Abstract
Objective
Hot flashes (HFs) are a common symptom in breast cancer survivors that can negatively impact quality of life. Preliminary data suggested that magnesium might be an effective, low-cost treatment for HFs with minimal side effects.
Methods
A four-arm, double-blind, placebo-controlled randomized trial was conducted. Postmenopausal women with a history of breast cancer and bothersome HFs were randomized into treatment groups of 800 or 1200 mg daily magnesium oxide, or corresponding placebo groups in 2:2:(1:1) ratios. HF frequency and scores (number times mean severity) were measured using a validated HF diary. A one-week baseline period preceded initiation of study medication. The primary endpoint was the intra-patient difference in average hot flash score between the baseline and the treatment periods, comparing each magnesium group to the combined placebo groups using a gate-keeping procedure. Results were analyzed using repeated measures and growth curve models on weekly HF score, based on a modified intent-to-treat principle.
Results
289 women enrolled between 12/2011 and 03/2013. The study groups were well balanced for baseline characteristics. Mean HF scores, frequencies, and associated changes during the treatment period were similar for each group. An increased incidence of diarrhea and a corresponding lower incidence of constipation were reported in magnesium arms compared to placebo. No statistically significant difference occurred in other toxicities or quality of life measures.
Conclusions
The results of this trial do not support the use of magnesium oxide for HFs.
Keywords: magnesium, hot flashes, breast cancer survivorship
Introduction
Hot flashes continue to be the most common symptom associated with menopause and can be experienced by about 75% of women1, 2. Although some might consider hot flashes to be a “benign” symptom, they can be a source of distress, can disrupt sleep, can negatively impact the ability to function in various life activities, and can cause changes to jobs or work schedules3. Estrogen-and/or progesterone-based therapy can offer an 80~90% reduction of hot flashes.4–7 However, hormone-based treatments are often not recommended for women with a history of breast cancer because of concerns of cancer recurrence and cancer-related risk factors such as a history of thrombotic events. Therefore, hot flashes in female breast cancer survivors are more difficult to treat than they are in other women8. Tamoxifen therapy is associated with hot flashes in over 50% of women and the incidence of hot flashes after treatment with aromatase inhibitors (AIs) has been reported to be 34 to 58%4, 9, 10. The most effective non-hormonal pharmacologic therapies, antidepressants and anticonvulsants, offer about a 50% reduction of hot flashes3, 9, 11, 12, but they do have some undesired side effects such as dizziness, dry mouth, trouble sleeping, somnolence and nausea.13 Furthermore, antidepressants have a stigma for many patients. While herbs and dietary supplements such as soy, black cohosh, flaxseed and vitamin E are popular hot flash remedies, to date, randomized placebo-controlled trials have not proven them to be effective14–18.
Magnesium, a mineral that has a long history of medicinal use, has been used to treat hypertension19, eclampsia20, and other cardiovascular21 and nerve disorders22. Currently, its most commonly recognized use is as a laxative, often used for preparing the bowel for surgery or diagnostic procedures.
The results of two pilot studies, using up to 1200 mg of daily magnesium oxide, suggested that this agent was associated with significant reductions in hot flash symptoms23, 24. One open label pilot study, using a magnesium oxide dose of up to 800 mg per day and validated methodologies25, supported that magnesium significantly reduced hot flash scores and frequency compared to baseline values. Of 25 patients, 14 patients (56%) experienced a >50% reduction in hot flash score, and 19 patients (76%) had a >25% hot flash reduction at the end of the 4 weeks of study treatment. The average weekly hot flash score decreased by 50.4% (p = 0.02).
A second open label study24 evaluated 400 mg of magnesium oxide three times per day for 4 weeks in 22 women undergoing treatment for breast cancer. Ten women (45%) reported having hot flashes resolve over this time and another 10 (45%) reported experiencing a reduction of 50% or more. The results from these two studies were comparable to the results of pilot studies of other agents that subsequently showed efficacy in phase III trials.
Several in vitro studies suggest possible relationship between the homeostasis of intracellular magnesium and estrogen and progesterone26–30. While the pathophysiology of hot flashes is still unclear, magnesium appeared to be a reasonable link between vasomotor symptoms and menopause. Magnesium oxide is inexpensive, generic, and readily available. In addition, no important side effects, aside from some diarrhea, have been found at the relevant dose ranges in patients with intact kidney function. Therefore, this current randomized, double-blinded, placebo-controlled trial was designed to definitively evaluate the efficacy of oral magnesium oxide for ameliorating hot flash symptoms in women with a history of breast cancer.
Methods
Postmenopausal women with a history of breast cancer who reported bothersome hot flashes, defined as greater than 28 times per week and of sufficient severity to make each patient desire therapeutic intervention, were included in this study. Other inclusion criteria included the presence of hot flashes for at least 30 days prior to study registration, preserved kidney function (calculated creatinine clearance greater than 30mL/minute), Eastern Cooperative Oncology Group performance status of 0 or 1, and the ability to complete questionnaires by themselves or with assistance.
Patients were excluded if they were receiving antineoplastic chemotherapy, estrogenic agents, progesterone analogs, androgens, gabapentin or antidepressants. Other exclusion criteria included a history of allergic or another adverse reaction to magnesium, concurrent use of magnesium for any indication, or any condition that might have affected magnesium levels, including diabetes, Crohn’s disease, diarrheal disease, alcohol abuse, or the use of diuretics, corticosteroids, or bile acid sequestrants. Patients participating in yoga or acupuncture for relief of hot flashes were also excluded. Each participant signed an institutional review board-approved, protocol-specific informed consent in accordance with federal and institutional guidelines.
Patients were stratified by age, concurrent anti-estrogenic therapy use, and daily frequency of hot flashes and then randomized into four double-blinded treatment groups of 800 mg or 1200 mg of daily magnesium oxide. The two placebo groups were assigned either 2 or 3 capsules, corresponding to the same number of capsules for each magnesium oxide dose (as each magnesium oxide capsule was 400 mg) in 2:2:(1:1) ratios.
Self-reported validated survey instruments25 and phone interviews were used to collect data on the frequency and severity of hot flashes as well as potential toxicities. The first week after enrollment was used to collect information on baseline characteristics of hot flashes and obtain data on symptoms that might have subsequently been construed as potential magnesium toxicities. These symptoms were queried on a symptom experience diary that asked patients to rate, on a 0–10 scale, the following: diarrhea, constipation, other gastrointestinal symptoms, constitutional symptoms, mood, concentration, and level of distress due to hot flashes. Patients started treatment after the baseline week at a dose of 1 tablet per day, which was titrated up by 1 tablet per week to a total of 2 or 3 tablets of allocated treatment per protocol. The same data that were collected in the baseline week were also collected in the same manner at the end of each treatment week. Serum magnesium concentrations were obtained prior to study medication and during the last week of treatment in the first 150 patients. The intra-personal changes in serum magnesium concentrations were compared among the 3 study arms. This trial was monitored at least twice annually by a Data and Safety Monitoring Board, a standing committee composed of individuals from within and outside the NCCTG/Alliance.
Statistical Analysis
Hot flashes were measured by the weekly average hot flash score25, which is a composite entity of both frequency and severity of hot flashes (number times mean severity). Patients were randomized using an established procedure of dynamic allocation31 that balanced the marginal distributions of stratification factors and the clinical site. We did not adjust for the site of enrollment in the statistical analysis as we did not observe an unusual imbalance. The modified intention-to-treat principle32 (that exclude cancellation, ineligible patients, and those who did not complete any post-baseline questionnaire for primary endpoint) was conducted for primary analysis. The primary endpoint was the intra-patient changes of weekly average hot flash scores from baseline, during the 8 weeks of treatment. Repeated measures models and growth curve models33 were used to examine the treatment effect of magnesium. To control for multiplicity from multiple treatment arms, a gatekeeper procedure,34 following a fixed-sequence hypothesis testing method, was used to examine the higher dose of magnesium vs. placebo first and then the lower dose of magnesium vs. placebo, if the former was statistically significant.
Secondary endpoints included the intra-patient changes of (1) the frequency of hot flashes, (2) toxicities including diarrhea collected using the CTCAE v4.0, (3) mood changes using the Profile of Mood States (POMS) and hot flash-related daily interference on activities collected using the Symptom Experience Questionnaire (SEQ) and the Hot Flash Related Daily Interference (HFRDI) scale, and (4) magnesium serum concentrations between magnesium oxide and placebo arms. All scales were converted to 0–100 where 100 represents the best quality of life (QOL), for ease of comparison of secondary endpoints35. The association of magnesium serum concentrations was explored for the first 150 patients only.
With a sample size of 80 patients per arm, the study had 80% power to detect a time-averaged clinically meaningful difference of 5.1 points (8.6 for either magnesium arms, 3.1 for placebo arms) in changes of hot flash scores (on 0–100 scale) using the repeated measures model at the two-sided 5% significance level. A moderate positive correlation of 0.5 was assumed between repeated measures of weekly hot flash scores over the 8 weeks. The sample size was inflated by 20% to account for patient ineligibility, cancellation, or major violations. Data collection and statistical analyses were conducted by the NCCTG/Alliance Statistics and Data Center. Data quality was ensured by review of data by the NCCTG/Alliance Statistics and Data Center and by the study chairperson following NCCTG/Alliance policies. The analyzed data set was locked on November 11, 2013.
Results
Baseline Characteristics
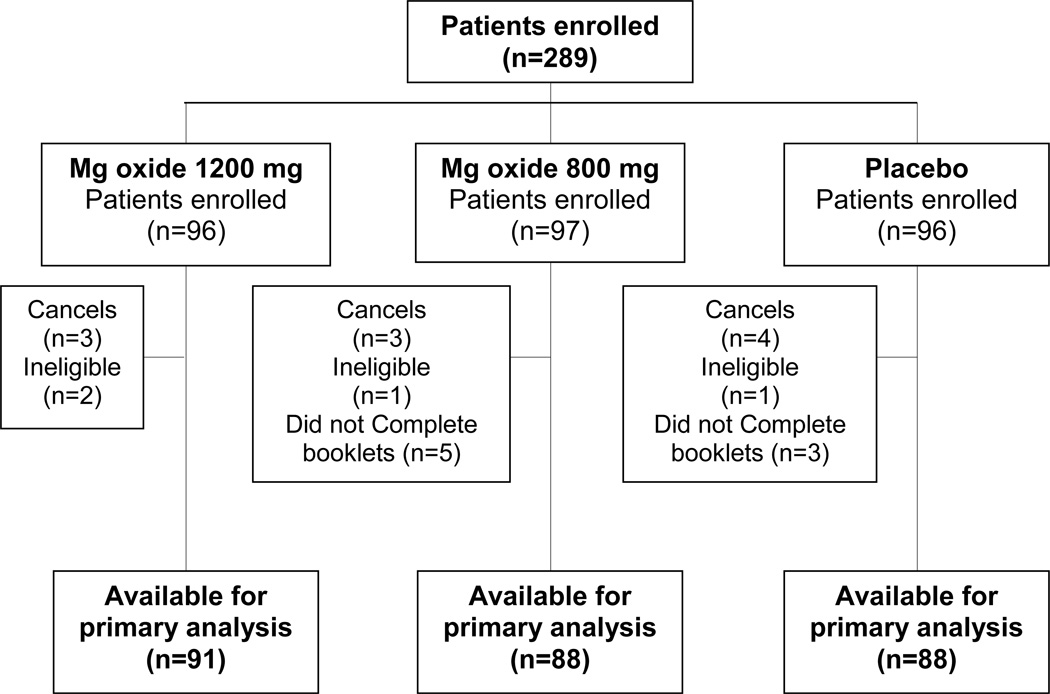
A total of 289 women were enrolled between 12/2011 and 03/2013, including 10 cancels and 4 ineligible patients, and additionally 8 patients who did not complete booklets for deriving the primary endpoint due to refusal, did not return booklet or dropping-out during cycle 1 because of an adverse event; see the CONSORT diagram in Figure 1. The study arms had reasonably well balanced patient baseline characteristics, as illustrated in Table 1. No crossovers or co-interventions were allowed during the treatment period for any of the arms, and we are not aware that any occurred.
Figure 1.
Consort diagram
Table 1.
Patient Characteristics
| Table 1: Baseline Patient Characteristics | |||||
|---|---|---|---|---|---|
| Magnesium 1200 mg/d (N=91) |
Magnesium 800 mg/d (N=93) |
Placebo (N=91) |
Total (N=275) |
P value | |
| Characteristic | N (%) | N (%) | N (%) | N (%) | |
| Age, years | 1.001 | ||||
| 18–49 | 15 (17%) | 15 (16%) | 15 (17%) | 45 (16%) | |
| >=50 | 76 (83%) | 78 (84%) | 76 (83%) | 230 (84%) | |
| Race | 0.522 | ||||
| White | 86 (95%) | 89 (96%) | 88 (97%) | 263 (96%) | |
| Black or African American | 4 (4%) | 2 (2%) | 3 (3%) | 9 (3%) | |
| Asian | 1 (1%) | 0 (0%) | 0 (0%) | 1 (0.4%) | |
| Not reported | 0 (0%) | 2 (2%) | 0 (0%) | 2 (1%) | |
| Current anti-estrogen therapy | 0.881 | ||||
| None | 15 (17%) | 15 (16%) | 15 (17%) | 45 (16%) | |
| Aromatase Inhibitor | 43 (47%) | 41 (44%) | 42 (46%) | 126 (46%) | |
| Tamoxifen | 33 (36%) | 37 (40%) | 34 (36%) | 104 (38%) | |
| Reported daily frequency of hot flashes | 0.052 | ||||
| 4–9 | 53 (58%) | 47 (50%) | 62 (68%) | 162 (59%) | |
| 10+ | 38 (42%) | 46 (50%) | 29 (32%) | 113 (41%) | |
| Use of oral calcium | 49 (54%) | 49 (53%) | 48 (53%) | 146 (53%) | 0.981 |
| Use of vitamin D | 57 (63%) | 56 (60%) | 57 (63%) | 170 (62%) | 0.931 |
| Baseline Serum Magnesium (mg/dL) | 0.183 | ||||
| N | 90 | 90 | 91 | 271 | |
| Mean (SD) | 2.0 (0.2) | 2.1 (0.2) | 2.0 (0.2) | 2.0 (0.2) | |
| Median | 2.0 | 2.1 | 2.0 | 2.0 | |
P-values calculated using
Chi-Square,
Fisher Exact, or
Kruskal Wallis methods
Efficacy
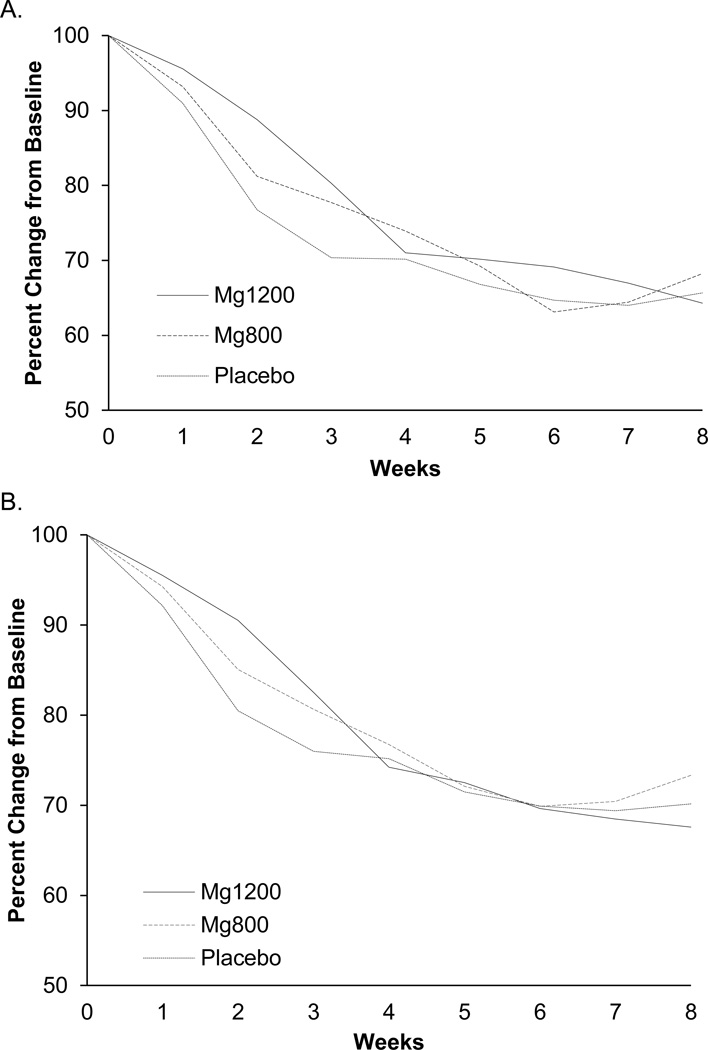
Following a modified intent-to-treat principle, 267 patients (92%) were available for the primary study analysis. Placebo arms were combined, as per the protocol plan, after determining that there were no differences between the two placebo arms during the protocol period. Mean hot flash scores and frequencies for each arm are shown in Table 2, with p-values comparing each treatment arm against the combined placebo arms. Changes in mean hot flash scores and frequencies over time are shown in Figure 2. All groups experienced reductions in hot flash scores and frequencies, but the degree of reduction in the treatment groups was similar to that of the placebo group.
Table 2.
Hot Flash Scores (A) and Frequencies (B) During Treatment
| A. Mean Hot Flash Scores | ||||||
|---|---|---|---|---|---|---|
| Placebo (N=91) | 1200 mg (N=88) | 800 mg (N=88) | ||||
| Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | |
| Baseline | 17.28 (10.84) | 83 | 15.35 (10.53) | 81 | 16.02 (9.62) | 81 |
| Week 1 | 15.52 (10.51) | 86 | 14.47 (11.10) | 78 | 14.28 (9.26) | 81 |
| Week 2 | 13.29 (9.46) | 85 | 12.59 (10.01) | 78 | 12.68 (8.87) | 79 |
| Week 3 | 12.34 (9.98) | 83 | 11.32 (10.72) | 76 | 12.29 (9.79) | 78 |
| Week 4 | 12.02 (9.57) | 82 | 10.14 (8.61) | 73 | 11.73 (10.35) | 78 |
| Week 5 | 11.63 (9.61) | 79 | 9.73 (9.19) | 67 | 11.77 (10.86) | 75 |
| Week 6 | 11.17 (8.78) | 79 | 9.44 (8.99) | 67 | 11.61 (10.46) | 71 |
| Week 7 | 10.71 (8.68) | 78 | 9.37 (9.24) | 67 | 11.92 (11.26) | 71 |
| Week 8 | 11.42 (10.32) | 79 | 9.06 (8.87) | 66 | 12.17 (10.88) | 69 |
| Repeated Measure (P-value) | Growth Curve Model (P-value) | |||||
| Mg 1200 Vs. Placebo | 0.67 | 0.90 | ||||
| Mg 800 Vs. Placebo | 0.13 | 0.26 | ||||
| B. Mean Hot Flash Frequencies | ||||||
|---|---|---|---|---|---|---|
| Placebo (N=91) | 1200 mg (N=88) | 800 mg (N=88) | ||||
| Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | |
| Baseline | 8.89 (4.52) | 83 | 7.55 (3.89) | 81 | 8.48 (4.34) | 81 |
| Week 1 | 8.04 (4.59) | 86 | 7.15 (4.13) | 78 | 7.83 (4.34) | 81 |
| Week 2 | 7.26 (4.65) | 85 | 6.51 (4.19) | 78 | 7.12 (4.18) | 79 |
| Week 3 | 6.97 (4.82) | 83 | 5.87 (4.21) | 76 | 6.73 (4.41) | 78 |
| Week 4 | 6.73 (4.44) | 82 | 5.34 (3.76) | 73 | 6.39 (4.51) | 78 |
| Week 5 | 6.45 (4.33) | 79 | 5.15 (4.00) | 67 | 6.31 (4.70) | 75 |
| Week 6 | 6.37 (4.30) | 79 | 4.95 (4.04) | 67 | 6.34 (4.56) | 71 |
| Week 7 | 6.18 (4.23) | 78 | 4.92 (4.13) | 67 | 6.43 (4.68) | 71 |
| Week 8 | 6.38 (4.67) | 79 | 4.88 (4.02) | 66 | 6.61 (4.58) | 69 |
| Repeated Measure (P-value) | Growth Curve Model (P-value) | |||||
| Mg 1200 Vs. Placebo | 0.55 | 0.80 | ||||
| Mg 800 Vs. Placebo | 0.25 | 0.39 | ||||
Figure 2.
Weekly mean changes, from baseline, for hot flash scores (A) and frequencies (B).
Analysis of changes in POMS and HFRDI scores did not show significant differences between treatment and placebo groups. No significant difference in serum magnesium levels was observed between any of the study arms. Furthermore, there was no statistically significant correlation between serum magnesium levels and change in hot flash symptoms.
Toxicity
There were no significant toxicity differences, measured by Common Terminology Criteria for Adverse Events (CTCAE), Version 4, between the three study arms. Nonetheless, symptom experience diary data revealed an increased incidence of diarrhea in the magnesium arms compared to placebo, and correspondingly, constipation was reported less frequently in the magnesium arms. These data are detailed in Table 3.
Table 3.
Common Toxicities assessed by Symptom Experience Questionnaire, Changes from Baseline to End of Treatment*
| Measure | Placebo | Magnesium 1200 mg/d |
Magnesium 800 mg/d |
|---|---|---|---|
| Diarrhea, mean (SD) | 0.4 (12.6) | − 14.3 (25.8) | − 4.5 (11.7) |
| Constipation, mean (SD) | 2.3 (15.2) | 7.3 (21.1) | 7.7 (20.6) |
Symptom is measured on a 0–100 scale with 100 being the best. Positive change indicates a decrease in symptoms.
Discussion
The results from this study do not support the study hypothesis that magnesium oxide would decrease hot flashes, despite pilot trial data suggesting that magnesium would be beneficial.
Serum magnesum levels were unchanged after treatment with magnesium, and were not associated with changes in hot flash scores. This was the case in another randomized trial, where oral magnesium was shown to be effective in controlling asthma related symptoms36. Considering that serum magnesium is tightly regulated by renal excretion, which can be significantly increased in the setting of high magnesium load, it is likely that serum magnesium is not an optimal surrogate for intracellular activity of magnesium, regardless of bioavailability of the agent.
The research group that conducted this current trial has conducted a variety of clinical trials based on anecdotal and/or pilot data suggesting benefits for different proposed treatments. Some of these trials have been positive, demonstrating benefit for megestrol acetate37, medroxyprogesterone acetate5, venlafaxine9, citalopram3, pregabalin38, fluoxetine39, and clonidine40. In contrast, this same research group has conducted other trials which have, unfortunately, not confirmed the study hypotheses, as was seen with the magnesium oxide in this current study. These negative trials included studies of flaxseed18, a soy product17, black cohosh16, and vitamin E15.
This series of trials illustrates the need to conduct well-designed, placebo-controlled trials to clarify the benefits and toxicities of agents that appear promising at the pilot study phase for the treatment of hot flashes.
Acknowledgments
Disclosures of funding: National Cancer Institute
Funding:
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-35431, CA-35267, CA-63848, CA-35113, CA-35195, CA-35101, CA-37417, CA-35090, CA-35448, CA-35119, CA-35103, CA-35415, and CA-63849. The study was also supported, in part, by grants from the National Cancer Institute (CA31946) to the Alliance for Clinical Trials in Oncology (Monica M. Bertagnolli, M.D., Chair) and to the Alliance Statistics and Data Center (Daniel J. Sargent, Ph.D., CA33601).The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Additional participating institutions include:
Marshfield Clinical Research Foundation, Minocqua, WI 54548 (Matthias Weiss, M.D.); Medical College of Georgia, Augusta, GA 30912 (Anand P. Jillella M.D.); Carle Cancer Center CCOP, Urbana, IL 61801 (Kendrith M. Rowland, Jr, M.D.); Missouri Valley Cancer Consortium, Omaha, NE 68106 (Gamini S. Soori, M.D.); St. Vincent Regional Cancer Center CCOP, Green Bay, WI 54303 (Anthony J. Jaslowski, M.D.); Hematology & Oncology of Dayton, Inc, Dayton, OH 45415 (Howard M. Gross, M.D.); Sanford Cancer Center Oncology Clinic, Sioux Falls, SD 57105 (Miroslaw Mazurczak, M.D.); Virginia Mason CCOP, Seattle, WA 98101 (Craig R. Nichols, M.D.); Geisinger Clinic & Medical Center CCOP, Danville, PA 17822 (Maged Khalil, M.D.); Iowa Oncology Research Association CCOP, Des Moines, IA 50309-1014 (Robert J. Behrens, M.D.); Meritcare Hospital CCOP, Fargo, ND 58122 (Preston D. Steen, M.D.); Lehigh Valley Hospital, Allentown, PA 18103 (Suresh Nair, M.D.); Upstate Carolina CCOP, Spartanburg, SC 29303 (James D. Bearden, III, M.D.); Columbus CCOP, Columbus, OH 53215 (J. Philip Kuebler, M.D., Ph.D.); Toledo Community Hospital Oncology Program CCOP, Toledo, OH 43623 (Rex B. Mowat, M.D.); Heartland Cancer Research CCOP, St. Louis, MO 63131 (Alan P. Lyss, M.D.); Montana Cancer Consortium, Billings, MT 59101 (Benjamin T. Marchello, M.D.); University of New Mexico, Albuquerque, NM 87131 (Zoneddy R. Dayao, M.D.); Northern Indiana Cancer Research Consortium CCOP, South Bend, IN 46601 (Robin T. Zon, M.D.); CentraCare Clinic, St. Cloud, MN 56301 (Donald J. Jurgens, M.D.); Cancer Care Associates, Tulsa, OK 74136 (Alan M. Keller, M.D.)
Footnotes
This study was orally presented at 2014 ASCO Annual Meeting and was the recipient of an ASCO Fellow Merit Award
Disclaimers: None
References
- 1.Stearns V, Ullmer L, Lopez JF, Smith Y, Isaacs C, Hayes D. Hot flushes. Lancet. 2002;360(9348):1851–1861. doi: 10.1016/s0140-6736(02)11774-0. [DOI] [PubMed] [Google Scholar]
- 2.Gracia CR, Freeman EW. Acute consequences of the menopausal transition: the rise of common menopausal symptoms. Endocrinology and metabolism clinics of North America. 2004;33(4):675–689. doi: 10.1016/j.ecl.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Barton DL, LaVasseur BI, Sloan JA, et al. Phase III, placebo-controlled trial of three doses of citalopram for the treatment of hot flashes: NCCTG trial N05C9. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(20):3278–3283. doi: 10.1200/JCO.2009.26.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molina JR, Barton DL, Loprinzi CL. Chemotherapy-induced ovarian failure: manifestations and management. Drug safety : an international journal of medical toxicology and drug experience. 2005;28(5):401–416. doi: 10.2165/00002018-200528050-00004. [DOI] [PubMed] [Google Scholar]
- 5.Bertelli G, Venturini M, Del Mastro L, et al. Intramuscular depot medroxyprogesterone versus oral megestrol for the control of postmenopausal hot flashes in breast cancer patients: a randomized study. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2002;13(6):883–888. doi: 10.1093/annonc/mdf151. [DOI] [PubMed] [Google Scholar]
- 6.MacGregor CA, Canney PA, Patterson G, McDonald R, Paul J. A randomised double-blind controlled trial of oral soy supplements versus placebo for treatment of menopausal symptoms in patients with early breast cancer. European journal of cancer. 2005;41(5):708–714. doi: 10.1016/j.ejca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Notelovitz M, Lenihan JP, McDermott M, Kerber IJ, Nanavati N, Arce J. Initial 17beta-estradiol dose for treating vasomotor symptoms. Obstetrics and gynecology. 2000;95(5):726–731. doi: 10.1016/s0029-7844(99)00643-2. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter JS, Johnson D, Wagner L, Andrykowski M. Hot flashes and related outcomes in breast cancer survivors and matched comparison women. Oncology nursing forum. 2002;29(3):E16–E25. doi: 10.1188/02.ONF.E16-E25. [DOI] [PubMed] [Google Scholar]
- 9.Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet. 2000;356(9247):2059–2063. doi: 10.1016/S0140-6736(00)03403-6. [DOI] [PubMed] [Google Scholar]
- 10.Morales AJ, Nolan JJ, Nelson JC, Yen SS. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. The Journal of clinical endocrinology and metabolism. 1994;78(6):1360–1367. doi: 10.1210/jcem.78.6.7515387. [DOI] [PubMed] [Google Scholar]
- 11.Loprinzi CL, Diekmann B, Novotny PJ, Stearns V, Sloan JA. Newer antidepressants and gabapentin for hot flashes: a discussion of trial duration. Menopause. 2009;16(5):883–887. doi: 10.1097/gme.0b013e31819c46c7. [DOI] [PubMed] [Google Scholar]
- 12.Ensrud KE, Joffe H, Guthrie KA, et al. Effect of escitalopram on insomnia symptoms and subjective sleep quality in healthy perimenopausal and postmenopausal women with hot flashes: a randomized controlled trial. Menopause. 2012;19(8):848–855. doi: 10.1097/gme.0b013e3182476099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barton D, Loprinzi CL. Making sense of the evidence regarding nonhormonal treatments for hot flashes. Clinical journal of oncology nursing. 2004;8(1):39–42. doi: 10.1188/04.CJON.39-42. [DOI] [PubMed] [Google Scholar]
- 14.Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA : the journal of the American Medical Association. 2006;295(17):2057–2071. doi: 10.1001/jama.295.17.2057. [DOI] [PubMed] [Google Scholar]
- 15.Barton DL, Loprinzi CL, Quella SK, et al. Prospective evaluation of vitamin E for hot flashes in breast cancer survivors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1998;16(2):495–500. doi: 10.1200/JCO.1998.16.2.495. [DOI] [PubMed] [Google Scholar]
- 16.Pockaj BA, Gallagher JG, Loprinzi CL, et al. Phase III double-blind, randomized, placebo-controlled crossover trial of black cohosh in the management of hot flashes: NCCTG Trial N01CC1. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(18):2836–2841. doi: 10.1200/JCO.2005.05.4296. [DOI] [PubMed] [Google Scholar]
- 17.Quella SK, Loprinzi CL, Barton DL, et al. Evaluation of soy phytoestrogens for the treatment of hot flashes in breast cancer survivors: A North Central Cancer Treatment Group Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18(5):1068–1074. doi: 10.1200/JCO.2000.18.5.1068. [DOI] [PubMed] [Google Scholar]
- 18.Pruthi S, Qin R, Terstreip SA, et al. A phase III, randomized, placebo-controlled, double-blind trial of flaxseed for the treatment of hot flashes: North Central Cancer Treatment Group N08C7. Menopause. 2012;19(1):48–53. doi: 10.1097/gme.0b013e318223b021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houston MC, Harper KJ. Potassium, magnesium, and calcium: their role in both the cause and treatment of hypertension. Journal of clinical hypertension. 2008;10(7) Suppl 2:3–11. doi: 10.1111/j.1751-7176.2008.08575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstetrics and gynecology. 2003;102(1):181–192. doi: 10.1016/s0029-7844(03)00475-7. [DOI] [PubMed] [Google Scholar]
- 21.Gums JG. Magnesium in cardiovascular and other disorders. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2004;61(15):1569–1576. doi: 10.1093/ajhp/61.15.1569. [DOI] [PubMed] [Google Scholar]
- 22.Nechifor M. Magnesium in major depression. Magnesium research : official organ of the International Society for the Development of Research on Magnesium. 2009;22(3):163S–166S. [PubMed] [Google Scholar]
- 23.Park H, Parker GL, Boardman CH, Morris MM, Smith TJ. A pilot phase II trial of magnesium supplements to reduce menopausal hot flashes in breast cancer patients. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2011;19(6):859–863. doi: 10.1007/s00520-011-1099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrada J, Gupta A, Campos-Gines AF, et al. Oral magnesium oxide for treatment of hot flashes in women undergoing treatment for breast cancer: A pilot study. Chicago: 2010 ASCO Annual Meeting; 2010. [Abstract]. [Google Scholar]
- 25.Sloan JA, Loprinzi CL, Novotny PJ, Barton DL, Lavasseur BI, Windschitl H. Methodologic lessons learned from hot flash studies. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19(23):4280–4290. doi: 10.1200/JCO.2001.19.23.4280. [DOI] [PubMed] [Google Scholar]
- 26.Alexander RT, Hoenderop JG, Bindels RJ. Molecular determinants of magnesium homeostasis: insights from human disease. Journal of the American Society of Nephrology : JASN. 2008;19(8):1451–1458. doi: 10.1681/ASN.2008010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groenestege WM, Hoenderop JG, van den Heuvel L, Knoers N, Bindels RJ. The epithelial Mg2+ channel transient receptor potential melastatin 6 is regulated by dietary Mg2+ content and estrogens. Journal of the American Society of Nephrology : JASN. 2006;17(4):1035–1043. doi: 10.1681/ASN.2005070700. [DOI] [PubMed] [Google Scholar]
- 28.O'Shaughnessy A, Muneyyirci-Delale O, Nacharaju VL, Dalloul M, Altura BM, Altura BT. Circulating divalent cations in asymptomatic ovarian hyperstimulation and in vitro fertilization patients. Gynecologic and obstetric investigation. 2001;52(4):237–242. doi: 10.1159/000052982. [DOI] [PubMed] [Google Scholar]
- 29.Pitkin RM, Reynolds WA, Williams GA, Hargis GK. Calcium-regulating hormones during the menstrual cycle. The Journal of clinical endocrinology and metabolism. 1978;47(3):626–632. doi: 10.1210/jcem-47-3-626. [DOI] [PubMed] [Google Scholar]
- 30.Muneyyirci-Delale O, Nacharaju VL, Dalloul M, Altura BM, Altura BT. Serum ionized magnesium and calcium in women after menopause: inverse relation of estrogen with ionized magnesium. Fertility and sterility. 1999;71(5):869–872. doi: 10.1016/s0015-0282(99)00065-5. [DOI] [PubMed] [Google Scholar]
- 31.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–115. [PubMed] [Google Scholar]
- 32.Abraha I, Montedori A. Modified intention to treat reporting in randomised controlled trials: systematic review. Bmj. 2010;340:c2697. doi: 10.1136/bmj.c2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diggle P, Heagerty P, Liang K-Y, Zeger S. Analysis of Longitudinal Data. 2nd ed. England: Oxford University Press; 2002. [Google Scholar]
- 34.Westfall PH, Krishen A. Optimally weighted, fixed sequence and gatekeeper multiple testing procedures. J Stat Plan Inference. 2001;99(1):25–40. [Google Scholar]
- 35.Sloan JA, Dueck A. Issues for statisticians in conducting analyses and translating results for quality of life end points in clinical trials. Journal of biopharmaceutical statistics. 2004;14(1):73–96. doi: 10.1081/BIP-120028507. [DOI] [PubMed] [Google Scholar]
- 36.Kazaks AG, Uriu-Adams JY, Albertson TE, Shenoy SF, Stern JS. Effect of oral magnesium supplementation on measures of airway resistance and subjective assessment of asthma control and quality of life in men and women with mild to moderate asthma: a randomized placebo controlled trial. The Journal of asthma : official journal of the Association for the Care of Asthma. 2010;47(1):83–92. doi: 10.3109/02770900903331127. [DOI] [PubMed] [Google Scholar]
- 37.Loprinzi CL, Michalak JC, Quella SK, et al. Megestrol acetate for the prevention of hot flashes. The New England journal of medicine. 1994;331(6):347–352. doi: 10.1056/NEJM199408113310602. [Clinical Trial Randomized Controlled Trial Research Support, U.S. Gov't, P.H.S.]. [DOI] [PubMed] [Google Scholar]
- 38.Loprinzi CL, Qin R, Balcueva EP, et al. Phase III, randomized, double-blind, placebo-controlled evaluation of pregabalin for alleviating hot flashes, N07C1. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(4):641–647. doi: 10.1200/JCO.2009.24.5647. [Clinical Trial, Phase III Randomized Controlled Trial Research Support, N.I.H., Extramural]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20(6):1578–1583. doi: 10.1200/JCO.2002.20.6.1578. [DOI] [PubMed] [Google Scholar]
- 40.Goldberg RM, Loprinzi CL, O'Fallon JR, et al. Transdermal clonidine for ameliorating tamoxifen-induced hot flashes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1994;12(1):155–158. doi: 10.1200/JCO.1994.12.1.155. [Clinical Trial Randomized Controlled Trial Research Support, U.S. Gov't, P.H.S.]. [DOI] [PubMed] [Google Scholar]