, it has been unclear as to whether this contributes to activity of this drug. FRα-mediated pemetrexed activity was not observed with either a 1- or 5-day exposure to the drug in the current study. Nor was FRα-mediated pemetrexed activity significantly influenced by the presence or absence of PCFT. An analysis of the pH-dependent dissociation of the antifolates established that the failure of FRα to augment the activity of pemetrexed was due to the failure of this drug to dissociate from the receptor at pH relevant to what occurs in endosomes during their cycling. The data suggest that competitive binding analyses at neutral pH do not provide the critical information regarding FR delivery of antifolates since pemetrexed’s binding properties in this assay were comparable to that of ZD9331 and weaker than that of folic acid and lomotrexol under these conditions. Rather, an analysis of its dissociation from FRα as a function of pH was much more informative and correlated with the lack of impact of FRα on the activity of pemetrexed in intact HeLa cells. In this case, dissociation was slowest at pH 5.0, at both 0 and 37 °C, although the relative affinity of pemetrexed for FRα at 37 °C was far less than at 0 °C [40]. Hence, for pemetrexed, little or no free drug apparently becomes available to exit the endosomes by PCFT or any other mechanism(s) even with prolong exposure to the drug. Hence, the data indicate that folate receptor-mediated endocytosis does not contribute to the pharmacological activity of pemetrexed in these HeLa cell lines even under conditions in which this protein is highly expressed and the endocytic mechanism is intact and mediates the activities of other antifolates.
Acknowledgments
This work was supported by grants from the National Institutes of Health (CA-82621 and CA-013330).
Abbreviations
- PCFT
Proton-coupled folate transporter
- RFC
Reduced folate carrier
- FR
Folate receptor
- GARFT
Glycinamide ribonucleotide formyltransferase
- 5-CHO-THF
5-Formyltetrahydrofolate
- DAVLBH
Desacetylvinblastine monohydrazide
- HBS
HEPES-buffered saline
- MBS
MES-buffered saline
Footnotes
Conflict of interest No conflict to disclose.
References
- 1.Zhao R, Diop-Bove N, Visentin M, Goldman ID. Mechanisms of membrane transport of folates into cells and across epithelia. Annu Rev Nutr. 2011;31:177–201. doi: 10.1146/annurev-nutr-072610-145133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desmoulin SK, Hou Z, Gangjee A, Matherly LH. The human proton-coupled folate transporter: biology and therapeutic applications to cancer. Cancer Biol Ther. 2012;13:1355–1373. doi: 10.4161/cbt.22020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visentin M, Diop-Bove N, Zhao R, Goldman ID. The intestinal absorption of folates. Annu Rev Physiol. 2014;76:251–274. doi: 10.1146/annurev-physiol-020911-153251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao R, Goldman ID. Folate and thiamine transporters mediated by facilitative carriers (SLC19A1-3 and SLC46A1) and folate receptors. Mol Aspects Med. 2013;34:373–385. doi: 10.1016/j.mam.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamen BA, Smith AK. A review of folate receptor alpha cycling and 5-methyltetrahydrofolate accumulation with an emphasis on cell models in vitro. Adv Drug Deliv Rev. 2004;56:1085–1097. doi: 10.1016/j.addr.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Elnakat H, Ratnam M. Distribution, functionality and gene regulation of folate receptor isoforms: implications in targeted therapy. Adv Drug Deliv Rev. 2004;56:1067–1084. doi: 10.1016/j.addr.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem. 2005;338:284–293. doi: 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 8.Gibbs DD, Theti DS, Wood N, Green M, Raynaud F, Valenti M, Forster MD, Mitchell F, Bavetsias V, Henderson E, Jackman AL. BGC 945, a novel tumor-selective thymidylate synthase inhibitor targeted to alpha-folate receptor-overexpressing tumors. Cancer Res. 2005;65:11721–11728. doi: 10.1158/0008-5472.CAN-05-2034. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Cherian C, Desmoulin SK, Polin L, Deng Y, Wu J, Hou Z, White K, Kushner J, Matherly LH, Gangjee A. Synthesis and antitumor activity of a novel series of 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl antifolate inhibitors of purine biosynthesis with selectivity for high affinity folate receptors and the proton-coupled folate transporter over the reduced folate carrier for cellular entry. J Med Chem. 2010;53:1306–1318. doi: 10.1021/jm9015729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Desmoulin SK, Cherian C, Polin L, White K, Kushner J, Fulterer A, Chang MH, Mitchell-Ryan S, Stout M, Romero MF, Hou Z, Matherly LH, Gangjee A. Synthesis, biological, and antitumor activity of a highly potent 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl antifolate inhibitor with proton-coupled folate transporter and folate receptor selectivity over the reduced folate carrier that inhibits beta-glycinamide ribonucleotide formyltransferase. J Med Chem. 2011;54:7150–7164. doi: 10.1021/jm200739e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Cherian C, Orr S, Mitchell-Ryan S, Hou Z, Raghavan S, Matherly LH, Gangjee A. Tumor-targeting with novel non-benzoyl 6-substituted straight chain pyrrolo[2,3-d]pyrimidine antifolates via cellular uptake by folate receptor alpha and inhibition of de novo purine nucleotide biosynthesis. J Med Chem. 2013;56:8684–8695. doi: 10.1021/jm401139z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006;127:917–928. doi: 10.1016/j.cell.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 13.Zhao R, Min SH, Qiu A, Sakaris A, Goldberg GL, Sandoval C, Malatack JJ, Rosenblatt DS, Goldman ID. The spectrum of mutations in the PCFT gene, coding for an intestinal folate transporter, that are the basis for hereditary folate malabsorption. Blood. 2007;110:1147–1152. doi: 10.1182/blood-2007-02-077099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diop-Bove N, Kronn D, Goldman ID. Hereditary folate malabsorption. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, editors. GeneReviews [Internet] Unverisity of Washington; Seattle, Seattle: 2014. [PubMed] [Google Scholar]
- 15.Cario H, Bode H, Debatin KM, Opladen T, Schwarz K. Congenital null mutations of the FOLR1 gene: a progressive neurologic disease and its treatment. Neurology. 2009;73:2127–2129. doi: 10.1212/WNL.0b013e3181c679df. [DOI] [PubMed] [Google Scholar]
- 16.Steinfeld R, Grapp M, Kraetzner R, Dreha-Kulaczewski S, Helms G, Dechent P, Wevers R, Grosso S, Gartner J. Folate receptor alpha defect causes cerebral folate transport deficiency: a treatable neurodegenerative disorder associated with disturbed myelin metabolism. Am J Hum Genet. 2009;85:354–363. doi: 10.1016/j.ajhg.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grapp M, Just IA, Linnankivi T, Wolf P, Lucke T, Hausler M, Gartner J, Steinfeld R. Molecular characterization of folate receptor 1 mutations delineates cerebral folate transport deficiency. Brain. 2012;135:2022–2031. doi: 10.1093/brain/aws122. [DOI] [PubMed] [Google Scholar]
- 18.Zhao R, Min SH, Wang Y, Campanella E, Low PS, Goldman ID. A role for the proton-coupled folate transporter (PCFT—SLC46A1) in folate receptor-mediated endocytosis. J Biol Chem. 2009;284:4267–4274. doi: 10.1074/jbc.M807665200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia W, Low PS. Folate-targeted therapies for cancer. J Med Chem. 2010;53:6811–6824. doi: 10.1021/jm100509v. [DOI] [PubMed] [Google Scholar]
- 20.Vlahov IR, Santhapuram HK, Kleindl PJ, Howard SJ, Stanford KM, Leamon CP. Design and regioselective synthesis of a new generation of targeted chemotherapeutics. Part 1: EC145, a folic acid conjugate of desacetylvinblastine monohydrazide. Bioorg Med Chem Lett. 2006;16:5093–5096. doi: 10.1016/j.bmcl.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 21.Naumann RW, Coleman RL, Burger RA, Sausville EA, Kutarska E, Ghamande SA, Gabrail NY, Depasquale SE, Nowara E, Gilbert L, Gersh RH, Teneriello MG, Harb WA, Konstantinopoulos PA, Penson RT, Symanowski JT, Lovejoy CD, Leamon CP, Morgenstern DE, Messmann RA. PRECEDENT: a randomized phase II trial comparing vintafolide (EC145) and pegylated liposomal doxorubicin (PLD) in combination versus PLD alone in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2013;31:4400–4406. doi: 10.1200/JCO.2013.49.7685. [DOI] [PubMed] [Google Scholar]
- 22.Dhawan D, Ramos-Vara JA, Naughton JF, Cheng L, Low PS, Rothenbuhler R, Leamon CP, Parker N, Klein PJ, Vlahov IR, Reddy JA, Koch M, Murphy L, Fourez LM, Stewart JC, Knapp DW. Targeting folate receptors to treat invasive urinary bladder cancer. Cancer Res. 2013;73:875–884. doi: 10.1158/0008-5472.CAN-12-2101. [DOI] [PubMed] [Google Scholar]
- 23.Zhao R, Diop-Bove N, Goldman ID. Enhanced receptor-mediated endocytosis and cytotoxicity of a folic acid-desacetylvinblastine monohydrazide conjugate in a pemetrexed-resistant cell line lacking folate-specific facilitative carriers but with increased folate receptor expression. Mol Pharmacol. 2014;85:310–321. doi: 10.1124/mol.113.089110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao R, Gao F, Hanscom M, Goldman ID. A prominent low-PH methotrexate transport activity in human solid tumor cells: contribution to the preservation of methotrexate pharmacological activity in HeLa cells lacking the reduced folate carrier. Clin Cancer Res. 2004;10:718–727. doi: 10.1158/1078-0432.ccr-1066-03. [DOI] [PubMed] [Google Scholar]
- 25.Zhao R, Chattopadhyay S, Hanscom M, Goldman ID. Antifolate resistance in a HeLa cell line associated with impaired transport independent of the reduced folate carrier. Clin Cancer Res. 2004;10:8735–8742. doi: 10.1158/1078-0432.CCR-04-0932. [DOI] [PubMed] [Google Scholar]
- 26.Diop-Bove NK, Wu J, Zhao R, Locker J, Goldman ID. Hypermethylation of the human proton-coupled folate transporter (SLC46A1) minimal transcriptional regulatory region in an antifolate-resistant HeLa cell line. Mol Cancer Ther. 2009;8:2424–2431. doi: 10.1158/1535-7163.MCT-08-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westerhof GR, Schornagel JH, Kathmann I, Jackman AL, Rosowsky A, Forsch RA, Hynes JB, Boyle FT, Peters GJ, Pinedo HM, Jansen G. Carrier- and receptor-mediated transport of folate antagonists targeting folate-dependent enzymes: correlates of molecular-structure and biological activity. Mol Pharmacol. 1995;48:459–471. [PubMed] [Google Scholar]
- 28.Theti DS, Jackman AL. The role of alpha-folate receptor-mediated transport in the antitumor activity of antifolate drugs. Clin Cancer Res. 2004;10:1080–1089. doi: 10.1158/1078-0432.ccr-03-0157. [DOI] [PubMed] [Google Scholar]
- 29.Zhao R, Gao F, Goldman ID. Marked suppression of the activity of some, but not all, antifolate compounds by augmentation of folate cofactor pools within tumor cells. Biochem Pharmacol. 2001;61:857–865. doi: 10.1016/s0006-2952(01)00532-9. [DOI] [PubMed] [Google Scholar]
- 30.Jackman AL, Kimbell R, Aherne GW, Brunton L, Jansen G, Stephens TC, Smith MN, Wardleworth JM, Boyle FT. Cellular pharmacology and in vivo activity of a new anticancer agent, ZD9331: a water-soluble, nonpolyglutamatable, quinazoline-based inhibitor of thymidylate synthase. Clin Cancer Res. 1997;3:911–921. [PubMed] [Google Scholar]
- 31.Zhao R, Hanscom M, Chattopadhyay S, Goldman ID. Selective preservation of pemetrexed pharmacological activity in HeLa cells lacking the reduced folate carrier; association with the presence of a secondary transport pathway. Cancer Res. 2004;64:3313–3319. doi: 10.1158/0008-5472.can-03-3953. [DOI] [PubMed] [Google Scholar]
- 32.Chattopadhyay S, Zhao R, Krupenko SA, Krupenko N, Goldman ID. The inverse relationship between reduced folate carrier function and pemetrexed activity in a human colon cancer cell line. Mol Cancer Ther. 2006;5:438–449. doi: 10.1158/1535-7163.MCT-05-0243. [DOI] [PubMed] [Google Scholar]
- 33.Andreassi JL, Moran RG. Mouse folylpoly-gamma-glutamate synthetase isoforms respond differently to feedback inhibition by folylpolyglutamate cofactors. Biochemistry. 2002;41:226–235. doi: 10.1021/bi015644d. [DOI] [PubMed] [Google Scholar]
- 34.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S, Manegold C, Niyikiza C, Paoletti P. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 35.Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, Von Pawel J, Gatzemeier U, Tsao TC, Pless M, Muller T, Lim HL, Desch C, Szondy K, Gervais R, Shaharyar Manegold C, Paul S, Paoletti P, Einhorn L, Bunn PA., Jr Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 36.Scagliotti GV, Parikh P, Von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, Lee JS, Mellemgaard A, Park K, Patil S, Rolski J, Goksel T, De Marinis F, Simms L, Sugarman KP, Gandara D. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advancedstage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 37.Bueno R, Appasani K, Mercer H, Lester S, Sugarbaker D. The alpha folate receptor is highly activated in malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2001;121:225–233. doi: 10.1067/mtc.2001.111176. [DOI] [PubMed] [Google Scholar]
- 38.Christoph DC, Asuncion BR, Hassan B, Tran C, Maltzman JD, O’Shannessy DJ, Wynes MW, Gauler TC, Wohlschlaeger J, Hoiczyk M, Schuler M, Eberhardt WE, Hirsch FR. Significance of folate receptor alpha and thymidylate synthase protein expression in patients with non-small-cell lung cancer treated with pemetrexed. J Thorac Oncol. 2013;8:19–30. doi: 10.1097/JTO.0b013e31827628ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nunez MI, Behrens C, Woods DM, Lin H, Suraokar M, Kadara H, Hofstetter W, Kalhor N, Lee JJ, Franklin W, Stewart DJ, Wistuba II. High expression of folate receptor alpha in lung cancer correlates with adenocarcinoma histology and EGFR [corrected] mutation. J Thorac Oncol. 2012;7:833–840. doi: 10.1097/JTO.0b013e31824de09c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leamon CP, You F, Santhapuram HK, Fan M, Vlahov IR. Properties influencing the relative binding affinity of pteroate derivatives and drug conjugates thereof to the folate receptor. Pharm Res. 2009;26:1315–1323. doi: 10.1007/s11095-009-9840-3. [DOI] [PubMed] [Google Scholar]