Abstract
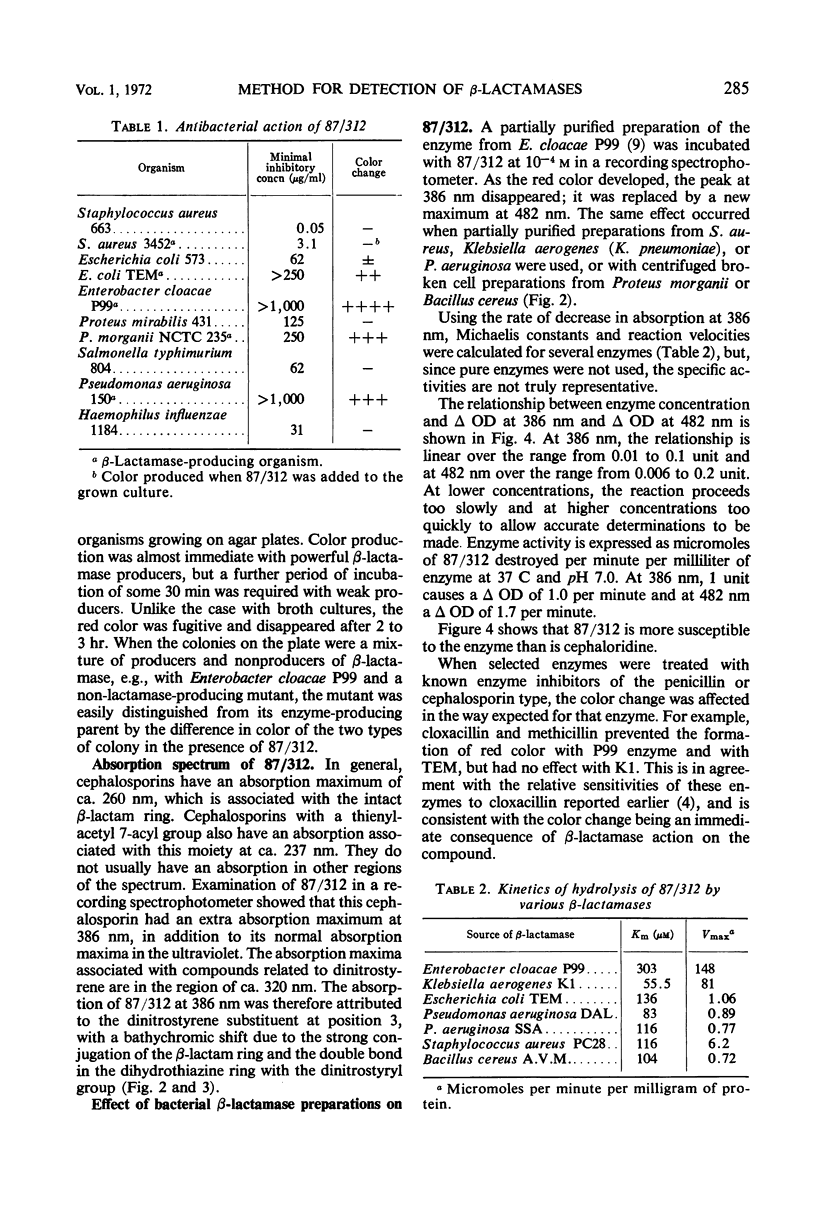
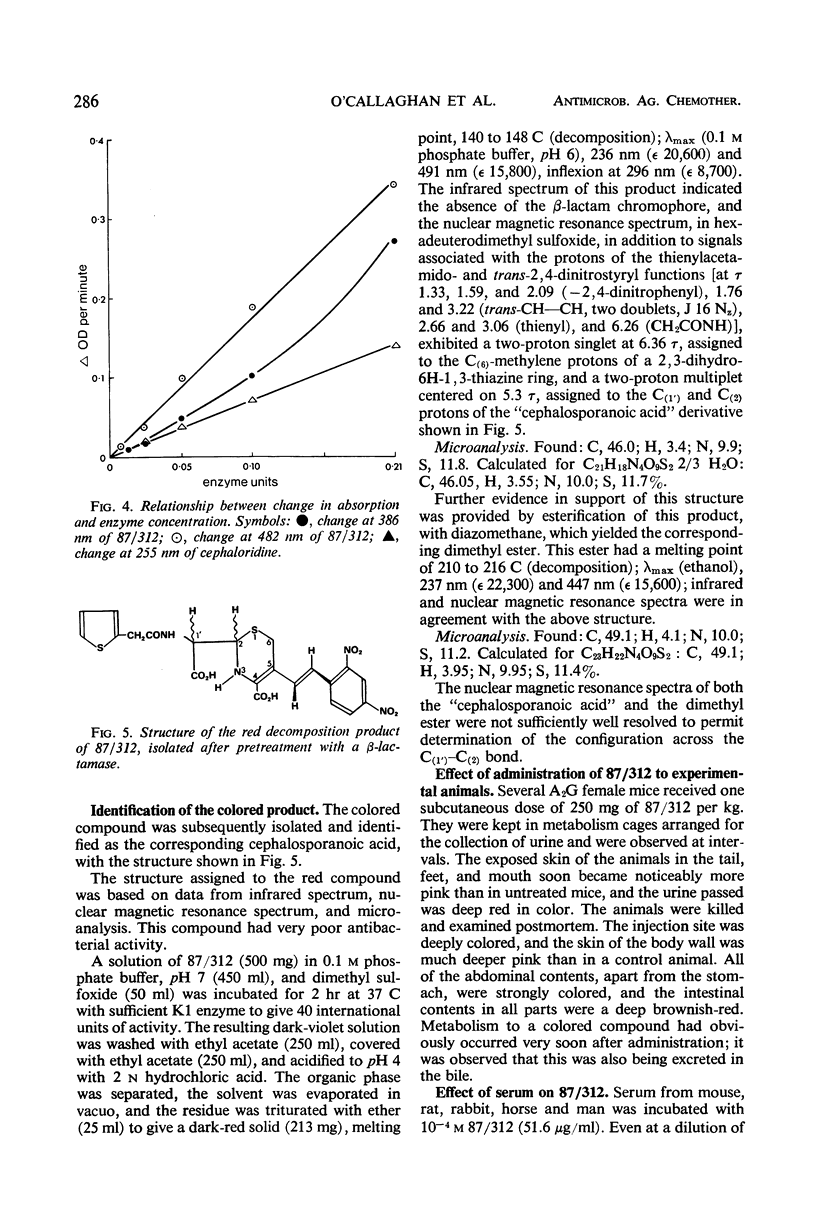
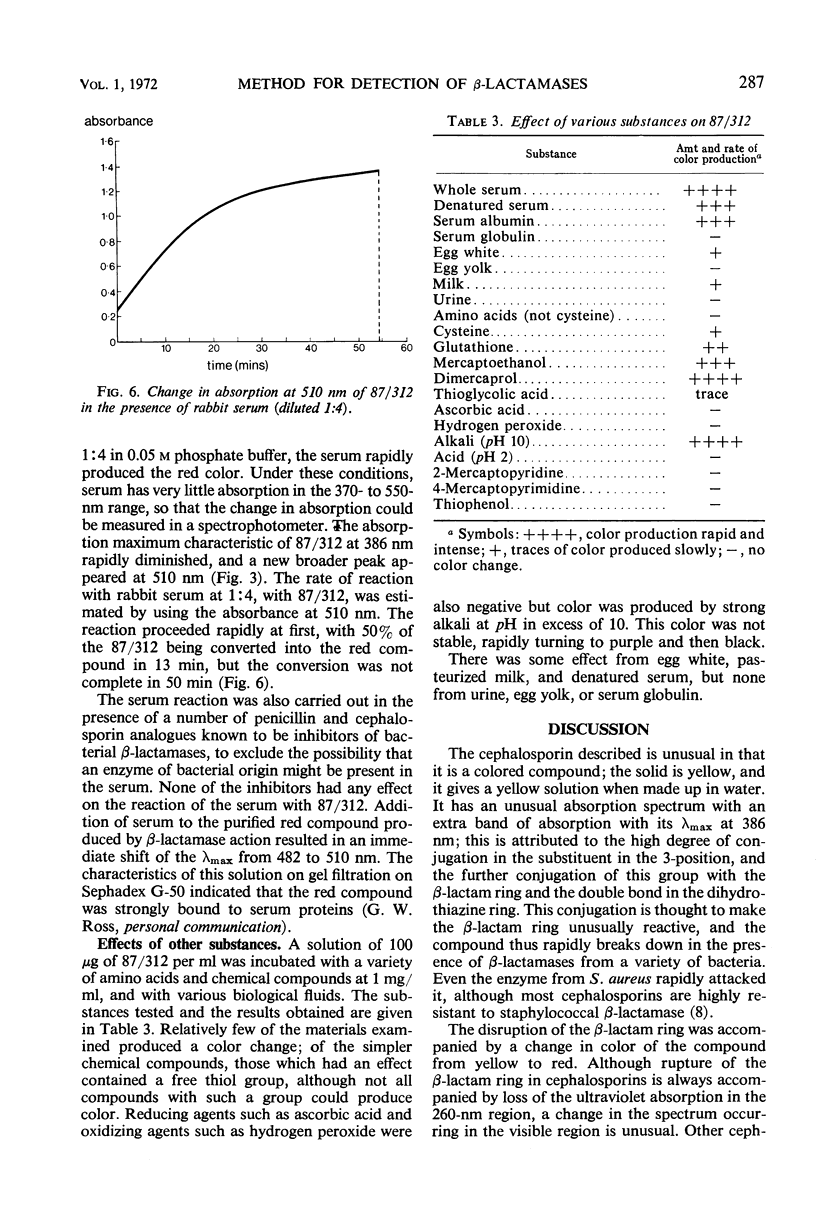
A new cephalosporin with a highly reactive β-lactam ring was found to give an immediate color change in the presence of β-lactamases from many bacteria, including staphylococci, Bacillus species, Enterobacteriaceae, and Pseudomonas. The reaction is confined to organisms producing β-lactamases, but it is sufficiently sensitive to indicate the presence of this enzyme is small amounts in strains previously considered not to produce it. The compound has an unusual ultraviolet spectrum, and the color change can be followed quantitatively by measuring changes in absorption which occur in the 380- to 500-nm region, where cephalosporins normally have no absorption. The development of color is thought to be a consequence of the β-lactam ring being unusually highly conjugated with the 3-substituent. Although in the bacteria only β-lactamases produce this color change, it was found that serum and tissues from experimental animals also rapidly produced the colored breakdown product, which was then excreted in the urine. The mechanism of the mammalian breakdown was considered to be different from that found in bacteria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P., Meadway R. J. Chemical structure of bacterial penicillinases. Nature. 1969 Apr 5;222(5188):24–26. doi: 10.1038/222024a0. [DOI] [PubMed] [Google Scholar]
- HAMILTON-MILLER J. M., SMITH J. T., KNOX R. The estimation of penicillins and penicillin destruction. J Pharm Pharmacol. 1963 Feb;15:81–91. doi: 10.1111/j.2042-7158.1963.tb12751.x. [DOI] [PubMed] [Google Scholar]
- Jack G. W., Richmond M. H. A comparative study of eight distinct beta-lactamases synthesized by gram-negative bacteria. J Gen Microbiol. 1970 Apr;61(1):43–61. doi: 10.1099/00221287-61-1-43. [DOI] [PubMed] [Google Scholar]
- Jack G. W., Richmond M. H. Comparative amino acid contents of purified beta-lactamases from enteric bacteria. FEBS Lett. 1970 Dec 23;12(1):30–32. doi: 10.1016/0014-5793(70)80587-7. [DOI] [PubMed] [Google Scholar]
- Knüsel F., Konopka E. A., Gelzer J., Rosselet A. Antimicrobial studies in vitro with CIBA 36278-Ba, a new cephalosporin derivative. Antimicrob Agents Chemother (Bethesda) 1970;10:140–149. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- O'Callaghan C. H., Kirby S. M. Some cephalosporins in clinical use and their structure-activity relationships. Postgrad Med J. 1970 Oct;(Suppl):9–13. [PubMed] [Google Scholar]
- O'Callaghan C. H., Muggleton P. W., Ross G. W. Effects of beta-lactamase from gram-negative organisms on cephalosporins and penicillins. Antimicrob Agents Chemother (Bethesda) 1968;8:57–63. [PubMed] [Google Scholar]
- Sabath L. D., Jago M., Abraham E. P. Cephalosporinase and penicillinase activities of a beta-lactamase from Pseudomonas pyocyanea. Biochem J. 1965 Sep;96(3):739–752. doi: 10.1042/bj0960739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shraer D. P., Simonova O. D. Dinamika beta-laktamazopodobnoi aktivnosti pochek belykh myshei v usloviiakh stafilokokkovoi infektsii i pri vozdeistvii penitsillinami. Antibiotiki. 1970 Mar;15(3):254–257. [PubMed] [Google Scholar]
- Sullivan H. R., McMahon R. E., Heiney R. E. The fate of parenteral cephalothin and related antibiotics in the rat. The nature of a minor long-lived metabolite. J Antibiot (Tokyo) 1971 Jun;24(6):375–382. doi: 10.7164/antibiotics.24.375. [DOI] [PubMed] [Google Scholar]



