Abstract
Asparagine linked glycosylation of proteins is an essential protein modification reaction in most eukaryotic organisms. N-linked oligosaccharides are important for protein folding and stability, biosynthetic quality control, intracellular traffic and the physiological function of many N-glycosylated proteins. In metazoan organisms, the oligosaccharyltransferase is composed of a catalytic subunit (STT3A or STT3B) and a set of accessory subunits. Duplication of the catalytic subunit gene allowed cells to evolve OST complexes that act sequentially to maximize the glycosylation efficiency of the large number of proteins that are glycosylated in metazoan organisms. We will summarize recent progress in understanding the mechanism of (a) cotranslational glycosylation by the translocation channel associated STT3A complex, (b) the role of the STT3B complex in mediating cotranslational or posttranslocational glycosylation of acceptor sites that have been skipped by the STT3A complex, and (c) the role of the oxidoreductase MagT1 in STT3B-dependent glycosylation of cysteine-proximal acceptor sites.
Keywords: asparagine linked glycosylation, endoplasmic reticulum, oligosaccharyltransferase, congenital disorders of glycosylation
1. Introduction
Asparagine-linked glycosylation of proteins in the rough endoplasmic reticulum (RER) is one of the most common protein modification reactions in eukaryotic cells. Most proteins that enter the secretory pathway are N-glycosylated including secretory proteins, the majority of cellular integral membrane proteins and the lumenal resident proteins of the endoplasmic reticulum (ER), Golgi, nuclear envelope and lysosome. Asparagine linked oligosaccharides perform important roles in protein folding, quality control, and sorting events in the secretory pathway [1]. In the RER, N-linked oligosaccharides are essential for the calnexin-calreticulin chaperone cycle that promotes protein folding and entry into ER-to-Golgi transport vesicles (as reviewed by [2]). After trimming by mannosidases in the ER, high mannose oligosaccharides direct misfolded proteins into the ER associated degradation (ERAD) pathway [3]. Thus, the biosynthetic pathway of many cellular proteins is dependent upon the accurate and efficient addition of N-linked oligosaccharides.
The oligosaccharyltransferase (OST) catalyzes the transfer of a preassembled oligosaccharide from a lipid linked oligosaccharide (LLO) donor onto the asparagine residue of glycosylation acceptor sites (typically N-X-T/S, where X≠P) or sequons in newly synthesized proteins. N-linked glycosylation is an ancient and conserved pathway that is present in most archaebacteria, a subset of eubacteria, and almost all eukaryotes. Unlike mammalian organisms which have several thousand N-glycosylated proteins [4], N-glycosylation in bacteria is limited to a small number of cell surface proteins including the S-layer glycoprotein, archaellins and pilin [5]. Archaebacterial and eubacterial OSTs are single subunit enzymes designated as AglB and PglB respectively. Although certain eubacterial OSTs recognize an extended acceptor site (D/E-Z-N-X-S/T, where X, Z!P [6]) the conservation of the hydroxyamino acid residue (T/S) at the +2 position relative to asparagine is indicative of conservation of the acceptor peptide-binding site.
The catalytic subunit (STT3) of the eukaryotic OST complex is homologous to AglB and PglB [7, 8]. Eukaryotic OST complexes range in size between the single subunit OSTs of trypanosomes and the heterooctameric OSTs that are present in higher eukaryotes [9]. The multi-subunit OSTs of fungi and metazoan organisms allow more precise selection of the fully assembled oligosaccharide donor [10-12]. With the exception of many protist organisms, most eukaryotes synthesize the dolichol pyrophosphate (Dol-PP) linked oligosaccharide Dol-PPGlcNAc2Man9Glc3 as the oligosaccharide donor. Metazoan organisms express two STT3 proteins (STT3A and STT3B) that are incorporated into distinct OST complexes that have partially overlapping roles in N-linked glycosylation [11, 13, 14].
The physiological significance of N-linked glycosylation is highlighted by the family of human diseases referred to as congenital disorders of glycosylation (CDG, as reviewed by [15, 16]). Mutations that cause defects in the assembly of the LLO donor, or reduce the transfer of the oligosaccharide onto proteins in the endoplasmic reticulum are grouped as CDG-1. Mutations that impact the processing of protein bound oligosaccharides in the Golgi constitute CDG-2. The CDG-1 family of diseases is characterized by hypoglycosylation of cellular glycoproteins resulting in multi-system pathological abnormalities.
2. Mammalian oligosaccharyltransferases
The two mammalian OST complexes, which we will refer to as the STT3A complex and the STT3B complex are composed of one copy of a catalytic subunit (STT3A or STT3B), a shared set of five non-catalytic subunits (ribophorin I, ribophorin II, OST48, DAD1 and OST4) as well as isoform specific subunits (MagT1, TUSC3, KCP2 and DC2).
In recent years, insight into the in vivo roles of the mammalian OST complexes has been obtained by siRNA-mediated depletion of OST subunits in cultured cells, and by the analysis of fibroblasts from human patients with congenital disorders of glycosylation (CDG-1). First, we will briefly summarize results concerning OST subunits that are shared by the both the STT3A and STT3B complexes before embarking on a more detailed discussion of the in vivo roles of the STT3A and STT3B complexes.
One copy of each of the shared subunits is present in both the STT3A and STT3B complexes. Yeast homologues of ribophorin I (Ost1p), ribophorin II (Swp1p), OST48 (Wbp1p), DAD1 (Ost2p) are encoded by essential yeast genes and are necessary for the stability and function of the OST complex [17-19]. Yeast ost4Δ mutants are temperature sensitive for growth and have a defect in N-glycosylation linked to a reduced association between the oxidoreductase subunits (Ost3p or Ost6p) and STT3 [20-22].
A mutation in the human DDOST gene, encoding OST48, causes a form of CDG-1 [23]. The DDOST mutation reduces the expression of OST48 and caused a general deficiency in N-linked glycosylation. At the restrictive temperature for tsBN7 cells, a point mutation in DAD1 destabilizes the OST complex, causing a glycosylation defect that results in apoptosis [24]. siRNA mediated depletion of OST48 or DAD1 destabilizes the STT3A and STT3B complexes and causes hypoglycosylation [25]. Depletion of OST4 by siRNA treatment causes a reduction in the steady state levels of the STT3A complex and a defect in glycosylation of prosaposin [26]. A homozygous point mutation in the ribophorin II gene has been identified in a CDG-1 patient, suggesting that a ribophorin II mutation causes a general glycosylation defect [27].
Conflicting results have been reported concerning ribophorin I. Depletion of ribophorin I by siRNA causes a reduction in both STT3A and STT3B, and a general defect in protein glycosylation that mimics simultaneous depletion of STT3A and STT3B [13]. Other investigators have concluded that ribophorin I depletion causes selective hypoglycosylation of specific glycoproteins [28, 29] and have proposed that ribophorin I delivers substrates to the active site of the OST complex. Current cryoelectron microscopy derived structures of the OST lack sufficient resolution to indicate how ribophorin I might impact substrate delivery [30, 31].
Malectin, an ER-localized lectin that binds the terminal Glc α-1,3 Glc disaccharide on GlcNAc2Man9Glc2 [32, 33], is thought to interact with the OST complex via ribophorin I [34]. Malectin coprecipitates with both the STT3A and STT3B complexes [35], but may not be present in equal stoichiometry to the core subunits of the STT3A and STT3B complexes. The association of malectin with the OST is an enigma given that malectin is proposed to be involved in a quality control pathway for malfolded glycoproteins [36, 37].
MagT1 and TUSC3 are orthologs of the Ost3p and Ost6p subunits of the yeast OST [9]. Human STT3B complexes contain either MagT1 or TUSC3 [35], just as the yeast OST complex contains either OST3 or OST6 [21, 22, 38]. The structures of the lumenal domains of Ost6p and TUSC3 have been solved revealing a thioredoxin fold with an active site CXXC motif that is required for function [39, 40]. Phylogenetic analysis of eukaryotic STT3 proteins revealed that fungal Stt3 proteins fall within the STT3B clade consistent with the presence of an oxidoreductase subunit in the yeast OST and mammalian STT3B complexes [14, 35].
KCP2 and DC2 were first identified as subunits of the STT3A complex when ribosome associated membrane proteins (RAMPs) were resolved by two dimensional blue-native gel electrophoresis [41]. Subsequent studies using cultured cells demonstrated that KCP2 is needed for full activity of the STT3A complex [25, 42]. Although DC2 reportedly assembles into STT3A and STT3B complexes [42], we favor the view that DC2 is an STT3A specific subunit based on the absence of readily identifiable DC2 and KCP2 orthologs in fungal organisms.
3. Cotranslational glycosylation by the STT3A complex
Although N-glycosylation is frequently referred to as a posttranslational modification, it has long been known that vacant acceptor sites in fully-folded proteins cannot be modified by the OST. Hence, glycosylation of proteins is spatially restricted to the lumen of the RER and temporally restricted by the formation of protein secondary and tertiary structures that prevent access of acceptor sequons to the enzyme active site. The earliest evidence that most glycoproteins are modified by a cotranslational process was obtained in radiolabeling experiments of intact cells, where it was observed that newly synthesized proteins were fully glycosylated after a relatively brief pulse. Using short pulses (90 sec) and two-dimensional gel electrophoretic analysis of nascent polypeptides, the Helenius lab showed that glycans are added in a highly synchronized process to the elongating influenza hemagglutinin protein before disulfide bond formation [43]. Acceptor sites in a nascent polypeptide first have access to the OST active site when the asparagine residue in a sequon is 65-75 residues from the peptidyltransferase site on the translating ribosome [44, 45]. The 65-75 residues of polypeptide are in an extended conformation, first passing through the ribosome exit tunnel in the large ribosomal subunit (~35 amino acid residues), then traversing the membrane through the central pore in the protein translocation channel (Sec61 complex, ~25 amino acid residues) before having access to the OST active site. The OST active site, which is located approximately 30Å from the lumenal surface of the membrane [46, 47], is quite near (~20-30Å) the lumenal face of the protein translocation channel. Photocrosslinking experiments employing nascent polypeptides with a photoreactive amino acid at the X-position of a cryptic glycosylation site (QXT instead of NXT) exclusively yielded crosslinks to STT3A when the QXT site was located 70-90 residues from the peptidyltransferase site on the ribosome [48].
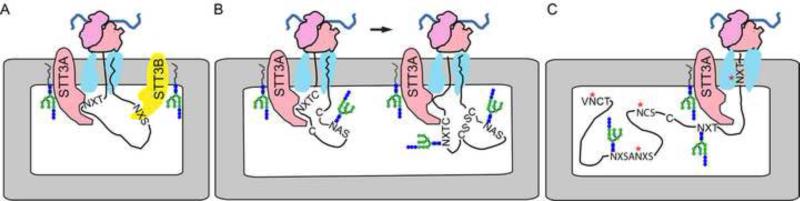
Biochemical evidence that the oligosaccharyltransferase is associated with membrane bound ribosomes and the protein translocation channel was deduced from cell fractionation experiments showing a 1:1 correlation between the content of membrane bound ribosomes and ribophorin I [49]. Antibodies that recognize the cytoplasmic C-terminus of ribophorin I block protein translocation by sterically interfering with ribosome binding to the protein translocation channel [50]. Detergent treatment of microsomal membranes yields a ribosome-associated membrane protein (RAMP) fraction that includes the protein translocation channel as well as ribophorin I, ribophorin II and OST48 [41, 51-53]. Mass spectrometric analysis revealed that the RAMP fraction contains the STT3A complex [41]. Differential treatment of microsomes with digitonin at increasing salt concentrations preferentially solubilizes the STT3B complex while leaving the STT3A complex in a RAMP fraction together with the protein translocation channel [13]. Taken together these results indicate that the STT3A complex is associated with the protein translocation channel, while the STT3B complex localizes to the RER but is not directly associated with the Sec61 complex (Fig. 1A)
Fig. 1.
Cotranslational glycosylation of proteins by the STT3A complex. (A) The STT3A complex is associated with the protein translocation channel and scans the growing polypeptide for glycosylation acceptor sites. The STT3B complex occupies a more distal position, but is able to modify skipped glycosylation sites by a cotranslational or posttranslocational mechanism. (B) Cotranslational scanning of the nascent polypeptide allows efficient glycosylation of acceptor sites in cysteine-rich proteins before disulfide bond formation stabilizes protein tertiary structure. (C) Examples of N-terminal, internal and extreme C-terminal acceptor sites (red asterisks) that have been skipped by the STT3A complex.
Blue native gel electrophoresis of microsomal membrane proteins can resolve the OST into 470 and 500 kD STT3A complexes and a 550 kD STT3B complex [25]. Although the 470 kD STT3A complex lacks KCP2 [25], it is not clear whether KCP2 dissociates from the 500 kD complex during RAMP isolation and electrophoresis, or instead indicates that cells assemble an alternative version of the STT3A complex. We favor the former explanation as STT3A complexes isolated by a conventional protein purification procedure lacked DC2 and KCP2 [11] indicating that these small subunits can dissociate from the STT3A complex in detergent solution.
Localization of the STT3A complex adjacent to the translocation channel allows the OST to scan the growing nascent polypeptide for the presence of acceptor sites and transfer oligosaccharides before protein folding occurs (Fig. 1A). An important question is whether cotranslational glycosylation by the STT3A complex is important for high occupancy of acceptor sites in mammalian glycoproteins. Specific depletion of the STT3A complex in HeLa cells by siRNA treatment reduces glycosylation of specific proteins [13, 25]. Human glycoprotein substrates that are particularly sensitive to STT3A depletion include prosaposin, progranualin and transferrin [13, 14, 54]. Acceptor sites that are strongly dependent upon the STT3A complex must correspond to suboptimal substrates for the STT3B complex. Prosaposin and progranulin are polyproteins consisting of small cysteine-rich autonomous folding domains separated by spacer segments [55, 56]. Alkylation with PEG-maleimide indicates that the 32 cysteine residues in prosaposin are oxidized within a few minutes after synthesis in HeLa cells [13], consistent with rapid folding of the four 82 residue saposin domains. Transferrin is a cysteine-rich protein and appears to fold quickly based upon relatively rapid acquisition of complex oligosaccharides in the Golgi [57]. Cotranslational glycosylation of nascent proteins by the STT3A complex provides a mechanism to glycosylate acceptor sites that are near cysteine residues (Fig. 1B) before disulfide bond formation stabilizes secondary or tertiary protein structures that would prevent access to the OST active site [35]. Hypoglycosylation of transferrin, as detected by isoelectric focusing (IEF), is the standard diagnostic marker for CDG-1 [58]. The obligate cotranslational glycosylation of transferrin by the STT3A complex causes transferrin to be prone to hypoglycosylation when the assembly pathway for the lipid-linked oligosaccharide is perturbed.
Crystal structures of monomeric bacterial OSTs have been solved in the presence (C. lari PglB, [47]) and absence (A. fulgidus AglB, [59]) of acceptor peptides. The requirement for a hydroxyamino acid at the +2 position relative to asparagine and the preference for NXT sequons relative to NXS sequons is explained by the structure of the peptide-binding pocket [47]. Interestingly, the asparagine residue in the N-X-T/S acceptor site projects through a narrow porthole in the active site that is closed by a flexible loop linking two of the PglB transmembrane spans. Thus, binding of an acceptor site to the OST active site and glycopeptide product release requires conformation changes in the OST [47].
The location of the STT3A complex adjacent to the protein translocation channel raises the possibility that acceptor sites in a nascent glycoprotein are recognized by an N-terminal to C-terminal scanning mechanism instead of a stochastic binding mechanism. Insight into this question has been provided by bioinformatic and biochemical analysis of closely spaced acceptor sites in glycoproteins [60]. STT3B independent glycosylation of adjacent sites (N-X-T/S-N-XT/S) or gap-1 sites (N-X-T/S-Z-N-X-T/S) is efficient when both sites have threonine as the +2 residue. Sequon skipping by STT3A, and incomplete modification by STT3B occurs when closely spaced sites contain serine as the +2 residue [60]. The analysis of reporter proteins that had tandem arrays of gap1 sites (e.g. (N-X-T-A)N) provided evidence for an N-terminal to C-terminal scanning mechanism by STT3A. The third sequon in arrays containing either 4 or 5 sequons was uniformly skipped, presumably due to steric constraints. Uniform skipping of the third sequon can only be explained by an N-terminal to C-terminal mechanism of acceptor site modification [60].
HeLa cells that have been treated with siRNAs specific for STT3A, but not STT3B, express higher amounts of the lumenal ER chaperone BiP [13] consistent with induction of the unfolded protein response (UPR) pathway in response to hypoglycosylation of proteins. This observation suggests that the STT3A complex is responsible for glycosylating most acceptor sites as the nascent polypeptide enters the ER lumen. The STT3A complex displays a higher stringency than the STT3B complex in selection of the fully assembled oligosaccharide donor [11] thereby assuring that most newly synthesized glycoproteins will carry the correct glycans for entry into the glycoprotein quality control pathways that are directed by ER-localized glycosidases and lectin chaperones.
An important role for the STT3A complex in human health was provided by the analysis of fibroblasts from a STT3A-CDG patient [54]. The V626A mutation in STT3A reduces stable expression of STT3A. The patient has an abnormal IEF pattern for transferrin, and has severe CDG symptoms [54]. Pulse labeling of the patients fibroblasts revealed defects in N-linked glycosylation that were very similar to results obtained in STT3A depleted HeLa cells.
4. Posttranslocational glycosylation by the STT3B complex
Glycosylation sites that are poorly modified in STT3B depleted cells relative to untreated cells must correspond to acceptor sites that are skipped at a high frequency by the STT3A complex (Fig. 1C) necessitating glycosylation by STT3B. Acceptor sites that have been observed to be skipped by the STT3A complex include the following: (a) acceptor sites that are within five residues of the signal sequence cleavage site, (b) acceptor sites located in the C-terminal 50 residues of proteins, (c) closely spaced NXS acceptor sites, (d) NXS sites in very small type I membrane proteins, (e) internal acceptor sites with non-optimal sequons including a subset of acceptor sites that are close to cysteine residues.
The analysis of glycosylation in STT3B depleted cells indicates that the major cellular role for the STT3B complex is to maximize sequon occupancy in glycoproteins by modification of sites that are skipped by the STT3A complex. STT3B can modify skipped sites by cotranslational (Fig. 1A) or posttranslocational pathways (Fig. 2). We will discuss the basis for site skipping by STT3A in more detail and summarize current knowledge concerning the STT3B dependent pathway for modification of skipped sequons.
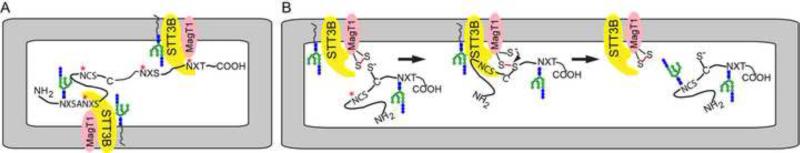
Fig. 2.
Posttranslocational glycosylation of skipped acceptor sites by the STT3B complex. (A) The STT3B complex mediates posttranslocational glycosylation of acceptor sites that are skipped by the STT3A complex. The MagT1 subunit of the STT3B complex in necessary for glycosylation of all tested STT3B dependent sites including those adjacent to cysteine residues. (B) The oxidized form of MagT1 is proposed to form a mixed disulfide with an acceptor site proximal free cysteine residue thereby delaying disulfide bond formation and promoting acceptor site recognition by the STT3B active site. Disulfide chemistry releases the nascent glycoprotein to regenerate the oxidized form of MagT1.
Bioinformatics analysis indicated that the N-terminal and C-terminal regions of glycoproteins contain fewer N-linked glycans that internal regions [61, 62]. The N-terminal region of glycoproteins have a lower glycan density in part due to the absence of acceptor sites in N-terminal signal sequences and the membrane spanning segments of type 2 (Ncyt-Clum) membrane proteins. Glycosylation acceptor sites that are within 5 residues of the signal sequence are not accessible to the OST active site prior to signal sequence cleavage [13, 63, 64] hence these sites are frequently skipped by the STT3A complex that is scanning the growing chain (Fig. 1A).
Analysis of large glycopeptide databases [4, 65] revealed that the C-terminal 65-75 residues of metazoan glycoproteins contain a lower glycan density than internal segments. Moreover, glycopeptides from the C-terminal region of proteins have a higher NXT:NXS ratio than internal segments of proteins [14]. The C-terminal reduction in glycan density is caused by a lower density of acceptor sequons and a reduced efficiency of acceptor site modification [14]. The C-terminal region of low glycan density correlates nicely with the experimentally determined distance between the OST active site and the peptidyltransferase site on the ribosome [14, 44, 45].
Following chain termination, the rate at which an acceptor site will move past the STT3A complex will no longer be limited by the protein synthesis rate, which in mammalian cells is approximately six residues per second [66]. Glycosylation of extreme C-terminal sites is strongly dependent upon STT3B (Fig. 2A), indicating that rapid movement of acceptor sites past STT3A promotes acceptor site skipping, particularly for sites located in the C-terminal 50 residues of proteins [14]. The rates and efficiency of posttranslocational glycosylation are higher for NXT sequons than for NXS sequons consistent with the higher affinity of the OST active site for NXT sequons [67] and the elevated NXT:NXS ratio among extreme C-terminal glycopeptides. Posttranslocational glycosylation of an extreme C-terminal site in a typical 50 kD soluble protein is slow (t1/2 of 3-10 min) relative to the time required for synthesis (~ 70 sec) but more rapid than the t1/2 for ER to Golgi transport. Posttranslocational glycosylation is likely limited by the rates of competing reactions including protein folding and diffusion of the glycoprotein away from the lumenal surface of the RER membrane. Posttranslocational glycosylation by the STT3B complex does not involve a polypeptide scanning mechanism (Fig. 2A) as extreme C-terminal sites in a single protein are modified at different rates [14, 60].
Extreme C-terminal sites that are glycosylated by STT3B have been identified in type II membrane proteins [14] and multi-spanning membrane proteins [68]. Glycosylation of extreme C-terminal sites in type II membrane proteins was less sensitive to STT3B depletion suggesting that protein retention in the vicinity of the protein translocation channel reduces acceptor site skipping by STT3A [14]. Glycosylation sites that are inserted into C-terminal epitope tags of membrane and soluble proteins are modified with high efficiency [69] by the STT3B complex [14].
Certain glycosylation sites in low molecular weight type I (Nlum-Ccyt) membrane proteins are posttranslocationally glycosylated. This class of posttranslocational glycosylation site was first identified in KCNE1 [70], a potassium channel β-subunit with two glycosylated asparagine residues (N5 and N26) located in the 43 residue lumenal domain. Cotranslational glycosylation of the N5 site promotes posttranslocational glycosylation of the N26 site. Replacing the N26MS site with a N26MT site allowed cotranslational glycosylation of both sites [70]. Further analysis of four other KCNE family members showed that cotranslational glycosylation of type I membrane proteins is favored by the presence of longer cytoplasmic domains (>100 residues) and NXT acceptor sites [71]. Thus, posttranslocational glycosylation of small type I membrane proteins by the STT3B complex is dependent upon sequon skipping by STT3A, which is enhanced by suboptimal acceptor sites and a brief residence time within the protein translocation channel [71, 72].
A fourth class of STT3B dependent sequon is exemplified by the posttranslocational glycosylation sites in blood coagulation factor VII [13, 73] and hemopexin [60]. These acceptor sites are skipped at a high frequency (>50%) by the STT3A complex despite being located more than 75 residues from the C-terminus of a protein (Fig. 1C). Protein sequence factors that promote skipping of internal acceptor sites by STT3A complex include the following: (a) close spacing between NXS acceptor sites [60], (b) NXS sites that have bulky hydrophobic or acidic X residues [72, 74, 75], (c) a subset of NCT/S sites, (d) and NXT/S sites that are closely bracketed by a disulfide in the folded protein (Fig. 1C). Additional local sequence context factors promote acceptor sequon skipping by the oligosaccharyltransferase, as determined by bioinformatic analysis of modified and unmodified acceptor sites [61, 62]. Although the clearest example is the strong bias against proline residues at the +3 position relative to asparagine in glycosylated acceptor sites [61, 62], statistical analysis of glycopeptide databases suggests that additional nearby residues influence glycosylation efficiency [62].
Due to a higher affinity for the OST active site [76], NXT sites are more efficiently glycosylated than NXS sites which in turn are much more efficiently glycosylated than NXC sites [77]. Experimentally verified NXC sites that are glycosylated are relatively uncommon [4, 78] and are prone to low occupancy by glycans [79]. Skipping of internal glycosylation sites by the STT3A complex can be reduced or eliminated by acceptor site optimization. Replacing the STT3B dependent NCS sequon in hemopexin with a NCT site eliminated sequon skipping by STT3A [60]. Elimination of the cysteine residues that bracket the N360IT site in factor VII reduces, but does not eliminate, sequon skipping by STT3B [35] indicating that multiple factors promote skipping of internal sites.
The physiological importance of posttranslocational glycosylation role was revealed by the discovery that reduced expression of STT3B can cause a newly discovered form of CDG-1 [54]. An important open question is whether recognition of acceptor sites by the STT3B complex occurs simply by diffusion, or whether unfolded proteins are delivered to the STT3B complex by lumenal chaperones or ER lectins. Glycosylation of the STT3B dependent sequons in factor VII, KNCE1 and β-glucuronidase decreases when the cotranslational glycosylation sites in the same proteins are eliminated [13, 14, 70] indicating that the glycosylation status of the substrate impacts delivery to the STT3B complex.
A role for the STT3B complex in the ER associated degradation (ERAD) pathway was recently described [80]. A folding defective form of transthyretin undergoes slow postranslocational glycosylation by STT3B at a normally unmodified C-terminal glycosylation site prior to entry into the glycan dependent branch of the ERAD pathway. It will be interesting to see if transthyretin glycosylation by STT3B is a unique example or a more general mechanism for targeting malfolded proteins into the ERAD pathway.
5. Posttranslocational glycosylation of cysteine proximal acceptor sites
The initially described roles for MagT1 and TUSC3 in protein glycosylation [11], became controversial when several groups reported that these proteins were cell-surface proteins needed for magnesium transport activity [81, 82]. However, cell-surface biotinylation and immunofluorescence microscopy experiments iestablished that MagT1 is an RER protein [35].
The observation that TUSC3 expression is more limited than MagT1 expression [82, 83] allowed the analysis of MagT1 function in HeLa cells, which do not express TUSC3. Glycosylation of all tested STT3B-dependent acceptor sites was reduced in MagT1-depleted HeLa cells [35]. The MagT1 depletion induced hypoglycosylation can be suppressed by expression of TUSC3. Thus, as expected based on the high sequence identity, TUSC3 and MagT1 have overlapping functions.
Why does the oligosaccharyltransferase have an accessory subunit with oxidoreducatase activity? Results obtained in the yeast experimental system indicated that deletion of the MagT1 orthologs (Ost3p or Ost6p) selectively reduces glycosylation of a subset of acceptor sites that on average are closer to a cysteine residue in the protein sequence [39, 84]. Ost3p and Ost6p are proposed to form mixed disulfides with free thiols in glycoprotein substrates, thereby delaying disulfide bond formation until nearby acceptor sites have been glycosylated [39]. In vivo, the majority of MagT1 is in the oxidized state [35], consistent with formation of mixed disulfides with protein thiols. Indeed, glycosylation of the STT3B dependent site in factor VII is dependent upon both active site cysteine residues in MagT1. Reaction of MagT1 or TUSC3 with free thiols in nascent glycoproteins should be the predominant pathway by which MagT1 facilitates N-glycosylation by the STT3B complex (Fig. 2B).
Analysis of the N29CT glycosylation site in cathepsin C was particularly informative. This acceptor site, which is four residues from the signal sequence ceases to be STT3B dependent under the following conditions: (a) cells are pulse labeled in the presence of sufficient dithiothreitol to reduce the RER lumen, (b) the cysteine residue in the sequon is replaced by serine, or (c) the distance between the signal sequence and the acceptor sequence is increased by 25 residues [35]. Thus, skipping of the N29CT site by STT3A is dependent upon the location next to the signal sequence, while MagT1 is required because C30 can form a disulfide that interferes with glycosylation of N29. Glycosylation of the N29CT site in cathepsin C is partially rescued by a MagT1 mutant containing a single active cysteine residue, indicating that a transiently reduced form of MagT1 can react with a non-native disulfide in cathepsin C to allow access of STT3B to the glycosylation site [35].
Both the yeast (Ost3p/Ost6p) and mammalian (MagT1/TUSC3) proteins have oxidoreductase dependent and independent functions. As shown first in the yeast system [39], mutation of the active site disulfide (CXXC motif) in Ost3p or Ost6p causes a less severe defect in glycosylation than the corresponding deletion mutant. Moreover, siRNA mediated depletion of MagT1 interferes with STT3B-dependent glycosylation of an SHBG derivative that lacks cysteine residues near the extreme C-terminal glycosylation sites [35]. Conceivably, the peptide bonding pocket of the oxidoreductase may help direct acceptor sites to the OST active site [40] regardless of whether cysteine chemistry is involved.
In mammalian glycoproteins, the presence of cysteine as the X residue in a sequon or the presence of a bracketing disulfide cannot be used to predict whether a sequon is MagT1 and STT3B dependent. Instead, sequon skipping by the STT3A complex is a prerequisite for MagT1 or TUSC3 dependent glycosylation sites.
Mutations in the human MagT1 gene cause an X-linked immunodeficiency disease (XMEN) characterized by chronic Epstein-Barr virus infections [85]. An earlier report that a point mutation (V311G) in MagT1 can cause X-linked mental retardation (XLMR, [83]) has been challenged by more recent evidence that the V311G variant of MagT1 is present in unaffected individuals [86]. Mutations in TUSC3 cause non-syndromic autosomal recessive mental retardation (ARMR) a disease characterized by intellectual impairment [83, 87, 88]. Consistent with transferrin being a STT3A dependent substrate, TUSC3-ARMR patients do not hypoglycosylate transferrin [83]. TUSC3-ARMR and MagT1-XMEN patients have milder symptoms than a STT3B-CDG patient [54], presumably because MagT1 and TUSC3 are semi-redundant in tissues where both mRNAs are expressed.
6. Conclusions and perspectives
Duplication of the STT3 gene gave rise to a second oligosaccharyltransferase complex in metazoan cells that allowed a division of labor between the translocation channel associated STT3A complex that scans the growing polypeptide chain for acceptor sites, and the STT3B complex that modifies acceptor sites that have been skipped by STT3A. As such, the two complexes cooperate to maximize glycosylation site occupancy in newly synthesized glycoproteins.
The concept of site skipping by the STT3A complex sheds light on the longstanding enigma that certain acceptor sites in glycoproteins show partial glycan occupancy. Postranslocational glycosylation of skipped sites by the STT3B complex is limited by protein folding rates and movement of the acceptor site away from the lumenal surface of the RER. We suspect that sequon skipping by the STT3A complex is more prevalent than suggested by current experiments that rely on siRNA-mediated depletion of the STT3B complex.
Incomplete glycosylation site occupancy can also be caused when the pool of Dol-PPGlcNAc2Man9Glc3 is reduced as observed in ALG7-CDG fibroblasts [89], or when LLO assembly is blocked at a late stage leading to the accumulation of truncated oligosaccharide donors (e.g., ALG6-CDG fibroblasts [90, 91]). The stringent selection of the LLO donor by the STT3A complex is predicted to enhance site skipping in cells with LLO assembly defects. The reduced stringency of LLO selection by the STT3B complex may partially compensate for sequon skipping in proteins that are good STT3B substrates. As the STT3B:STT3A ratio may vary between human tissues, the severity of protein hypoglycosylation that is caused by defects in oligosaccharide donor assembly may show tissue specific differences.
Acknowledgements
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number GM43687.
Abbreviations
- ARMR
autosomal recessive mental retardation
- CDG
congenital disorders of glycosylation
- ERAD
ER-associated degradation
- IEF
isoelectric focusing
- LLO
lipid linked oligosaccharide
- OST
oligosaccharyltransferase
- RAMP
ribosome associated membrane proteins
- RER
rough endoplasmic reticulum
- SHBG
sex hormone binding globulin
- XLMR
X-linked mental retardation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Helenius A, Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem. 2004;73:1019–49. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- 2.Hebert DN, Molinari M. In and out of the ER: protein folding, quality control, degradation, and related human diseases. Physiol Rev. 2007;87:1377–408. doi: 10.1152/physrev.00050.2006. [DOI] [PubMed] [Google Scholar]
- 3.Aebi M, Bernasconi R, Clerc S, Molinari M. N-glycan structures: recognition and processing in the ER. Trends Biochem Sci. 2010;35:74–82. doi: 10.1016/j.tibs.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Zielinska DF, Gnad F, Wisniewski JR, Mann M. Precision mapping of an in vivo N-glycoproteome reveals rigid topological and sequence constraints. Cell. 2010;141:897–907. doi: 10.1016/j.cell.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Jarrell KF, Ding Y, Meyer BH, Albers SV, Kaminski L, Eichler J. N-linked glycosylation in Archaea: a structural, functional, and genetic analysis. Microbiol Mol Biol Rev. 2014;78:304–41. doi: 10.1128/MMBR.00052-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kowarik M, Young NM, Numao S, Schulz BL, Hug I, Callewaert N, et al. Definition of the bacterial N-glycosylation site consensus sequence. Embo J. 2006;25:1957–66. doi: 10.1038/sj.emboj.7601087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spirig U, Glavas M, Bodmer D, Reiss G, Burda P, Lippuner V, et al. The STT3 protein is a component of the yeast oligosaccharyltransferase complex. Mol Gen Genet. 1997;256:628–37. doi: 10.1007/s004380050611. [DOI] [PubMed] [Google Scholar]
- 8.Szymanski CM, Yao R, Ewing CP, Trust TJ, Guerry P. Evidence for a system of general protein glycosylation in Campylobacter jejuni. Mol Microbiol. 1999;32:1022–30. doi: 10.1046/j.1365-2958.1999.01415.x. [DOI] [PubMed] [Google Scholar]
- 9.Kelleher DJ, Gilmore R. An evolving view of the eukaryotic oligosaccharyltransferase. Glycobiology. 2006;16:47–62. doi: 10.1093/glycob/cwj066. [DOI] [PubMed] [Google Scholar]
- 10.Karaoglu D, Kelleher DJ, Gilmore R. Allosteric regulation provides a molecular mechanism for preferential utilization of the fully assembled dolichol-linked oligosaccharide by the yeast oligosaccharyltransferase. Biochemistry. 2001;40:12193–206. doi: 10.1021/bi0111911. [DOI] [PubMed] [Google Scholar]
- 11.Kelleher DJ, Karaoglu D, Mandon EC, Gilmore R. Oligosaccharyltransferase isoforms that contain different catalytic STT3 subunits have distinct enzymatic properties. Mol Cell. 2003;12:101–11. doi: 10.1016/s1097-2765(03)00243-0. [DOI] [PubMed] [Google Scholar]
- 12.Kelleher DJ, Banerjee S, Cura AJ, Samuelson J, Gilmore R. Dolichol-linked oligosaccharide selection by the oligosaccharyltransferase in protist and fungal organisms. J Cell Biol. 2007;177:29–37. doi: 10.1083/jcb.200611079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruiz-Canada C, Kelleher DJ, Gilmore R. Cotranslational and posttranslational N-glycosylation of polypeptides by distinct mammalian OST isoforms. Cell. 2009;136:272–83. doi: 10.1016/j.cell.2008.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shrimal S, Trueman SF, Gilmore R. Extreme C-terminal sites are posttranslocationally glycosylated by the STT3B isoform of the OST. J Cell Biol. 2013;201:81–95. doi: 10.1083/jcb.201301031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haeuptle MA, Hennet T. Congenital disorders of glycosylation: an update on defects affecting the biosynthesis of dolichol-linked oligosaccharides. Hum Mutat. 2009;30:1628–41. doi: 10.1002/humu.21126. [DOI] [PubMed] [Google Scholar]
- 16.Freeze HH. Understanding human glycosylation disorders: biochemistry leads the charge. J Biol Chem. 2013;288:6936–45. doi: 10.1074/jbc.R112.429274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silberstein S, Collins PG, Kelleher DJ, Rapiejko PJ, Gilmore R. The alpha subunit of the Saccharomyces cerevisiae oligosaccharyltransferase complex is essential for vegetative growth of yeast and is homologous to mammalian ribophorin I. J Cell Biol. 1995;128:525–36. doi: 10.1083/jcb.128.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silberstein S, Collins PG, Kelleher DJ, Gilmore R. The essential OST2 gene encodes the 16-kD subunit of the yeast oligosaccharyltransferase, a highly conserved protein expressed in diverse eukaryotic organisms. J Cell Biol. 1995;131:371–83. doi: 10.1083/jcb.131.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.te Heesen S, Knauer R, Lehle L, Aebi M. Yeast Wbp1p and Swp1p form a protein complex essential for oligosaccharyl transferase activity. EMBO J. 1993;12:279–84. doi: 10.1002/j.1460-2075.1993.tb05654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chi JH, Roos J, Dean N. The OST4 gene of Saccharomyces cerevisiae encodes an unusually small protein required for normal levels of oligosaccharyltransferase activity. J Biol Chem. 1996;271:3132–40. doi: 10.1074/jbc.271.6.3132. [DOI] [PubMed] [Google Scholar]
- 21.Karaoglu D, Kelleher DJ, Gilmore R. The highly conserved Stt3 protein is a subunit of the yeast oligosaccharyltransferase and forms a subcomplex with Ost3p and Ost4p. J Biol Chem. 1997;272:32513–20. doi: 10.1074/jbc.272.51.32513. [DOI] [PubMed] [Google Scholar]
- 22.Spirig U, Bodmer D, Wacker M, Burda P, Aebi M. The 3.4 kDa Ost4 protein is required for the assembly of two distinct oligosaccharyltransferase complexes in yeast. Glycobiology. 2005;15:1396–406. doi: 10.1093/glycob/cwj025. [DOI] [PubMed] [Google Scholar]
- 23.Jones MA, Ng BG, Bhide S, Chin E, Rhodenizer D, He P, et al. DDOST mutations identified by whole-exome sequencing are implicated in congenital disorders of glycosylation. Am J Hum Genet. 2012;90:363–8. doi: 10.1016/j.ajhg.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanjay A, Fu J, Kreibich G. DAD1 is required for the function and the structural integrity of the oligosaccharyltransferase complex. J Biol Chem. 1998;273:26094–9. doi: 10.1074/jbc.273.40.26094. [DOI] [PubMed] [Google Scholar]
- 25.Roboti P, High S. The oligosaccharyltransferase subunits OST48, DAD1 and KCP2 function as ubiquitous and selective modulators of mammalian N-glycosylation. J Cell Sci. 2012;125:3474–84. doi: 10.1242/jcs.103952. [DOI] [PubMed] [Google Scholar]
- 26.Dumax-Vorzet A, Roboti P, High S. OST4 is a subunit of the mammalian oligosaccharyltransferase required for efficient N-glycosylation. J Cell Sci. 2013;126:2595–606. doi: 10.1242/jcs.115410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vleugels W, Schollen E, Foulquier F, Matthijs G. Screening for OST deficiencies in unsolved CDG-I patients. Biochem Biophys Res Commun. 2009;390:769–74. doi: 10.1016/j.bbrc.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 28.Wilson CM, High S. Ribophorin I acts as a substrate-specific facilitator of N-glycosylation. J Cell Sci. 2007;120:648–57. doi: 10.1242/jcs.000729. [DOI] [PubMed] [Google Scholar]
- 29.Wilson CM, Roebuck Q, High S. Ribophorin I regulates substrate delivery to the oligosaccharyltransferase core. Proc Natl Acad Sci U S A. 2008;105:9534–9. doi: 10.1073/pnas.0711846105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, Chavan M, Schindelin H, Lennarz WJ. Structure of the oligosaccharyl transferase complex at 12 A resolution. Structure. 2008;16:432–40. doi: 10.1016/j.str.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 31.Pfeffer S, Dudek J, Gogala M, Schorr S, Linxweiler J, Lang S, et al. Structure of the mammalian oligosaccharyl-transferase complex in the native ER protein translocon. Nat Commun. 2014;5:3072. doi: 10.1038/ncomms4072. [DOI] [PubMed] [Google Scholar]
- 32.Schallus T, Jaeckh C, Feher K, Palma AS, Liu Y, Simpson JC, et al. Malectin: a novel carbohydrate-binding protein of the endoplasmic reticulum and a candidate player in the early steps of protein N-glycosylation. Mol Biol Cell. 2008;19:3404–14. doi: 10.1091/mbc.E08-04-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schallus T, Feher K, Sternberg U, Rybin V, Muhle-Goll C. Analysis of the specific interactions between the lectin domain of malectin and diglucosides. Glycobiology. 2010;20:1010–20. doi: 10.1093/glycob/cwq059. [DOI] [PubMed] [Google Scholar]
- 34.Qin SY, Hu D, Matsumoto K, Takeda K, Matsumoto N, Yamaguchi Y, et al. Malectin forms a complex with ribophorin I for enhanced association with misfolded glycoproteins. J Biol Chem. 2012;287:38080–9. doi: 10.1074/jbc.M112.394288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cherepanova NA, Shrimal S, Gilmore R. Oxidoreductase activity is necessary for N-glycosylation of cysteine-proximal acceptor sites in glycoproteins. J Cell Biol. 2014;206:525–39. doi: 10.1083/jcb.201404083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, Hu D, Yabe R, Tateno H, Qin SY, Matsumoto N, et al. Role of malectin in Glc(2)Man(9)GlcNAc(2)-dependent quality control of alpha1-antitrypsin. Mol Biol Cell. 2011;22:3559–70. doi: 10.1091/mbc.E11-03-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galli C, Bernasconi R, Solda T, Calanca V, Molinari M. Malectin participates in a backup glycoprotein quality control pathway in the mammalian ER. PLoS One. 2011;6:e16304. doi: 10.1371/journal.pone.0016304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knauer R, Lehle L. The oligosaccharyltransferase complex from Saccharomyces cerevisiae. Isolation of the OST6 gene, its synthetic interaction with OST3, and analysis of the native complex. J Biol Chem. 1999;274:17249–56. doi: 10.1074/jbc.274.24.17249. [DOI] [PubMed] [Google Scholar]
- 39.Schulz BL, Stirnimann CU, Grimshaw JP, Brozzo MS, Fritsch F, Mohorko E, et al. Oxidoreductase activity of oligosaccharyltransferase subunits Ost3p and Ost6p defines site-specific glycosylation efficiency. Proc Natl Acad Sci USA. 2009;106:11061–6. doi: 10.1073/pnas.0812515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohorko E, Owen RL, Malojcic G, Brozzo MS, Aebi M, Glockshuber R. Structural basis of substrate specificity of human oligosaccharyl transferase subunit N33/Tusc3 and its role in regulating protein N-glycosylation. Structure. 2014;22:590–601. doi: 10.1016/j.str.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Shibatani T, David LL, McCormack AL, Frueh K, Skach WR. Proteomic analysis of mammalian oligosaccharyltransferase reveals multiple subcomplexes that contain Sec61, TRAP, and two potential new subunits. Biochemistry. 2005;44:5982–92. doi: 10.1021/bi047328f. [DOI] [PubMed] [Google Scholar]
- 42.Roboti P, High S. Keratinocyte-associated protein 2 is a bona fide subunit of the mammalian oligosaccharyltransferase. J Cell Sci. 2012;125:220–32. doi: 10.1242/jcs.094599. [DOI] [PubMed] [Google Scholar]
- 43.Chen W, Helenius J, Braakman I, Helenius A. Cotranslational folding and calnexin binding during glycoprotein synthesis. Proc Natl Acad Sci USA. 1995;92:6229–33. doi: 10.1073/pnas.92.14.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitley P, Nilsson IM, von Heijne G. A nascent secretory protein may traverse the ribosome/endoplasmic reticulum translocase complex as an extended chain. J Biol Chem. 1996;271:6241–4. doi: 10.1074/jbc.271.11.6241. [DOI] [PubMed] [Google Scholar]
- 45.Deprez P, Gautschi M, Helenius A. More than one glycan is needed for ER glucosidase II to allow entry of glycoproteins into the calnexin/calreticulin cycle. Mol Cell. 2005;19:183–95. doi: 10.1016/j.molcel.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 46.Nilsson I, von Heijne G. Determination of the distance between the oligosaccharyltransferase active site and the endoplasmic reticulum membrane. J Biol Chem. 1993;268:5798–801. [PubMed] [Google Scholar]
- 47.Lizak C, Gerber S, Numao S, Aebi M, Locher KP. X-ray structure of a bacterial oligosaccharyltransferase. Nature. 2011;474:350–5. doi: 10.1038/nature10151. [DOI] [PubMed] [Google Scholar]
- 48.Nilsson I, Kelleher DJ, Miao Y, Shao Y, Kreibich G, Gilmore R, et al. Photocross-linking of nascent chains to the STT3 subunit of the oligosaccharyltransferase complex. J Cell Biol. 2003;161:715–25. doi: 10.1083/jcb.200301043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marcantonio EE, Amar-Costesec A, Kreibich G. Segregation of the polypeptide translocation apparatus to regions of the endoplasmic reticulum containing ribophorins and ribosomes. II. Rat liver microsomal subfractions contain equimolar amounts of ribophorins and ribosomes. J Cell Biol. 1984;99:2254–9. doi: 10.1083/jcb.99.6.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu Y, Sabatini DD, Kreibich G. Antiribophorin antibodies inhibit the targeting to the ER membrane of ribosomes containing secretory polypeptides. J Cell Biol. 1990;111:1335–42. doi: 10.1083/jcb.111.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Görlich D, Prehn S, Hartmann E, Kalies K-U, Rapoport TA. A mammalian homologue of Sec61p and SecYp is associated with ribosomes and nascent polypeptides during translocation. Cell. 1992;71:489–503. doi: 10.1016/0092-8674(92)90517-g. [DOI] [PubMed] [Google Scholar]
- 52.Wang L, Dobberstein B. Oligomeric complexes involved in translocation of proteins across the membrane of the endoplasmic reticulum. FEBS Lett. 1999;457:316–22. doi: 10.1016/s0014-5793(99)01075-3. [DOI] [PubMed] [Google Scholar]
- 53.Menetret JF, Hegde RS, Heinrich SU, Chandramouli P, Ludtke SJ, Rapoport TA, et al. Architecture of the ribosome-channel complex derived from native membranes. J Mol Biol. 2005;348:445–57. doi: 10.1016/j.jmb.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 54.Shrimal S, Ng BG, Losfeld ME, Gilmore R, Freeze HH. Mutations in STT3A and STT3B cause two congenital disorders of glycosylation. Hum Mol Genet. 2013;22:4638–45. doi: 10.1093/hmg/ddt312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kishimoto Y, Hiraiwa M, O'Brien JS. Saposins: structure, function, distribution, and molecular genetics. J Lipid Res. 1992;33:1255–67. [PubMed] [Google Scholar]
- 56.Bateman A, Bennett HP. Granulins: the structure and function of an emerging family of growth factors. J Endocrinol. 1998;158:145–51. doi: 10.1677/joe.0.1580145. [DOI] [PubMed] [Google Scholar]
- 57.Nakada H, Kohno H, Kawasaki T, Tashiro Y. Intracellular forms of transferrin oligosaccharide chains in rat liver. Eur J Biochem. 1983;136:259–65. doi: 10.1111/j.1432-1033.1983.tb07736.x. [DOI] [PubMed] [Google Scholar]
- 58.Freeze HH, Aebi M. Altered glycan structures: the molecular basis of congenital disorders of glycosylation. Curr Op Struct Biol. 2005;15:490–8. doi: 10.1016/j.sbi.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 59.Matsumoto S, Shimada A, Nyirenda J, Igura M, Kawano Y, Kohda D. Crystal structures of an archaeal oligosaccharyltransferase provide insights into the catalytic cycle of N-linked protein glycosylation. Proc Natl Acad Sci U S A. 2013;110:17868–73. doi: 10.1073/pnas.1309777110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shrimal S, Gilmore R. Glycosylation of closely spaced acceptor sites in human glycoproteins. J Cell Sci. 2013;126:5513–23. doi: 10.1242/jcs.139584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gavel Y, Von Heijne G. Sequence differences between glycosylated and nonglycosylated Asn-X-Thr/Ser acceptor sites: implications for protein engineering. Protein Eng. 1990;3:433–42. doi: 10.1093/protein/3.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ben-Dor S, Esterman N, Rubin E, Sharon N. Biases and complex patterns in the residues flanking protein N-glycosylation sites. Glycobiology. 2004;14:95–101. doi: 10.1093/glycob/cwh004. [DOI] [PubMed] [Google Scholar]
- 63.Chen X, VanValkenburgh C, Liang H, Fang H, Green N. Signal peptidase and oligosaccharyltransferase interact in a sequential and dependent manner within the endoplasmic reticulum. J Biol Chem. 2001;276:2411–6. doi: 10.1074/jbc.M007723200. [DOI] [PubMed] [Google Scholar]
- 64.Tanaka K, Kitagawa Y, Kadowaki T. Drosophila segment polarity gene product porcupine stimulates the posttranslational N-glycosylation of wingless in the endoplasmic reticulum. J Biol Chem. 2002;277:12816–23. doi: 10.1074/jbc.M200187200. [DOI] [PubMed] [Google Scholar]
- 65.Zielinska DF, Gnad F, Schropp K, Wisniewski JR, Mann M. Mapping N-glycosylation sites across seven evolutionarily distant species reveals a divergent substrate proteome despite a common core machinery. Mol Cell. 2012;46:542–8. doi: 10.1016/j.molcel.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 66.Hershey JWB. Translational control in mammalian cells. Annu Rev Biochem. 1991;60:717–55. doi: 10.1146/annurev.bi.60.070191.003441. [DOI] [PubMed] [Google Scholar]
- 67.Bause E. Model studies on N-glycosylation of proteins. Biochem Soc Trans. 1984;12:514–7. doi: 10.1042/bst0120514. [DOI] [PubMed] [Google Scholar]
- 68.Suzuki S, Shuto T, Sato T, Kaneko M, Takada T, Suico MA, et al. Inhibition of post-translational N-glycosylation by HRD1 that controls the fate of ABCG5/8 transporter. Sci Rep. 2014;4:4258. doi: 10.1038/srep04258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pult F, Fallah G, Braam U, Detro-Dassen S, Niculescu C, Laube B, et al. Robust post-translocational N-glycosylation at the extreme C-terminus of membrane and secreted proteins in Xenopus laevis oocytes and HEK293 cells. Glycobiology. 2011;21:1147–60. doi: 10.1093/glycob/cwr013. [DOI] [PubMed] [Google Scholar]
- 70.Bas T, Gao GY, Lvov A, Chandrasekhar KD, Gilmore R, Kobertz WR. Post-translational N-glycosylation of type I transmembrane KCNE1 peptides: implications for membrane protein biogenesis and disease. J Biol Chem. 2011;286:28150–9. doi: 10.1074/jbc.M111.235168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Malaby HL, Kobertz WR. Molecular determinants of co- and post-translational N-glycosylation of type I transmembrane peptides. Biochem J. 2013;453:427–34. doi: 10.1042/BJ20130028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Malaby HL, Kobertz WR. The middle x residue influences cotranslational N-glycosylation consensus site skipping. Biochemistry. 2014;53:4884–93. doi: 10.1021/bi500681p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bolt G, Kristensen C, Steenstrup TD. Posttranslational N-glycosylation takes place during the normal processing of human coagulation factor VII. Glycobiology. 2005;15:541–7. doi: 10.1093/glycob/cwi032. [DOI] [PubMed] [Google Scholar]
- 74.Shakin-Eshleman SH, Spitalnik SL, Kasturi L. The amino acid at the X position of an Asn-X-Ser sequon is an important determinant of N-linked core-glycosylation efficiency. J Biol Chem. 1996;271:6363–6. doi: 10.1074/jbc.271.11.6363. [DOI] [PubMed] [Google Scholar]
- 75.Kasturi L, Chen H, Shakin-Eshleman SH. Regulation of N-linked core glycosylation: use of a site-directed mutagenesis approach to identify Asn-Xaa-Ser/Thr sequons that are poor oligosaccharide acceptors. Biochem J. 1997;323(Pt 2):415–9. doi: 10.1042/bj3230415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bause E, Legler G. The role of the hydroxy amino acid in the triplet sequence asn-xaathr(ser) for the N-glycosylation step during glycoprotein biosynthesis. Biochem J. 1981;195:639–44. doi: 10.1042/bj1950639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kasturi L, Eshleman JR, Wunner WH, Shakin-Eshleman SH. The hydroxy amino acid in an Asn-X-Ser/Thr sequon can influence N-linked core glycosylation efficiency and the level of expression of a cell surface glycoprotein. J Biol Chem. 1995;270:14756–61. doi: 10.1074/jbc.270.24.14756. [DOI] [PubMed] [Google Scholar]
- 78.Titani K, Kumar S, Takio K, Ericsson LH, Wade RD, Ashida K, et al. Amino acid sequence of human von Willebrand factor. Biochemistry. 1986;25:3171–84. doi: 10.1021/bi00359a015. [DOI] [PubMed] [Google Scholar]
- 79.Vance BA, Wu W, Ribaudo RK, Segal DM, Kearse KP. Multiple dimeric forms of human CD69 result from differential addition of N-glycans to typical (Asn-X-Ser/Thr) and atypical (Asn-X-cys) glycosylation motifs. J Biol Chem. 1997;272:23117–22. doi: 10.1074/jbc.272.37.23117. [DOI] [PubMed] [Google Scholar]
- 80.Sato T, Sako Y, Sho M, Momohara M, Suico MA, Shuto T, et al. STT3B-dependent posttranslational N-glycosylation as a surveillance system for secretory protein. Mol Cell. 2012;47:99–110. doi: 10.1016/j.molcel.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 81.Goytain A, Quamme GA. Identification and characterization of a novel mammalian Mg2+ transporter with channel-like properties. BMC Genomics. 2005;6:48. doi: 10.1186/1471-2164-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou H, Clapham DE. Mammalian MagT1 and TUSC3 are required for cellular magnesium uptake and vertebrate embryonic development. Proc Natl Acad Sci USA. 2009;106:15750–5. doi: 10.1073/pnas.0908332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Molinari F, Foulquier F, Tarpey PS, Morelle W, Boissel S, Teague J, et al. Oligosaccharyltransferase-subunit mutations in nonsyndromic mental retardation. Am J Hum Genet. 2008;82:1150–7. doi: 10.1016/j.ajhg.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schulz BL, Aebi M. Analysis of glycosylation site occupancy reveals a role for Ost3p and Ost6p in site-specific N-glycosylation efficiency. Mol Cell Proteomics. 2009;8:357–64. doi: 10.1074/mcp.M800219-MCP200. [DOI] [PubMed] [Google Scholar]
- 85.Li FY, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, Douek DC, et al. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature. 2011;475:471–6. doi: 10.1038/nature10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Piton A, Redin C, Mandel JL. XLID-causing mutations and associated genes challenged in light of data from large-scale human exome sequencing. Am J Hum Genet. 2013;93:368–83. doi: 10.1016/j.ajhg.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garshasbi M, Hadavi V, Habibi H, Kahrizi K, Kariminejad R, Behjati F, et al. A defect in the TUSC3 gene is associated with autosomal recessive mental retardation. Am J Hum Genet. 2008;82:1158–64. doi: 10.1016/j.ajhg.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Garshasbi M, Kahrizi K, Hosseini M, Nouri Vahid L, Falah M, Hemmati S, et al. A novel nonsense mutation in TUSC3 is responsible for non-syndromic autosomal recessive mental retardation in a consanguineous Iranian family. Am J Med Genet A. 2011;155A:1976–80. doi: 10.1002/ajmg.a.34077. [DOI] [PubMed] [Google Scholar]
- 89.Wu X, Rush JS, Karaoglu D, Krasnewich D, Lubinsky MS, Waechter CJ, et al. Deficiency of UDP-GlcNAc:Dolichol Phosphate N-Acetylglucosamine-1 Phosphate Transferase (DPAGT1) Causes a Novel Congenital Disorder of Glycosylation Type Ij. Hum Mutat. 2003;22:144–50. doi: 10.1002/humu.10239. [DOI] [PubMed] [Google Scholar]
- 90.Westphal V, Schottstadt C, Marquardt T, Freeze HH. Analysis of multiple mutations in the hALG6 gene in a patient with congenital disorder of glycosylation Ic. Mol Genet Metab. 2000;70:219–23. doi: 10.1006/mgme.2000.3017. [DOI] [PubMed] [Google Scholar]
- 91.Imbach T, Grunewald S, Schenk B, Burda P, Schollen E, Wevers RA, et al. Multi-allelic origin of congenital disorder of glycosylation (CDG)-Ic. Hum Genet. 2000;106:538–45. doi: 10.1007/s004390000293. [DOI] [PubMed] [Google Scholar]