CNS manifestations of heroin include toxic leukoencephalopathy, strokes, seizures, and movement disorders.1 Oral2,3 and IV4 opiate intoxications may trigger obstructive hydrocephalus. We describe refractory hydrocephalus with a communicating element as a new complication of heroin inhalation while “chasing the dragon.” We discuss the putative mechanisms for communicating hydrocephalus, and its successful management with a ventriculo-peritoneal shunt (VPS).
Case report.
A 25-year-old right-handed woman with a 2-year history of heroin inhalation and bipolar disorder was found comatose in her parked car. She was intubated at the scene for airway protection. Her friends confirmed that she had never used IV heroin previously, but had pursued heroin inhalation in the preceding hours. In the emergency room, her initial Glasgow Coma Scale score (GCS) was 6 (E1V1M4). On admission, she was afebrile with tachycardia (109 bpm) and systolic hypertension (168/44 mm Hg). Her general examination was unremarkable. On neurologic examination she was unresponsive to verbal stimuli, but withdrew from noxious stimuli in all extremities. Pupils were both 4 mm in diameter and reactive with preserved corneal, cough, and gag reflexes. There were bilateral Babinski signs.
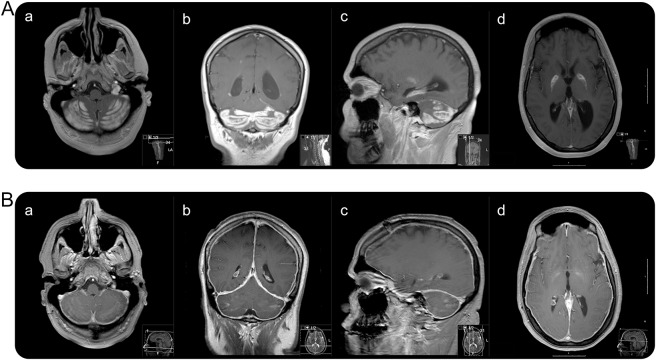
Routine laboratory studies and immunologic, infectious, and paraneoplastic tests were negative (see tables e-1, e-2, and e-3 and appendix e-1 on the Neurology® Web site at Neurology.org). Lumbar puncture was normal except for an elevated opening pressure of 43 cm H2O and elevated myelin basic protein (10 n/mL). Urine opiates were positive. Neuroimaging studies at admission showed enlarged ventricles, communicating ventriculomegaly, and cerebellar enhancement (figure 1).
Figure 1. MRI at admission and 3-month follow-up.
(A) Initial T1-weighted imaging with contrast: axial (a), coronal (b), and sagittal (c) show cerebellar involvement. Axial (d) demonstrates globi pallidi involvement. (B) Three-month follow-up T1-weighted imaging with contrast: axial (a), coronal (b), and sagittal (c) show interval resolution of contrast enhancement. Axial (d) demonstrates resolution of globi pallidi enhancement. Note diffuse pachymeningeal enhancement.
An external ventricular drain (EVD) was emergently placed. She then opened her eyes spontaneously, began speaking, was oriented to self, and moved all extremities distally, spontaneously, and to command. She developed a left hand high-amplitude 4-Hz resting tremor and noticeable cogwheeling. EEG showed diffuse slowing without epileptiform activity.
The patient was started on acetazolamide 500 mg q12h to aid in the treatment of hydrocephalus. Once infectious etiologies were ruled out, dexamethasone therapy was initiated. She became obtunded when her intracranial pressures elevated during multiple EVD clamping trials. Serial CT scans (figure e-1) and a second MRI did not show occlusion of the ventricular system, but improvement in her cerebellar radiologic changes while drained through the EVD.
She received VPS placement after 13 days of hospitalization. Upon discharge to a rehabilitation facility, her GCS was 15 and her modified Rankin Scale score was 5 (bedridden and required constant nursing attention).
After 3 months, her examination had significantly improved (GCS of 15 and modified Rankin Scale score of 3). She reported a headache with low-pressure characteristics. Her dysphagia had resolved. There were executive dysfunction, attentional, registration, and short-term memory deficits. Her language was intact. Her resting tremor had resolved. She was able to walk without assistance. There was an overall 4/5 weakness. A brain MRI showed large improvement and increased pachymeningeal enhancement in postgadolinium T1 sequences (figure 1).
Discussion.
We report a woman who developed hydrocephalus with a communicating element to it in the setting of acute opiate intoxication. Our pathophysiologic hypothesis contemplates 2 mechanisms concerning our patient's hydrocephalus. First, the extent of the inflammation at the cerebellar and 4th ventricular level did not fully account for her refractory hydrocephalus (figure 1 and figure e-1). In addition, her hydrocephalus did not improve along with her cerebellar edema. These factors argue for a communicating mechanism. Certainly, a relative obstructive component may have occurred in the setting of a partial narrowing, yet not occlusion, of the 4th ventricle lumen in the initial phase of cerebellar edema. Indeed, the rapid hydrocephalus development suggests this mechanism.
There are 3 explanations for the communicating hydrocephalus. First, there was a local inflammatory response with a radiologic correlate (figure 1) that preceded systemic inflammation.5 Secondly, granular ependymitis prompted arachnoid villi congestion.6 Finally, heroin inhalation triggers small vessel changes, which might involve enhanced blood–brain barrier permeability.7
The unusual cerebellar and globi pallidi involvement with cerebral hemisphere sparing is noteworthy. The patient had used inhaled heroin for 2 years, which explains the relatively limited, yet clinically substantial, chronic heroin white matter radiographic abnormalities.
Our patient's examination at 3-month follow-up showed improvement. However, she sustained cognitive deficits and a low-pressure headache accompanied by pachymeningeal enhancement due to an overfunctioning VPS. While we cannot substantiate to what extent her clinical and radiologic improvement is attributable to the aggressive surgical management, we speculate that a rapid recognition of communicating hydrocephalus with early EVD placement and subsequent shunting may have played a critical role.
Supplementary Material
Acknowledgments
Acknowledgement: The authors thank Professor Robert Daroff (Case Western Reserve University) for critical appraisal of the manuscript.
Footnotes
Supplemental data at Neurology.org
Author contributions: Ciro Ramos-Estebanez contributed to the conceptualization of the manuscript, its writing, and revisions for intellectual content. Drs. Bui and Pace contributed to writing the manuscript. Drs. Manjila, Gokhale, Shun, and Sloan revised the manuscript for intellectual content.
Study funding: No targeted funding reported.
Disclosure: D. Bui, J. Pace, S. Manjila, S. Gokhale, and M. Shun report no disclosures relevant to the manuscript. A. Sloan is the Peter D. Cristal Associate Professor of Neurological Surgery, Case Western Reserve University School of Medicine, Director of the Brain Tumor & Neuro-Oncology Center, University Hospitals Neurological Institute, and Ireland Cancer Center of the Case Comprehensive Cancer Center. He belongs to the scientific advisory board of “Surgical Theater,” which is a patented and FDA-approved surgical planner. This does not represent a conflict of interest in the present manuscript. C. Ramos-Estebanez reports no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
References
- 1.Filley CM, Kleinschmidt-DeMasters BK. Toxic leukoencephalopathy. N Engl J Med 2001;345:425–443. [DOI] [PubMed] [Google Scholar]
- 2.Morales Odia Y, Jinka M, Ziai WC. Severe leukoencephalopathy following acute oxycodone intoxication. Neurocrit Care 2010;13:93–97. [DOI] [PubMed] [Google Scholar]
- 3.Mills F, MacLennan SC, Devile CJ, Saunders DE. Severe cerebellitis following methadone poisoning. Pediatr Radiol 2008;38:227–229. [DOI] [PubMed] [Google Scholar]
- 4.Long H, Zhou J, Zhou X, Xie Y, Xiao B. Acute hydrocephalus following heroin induced leukoencephalopathy. Neurol Sci 2013;34:1031–1032. [DOI] [PubMed] [Google Scholar]
- 5.Long H, Deore K, Hoffman RS, Nelson LS. A fatal case of spongiform leukoencephalopathy linked to “chasing the dragon.” J Toxicol Clin Toxicol 2003;41:887–891. [DOI] [PubMed] [Google Scholar]
- 6.Haberland C. Clinical Neuropathology: Text and Color Atlas. New York: Demos Medical Publishing LLC; 2007. [Google Scholar]
- 7.Yin R, Lu C, Chen Q, Fan J, Lu J. Microvascular damage is involved in the pathogenesis of heroin induced spongiform leukoencephalopathy. Int J Med Sci 2013;10:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.