Abstract
Rates of depression are high among individuals living with HIV. Accurate assessment of depressive symptoms among this population is important for ensuring proper diagnosis and treatment. The Beck Depression Inventory-II (BDI-II) is a widely used measure for assessing depression, however its psychometric properties have not yet been investigated for use with HIV-positive populations in the U.S. The current study was the first to assess the psychometric properties of the BDI-II among a large cohort of HIV-positive participants sampled at multiple sites across the U.S. as part of the CNS HIV Antiretroviral Therapy Effects Research (CHARTER) study. The BDI-II test scores showed good internal consistency (α = 0.93) and adequate test-retest reliability (ICC = 0.83) over a 6-month period. Using a ‘gold standard’ of major depressive disorder determined by the Composite International Diagnostic Interview (CIDI), sensitivity and specificity were maximized at a total cut-off score of 17 and a Receiver Operating Characteristic (ROC) analysis confirmed that the BDI-II is an adequate diagnostic measure for the sample (AUC = 0.83). The sensitivity and specificity of each score are provided graphically. Confirmatory factor analyses confirmed the best fit for a 3-factor model over 1-factor and 2-factor models and models with a higher-order factor included. The results suggest that the BDI-II is an adequate measure for assessing depressive symptoms among U.S. HIV-positive patients. Cut-off scores should be adjusted to enhance sensitivity or specificity as needed and the measure can be differentiated into cognitive, affective, and somatic depressive symptoms.
Keywords: HIV, AIDS, Depression, BDI-II, Psychometric, Validation
Introduction
The rates of depression in HIV-positive populations are double that of the general population (Ciesla & Roberts, 2001; Penzak, Reddy, & Grimsley, 2000). The consequences of depression in HIV are profound and include worse medication adherence (Ammassari et al., 2004; Patterson, Swindells, & Mohr, 1999; Singh & Squier, 1996), alterations in the immune system (Leserman, 2003; Lyketsos, Hoover, & Guccione, 1993; Patterson et al., 1999), higher rates of risky sexual and drug use behaviors (Hutton, Lyketsos, Thompson, & Erbelding, 2004; Perdue, Hagan, Thiede, & Valleroy, 2003), and premature death (Pyne et al., 2008). The BDI-II, one of the most widely used self-report measures for assessing depression among the general population, frequently serves as an index of depressive symptoms in research with HIV-infected samples (Berger-Greenstein et al., 2007; Kagee, Nel, & Saal, 2013; Rosenbloom et al., 2007).
Despite its wide use, only two studies to date examined the psychometric properties of the BDI-II among individuals with HIV/AIDS. Lipps and colleagues (Lipps et al., 2010) administered the BDI-II to 191 HIV-positive individuals recruited through HIV clinics in Kingston and St. Andrew, Jamaica. The BDI-II test scores showed good internal consistency with a cronbach’s coefficient alpha of 0.89, and the score interpretations had good convergent validity (r = 0.74) with the Center for Epidemiological Studies -Depression Scale (Lipps et al., 2010) and discriminant validity (r = −0.42) with the Social Provisions Scale (Cutrona & Russell, 1987). A three-factor solution of cognitive, affective, and somatic symptoms emerged using a principal components analysis with oblique rotation. More recently, Kagee, Nel, and Saal (2013) administered the BDI-II to a group of 185 HIV-positive South Africans and found good internal consistency (α = .90) and a three-factor solution of cognitive, affective, and somatic symptoms using principal components analysis with orthogonal rotation. Previous research suggests that the experience of living with HIV and depressive symptoms are influenced by cultural factors such as stigma, cultural beliefs, and collective knowledge (Airhihenbuwa, Ford, & Iwelunmor, 2013; Cain et al., 2013; Walsh & Cross, 2013). These cultural factors may influence the validity of the BDI-II among varying populations and geographic regions, thus warranting a thorough examination of the BDI-II among a U.S. population as well.
Studies exploring the factor structure of the BDI-II among HIV seronegative samples have consistently found 2- and 3-factors solutions using both exploratory and confirmatory factor analyses (CFA) (Al-Musawi, 2001; Dozois, Dobson, & Ahnberg, 1998; Vanheule, Desmet, Groenvynck, Rosseel, & Fontaine, 2008). There is also support for an underlying, higher-order factor often labeled general depression or general distress (Steer, Clark, Beck, & Ranieri, 1998). No study to date has examined the factor structure of the BDI-II among an adequately sized sample of HIV-positive patients within the U.S..
The diagnostic value of the cut-off scores outlined by Beck and colleagues (Beck, Steer, & Brown, 1996) has not been validated within an HIV-positive population. Commonly used diagnostic values include sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). Sensitivity is the proportion of participants diagnosed with MDD that are correctly identified by the BDI-II cut-off score. Specificity is the proportion of participants not diagnosed with MDD that are correctly identified by the BDI-II cut-off score. Positive predictive value is the probability that a participant has MDD given that they are above the designated BDI-II cut off score. Negative predictive value is the probability that a participant does not have MDD given that they are below the designated BDI-II cut off score.
In the original validation, Beck and colleagues (Beck et al., 1996) conducted Receiver Operating Characteristics (ROC) analyses with data collected from psychiatric outpatients. The calculated Area Under the Curve (AUC) value, which is an index of the amount of diagnostic information provided by the measure, ranges from 0.0 (no information) to 1.0 (perfect diagnostic accuracy) and values .80 and above represent a useful screening instrument (Arnau, Meagher, Norris, & Bramson, 2001; Holmes, 1998). Beck and colleagues conducted 3 ROC analyses comparing categories of depression severity. They used the cut-off score in each analysis that best maximized sensitivity and specificity to develop the commonly used classifications, however they did not report specific diagnostic values for each.
Sensitivity and specificity varies for BDI-II cut-off scores depending on the population sampled. An ROC analysis with BDI-II scores collected from a sample of primary care patients showed an AUC of .96, indicating excellent predictive validity. A cut-off score of 18 provided the best balance between sensitivity (94%) and specificity (92%). An AUC of .91 was determined with BDI-II scores collected from African American primary care patients and a cut-off score of 14 provided the best balance between sensitivity (88%) and specificity (84%) (Dutton et al., 2004). The appropriate cut-off score for use with HIV-positive individuals has not yet been determined.
The current study examined the psychometric properties of the BDI-II among a large, sample of HIV-positive patients recruited in the United States. The findings provide information on the reliability, factor structure, and diagnostic characteristics of the BDI-II for use with HIV-positive populations.
Methods
A cross-sectional examination was conducted using data collected from the CHARTER study to assess the psychometric properties of the BDI-II among a large, U.S. based sample of individuals with HIV/AIDS. The CHARTER study is a multi-site, prospective, observational study funded by the National Institute of Mental Health and the National Institute of Neurological Disorders and Stroke and was conducted at the following six North American medical centers from 2003 to 2007: Johns Hopkins University, Baltimore, MD; The Mount Sinai School of Medicine, New York, NY; University of California, San Diego, San Diego, CA; University of Texas, Medical Branch, Galveston, TX; University of Washington, Seattle, WA; and Washington University, St. Louis, MO. Local Institutional review boards approved research at each site and all participants provided written informed consent. Participants were eligible to participate in the CHARTER study if they were HIV-positive, able to provide written informed consent, and able to complete baseline assessments. The minimal exclusion criteria included only the inability to complete assessments. Of the 1,900 patients asked to participate, 78.1% agreed. The 317 patients that refused had lower education levels, higher rates of female gender, and nonwhite ethnicity compared to the study participants. The total sample collected at time 1 consisted of 1,583 HIV-positive individuals from diverse backgrounds recruited to be reflective of patients at university-affiliated HIV treatment centers. A smaller group of participants (n=698) returned for follow-up data collection 6 months later. These participants were selected on the basis of identified groups of interest with characteristics whose long-term follow-up would shed additional light on important questions in regards to neurological function in the context of HAART. Participants completed thorough neuromedical and neurobehavioral assessments at each time point. Only the measures of mood and depression were used for the current investigation.
Measures
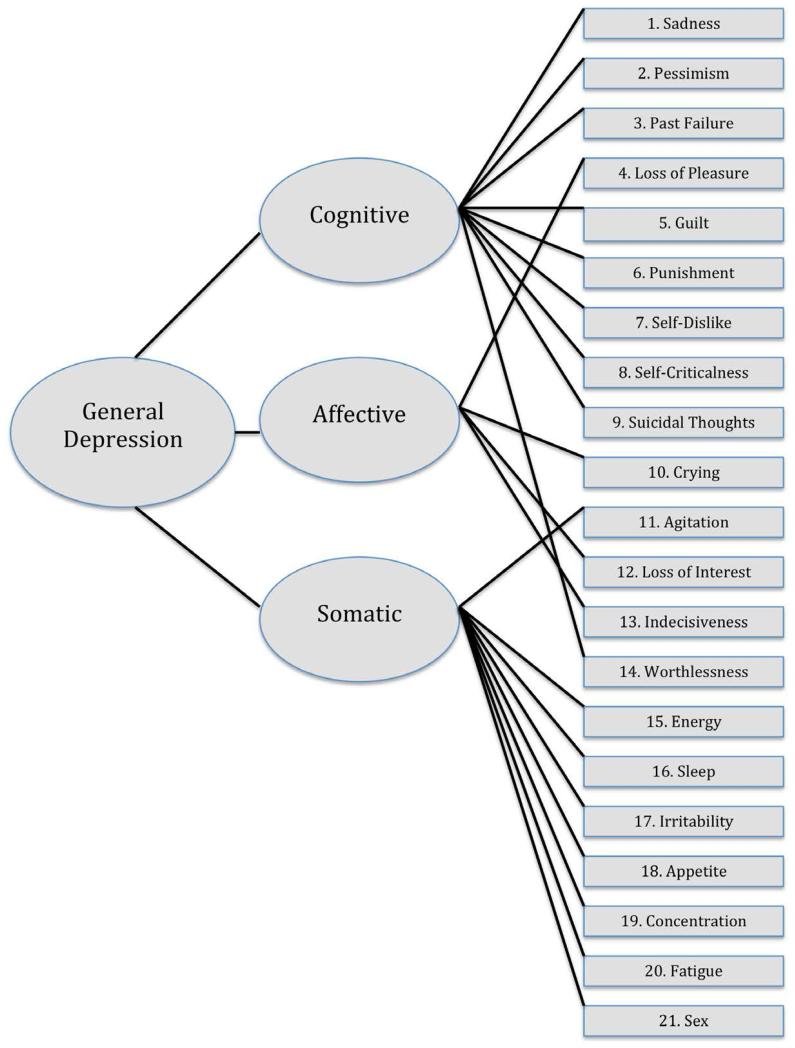
The BDI-II (Beck et al., 1996) is a measure of self-reported depression and mood. The items include an assessment of 21 behavioral and observable symptom areas covering a range of emotional, cognitive, motivational, and somatic components of clinical depression designed to reflect the depressive episode criteria outlined in the DSM-IV (American Psychiatric Association, 1994; Beck & Beamesderfer, 1974). It can be conducted with persons 13 to 80 years of age, requires approximately 5 to 10 minutes to complete, and can be read to respondents who may have difficulty reading independently. The BDI-II is scored by summing the ratings, which are each scored on a 4-point scale ranging from 0 to 3, with a range from 0 to 63. This measure was designed as a tool for determining depressive symptom type and severity and not as a diagnostic tool (Beck et al., 1996). However, the BDI-II is often used as a diagnostic or screening tool with the cutoff-scores determined during the original validation with psychiatric outpatients: 0-13 minimal, 14-19 mild, 20-28 moderate, 29-63 severe. The complete manual and scoring kit, including 25 record forms, can be purchased from Pearson (San Antonia, TX) for $125.00. Pearson also offers a web-based scoring and reporting platform, Q-global™, that produces interpretive reports for $3.00 each.
The Composite International Diagnostic Interview (Robins et al., 1988) is a structured interview designed to assess the quality and severity of substance use disorders and mental disorders as outlined in the International Classification of Diseases (9th revision) and the Diagnostic and Statistical Manual of Mental Disorders III. Reliability and validity have been shown across cultures around the world (Robins et al., 1988). For the current study, all assessors were trained and certified by the Psychiatric Coordinator at the Coordinating Center, and provided tape recordings of their first 15 CIDI interviews, which to the protocol and reliability. All assessors were re-certified on CIDI administration on an annual basis.
Statistical Analyses
All statistical analyses were conducted using SPSS 20 (IBM Corp, Released 2011) or Mplus Version 6 (Muthén & Muthén, 1998-2012). Chi-square tests of independence and independent t-tests were conducted to identify significant differences between the time 1 and 2 samples. Internal consistency was examined using cronbach’s coefficient alpha. Intraclass correlation coefficients were used to assess test-retest reliability for the BDI-II total score and individual items. Logistic regression assessed the predictive validity of the BDI-II test score interpretations. Current major depressive disorder (MDD) at time 2 was regressed onto BDI-II total score at time 1, while controlling for current MDD at time 1.
Sensitivity, specificity, PPV, and NPV were calculated to examine the BDI-II as a tool for screening and diagnosing MDD among HIV-infected individuals. ROC curves and AUC values were calculated and the ‘gold standard’ of MDD for the current sample was determined by the CIDI. The ROC analyses were conducted with time 1 data only.
CFA tested one-, two-, and three-factor solutions to account for variation in individual BDI-II items. The two- and three-factors solutions were also conducted with a higher-order factor included in the model to assess for improvement in fit. The CFAs were run with time 1 and 2 data to assess for reliability across samples. For the CFAs, weighted least squares with parameter estimates (WLSMV) were used to calculate chi-square test statistics (χ2) root mean square error of approximation (RMSEA), comparative fit index (CFI), Tucker-Lewis fit index (TFI), and goodness of fit index (GFI).
Chi-square difference tests, a common method of comparing nested models, cannot be used to compare the present models for two reasons; 1) WLSMV produces a between-model value that is not distributed as chi-square (Byrne, 2012, p.139) and, 2) Mplus does not deem the current models as nested., which Brown (2006, p.164) explains concisely. Thus, the CFAs were rerun using maximum likelihood parameter estimates (MLR) that are robust to non-normality and non-independence of observations to allow for comparison across models using Akaike Information Criterion values (AIC). AIC was used to compare the overall fit of competing, non-nested models, with lower values indicating a better fit (Brown, 2006, p.180).
The factors tested in the current analyses were pre-specified based on the two-factor model derived by Beck and colleagues (Beck et al., 1996) with a sample of 277 psychiatric outpatients using promax-rotated iterated-principal factor analysis, and the three-factor model derived by Buckley and colleagues (Buckley, Parker, & Heggie, 2001) using CFAs with a sample of 416 men admitted to a residential substance use program at a Veteran’s Affairs Medical Center. The models are displayed in Figures 1 and 2, respectively. These two models were chosen for the current study based on evidence from a large comparison study conducted by Vanheule and colleagues (Vanheule et al., 2008), who found these two models to have the best fit when compared to five alternative two-factor models and four alternative three-factor models across clinical and non-clinical adult samples.
Figure 1.
Two-Factor Model derived from Beck et al., 1996 with a higher-order factor.
Figure 2.
Three-Factor Model derived from Buckley et al., 2001 with a higher-order factor.
All secondary data analyses were approved by the University at Albany, State University of New York Institutional Review Board.
Results
The demographic, disease, and medical comorbidity characteristics of the time 1 and 2 samples are displayed in Table 1. The time 2 sample was significantly older, had higher CD4 absolute cell counts, lower viral load counts, less medical comorbidities, and less HIV-associated neurocognitive impairment. Current and lifetime psychiatric and substance use diagnoses are displayed in Table 2. The time 1 sample had significantly more diagnoses of current MDD. Statistics for the BDI-II items and total scores for time 1 and time 2 data are displayed in Table 3. The time 1 sample had significantly higher BDI-II scores (t(2261)= 3.85, p<.01). The mean BDI-II total score found among the time 1 sample was similar to other samples of individuals living with HIV (Lipps et al., 2010; Rosenbloom et al., 2007) and lower than HIV-positive participants with significant substance use and psychiatric morbidities (Berger-Greenstein et al., 2007). Internal consistency was good for time 1 (α = 0.93) and time 2 (α = 0.94). Test-retest reliability was adequate for the total score (ICC = 0.83) considering depressive symptoms were expected to change over the 6-month period between BDI-II administrations. Intraclass correlation coefficients varied for each BDI-II item with the lowest reliability coefficient for changes in appetite (ICC = 0.48) and the highest for loss of interest in sex (ICC = 0.75).
Table 1. Characteristics of Time 1 and 2 Samples.
| Time 1 (N= 1,583) |
Time 2 (N= 698) |
||
|---|---|---|---|
| n (%) or M (SD) | n (%) or M (SD) | p | |
| Gender | 0.26 | ||
| Male | 1216 (76.8%) | 551 (78.9%) | |
| Female | 367 (23.2%) | 147 (12.1%) | |
| Race | 0.29 | ||
| Black | 760 (48.0%) | 301 (43.1%) | |
| White | 633 (40%) | 303 (43.4%) | |
| Hispanic | 149 (9.4%) | 76 (10.9%) | |
| Other | 40 (2.5%) | 17 (2,4%) | |
| Age (years) | 43.09 (8.5) | 43.95 (8.5) | 0.03 |
| Years of Education Completed | 12.53 (2.6) | 12.73 (2.6) | 0.08 |
| Disease Status | |||
| AIDS | 991 (62.7%) | 433 (62.0%) | 0.77 |
| CD4 Absolute | 463.15 (287.9) | 489.33 (266.8) | 0.04 |
| Viral Load (Log) | 2.87 (1.31) | 2.64 (1.2) | 0.01 |
| Comorbidity Status | |||
| Incidental | 867 (54.8%) | 421 (60.3%) | 0.03 |
| Contributing | 477 (30.1%) | 194 (27.8%) | |
| Confounding | 239 (15.1%) | 83 (11.9%) | |
| HAND Categories | 0.01 | ||
| No Impairment | 718 (53.4%) | 382 (62.1%) | |
| Asymptomatic | 465 (34.6%) | 119 (19.3%) | |
| Mild | 122 (9.1%) | 94 (15.3%) | |
| Dementia | 39 (2.9%) | 20 (3.3%) |
Note. AIDS status was determined using CDC criteria (Castro et al., 1992); Details regarding medical comorbidity categories can be found in Antinori et al., 2007; HAND= HIV associated neurocognitive disorders; HAND catories were only computed for the participants with incidental and contributing medical comorbidity statuses as per guidelines in Antinori et al., 2007.
Table 2. Psychiatric and Substance Use Diagnoses of Time 1 and 2 Samples.
| Time 1 (N= 1,583) |
Time 2 (N= 698) |
||
|---|---|---|---|
| n (%) | n (%) | p | |
| Psychiatric | |||
| Current MDD | 227 (14.5%) | 79 (11.4%) | 0.05 |
| Lifetime MDD | 802 (51.2%) | 380 (54.9%) | 0.10 |
| Current Dysthymia | 13 (0.8%) | 2 (0.3%) | 0.33 |
| Lifetime Dysthymia | 34 (2.1%) | 24 (3.4%) | 0.19 |
| Substance Use | |||
| Any Current Substance Use | 50 (3.2%) | 22 (3.2%) | 0.94 |
| Any Lifetime Substance Use | 1152 (72.8%) | 509 (72.9%) | 0.94 |
| Current Alcohol | 25 (1.6%) | 10 (1.4%) | 0.91 |
| Lifetime Alcohol | 868 (54.8%) | 387 (55.4%) | 0.92 |
| Current Cannabis | 17 (1.1%) | 4 (0.6%) | 0.48 |
| Lifetime Cannabis | 471 (29.8%) | 204 (29.2%) | 0.91 |
| Current Cocaine | 0 (0.0%) | 0 (0.0%) | N/A |
| Lifetime Cocaine | 676 (42.7%) | 289 (41.4%) | 0.78 |
| Current Methamphetamine | 10 (0.6%) | 8 (1.1%) | 0.42 |
| Lifetime Methamphetamine | 271 (17.1%) | 122 (17.5%) | 0.93 |
| Current Hallucinogen | 0 (0.0%) | 0 (0.0%) | N/A |
| Lifetime Hallucinogen | 120 (7.6%) | 46 (6.6%) | 0.66 |
| Current Inhalant | 0 (0.0%) | 0 (0.0%) | N/A |
| Lifetime Inhalant | 55 (3.5%) | 22 (3.2% | 0.87 |
| Current Opioid | 2 (0.1%) | 0 (0.0%) | 0.61 |
| Lifetime Opioid | 269 (17.0%) | 119 (17.0%) | 0.94 |
| Current PCP | 0 (0.0%) | 0 (0.0%) | N/A |
| Lifetime PCP | 44 (2.8%) | 21 (3.0%) | 0.90 |
| Current Sedative | 0 (0.0%) | 0 (0.0%) | N/A |
| Lifetime Sedative | 123 (7.8%) | 50 (7.2%) | 0.83 |
| Current Other | 0 (0.0%) | 0 (0.0%) | N/A |
| Lifetime Other | 36 (2.3%) | 18 (2.6%) | 0.86 |
Note. MDD= major depressive disorder; PCP= phencyclidine
Table 3. Beck Depression Inventory-II Statistics.
| Time 1 Sample | Time 2 Sample | ||||||
|---|---|---|---|---|---|---|---|
| BDI-II Item | n | M (SD) | Range | n | M (SD) | Range | Test-retest r |
| Sadness | 1579 | 0.45 (0.66) | 0-3 | 698 | 0.40 (0.62) | 0-3 | 0.60*** |
| Pessimism | 1579 | 0.55 (0.76) | 0-3 | 698 | 0.48 (0.68) | 0-3 | 0.65*** |
| Past-Failure | 1579 | 0.77 (0.86) | 0-3 | 698 | 0.59 (0.77) | 0-3 | 0.74*** |
| Loss of Pleasure | 1579 | 0.71 (0.75) | 0-3 | 698 | 0.63 (0.72) | 0-3 | 0.73*** |
| Guilty Feelings | 1579 | 0.57 (0.67) | 0-3 | 698 | 0.47 (0.66) | 0-3 | 0.69*** |
| Punishment Feelings | 1579 | 0.49 (0.93) | 0-3 | 698 | 0.38 (0.82) | 0-3 | 0.59*** |
| Self-Dislike | 1579 | 0.66 (0.89) | 0-3 | 698 | 0.51 (0.79) | 0-3 | 0.64*** |
| Self-Criticalness | 1578 | 0.64 (0.85) | 0-3 | 698 | 0.55 (0.81) | 0-3 | 0.58*** |
| Suicidal Thoughts or Wishes | 1578 | 0.23 (0.47) | 0-3 | 698 | 0.20 (0.41) | 0-2 | 0.70*** |
| Crying | 1578 | 0.55 (0.90) | 0-3 | 698 | 0.46 (0.83) | 0-3 | 0.53*** |
| Agitation | 1579 | 0.57 (0.79) | 0-3 | 697 | 0.47 (0.71) | 0-3 | 0.54*** |
| Loss of Interest | 1579 | 0.70 (0.86) | 0-3 | 698 | 0.61 (0.80) | 0-3 | 0.70*** |
| Indecisiveness | 1579 | 0.58 (0.80) | 0-3 | 698 | 0.46 (0.72) | 0-3 | 0.71*** |
| worthlessness | 1578 | 0.42 (0.71) | 0-3 | 698 | 0.38 (0.68) | 0-3 | 0.69*** |
| Loss of Energy | 1579 | 0.92 (0.71) | 0-3 | 698 | 0.84 (0.71) | 0-3 | 0.70*** |
| Changes in Sleeping Pattern | 1572 | 1.16 (0.95) | 0-3 | 696 | 1.05 (0.95) | 0-3 | 0.54*** |
| Irritability | 1579 | 0.58 (0.76) | 0-3 | 698 | 0.50 (0.72) | 0-3 | 0.62*** |
| Changes in Appetite | 1571 | 0.87 (0.88) | 0-3 | 697 | 0.79 (0.87) | 0-3 | 0.48*** |
| Concentration Difficulty | 1579 | 0.77 (0.76) | 0-3 | 698 | 0.63 (0.71) | 0-3 | 0.70*** |
| Tiredness or Fatigue | 1579 | 0.89 (0.79) | 0-3 | 698 | 0.82 (0.77) | 0-3 | 0.71*** |
| Loss of Interest in Sex | 1579 | 0.90 (0.97) | 0-3 | 698 | 0.84 (0.95) | 0-3 | 0.75*** |
| Total | 1566 | 13.94 (10.81) | 0-58 | 694 | 12.07 (10.51) | 0-56 | 0.83*** |
Note.
p<.05
p<.01
p<.001
Logistic regression analyses indicated that BDI-II total score at time 1 successfully distinguished between those with and without a diagnosis of MDD at time 2, χ2 (2, 697) = 81.30, p < .001. The odds of being diagnosed with MDD at time 2 were 1.1 greater with every one-unit increase in BDI-II score at time 1. Classification however, was unimpressive, with 11% of those with MDD and 99% of those without MDD correctly predicted, for an overall success rate of 89%.
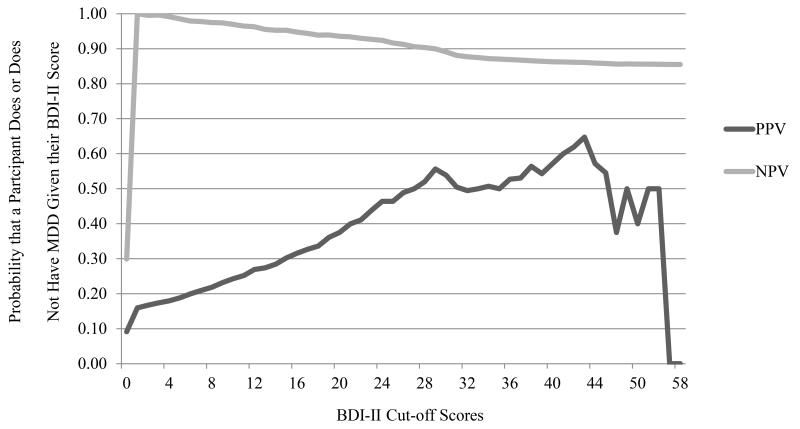
The sensitivity, specificity, PPV, and NPV at each BDI-II value are displayed in Figures 3 and 4, respectively. The BDI-II had equal sensitivity and specificity (74%) at a cut-off of 17, meaning that 74% of those with depression and without depression were correctly identified using a cut-off of 17. Sensitivity increased to 90% at a cut-off of 10; however, specificity decreased to 52%, suggesting that approximately half of those without depression will be suspected to be depressed based on the BDI-II cut-off. The ROC analysis revealed an AUC of 0.83, which denotes the BDI-II as an adequate diagnostic measure for this sample, albeit less accurate than the previously mentioned studies. Excluding somatic and affective depressive symptoms reduced the diagnostic value of the measure with an AUC of 0.82 for both ROC analyses.
Figure 3.
Sensitivity and Specificity of the BDI-II. Sensitivity is the proportion of participants diagnosed with MDD that are correctly identified by the BDI-II cut-off score. Specificity is the proportion of participants not diagnosed with MDD that are correctly identified by the BDI-II cut-off score. Correctly diagnosed/ not diagnosed was determined by Composite International Diagnostic Interview.
Figure 4.
Positive and negative predictive value of the BDI-II. Positive predictive value (PPV) is the probability that a participant has MDD given that they are above the designated BDI-II cut off score. Negative predictive value (NPV) is the probability that a participant does not have MDD given that they are below the designated BDI-II cut off score. Correctly diagnosed/ not diagnosed was determined by Composite International Diagnostic Interview.
CFAs were used to assess the fit of the previously described models of the BDI-II items. The CFAs revealed adequate fit for all models (see Table 4) based on various fit indices. The 3-factor model proposed by Buckley and colleagues (2001) was significantly better than the two-factor model proposed by Beck and colleagues (1996) and the one-factor model indicative of no subscales, as evidenced by its low AIC value. The addition of a higher-order factor to the model did not improve fit for the three-factor or two-factor models. These findings were consistent across the time1 and 2 samples. All three factors were strongly, positively correlated, with the cognitive and affective subscales showing the strongest correlation (r = .0.76), followed by somatic and affective (r = 0.75), and cognitive and somatic (r = 0.68).
Table 4. Confirmatory Factor Analysis of the BDI-II Factor Structure in Time 1 and 2 Samples.
| χ 2 | df | RMSEA (<.06) | CFI (>.95) | TLI (>.95) | AIC | |
|---|---|---|---|---|---|---|
| Time 1 Sample | ||||||
| 1-factor | 2211.2*** | 189 | 0.08 | 0.94 | 0.94 | 54081.1 |
| 2-factor | 1878.8*** | 188 | 0.08 | 0.95 | 0.95 | 53856.5 |
| 3-factor | 1620.5*** | 186 | 0.07 | 0.96 | 0.96 | 53704.8 |
| Higher order 2-factor | 1878.8*** | 188 | 0.08 | 0.95 | 0.95 | 53856.5 |
| Higher order 3-factor | 1827.3*** | 187 | 0.08 | 0.95 | 0.95 | 53846.4 |
| Time 2 Sample | ||||||
| 1-factor | 1149.50*** | 189 | 0.09 | 0.95 | 0.95 | 21505.20 |
| 2-factor | 984.19*** | 188 | 0.08 | 0.96 | 0.96 | 21398.00 |
| 3-factor | 836.83*** | 186 | 0.07 | 0.97 | 0.96 | 21328.60 |
| Higher order 2-factor | 984.19*** | 188 | 0.08 | 0.96 | 0.96 | 21398.00 |
| Higher order 3-factor | 941.36*** | 187 | 0.08 | 0.96 | 0.96 | 21385.80 |
Note.
p<.05.
p < .01.
p < .001.
Parenthetical values indicate good model fit based on Hu and Benter (1999).
Discussion
The current study evaluated the psychometric properties of the BDI-II among a large, diverse sample of HIV-positive individuals from the U.S. The BDI-II scores showed good internal consistency, test-retest reliability, and predictive validity. Using CFAs, results supported the fit of a three-factor model of depression over one-factor and two-factor models, and found the addition of a higher-order general depression factor did not improve the fit. The sensitivity, specificity, PPV, and NPV of the BDI-II were determined and graphed for future use by clinicians and researchers who may choose to use various cut-off scores depending on the function of the screening method.
Implications
This is the first study to validate the BDI-II for use among HIV-positive individuals within the U.S., which supplements the previous studies conducted with Jamaican and South African individuals living with HIV/AIDS. Major depression is prevalent among individuals with HIV-infection (Ciesla & Roberts, 2001; Maj et al., 1994; Penzak et al., 2000) and has been associated with poor HAART adherence (Ammassari et al., 2004) and higher rates of morbidity and mortality (Leserman, 2003).
Although the BDI-II was not designed to be used as a diagnostic tool for major depression (Beck et al., 1996), it is commonly employed as a screening assessment in HIV clinics to determine which patients will be referred for further evaluation by a mental health professional. The diagnostic value of the BDI-II was lower among the current sample than other samples of psychiatric and primary care outpatients (Beck et al., 1996). With post-hoc ROC analyses, we tested the hypothesis that somatic or affective symptoms, which are associated with both depression and HIV illness, would reduce the diagnostic value of the BDI-II, however this was not supported and the diagnostic value actually decreased with their exclusion.
If the BDI-II is being used to determine the need for mental health triage for HIV-positive patients, clinicians may wish to increase their sensitivity at the expense of specificity, to ensure that truly depressed patients are not overlooked. Using the cut-off score of 14 derived in the original BDI-II validation study provides a sensitivity of 81% and a cut-off score of 10 provides a sensitivity of 90%. If a clinician is hoping to maximize both sensitivity and specificity for the measure, than a cut-off of 17 should be used. Alternatively, researchers may wish to increase their specificity if they aim to recruit truly depressed participants. If this is the case, a cut-off of 25 provides specificity at 90% and approximately 46% of those identified will actually have MDD. It is always recommended that that the presence of MDD be confirmed with further assessment and ideally a structured or semi-structured diagnostic interview based on DSM-V or ICD-10 criteria.
This was the first study to examine the factor structure of the BDI-II among a large and diverse HIV-positive sample collected in the United States. The 3-factor model of the BDI-II factor structure confirmed as the best model fit in the present study was labeled in previous research as cognitive, affective, and somatic depressive symptoms (Buckley et al., 2001). This finding underlines the multidimensional nature of depressive symptoms and the 3-factor solution supported among the current sample is consistent with the solutions found in previous studies conducted with HIV-positive participants (Lipps et al., 2010). Differences in item factor loadings may result from demographic differences between the two samples. In our own review of the factor loadings found in the many studies conducted on the BDI and BDI-II, it was rare for two studies to find the same exact factor structure if exploratory factor analyses were conducted; however 2-factor and 3-factor solutions consistently emerged. Although the addition of a higher-order factor to this model did not improve the model fit among the current sample, it also did not decrease the fit of the model in a meaningful way. This suggests that a higher-order factor may still be relevant for the population.
Limitations
The current data were collected at multiple sites across the United States, suggesting that the findings are likely generalizable to many areas in the country. However, data collection began nearly a decade ago and certain regions and ethnic groups were not well represented in the sample. For example, it is possible that these results do not adequately represent Hispanics and Latinos, who account for a slowly increasing number of HIV diagnoses in the U.S. (22% in 2011), especially in Massachusetts and Louisiana—two states not represented in the current study (CDC, 2011).
Further, the CHARTER study did not include multiple self-report measures of depression and therefore did not allow for an examination of convergent and discriminant validity. The BDI-II had good convergent and discriminant validity among a sample of Jamaican participants with HIV-infection (Lipps et al., 2010) and in other validation studies of various psychiatric and general populations (Beck et al., 1996).
It is of note that the time 2 sample in the current study was selected for specified disease characteristics of interest to the researchers. Although, these participants were not selected based on depression ratings, the time 2 participants had lower BDI-II scores and less MDD diagnoses than the time 1 participants. This may have lowered the test-retest reliability found in the current study and therefor should be interpreted with caution.
The results of the current study suggest that the BDI-II is an adequate measure for assessing depressive symptoms among HIV-positive individuals in the U.S. In light of advances in the treatment of HIV and depression, future studies should continue to validate the BDI-II for use among HIV-positive populations. Clinics should stay abreast of empirical studies and use information regarding sensitivity, specificity, and factor structure to increase their accuracy when measuring depressive symptoms among their patients.
Acknowledgments
This research was supported in part by NIMH/NINDS (HHS-N-271-2010-00036C and CHARTER As A Resource NIH/NIMH/NONDS (HHS-N-270-2010-00030C). CHARTER is affiliated with The Johns Hopkins University, Mount Sinai School of Medicine, University of California, San Diego, University of Texas, Galveston, University of Washington, Seattle, Washington University, St. Louis, and is headquartered at the University of California, San Diego, and includes: Director: Igor Grant, MD; Co-Directors: J. Allen McCutchan, M.D., Ronald J. Ellis, M.D., Ph.D., Thomas D. Marcotte, Ph.D.; Center Manager: Donald Franklin, Jr.,; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), J. Allen McCutchan, M.D., Terry Alexander, R.N.; Laboratory, Pharmacology and Immunology Component: Scott Letendre, M.D. (P.I.), Edmund Capparelli, Pharm.D., Janis Durelle; Neurobehavioral Component: J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Matthew Dawson; Virology Component: Joseph K. Wong, M.D. (P.I.); Imaging Component: Terry Jernigan, Ph.D. (Co-P.I.), Michael J. Taylor, Ph.D. (Co-P.I.), Rebecca Theilmann, Ph.D.; Data Management Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman,; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Christopher Ake, Ph.D., Florin Vaida, Ph.D.; Protocol Coordinating Component: Thomas D. Marcotte, Ph.D. (P.I.), Rodney von Jaeger, M.P.H.; Johns Hopkins University Site: Justin McArthur (P.I.), Gilbert Mbeo, MBChB; Mount Sinai School of Medicine Site: Susan Morgello, M.D. (Co-P.I.) and David Simpson, M.D. (Co-P.I.), Letty Mintz, N.P.; University of California, San Diego Site: J. Allen McCutchan, M.D. (P.I.), Susan Ueland, R.N.; University of Washington, Seattle Site: Ann Collier, M.D. (Co-P.I.), Trudy Jones, M.N., A.R.N.P.; University of Texas, Galveston Site: Benjamin Gelman, M.D., Ph.D. (P.I.), Eleanor Heckendorn, R.N., B.S.N.; and Washington University, St. Louis Site: David Clifford, M.D. (P.I.), Muhammad Al-Lozi, M.D., Mengesha Teshome, M.D. The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government, UCSD or CHARTER. In addition, the authors would like to thank Dr. Ann Collier of the University of Washington School of Medicine for her insights and helpful feedback.
Footnotes
This manuscript has not been previously published nor is it under consideration for publication elsewhere. No financial conflicts of interest exist in relation to this research and study procedures were approved by the University at Albany, State University of New York Institutional Review Board.
References
- Airhihenbuwa CO, Ford CL, Iwelunmor JI. Why Culture Matters in Health Interventions: Lessons From HIV/AIDS Stigma and NCDs. Health, Education, and Behavior. 2013 doi: 10.1177/1090198113487199. doi: 10.1177/1090198113487199. [DOI] [PubMed] [Google Scholar]
- Al-Musawi NM. Psychometric properties of the Beck Depression Inventory-II with university students in Bahrain. Journal of Personality Assessment. 2001;77(3):568–579. doi: 10.1207/S15327752JPA7703_13. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th Edition ed. Washington, DC: 1994. [Google Scholar]
- Ammassari A, Antinori A, Aloisi MS, Trotta MP, Murri R, Bartoli L, Starace F. Depressive symptoms, neurocognitive impairment, and adherence to highly active antiretroviral therapy among HIV-infected persons. Psychosomatics. 2004;45(5):394–402. doi: 10.1176/appi.psy.45.5.394. [DOI] [PubMed] [Google Scholar]
- Arnau RC, Meagher MW, Norris MP, Bramson R. Psychometric evaluation of the Beck Depression Inventory-II with primary care medical patients. Health Psychology. 2001;20(2):112–119. doi: 10.1037//0278-6133.20.2.112. doi: 10.1037//0278-6133.20.2.112. [DOI] [PubMed] [Google Scholar]
- Beck AT, Beamesderfer A. Assessment of Depression: The Depression Inventory. Psychological Measurements in Psychopharmacology. 1974;7:151–169. doi: 10.1159/000395074. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory manual. 2nd ed. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Berger-Greenstein JA, Cuevas CA, Brady SM, Trezza G, Richardson MA, Keane TM. Major depression in patients with HIV/AIDS and substance abuse. AIDS Patient Care STDS. 2007;21(12):942–955. doi: 10.1089/apc.2006.0153. doi: 10.1089/apc.2006.0153. [DOI] [PubMed] [Google Scholar]
- Buckley TC, Parker JD, Heggie J. A psychometric evaluation of the BDI-II in treatment-seeking substance abusers. Journal of Substance Abuse Treatment. 2001;20:197–204. doi: 10.1016/s0740-5472(00)00169-0. [DOI] [PubMed] [Google Scholar]
- Cain R, Jackson R, Prentice T, Collins E, Mill J, Barlow K. The experience of HIV diagnosis among Aboriginal people living with HIV/AIDS and depression. Qual Health Res. 2013;23(6):815–824. doi: 10.1177/1049732313482525. doi: 10.1177/1049732313482525. [DOI] [PubMed] [Google Scholar]
- CDC . HIV Surveillance by Race/Ethnicity. National Center for HIV/AIDS Viral Hepatitis STD & TB Prevention; 2011. [Google Scholar]
- Ciesla JA, Roberts JE. Meta-Analysis of the relationship between HIV infection and risk for depressive disorders. American Journal of Psychiatry. 2001;158(5):725–730. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- Cutrona C, Russell D. The provisions of social relationships and adaptation to stress. Advances in Personal Relationships. 1987;1:37–67. [Google Scholar]
- Dozois DJA, Dobson KS, Ahnberg JL. A psychometric evaluation of the Beck Depression Inventory-II. Psychological Assessment. 1998;10(2):83–89. [Google Scholar]
- Dutton GR, Grothe KB, Jones GN, Whitehead D, Kendra K, Brantley PJ. Use of the Beck Depression Inventory-II wiht African American primary care patients. General Hospital Psychiatry. 2004;26:437–442. doi: 10.1016/j.genhosppsych.2004.06.002. doi: 10.1016/j.genhosppsych.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Holmes W. A short, psychiatric, case-finding measure for HIV seropositive outpatients. Medical Care. 1998;36:237–243. doi: 10.1097/00005650-199802000-00012. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6(1):1–55. [Google Scholar]
- Hutton HE, Lyketsos CM, Thompson RE, Erbelding EJ. Depression and HIV risk behaviors among patients in a sexually transimitted disease clinic. American Journal of Psychiatry. 2004;161:912–914. doi: 10.1176/appi.ajp.161.5.912. [DOI] [PubMed] [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 20.0. IBM Corp.; Armonk, NY: Released 2011. [Google Scholar]
- Kagee A, Nel A, Saal W. Factor structure of the Beck Depression Inventory-II among South Africans receiving antiretroviral therapy. AIDS Care. 2013:1–6. doi: 10.1080/09540121.2013.802278. doi: 10.1080/09540121.2013.802278. [DOI] [PubMed] [Google Scholar]
- Leserman J. HIV disease progression: Depression, stress, and possible mechanisms. Biological Psychiatry. 2003;54:295–306. doi: 10.1016/s0006-3223(03)00323-8. [DOI] [PubMed] [Google Scholar]
- Lipps GE, Lowe GA, De La Haye W, Longman-Mills S, Clarke TR, Barton EN, Bain B. Validation of the Beck Depression Inventory II in HIV-positive Patients. West Indian Medical Journal. 2010;59(4):374–379. [PubMed] [Google Scholar]
- Lyketsos CG, Hoover DR, Guccione M. Depressive symptoms as predictors of medical outcomes in HIV infection. Journal of American Medical Association. 1993;270:2563–2567. [PubMed] [Google Scholar]
- Maj M, Janssen R, Starace F, Zaudig M, Satz P, Sughondhabirom B, Sartorius N. WHO Neuropsychiatric AIDS Study, Cross-sectional Phase I. Archives of General Psychiatry. 1994;51(1):39–49. doi: 10.1001/archpsyc.1994.03950010039006. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. Sixth Edition Muthén & Muthén; Los Angeles, CA: 1998-2010. [Google Scholar]
- Patterson D, Swindells S, Mohr J. How much adherence is enough? A prospective study of adherence to protease inhibitor therapy using MEMS caps; Paper presented at the Conference on Retroviruses and Opportunistic Infections; Chicago, Illinois. 1999. [Google Scholar]
- Penzak SR, Reddy YS, Grimsley SR. Depression in patients with HIV infection. American Journal of Health Systems Pharmacy. 2000;57:376–386. doi: 10.1093/ajhp/57.4.376. [DOI] [PubMed] [Google Scholar]
- Perdue T, Hagan H, Thiede H, Valleroy L. Depression and HIV risk behavior among Seattle-area injection drug users and young men who have sex with men. AIDS Education and Prevention. 2003;15(1):81–92. doi: 10.1521/aeap.15.1.81.23842. [DOI] [PubMed] [Google Scholar]
- Pyne JM, Asch SM, Lincourt K, Kilbourne AM, Bowman C, Atkinson H, Gifford A. Quality indicators for depression care in HIV patients. AIDS Care. 2008;20(9):1075–1083. doi: 10.1080/09540120701796884. [DOI] [PubMed] [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, Towle LH. The Composite International Diagnostic Interview. Archives of General Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MJ, Sullivan E, Sassoon SA, O’Reilly A, Fama R, Kemper CA, Pfefferbaum A. Alcoholism, HIV infection, and their comorbidity: Factors affecting self-rated health-related quality of life. Journal of Studies on Alcohol and Drugs. 2007;68:115–125. doi: 10.15288/jsad.2007.68.115. [DOI] [PubMed] [Google Scholar]
- Singh N, Squier C. Determinants of compliance with antiretroviral therapy in patients with human immunodeficiency virus: Prospective assessment with implications for enhancing compliance. AIDS Care. 1996;8(3):261–270. doi: 10.1080/09540129650125696. [DOI] [PubMed] [Google Scholar]
- Steer RA, Clark DA, Beck AT, Ranieri WF. Common and specific dimensions of self-reported anxiety and depression: The BDI-II versus the BDI-IA. Behaviour Research and Therapy. 1998;37:183–190. doi: 10.1016/s0005-7967(98)00087-4. [DOI] [PubMed] [Google Scholar]
- Vanheule S, Desmet M, Groenvynck H, Rosseel Y, Fontaine J. The factor structure of the Beck Depression Inventory II: An evaluation. Assessment. 2008;15(2):177–187. doi: 10.1177/1073191107311261. doi: 10.1177/1073191107311261. [DOI] [PubMed] [Google Scholar]
- Walsh K, Cross W. Depression: Classification, Culture and the Westernisation of Mental Illness. In: Kocabasoglu N, editor. Mood Disorders. 2013. Retrieved from http://www.intechopen.com/books/mood-disorders/depression-classification-culture-and-the-westernisation-of-mental-illness. doi:10.5772/54176. [Google Scholar]