Abstract
New recommendations for birth cohort screening for hepatitis C virus (HCV) infection and the development of new, highly effective antiviral medications are expected to increase the demand for HCV treatment. In the past, antiviral therapy for HCV was almost exclusively prescribed by specialists in the field of gastroenterology and infectious diseases, meaning that people living in rural areas that are underserved by specialists may have poor access to treatment. We investigated the number and geographic distribution of medical providers who actively prescribed direct acting antiviral drugs for hepatitis C in Wisconsin during 2012. Using public health surveillance data and a state-wide prescription drug database, we found that there was 1 treatment provider for every 340 residents known to be living with HCV. However, 51 of 72 Wisconsin counties had no providers who provided HCV treatment in 2012. Scaling up antiviral treatment to address the epidemic of hepatitis C efficiently and equitably will require strategies to increase the number of treatment providers in rural communities. Providing education, training, and support to the primary care workforce serving rural communities should be considered a potentially effective and efficient approach to preventing future HCV-related illness.
Keywords: access to care, efficiency, medications, primary care, rural health
Background
Since approval of the first direct-acting antiviral drugs (DAAs) by the Food and Drug Administration in 2011, the treatment landscape for chronic hepatitis C virus (HCV) infection has rapidly evolved. Between 1998 and 2010, standard treatment for HCV genotype 1 was a 48-week course of oral ribavirin and weekly, subcutaneous injection of pegylated interferon. The rate of cure with this regimen was less than 50%, and treatment was poorly tolerated. Up to one third of patients discontinued treatment because of side effects such as depression and influenza-like symptoms.1 Newer, all-oral treatment regimens, including pan-genotypic DAAs are simpler to administer, better tolerated, and curative in over 90% of patients with substantially shorter treatment durations.2 These factors, together with national recommendations to improve detection of undiagnosed HCV infection through expanded routine screening,3,4 are expected to contribute to a steep rise in demand for HCV treatment in coming years, which will likely exceed the capacity of the specialty-trained physician workforce.5
In view of this trend, and in response to reports of increased HCV cases in rural areas,6 we evaluated the physician workforce engaged in HCV treatment during the first year of the DAA era. Using hepatitis surveillance data and prescription records from Wisconsin, we sought to determine whether patients living in rural areas are likely to be underserved because of a shortage of providers with experience treating hepatitis C infection. Wisconsin is a geographically diverse state with 1 large core metropolitan area, 1 medium-sized metropolitan area, and large land mass consisting of 24 small metropolitan and 47 nonmetropolitan counties with low population density, making it an appropriate setting in which to study rural-urban differences in health care utilization.
Methods
We searched a commercially available prescription database (IMS Health, Danbury, CT) linked to the American Medical Association Physician Masterfile to identify the names and practice addresses of providers who submitted more than one prescription for boceprevir or telaprevir to a retail or mail-order pharmacy in Wisconsin between January 1 and December 31, 2012. These 2 medications were selected because they were the only 2 DAAs approved for HCV treatment during that year. For each provider, the database also contained the area of specialization and the total number of prescriptions submitted for the 2 medications during 2012. Using the clinic location for all physicians identified who had a practice address in Wisconsin, we determined the number of HCV treatment providers practicing in each of the 72 counties in Wisconsin. We compared this with the estimated prevalence of HCV infection in every county. HCV prevalence is estimated by Wisconsin Division of Public Health on an ongoing basis through mandated, name and address–based reporting of HCV cases by providers and diagnostic laboratories. For this study, the prevalence during 2012 was determined by number of HCV cases reported as of December 31, 2012, then subtracting the number of cases known to have died or moved out of the state. The study was approved by the institutional review board at the University of Wisconsin–Madison.
Results
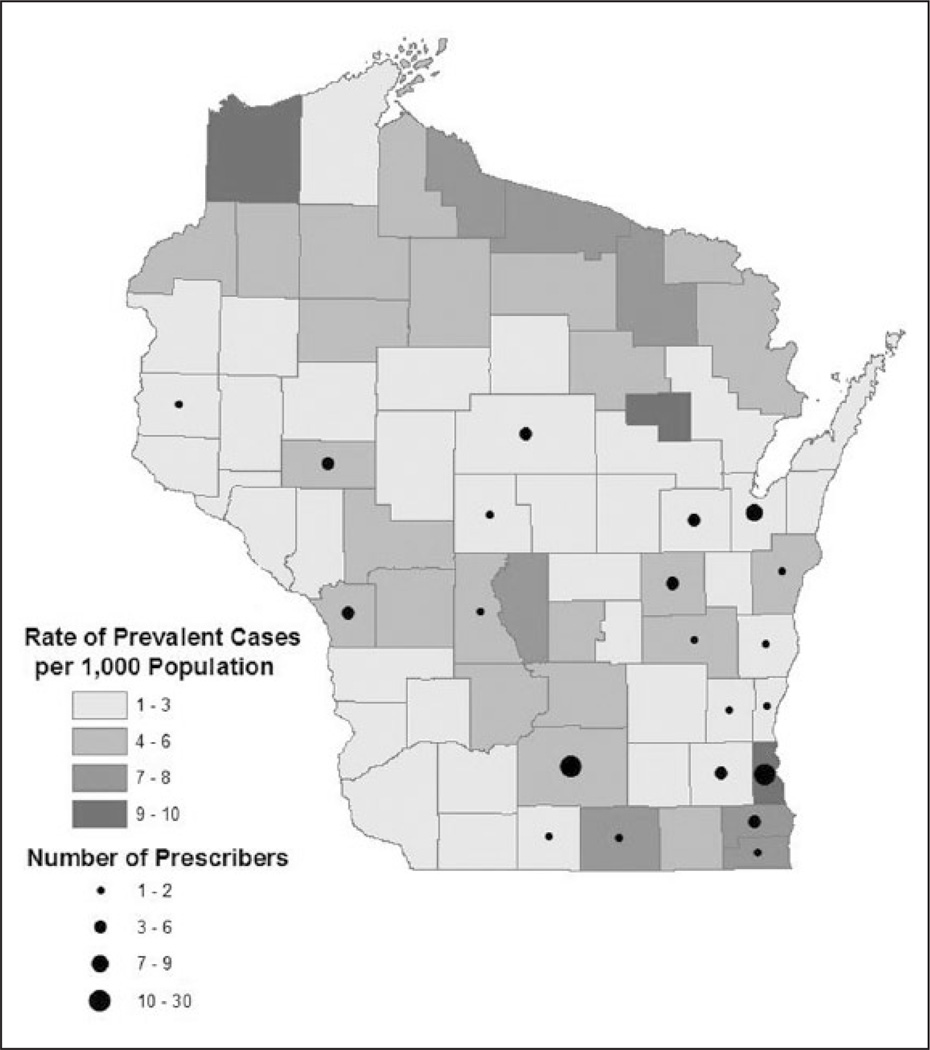
We identified 103 providers who submitted a total of 1053 prescriptions for telaprevir or boceprevir during 2012. These included 62 (60%) providers whose specialty was gastroenterology, 5 (5%) infectious diseases, 11 (11%) internal medicine, and 1 (1%) family medicine. Another 22 (21%) prescribers were nurse practitioners or physician assistants affiliated with gastroenterology/hepatology clinics. More than two thirds of the 72 counties had no providers prescribing DAAs during 2012 (Figure 1). Milwaukee and Dane County, the 2 most populous counties in Wisconsin, had 30 and 20 providers, respectively. These 50 providers accounted for 639 prescriptions, which was more than 61% of the DAAs prescribed in Wisconsin during 2012.
Figure 1.
Hepatitis C virus (HCV) prevalence and distribution of HCV treatment prescribers, Wisconsin, 2012.
Approximately 35 000 individuals were living with HCV in Wisconsin in 2012 based on statewide public health surveillance.7 Of known cases, only 22% resided in a large core metropolitan area. Approximately 5,000 HCV-infected individuals resided in a rural county with no provider who prescribed HCV treatment during 2012. Using these data, we estimate there was 1 treatment provider for every 340 known cases of HCV.
Discussion
Provider inexperience and limited access to specialists have been previously demonstrated to be important barriers to treatment of hepatitis C.8,9 Our findings suggest that there are large geographic areas with high burdens of HCV infection that are not served by providers specializing in HCV care. As the number of patients eligible for HCV treatment continues to increase, the provider workforce currently engaged in management of HCV infection will lack the capacity to provide care for all who would benefit from treatment.
Several approaches have been proposed and implemented to expand access to high-quality HCV treatment, including telemedicine10 and clinical decision support systems.11 Continued investment in these types of resources could allow primary care providers in rural communities to play a much larger role in providing antiviral treatment to their patients with chronic HCV infection. Through US guidelines recommending universal HCV screening for all adults born between 1945 and 1965, primary care providers are already being asked to play a central role in the nation’s approach to the hepatitis C epidemic. Ensuring that additional training and support is available to primary care providers interested in prescribing antiviral therapy would equip the health care system to more efficiently care for the aging, HCV-infected population. Enlisting primary care clinics as the front line of a “test-and-treat” approach to hepatitis C could be a cost-effective strategy for preventing HCV-related deaths in the coming decades.
Several limitations apply to these data. Some patients living in counties bordering adjacent states may receive HCV treatment from providers practicing outside Wisconsin who were not captured in the pharmacy database. Patients treated for a subtype of HCV other than genotype 1 may have been prescribed HCV treatment that did not include boceprevir or telaprevir. However, because HCV genotype 1 comprises the majority of cases in Wisconsin, we believe it is unlikely that any provider with substantial expertise in treating HCV was not captured by our search strategy. The true prevalence of HCV in Wisconsin is not known, but because of underdiagnosis, is understood to be substantially higher than the number of reported cases.3,4 Our study therefore likely underestimates the future need for HCV treatment, and by extension, the magnitude of the shortage of HCV treatment providers.
Treatment for hepatitis C continues to evolve and improve at a rapid pace. Telaprevir and boceprevir, the only DAAs in use during the year data were collected for this study, are already obsolete, having been replaced by drugs that are even more effective and well tolerated.12 With these advances, it will become increasingly feasible for primary care providers to become more involved with all aspects of HCV treatment. By engaging primary care workforce in rural communities, the US health care system will be much better equipped to respond to the HCV epidemic.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by the Wisconsin Division of Public Health, The Department of Medicine at the Medical College of Wisconsin, and National Institutes of Health grant number K23DA032306.
Biographies
Ryan P. Westergaard is an assistant professor of Medicine and Population Health Sciences at the University of Wisconsin-Madison.
Lauren J. Stockman is an epidemiologist with the Wisconsin Division of Public Health.
Heather A. Hyland is a medical student at the Medical College of Wisconsin.
Sheila M. Guilfoyle is the Viral Hepatitis Coordinator for the Wisconsin Division of Public Health.
John J. Fangman is an associate professor of Medicine and Chief of the Division of Infectious Diseases at the Medical College of Wisconsin.
James M. Vergeront is the Director of the Wisconsin AIDS/HIV Program in the Division of Public Health.
Footnotes
The statements, findings, conclusions, views, and opinions contained and expressed in this article are based in part on data obtained under license from the following IMS Health Incorporated information service(s): National Prescription Audit, January 2012–December 2012, IMS Health Incorporated. All rights reserved. Such statements, findings, conclusions, views, and opinions are not necessarily those of IMS Health Incorporated or any of its affiliated or subsidiary entities.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon α-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 2.Afdhal N, Reddy KR, Nelson DR, et al. ION-2 Investigators. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483–1493. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 3.Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61(RR-4):1–32. [PubMed] [Google Scholar]
- 4.Moyer VA US Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013 Sep 3;159(5):349–357. doi: 10.7326/0003-4819-159-5-201309030-00672. [DOI] [PubMed] [Google Scholar]
- 5.Liang TJ, Ghany MG. Current and future therapies for hepatitis C virus infection. N Engl J Med. 2013;368:1907–1917. doi: 10.1056/NEJMra1213651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Notes from the field: hepatitis C virus infections among young adults—rural Wisconsin, 2010. MMWR Morb Mortal Wkly Rep. 2012;61(19):358. [PubMed] [Google Scholar]
- 7.Wisconsin Department of Health Services. [Accessed September 29,2014];Epidemiologic profile of hepatitis C virus (HCV) in Wisconsin. http://www.dhs.wisconsin.gov/publications/P0/p00860.pdf.
- 8.Morrill JA, Shrestha M, Grant RW. Barriers to the treatment of hepatitis C. Patient, provider, and system factors. J Gen Intern Med. 2005;20:754–758. doi: 10.1111/j.1525-1497.2005.0161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGowan CE, Monis A, Bacon BR, et al. A global view of hepatitis C: physician knowledge, opinions, and perceived barriers to care. Hepatology. 2013;57:1325–1332. doi: 10.1002/hep.26246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199–2207. doi: 10.1056/NEJMoa1009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fathauer L, Meek J. Initial implementation and evaluation of a hepatitis C treatment clinical decision support system (CDSS): a nurse practitioner-driven quality improvement initiative. Appl Clin Inform. 2012;3:337–348. doi: 10.4338/ACI-2012-04-RA-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Afdhal NH, Zeuzem S, Schooley RT, et al. The new paradigm of hepatitis C therapy: integration of oral therapies into best practices. J Viral Hepat. 2013;20:745–760. doi: 10.1111/jvh.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]