Abstract
OBJECTIVE
The study aims are to evaluate cerebral background patterns using amplitude-integrated electroencephalography (aEEG) in newborns with critical congenital heart disease (CHD), determine if aEEG is predictive of preoperative brain injury and assess the incidence of preoperative seizures. We hypothesize that aEEG will show abnormal background patterns in the early preoperative period in infants with CHD that have preoperative brain injury on magnetic resonance imaging (MRI).
METHODS
Twenty-four newborns with CHD requiring surgery at less than 30 days of age were prospectively enrolled within the first three days of age at a tertiary care pediatric hospital. Infants had aEEG for 24 hours beginning close to birth and preoperative brain MRI. The aEEGs were read to determine if the background pattern was normal, mildly abnormal, or severely abnormal. The presence of seizures and sleep-wake cycling were noted. The preoperative brain MRIs were used for brain injury and brain atrophy assessment.
RESULTS
Fifteen of 24 infants had an abnormal aEEG at 0.71 [0–2] (mean [range]) days of age. In five infants the background pattern was severely abnormal (burst suppression and/or continuous low voltage). Of the 15 infants with an abnormal aEEG, 9 (60%) had brain injury. One infant, with brain injury, had a seizure on aEEG. A severely abnormal background pattern on aEEG was associated with brain atrophy (P = 0.03) and absent sleep-wake cycling (P = 0.022).
CONCLUSION
Background cerebral activity is abnormal on aEEG following birth in newborns with CHD that have findings of brain injury and/or brain atrophy on preoperative brain MRI.
Keywords: brain injury, seizures, electroencephalogram, neuromonitoring, newborn, congenital heart disease
INTRODUCTION
Newborns with congenital heart disease (CHD) that require surgery during the neonatal period are at risk for early brain injury, with up to 50% having brain injury on preoperative brain magnetic resonance imaging (MRI).1–3 Methods to identify brain injury and predict neurodevelopmental outcomes using non-invasive neurodiagnostic tests, such as electroencephalography (EEG), could improve clinical care of these newborns. In addition, recent guidelines by the American Clinical Neurophysiology Society recommends long-term EEG monitoring in infants with CHD that require early surgery.4
Amplitude-integrated EEG (aEEG) differs from a conventional EEG as it is a time compressed EEG recording using a limited number of channels. An aEEG is most commonly used in neonatal intensive care units and has been well studied in infants with hypoxic-ischemic encephalopathy.5–7 The advantages of aEEG are that it can function as a bedside brain monitor and can more easily be interpreted by non-neurologists using pattern recognition. The background pattern is an important feature of a newborn’s EEG providing a measure of brain function and relative brain maturity. A dysmature EEG background pattern (excessive discontinuity for postconceptual age) may indicate a higher risk for an impaired neurodevelopmental outcome.5, 8
Seizures can be seen preoperatively on EEG in newborns with CHD.9–12 In a study of preoperative aEEG in newborns with CHD, 45% had an abnormal background pattern and 14% had a severely abnormal pattern (burst suppression or flat trace) at some point during the recording, but these findings were not correlated with brain MRI.12 Similarly, in another study, only 55% of infants had a normal preoperative EEG.11 A study of aEEG postoperatively showed that a delayed recovery of the cerebral background pattern was associated with impaired motor outcomes at two years of age.13 Therefore, evaluation of an infant’s EEG can provide valuable neurodevelopmental prognostic information.
The objectives of our pilot study were to: 1.) evaluate cerebral background patterns using aEEG in newborns with critical CHD close to the time of birth, 2.) determine if background pattern on aEEG is a biomarker for preoperative brain injury on brain MRI, and 3.) assess the incidence of seizures in newborns with CHD in the early preoperative period. We hypothesized that aEEG will show abnormal background patterns in a significant number of newborns with CHD and that it will predict brain injury on MRI.
METHODS
Participants
Following Institutional Review Board approval, a prospective observational study was performed at Arkansas Children’s Hospital (ACH) between June 1, 2012 and October 31, 2013. Recruitment was done prenatally for fetuses with a prenatally-diagnosed heart disease that was expected to require surgery at less than 30 days of age from the Maternal-Fetal Medicine clinic at the University of Arkansas for Medical Sciences and the Bale Fetal Heart Center at ACH. Newborns that were not prenatally diagnosed, but that were admitted at three days of age or less and diagnosed as having CHD likely requiring surgery during the neonatal period were also enrolled. Infants with all types of CHD requiring surgery as neonates were included. Infants were excluded if their gestational age at birth was <36 weeks, if they had a major genetic syndrome, or if preoperative brain MRI was unable to be performed. Informed consent was obtained from the parents of each infant. Recorded baseline characteristics included demographics, birth history (birth weight, gestational age, and Apgar scores), type of CHD, and details pertaining to the first surgery including, age, type of surgery, and the Risk Adjustment for CHD Surgery (RACHS)-1 category.14 Sedative medications (morphine, midazolam, and lorazepam), the need for mechanical ventilation, and lowest blood glucose during the aEEG were noted.
Amplitude-integrated EEG
aEEG monitoring was performed as soon as possible after birth for 24 hours using the Olympic Brainz Monitor (Natus Medical Inc., San Carlos, CA). The aEEG was discontinued early for cases of a necessary medical procedure. The Olympic Brainz Monitor has four scalp electrodes placed at C4, P4, C3, and P3 locations of the international 10–20 EEG system and a ground electrode which allows for analysis of the right hemisphere, left hemisphere, and cross cerebral activity.
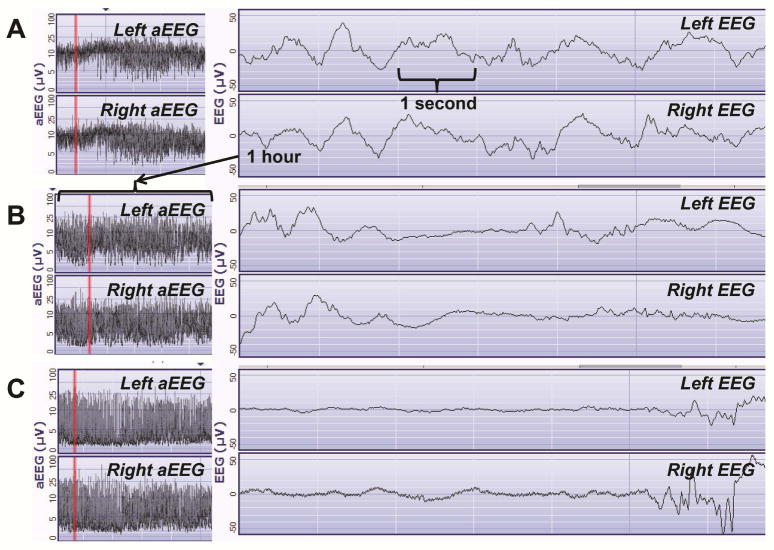
The aEEGs were de-identified and objectively read by a pediatric neurologist trained in aEEG interpretation. The 24-hour aEEG recording was interpreted in 3-hour blocks. The left, right, and cross cerebral channels were analyzed separately. The predominant background pattern in each aEEG block was scored as continuous normal voltage, discontinuous normal voltage, burst suppression, low voltage, or as isoelectric/flat trace per the pattern classification system.7, 15 An aEEG was considered normal when all aEEG blocks showed continuous normal voltage for the majority of the recording (Figure 1A). Mildly abnormal was considered when the tracing showed discontinuous normal voltage for the majority of at least one block during the recording and a severely abnormal background pattern was documented when the tracing showed burst suppression, continuous low voltage, or flat trace for at least one block of the recording (Figure 1B–C). Seizures were defined as an elevation of the upper and lower aEEG margins correlating with ≥10 seconds of rhythmic sharply contoured activity on the raw EEG trace. Sleep-wake cycling was described as sinusoidal variations with alternating narrow and broad aEEG tracings representing changing between discontinuous and more continuous background patterns.
Fig. 1. Sample aEEG and Corresponding Raw EEG in Newborns with CHD.
A: A normal aEEG showing continuous normal voltage and sleep-wake cycling in a 0 day old 39 week gestation newborn with hypoplastic left heart syndrome (Infant 2, Table 2). B: A mildly abnormal aEEG showing a discontinuous normal voltage pattern in a 1 day old 37 week gestation newborn with heterotaxy syndrome, double outlet right ventricle, and pulmonary stenosis (Infant 14, Table 2). C: A severely abnormal aEEG background pattern showing burst suppression in a 2 day old 37 week gestation newborn with hypoplastic left heart syndrome (Infant 20, Table 2).
Brain MR Imaging
Infants underwent a brain MRI, preoperatively, on a 1.5 Tesla Achieva scanner (Philips Healthcare, Best, Netherlands) using a neonatal MRI protocol that consisted of 3D T1 weighted images, axial T2 weighted images, T1 inversion recovery, fluid attenuation inversion recovery, diffusion weighted images, susceptibility weighted images, and diffusion tensor imaging. The MRIs were read by pediatric neuroradiologists and scored using the CHD MRI Injury score as reported previously.3 Brain injury was defined as an infarct, white matter injury, and/or deep gray matter injury. Brain atrophy was defined as enlargement of the extra-axial fluid spaces, dilatation of the lateral ventricles, and cortical sulcal prominence.
Statistical Analysis
Descriptive statistics for continuous variables (mean, median, range) and categorical variables (frequencies and percentages) were summarized. Comparisons between normal and abnormal aEEGs were performed with either Wilcoxon-Mann-Whitney test for continuous variables or Fisher’s Exact test for categorical variables. Bivariate associations between aEEG, brain injury, brain atrophy, sleep-wake cycling, and medications were assessed by logistic regressions. Odds ratio (OR) with 95% confidence intervals (CI) and the wald-type p-values were reported. Association between CHD MRI Injury score and aEEG findings were analyzed by Kruskal-Wallis test. All analyses were performed with the use of R software, version 3.0.2, and P-values of < 0.05 were considered to indicate statistical significance.
RESULTS
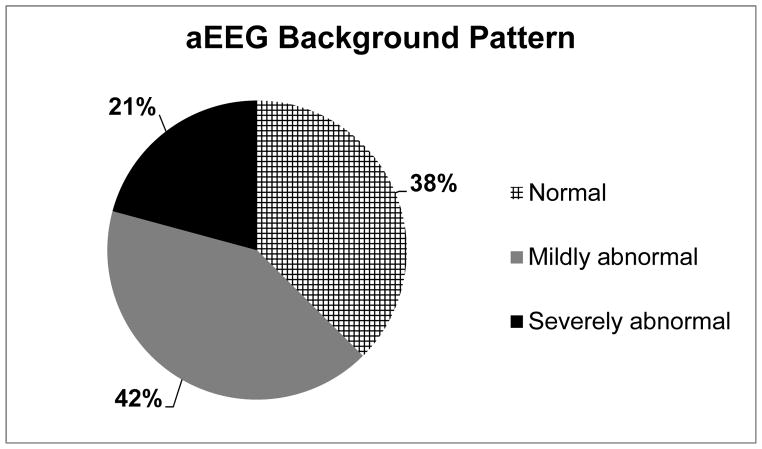
The demographic and clinical characteristics of the 24 infants are described in Table 1. An aEEG was performed on average for 22.3 [10.5–24] (mean [range]) hours at 0.71 [0–2] (mean [range]) days of age in the infants. Fifteen of 24 (62.5%) infants had an abnormal aEEG (Figure 2, Table 2). Infants with an abnormal aEEG had a lower 5-minute Apgar score (P =0.015), had CHD surgery at an older age (P =0.018), and were more likely to be male (P =0.047) compared to infants with a normal aEEG (Table 1). There was no difference in type of CHD, presence of sedative medications, need for mechanical ventilation or in the blood glucose during the aEEG between infants with a normal versus abnormal aEEG (Table 1). Five of 15 infants with an abnormal aEEG had a severely abnormal background pattern; burst suppression (n = 2), continuous low voltage (n = 4), and/or flat trace (n = 0). On average, 25% of the recording time was spent in the severely abnormal range for infants with a severely abnormal aEEG.
Table 1.
CHD infant characteristics according to aEEG background
| aEEG Normal† (N = 9) | aEEG Abnormal‡ (N = 15) | P | |
|---|---|---|---|
| Gestational Age (weeks) | 38.6 (36.9–39.6) | 38.0 (36.0–39.4) | .270 |
| Birth Weight (grams) | 3160.4 (2625.0–3515.0) | 3186.1 (1840.0–3990.0) | .318 |
| Apgar 1 minute | 8.1 (7.0–9.0) | 6.4 (1.0–9.0) | .083 |
| Apgar 5 minute | 8.8 (8.0–9.0) | 7.6 (3.0–9.0) | .015 |
| Male, (N, %) | 5 (55.6%) | 14 (93.3%) | .047 |
| d-Transposition of the great arteries (N, %) | 4 (44.4%) | 8 (53.3%) | 1 |
| Hypoplastic left heart syndrome (N, %) | 3 (33.3%) | 4 (26.7%) | 1 |
| Other CHD type (N, %) | 7 (77.8%) | 13 (86.7%) | .615 |
| aEEG (days of age) | 0.4 (0.0–1.0) | 0.9 (0.0–2.0) | .244 |
| Mechanical Ventilation during aEEG (N, %) | 2 (22.2%) | 8 (53.3%) | .210 |
| Sedation during aEEG (N, %) | 4 (44.4%) | 5 (33.3%) | .678 |
| Lowest Glucose during aEEG | 81.7 (59–125) | 83.6 (60–161) | 0.98 |
| Brain MRI (days of age) | 6.2 (1.0–13.0) | 10.0 (3.0–27.0) | .052 |
| Surgery (days of age) | 8.4 (2.0–26.0) | 12.9 (4.0–31.0) | .018 |
| RACHS-1 Category* | 1 | ||
| 1–2 | 1 (14.3%) | 1 (7.1%) | |
| 3–4 | 6 (85.7%) | 12 (85.7%) | |
| 5–6 | 1 (0.0%) | 1 (7.1%) | |
| Discharge (days of age) | 35.1 (4.0–83.0) | 50.4 (21.0–132.0) | .203 |
N (Col %) and Fisher’s Exact Test reported. Otherwise, Mean (Min, Max) and Wilcoxon-Mann-Whitney reported.
Specific Notes:
RACHS-1 category: A consensus-based method for CHD surgical risk. “1” is the lowest score associated with the least amount of risk of in-hospital mortality and “6” is the highest. Not all surgeries for CHD have a RACHS-1 category [14].
Normal aEEG: continuous normal voltage during the entire recording
Abnormal aEEG: either a mildly abnormal background pattern or a severely abnormal background pattern during at least one 3 hour block of the recording
Fig. 2. Background aEEG pattern in 24 CHD newborns.
An aEEG was considered normal when the background pattern was continuous normal voltage for the entire recording. Mildly abnormal was considered when the tracing showed discontinuous normal voltage during the recording and a severely abnormal background pattern was documented when the tracing showed burst suppression, continuous low voltage, or flat trace.
Table 2.
Infant CHD type, aEEG background and brain MRI findings
| Infant | CHD Type | aEEG* | CHD MRI Injury Score† | Focal Infarct | White Matter Injury‡ | Brain Atrophy (Yes/No) |
|---|---|---|---|---|---|---|
| 1 | Hypoplastic left heart syndrome | Normal | 6 | MCA (left, right) | – | Yes |
| 2 | Hypoplastic left heart syndrome | Normal | 1 | – | Minimal | No |
| 3 | Heterotaxy syndrome, double outlet right ventricle | Normal | 0 | – | – | No |
| 4 | d-Transposition of the great arteries | Normal | 0 | – | – | No |
| 5 | d-Transposition of the great arteries | Normal | 0 | – | – | No |
| 6 | Double outlet right ventricle with sub pulmonary stenosis | Normal | 2 | ACA (right, MCA (left) | – | No |
| 7 | Tetralogy of Fallot, pulmonary artery stenosis | Normal | 1 | – | – | No |
| 8 | Coarctation of aorta | Normal | 3 | – | – | Yes |
| 9 | Hypoplastic left heart syndrome, d-Transposition of the great arteries | Normal | 4 | – | – | No |
| 10 | d-Transposition of the great arteries | Mild | 0 | – | – | No |
| 11 | d-Transposition of the great arteries | Mild | 0 | – | – | No |
| 12 | d-Transposition of the great arteries | Mild | 3 | – | Minimal | No |
| 13 | d-Transposition of the great arteries | Mild | 3 | – | Moderate | No |
| 14 | Heterotaxy syndrome, double outlet right ventricle, pulmonary stenosis | Mild | 1 | PCA (right) | – | No |
| 15 | Hypoplastic left heart syndrome | Mild | 0 | – | – | No |
| 16 | Hypoplastic left heart syndrome | Mild | 5 | – | Minimal | Yes |
| 17 | Pulmonary atresia with intact ventricular septum | Mild | 4 | MCA (left) | – | Yes |
| 18 | Pulmonary atresia | Mild | 0 | – | – | No |
| 19 | d-Transposition of the great arteries | Mild | 3 | – | Moderate | No |
| 20 | Hypoplastic left heart syndrome | Severe | 6 | ACA (right), MCA (right) | – | Yes |
| 21 | d-Transposition of the great arteries | Severe | 2 | – | – | No |
| 22 | Hypoplastic left heart syndrome | Severe | 3 | – | – | Yes |
| 23 | d-Transposition of the great arteries | Severe | 6 | ACA (left) | Minimal | Yes |
| 24 | d-Transposition of the great arteries | Severe | 4 | – | Minimal | Yes |
Abbreviations:
ACA – anterior cerebral artery
MCA – middle cerebral artery
PCA – posterior cerebral artery
Specific Notes:
A normal aEEG showed continuous normal voltage, a mildly abnormal aEEG showed discontinuous normal voltage and a severely abnormal aEEG showed burst suppression, continuous low voltage or isoelectric/flat trace.
CHD MRI Injury Score: A detailed score based on conventional brain MRI for newborns with CHD in which distribution/severity of brain injury is quantified for 11 types of MRI findings. “0” represents a completely normal brain MRI [3].
White matter injury; Minimal is less than or equal to three areas of T1 signal abnormality on brain MRI measuring less than 2 mm in size, Moderate is greater than three areas of T1 signal abnormality or the areas measure greater than 2 mm but less than 5% of the hemisphere involved [3].
Brain injury, as defined by having a single focal infarct or multi-focal infarcts (n = 6) and/or white matter injury (n = 7) on preoperative brain MRI, was seen in 12 infants (50%, Table 2). Infants with brain injury had higher odds of having had an abnormal aEEG (OR = 3, 95% CI: 0.56–1.91), though not statistically significant due to small sample size (P = 0.213). Infants with a mildly abnormal aEEG had higher CHD MRI Injury scores compared to infants with a normal aEEG (median [25th–75th]) (2.0 [0.0–3.0] vs. 1.0 [0.0–3.0]). Infants with a severely abnormal aEEG had the highest CHD MRI injury scores (4.0 [3.0–6.0]).
Eight of the 24 (33%) infants’ had brain atrophy (Table 2). A severely abnormal aEEG background pattern was associated with brain atrophy (OR = 15, 95% CI: 1.67–343.39, P = 0.03), as 80% of infants with severe background abnormalities on aEEG also had brain atrophy. Three infants with brain atrophy and severe aEEG background abnormalities also had other forms of brain injury (focal infarct, n = 2 and/or white matter injury, n = 2), but these did not fit into a specific brain injury pattern.
One infant, that had a mildly abnormal background aEEG pattern and moderate white matter injury, had a subclinical seizure on the aEEG. Seizures were not found in the other infants. Sleep-wake cycling was present for at least half of the aEEG in eight infants. No association was found between the presence of sleep-wake cycling and brain injury. Infants with brain atrophy, however, were unlikely to have had sleep-wake cycling (OR = 0.065, 95% CI: 0.003–0.5, P = 0.022).
DISCUSSION
This study shows that close to 60% of newborns with CHD have abnormal aEEG background patterns on the first day of age. An abnormal aEEG background pattern correlates with brain injury on preoperative brain MRI and a severely abnormal aEEG background pattern is associated with brain atrophy. To our knowledge, this is the first study to correlate an early abnormal aEEG and brain injury detected on MRI in newborns with CHD that require neonatal surgery. These findings are important because routine neuromonitoring with EEG is not performed as a standard procedure in newborns with CHD that have surgery as neonates. Finding abnormal background EEG patterns, even in this limited time frame before surgery, can provide relevant information about the infants’ neurologic status that may correlate with neurodevelopmental outcome.
Fifty percent of our infants (n = 12) had mild to moderate brain injury scores based on the CHD MRI Injury score.3 Of these infants with brain injury, only 25% had a normal aEEG. We recently reported findings of widespread changes to the white matter tracts in infants with preoperative brain injury using diffusion tensor imaging.16 Having an abnormal aEEG indicates cerebral dysfunction, and given that 74% of the time the abnormal aEEG background was the same over the left and right cerebral hemispheres, this indicates abnormal function affecting the whole brain. Our diffusion tensor imaging analysis supports the aEEG findings, in that microstructural changes to the brain in infants with CHD and brain injury are diffuse.
Infants with CHD can have reduced brain volumes preoperatively.17–18 Brain atrophy, or reduced brain growth, represents a “global” brain abnormality. Fetal brain volume studies have shown reduced brain growth during the third trimester in some fetuses with CHD, a time in which brain development is typically rapid.17, 19 Reduced cerebral blood flow and oxygen delivery to the brain may be mechanisms for this finding.19–22 In our study, we found that infants with atrophy on brain MRI had severely abnormal aEEG background patterns (P = 0.03) and the absence of sleep-wake cycling (P = 0.02). The five infants with severely abnormal background aEEG patterns did not have findings consistent with hypoxic-ischemic encephalopathy at birth (mean [range] 5-minute Apgar 6.8 [3–9] vs. 8.4 [8–9], P = 0.2) to explain the background depression and they also did not have a difference in type of CHD and gestational age at birth compared to the other 19 infants. None of the five infants therefore qualified for hypothermia therapy,23 so the severely abnormal aEEG and lower Apgar score was not due to an acute hypoxic-ischemic mechanism. In studies of newborns with hypoxic-ischemic encephalopathy, early severe background aEEG abnormalities are associated with poor neurodevelopmental outcomes.6, 19, 24 Identification of an infant at high risk for neurodevelopmental impairments could help in discussing long-term neurologic outcomes with families even prior to surgical intervention.
A discontinuous aEEG, as was present in ten of our study infants, is a non-specific finding which can be seen due to brain immaturity, mild encephalopathy, metabolic abnormalities, and with medications. In addition, normal newborns can have periods of mild discontinuity during quiet sleep. Therefore, our study infants may have had some normal periods of discontinuity. On average, however the aEEG background was discontinuous for 46% of the recording in these ten infants recorded as having discontinuous normal voltage. Ten of 15 infants (67%) with an abnormal aEEG had no sedative medications during the recording compared to 5 of 9 infants (56%) with a normal aEEG, so medications may not have contributed much to the findings.25 Our infants also did not have hypoglycemia during the aEEG (Table 1). The infants with an abnormal aEEG may also have been more clinically unstable based on lower Apgar scores and later surgery due to time needed to stabilize them.
Our study found a subclinical seizure in only one infant with an average duration of aEEG recording of 22.3 hours, which was not confirmed by conventional EEG. This is a lower incidence of seizures compared to a similar study in which 19% of infants had seizures on preoperative aEEG, where infants were monitored for a mean of 54 hours.12 In that study, seizures were more common in infants with hypoplastic left heart syndrome, critical aortic valve stenosis, and aortic coarctation compared to infants with d-transposition of the great arteries, perhaps due to reduced systemic, and therefore cerebral perfusion.12 Infants with seizures may have significantly worse neurodevelopmental outcomes at age one year,26 so these early findings may pose significant neurodevelopmental consequences. Clearly, infants with CHD that require early surgery are at risk for seizures and would benefit from increased neuromonitoring.4
As seen in other studies,12 sleep-wake cycling was present in 50% of our infants at some point during the aEEG, with 33% of infants having sleep-wake cycling present for at least half of the recording. We did not find a correlation between the absence of sleep-wake cycling and brain injury as expected, which may be due to brain injury being fairly mild in most of the infants. The early return of sleep-wake cycling on aEEG in newborns with hypoxic-ischemic encephalopathy is associated with a more favorable outcome,27 so this finding in our infants may be associated with improved neurodevelopment. We did however, find sleep-wake cycling to be absent in all but one infant with brain atrophy.
This study is limited by the small sample size, but was appropriate for a pilot study.28 Due to different time points of study enrollment (prenatal and postnatal), there was some variability in the infants’ age at the start of the aEEG. An aEEG was selected for this study due to its ability to be used as a bedside brain monitor. However, it has inferior seizure detection compared to conventional EEG.29, 30 We likely would have identified seizures in more infants had conventional EEG been used and with longer duration. Another limitation of the aEEG monitor is that it does not have simultaneous video recording which would have allowed evaluation for environmental artifacts. Instead, nursing staff made notes on the monitor for clinical care such as respiratory treatments that can produce artifact on EEG. This study is also limited by the lack of a standardized neurologic exam during the aEEG. An exam was performed preoperatively though and there was no correlation between the neurologic exam and the earlier aEEG. We plan to follow the infants with a one year neurodevelopmental assessment. All but one infant survived to hospital discharge (Infant 20 in Table 2, aEEG sample Figure 1C).
In conclusion, aEEG abnormalities are detected in newborn infants with CHD and this correlates with brain injury and brain atrophy on preoperative MRI. The ability of a non-invasive and real-time neurodiagnostic test to inform about brain imaging abnormalities in newborns with CHD may enable improved neurologic focused preoperative clinical care that can hopefully lead to improved long-term neurodevelopmental outcomes.
Acknowledgments
Christopher J. Swearingen, PhD, Maria S. Melguizo, MS, and Chunqiao Luo, MS designed the research database and performed preliminary analyses. The authors appreciate the work of Nupur Chowdhury, CRRP, the ACH Research Institute research nurse coordinators, the EEG and MRI technicians, the cardiovascular intensive care unit nurses, the pediatric cardiothoracic surgery anesthesia department, and the ACH Angel One Transport service. We thank Natus Medical Inc. (San Carlos, CA) for providing the Olympic Brainz Monitor to use during the research study. We also greatly appreciate the infants and their families.
Funding: This study was supported by the University of Arkansas for Medical Sciences Center for Translational Neuroscience award from The National Institutes of Health (P20 GM103425 and P30 GM110702). REDCap receives institutional support from the University of Arkansas for Medical Sciences Translational Research Institute (UL1TR000039 and KL2TR000063 from the NIH). Dr. Mulkey also receives support from the Arkansas Children’s Hospital Research Institute and the Arkansas Biosciences Institute.
Abbreviations
- aEEG
amplitude-integrated electroencephalogram
- ACH
Arkansas Children’s Hospital
- ACA
anterior cerebral artery
- CHD
Congenital heart disease
- CI
confidence interval
- EEG
electroencephalography
- MCA
middle cerebral artery
- MRI
Magnetic resonance imaging
- OR
Odds ratio
- PCA
posterior cerebral artery
- RACHS-1
Risk Adjustment for CHD Surgery category
Footnotes
Conflict of Interest: Dr. Mulkey receives funding from The National Institutes of Health (P20 GM103425 and P30 GM110702), support from the Arkansas Children’s Hospital Research Institute, and the Arkansas Biosciences Institute. Natus Medical Inc. (San Carlos, CA) provided the Olympic Brainz Monitor to use during the study, but did not have input into the study design, data analysis, or the results reported in this study. All other authors do not have any conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sarah B. Mulkey, Email: mulkeysarah@uams.edu.
Shasha Bai, Email: SBai@uams.edu.
Raghu H. Ramakrishnaiah, Email: Raghuhr@uams.edu.
Charles M. Glasier, Email: GlasierCharlesM@uams.edu.
Renee A. Bornemeier, Email: BornemeierReneeA@ams.edu.
Michael L. Schmitz, Email: SchmitzMichaelL@uams.edu.
References
- 1.Mahle WT, Tavani F, Zimmerman RA, Nicolson SC, Galli KK, Gaynor JW, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106(12 Suppl 1):I109–14. [PubMed] [Google Scholar]
- 2.McQuillen PS, Barkovich AJ, Hamrick SE, Perez M, Ward P, Glidden DV, et al. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007;38(2 Suppl):736–41. doi: 10.1161/01.STR.0000247941.41234.90. [DOI] [PubMed] [Google Scholar]
- 3.Mulkey SB, Swearingen CJ, Melguizo MS, Schmitz ML, Ou X, Ramakrishnaiah RH, et al. Multi-Tiered Analysis of Brain Injury in Neonates With Congenital Heart Disease. Pediatr Cardiol. 2013;34:1772–84. doi: 10.1007/s00246-013-0712-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shellhaas RA, Chang T, Tsuchida T, Scher MS, Riviello JJ, Abend NS, et al. The American Clinical Neurophysiology Society’s Guideline on Continuous Electroencephalography Monitoring in Neonates. J Clin Neurophysiol. 2011;28:611–7. doi: 10.1097/WNP.0b013e31823e96d7. [DOI] [PubMed] [Google Scholar]
- 5.de Vries LS, Toet MC. Amplitude integrated electroencephalography in the full-term newborn. Clin Perinatol. 2006;33:619–32. doi: 10.1016/j.clp.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Hellstrom-Westas L, Rosen I, Svenningsen NW. Predictive value of early continuous amplitude integrated EEG recordings on outcome after severe birth asphyxia in full term infants. Arch Dis Child Fetal Neonatal Ed. 1995;72:F34–8. doi: 10.1136/fn.72.1.f34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toet MC, Hellstrom-Westas L, Groenendaal F, Eken P, de Vries LS. Amplitude integrated EEG 3 and 6 hours after birth in full term neonates with hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 1999;81:F19–23. doi: 10.1136/fn.81.1.f19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scher MS. Neurophysiological assessment of brain function and maturation. II. A measure of brain dysmaturity in healthy preterm neonates. Pediatr Neurol. 1997;16:287–95. doi: 10.1016/s0887-8994(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 9.Abend NS, Dlugos DJ, Clancy RR. A review of long-term EEG monitoring in critically ill children with hypoxic-ischemic encephalopathy, congenital heart disease, ECMO, and stroke. J Clin Neurophysiol. 2013;30:134–42. doi: 10.1097/WNP.0b013e3182872af9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunn JK, Beca J, Hunt RW, Olischar M, Shekerdemian LS. Perioperative amplitude-integrated EEG and neurodevelopment in infants with congenital heart disease. Intensive Care Med. 2012;38:1539–47. doi: 10.1007/s00134-012-2608-y. [DOI] [PubMed] [Google Scholar]
- 11.Limperopoulos C, Majnemer A, Rosenblatt B, Shevell MI, Rohlicek C, Tchervenkov C, et al. Association between electroencephalographic findings and neurologic status in infants with congenital heart defects. J Child Neurol. 2001;16:471–6. doi: 10.1177/088307380101600702. [DOI] [PubMed] [Google Scholar]
- 12.ter Horst HJ, Mud M, Roofthooft MT, Bos AF. Amplitude integrated electroencephalographic activity in infants with congenital heart disease before surgery. Early Hum Dev. 2010;86:759–64. doi: 10.1016/j.earlhumdev.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 13.Gunn JK, Beca J, Penny DJ, Horton SB, d’Udekem YA, Brizard CP, et al. Amplitude-integrated electroencephalography and brain injury in infants undergoing Norwood-type operations. Ann Thorac Surg. 2012;93:170–6. doi: 10.1016/j.athoracsur.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Jenkins KJ. Risk adjustment for congenital heart surgery: the RACHS-1 method. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2004;7:180–4. doi: 10.1053/j.pcsu.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Hellstrom-Westas L, Rosen I, de Vries LS, Greisen G. Amplitude-integrated EEG classification and interpretation in preterm and term infants. Neoreviews. 2006;7:e72–e87. [Google Scholar]
- 16.Mulkey SB, Ou X, Ramakrishnaiah RH, Glasier CM, Swearingen CJ, Melguizo MS, et al. White matter injury in newborns with congenital heart disease- A diffusion tensor imaging study. Ped Neurol. 2014;51:377–83. doi: 10.1016/j.pediatrneurol.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinton RB, Andelfinger G, Sekar P, Hinton AC, Gendron RL, Michelfelder EC, et al. Prenatal head growth and white matter injury in hypoplastic left heart syndrome. Pediatr Res. 2008;64:364–9. doi: 10.1203/PDR.0b013e3181827bf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Licht DJ, Shera DM, Clancy RR, Wernovsky G, Montenegro LM, Nicolson SC, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529–36. doi: 10.1016/j.jtcvs.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Limperopoulos C, Tworetzky W, McElhinney DB, Newburger JW, Brown DW, Robertson RL, Jr, et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121:26–33. doi: 10.1161/CIRCULATIONAHA.109.865568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berg C, Gembruch O, Gembruch U, Geipel A. Doppler indices of the middle cerebral artery in fetuses with cardiac defects theoretically associated with impaired cerebral oxygen delivery in utero: is there a brain-sparing effect? Ultrasound Obstet Gynecol. 2009;34:666–72. doi: 10.1002/uog.7474. [DOI] [PubMed] [Google Scholar]
- 21.Kaltman JR, Di H, Tian Z, Rychik J. Impact of congenital heart disease on cerebrovascular blood flow dynamics in the fetus. Ultrasound Obstet Gynecol. 2005;25:32–6. doi: 10.1002/uog.1785. [DOI] [PubMed] [Google Scholar]
- 22.Licht DJ, Wang J, Silvestre DW, Nicolson SC, Montenegro LM, Wernovsky G, et al. Preoperative cerebral blood flow is diminished in neonates with severe congenital heart defects. J Thorac Cardiovasc Surg. 2004;128:841–9. doi: 10.1016/j.jtcvs.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Mulkey SB, Fontenot E, Imamura M, Yap VL. Therapeutic hypothermia in a neonate with hypoxic-ischemic encephalopathy and transposition of the great arteries. Ther Hypotherm Temp Manage. 2011;1:205–8. doi: 10.1089/ther.2011.0016. [DOI] [PubMed] [Google Scholar]
- 24.Toet MC, Lemmers PM, van Schelven LJ, van BF. Cerebral oxygenation and electrical activity after birth asphyxia: their relation to outcome. Pediatrics. 2006;117:333–9. doi: 10.1542/peds.2005-0987. [DOI] [PubMed] [Google Scholar]
- 25.Olischar M, Davidson AJ, Lee KJ, Hunt RW. Effects of morphine and midazolam on sleep-wake cycling in amplitude-integrated electroencephalography in post-surgical neonates >/= 32 weeks of gestational age. Neonatology. 2012;101:293–300. doi: 10.1159/000334636. [DOI] [PubMed] [Google Scholar]
- 26.Bellinger DC, Jonas RA, Rappaport LA, Wypij D, Wernovsky G, Kuban KC, et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N Engl J Med. 1995;332:549–55. doi: 10.1056/NEJM199503023320901. [DOI] [PubMed] [Google Scholar]
- 27.Osredkar D, Toet MC, van Rooij LG, van Huffelen AC, Groenendaal F, de Vries LS. Sleep-wake cycling on amplitude-integrated electroencephalography in term newborns with hypoxic-ischemic encephalopathy. Pediatrics. 2005;115:327–32. doi: 10.1542/peds.2004-0863. [DOI] [PubMed] [Google Scholar]
- 28.Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharmaceut Statist. 2005;4:287–91. [Google Scholar]
- 29.Toet MC, van der Meij W, de Vries LS, Uiterwaal CS, van Huffelen KC. Comparison between simultaneously recorded amplitude integrated electroencephalogram (cerebral function monitor) and standard electroencephalogram in neonates. Pediatrics. 2002;109:772–9. doi: 10.1542/peds.109.5.772. [DOI] [PubMed] [Google Scholar]
- 30.Shellhaas RA, Soaita AI, Clancy RR. Sensitivity of amplitude-integrated electroencephalography for neonatal seizure detection. Pediatrics. 2007;120:770–7. doi: 10.1542/peds.2007-0514. [DOI] [PubMed] [Google Scholar]