Abstract
Background: Polyphenols are phytochemicals that possess antioxidant and anti-inflammatory properties and improve glucose metabolism in animal experiments, although data from prospective epidemiologic studies examining polyphenol intakes in relation to type 2 diabetes (T2D) risk are inconsistent.
Objectives: We examined urinary excretion of select flavonoid and phenolic acid metabolites, as biomarkers of intake, in relation to T2D risk.
Methods: Eight polyphenol metabolites (naringenin, hesperetin, quercetin, isorhamnetin, catechin, epicatechin, caffeic acid, and ferulic acid) were quantified in spot urine samples by liquid chromatography/mass spectrometry among 1111 T2D case-control pairs selected from the Nurses’ Health Study (NHS) and NHSII.
Results: Higher urinary excretion of hesperetin was associated with a lower T2D risk after multivariate adjustment: the OR comparing top vs. bottom quartiles was 0.68 (95% CI: 0.49, 0.96), although a linear trend was lacking (P = 0.30). The other measured polyphenols were not significantly associated with T2D risk after multivariate adjustment. However, during the early follow-up period [≤4.6 y (median) since urine sample collection], markers of flavanone intakes (naringenin and hesperetin) and flavonol intakes (quercetin and isorhamnetin) were significantly associated with a lower T2D risk. The ORs (95% CIs) comparing extreme quartiles were 0.61 (0.39, 0.98; P-trend: 0.03) for total flavanones and 0.55 (0.33, 0.92; P-trend: 0.04) for total flavonols (P-interaction with follow-up length: ≤0.04). An inverse association was also observed for caffeic acid during early follow-up only: the OR was 0.52 (95% CI: 0.32, 0.84; P-trend: 0.03). None of these markers was associated with T2D risk during later follow-up. Metabolites of flavan-3-ols and ferulic acid were not associated with T2D risk in either period.
Conclusions: These results suggest that specific flavonoid subclasses, including flavanones and flavonols, as well as caffeic acid, are associated with a lower T2D risk in relatively short-term follow-up but not during longer follow-up. Substantial within-person variability of the metabolites in single spot urine samples may limit the ability to capture associations with long-term disease risk.
Keywords: diabetes, nutrition, polyphenol, urinary biomarker, women
Introduction
Polyphenols is a collective term referring to thousands of plant-derived molecules that share a common structure consisting of multiple hydroxyl groups attached to aromatic rings (1). Of these polyphenols, several hundred are derived from diet and can be largely classified into 4 major groups: phenolic acids, flavonoids, lignans, and stilbenes (1). A wide spectrum of biological activities is associated with polyphenols in vitro including anti-inflammatory and antioxidant effects that are likely mediated through interactions with a range of cell signaling pathways, such as modulation of transcription factors and the subsequent expression of genes and microRNAs (2–4). Results from rodent models have provided corroborative results demonstrating that intake of specific flavonoid subclasses improves insulin resistance, enhances β cell function, and delays diabetes onset (5–7).
In contrast to data from animal experiments that suggest beneficial effects of these compounds on lowering diabetes risk, thus far only a limited number of prospective studies have been conducted to examine the associations between consumption of flavonoid subclasses or flavonoid-rich foods and type 2 diabetes (T2D)12 risk, and findings were inconsistent (8–13). In addition, data regarding other major classes of polyphenols, such as phenolic acids, are lacking. The scarcity of data is at least partially due to the complexity in accurately assessing intake of polyphenols from FFQs (1). Using biomarkers of polyphenol intake is an appealing approach because biomarkers are largely independent of measurement errors that may be associated with self-reported dietary intakes (14). Flavonoids and phenolic acids that occur in blood or urine as glucuronidated, sulfated, and/or methylated metabolites may serve as promising biomarkers for assessing their intakes (15), but to our knowledge, these biomarkers have not been studied in relation to risk of T2D.
We previously examined enterolignans, gut microbiota products of dietary lignans, in relation to diabetes risk in 2 well-characterized cohorts of US women, the Nurses’ Health Study (NHS) and NHSII (16). In the current analysis, we aimed to extend this research to metabolites of 3 specific flavonoid subclasses and 2 phenolic acids and evaluated the hypothesis that higher urinary excretions of these polyphenol metabolites are prospectively associated with a lower T2D risk.
Methods
Study population.
The NHS cohort was established in 1976 when 121,700 female registered nurses aged 30–55 y living in 1 of 11 US states responded to a questionnaire. A total of 116,430 younger female registered nurses aged 25–42 y were enrolled in 1989 using the same approach, and these women constituted the NHSII cohort. Similar data collection methods and participant follow-up strategies were applied in these 2 cohorts. Follow-up questionnaires are administered biennially to assess and update a multitude of variables, such as body weight and height, demographics, lifestyle practices, history of chronic diseases, and medication use. In addition, in the NHSII, 29,611 participants aged 32–52 y provided blood and urine samples from 1996 to 2001, and in the NHS, 18,743 participants aged 53–80 y provided samples from 2000 to 2002. In both cohorts, the samples were returned to our biorepository via overnight courier and were immediately processed upon arrival and aliquoted into cryotubes, which were stored in the vapor phase of liquid nitrogen freezers at ≤−130°C. Among participants who provided blood and urine samples, a high follow-up rate of >95% has been maintained.
In both cohorts, we conducted a prospective, nested case-control study of T2D among participants who provided urine samples and were free of self-reported diabetes, cardiovascular disease, and cancer at sample collection. During follow-up through June 2008 (NHS) or June 2007 (NHSII), we prospectively identified and confirmed 1111 T2D cases (NHS: 456; NHSII: 655) and randomly selected 1 control for each case (17). Cases and controls were matched for age at urine sample collection, month of sample collection, first morning urine (yes or no), race (white or other races), and menopausal status and postmenopausal hormone use (NHSII only). To avoid systematic measurement error, samples of each case-control pair were shipped in the same batch and analyzed in the same run in a random sequence under identical conditions.
The study protocol was approved by the institutional review board of the Brigham and Women’s Hospital and the Human Subjects Committee Review Board of Harvard School of Public Health.
Ascertainment of T2D.
We sent a validated supplemental questionnaire (18) to those who reported having a T2D diagnosis to confirm/refute the incidence. We used at least one of the following American Diabetes Association 1998 criteria to confirm a self-reported T2D diagnosis: 1) an elevated glucose concentration (fasting plasma glucose ≥7.0 mmol/L, random plasma glucose ≥11.1 mmol/L, or plasma glucose ≥11.1 mmol/L after an oral glucose load), and at least 1 symptom related to diabetes; 2) no symptoms, but elevated glucose concentrations on 2 separate occasions; or 3) treatment with insulin or oral hypoglycemic medication. Only confirmed T2D cases were included in the current study.
Assessment of diet.
Validated FFQs (14) were administered in 1984 and 1986 in the NHS and in 1991 in the NHSII. Extended FFQs were subsequently sent to participants quadrennially to update diet information. In these FFQs, we inquired about the average consumption frequency of 118–166 food items in the past year with a prespecified serving size for each item. The methodology for the assessment of flavonoid intake was previously described in detail elsewhere (19). Briefly, we derived intake of each subclass of flavonoids by multiplying the consumption frequency of each relevant food item by its contents of the flavonoids and then summing intake of flavonoids across all food items (8). We did not estimate the intake levels of phenolic acids because phenolic acids are not currently included in the Harvard food composition database.
Laboratory measurements.
In the current study, we used isotope dilution electrospray ionization orbitrap LC isotope dilution orbitrap MS after hydrolysis with glucuronidase and sulfatase and liquid-liquid extraction (20) to measure 8 metabolites that can be reliably measured in urine samples with reasonable reproducibility: naringenin and hesperetin (flavanones); quercetin and isorhamnetin (flavonols); catechin and epicatechin (flavan-3-ols); and caffeic acid and ferulic acid (phenolic acids). Urinary creatinine excretion was measured with use of a Roche-Cobas MiraPlus clinical chemistry autoanalyzer (Roche Diagnostics). Urinary excretions of polyphenols were adjusted for creatinine excretion in this analysis. The mean intra-assay CV was 22.8% for hesperetin, 9.0% for naringenin, 18.0% for quercetin, 21.5% for isorhamnetin, 24.3% for catechin, 13.6% for epicatechin, 10.5% for caffeic acid, 11.5% for ferulic acid, and 5.6% for creatinine. We observed higher interassay CVs compared to intra-assay CVs and thus used conditional logistic regression to model associations of interest to minimize the impact of high interassay CVs.
In a pilot study, we evaluated within-person stability of the polyphenols and calculated intraclass correlation coefficients (ICCs) between excretions in 2 urine samples collected 1–2 y apart from 58 NHSII participants. The ICCs ranged from low (0.03 for epicatechin, 0.10 for hesperetin, 0.11 for catechin, 0.13 for naringenin, 0.22 for isorhamnetin, and 0.35 for quercetin) to moderate/high (0.40 for caffeic acid and 0.87 for ferulic acid). Of note, as the timing of urine sample collections was likely independent of diet before the collections in these participants, the ICCs incorporated extraneous variability in polyphenol excretions resulting from these 2 factors (i.e., diet and timing of sample collection since last meal).
Statistical methods.
The missing percentages of covariates were relatively low. In the NHS, the percentage of missing data ranged from 0.1% for physical activity to 1.1% for BMI. In the NHSII, the percentage of missing data ranged from 0.2% for oral contraceptive use to 4.2% for BMI. Given the low percentages of missing data, we replaced missing values with case-control, status-specific medians for continuous variables or combined the missing category with the reference group for categorical variables.
We calculated Spearman partial correlation coefficient (rs) values among controls from both studies to evaluate the correlations between urinary metabolites of flavonoids and their intake levels. We controlled for age at sample collection, fasting status, smoking status, BMI, physical activity, total calorie intake, and cohort (NHS or NHSII). Because data on dietary intake of phenolic acids were not available, we used linear regression to identify significant food predictors of log-transformed levels of metabolites following the stepwise selection algorithm with multivariate adjustment of the aforementioned covariates. We then calculated rs values between metabolites and their food predictors.
We categorized the study population into quartiles according to the distribution of polyphenol excretions among controls. We used conditional logistic regression, in which matching factors are accounted for and data from matched case-control pairs are analyzed in the same strata, to model the associations of interest (17). To control for confounding, we adjusted for BMI, smoking status, physical activity, alcohol use, oral contraceptive use (NHSII only), postmenopausal hormone use (NHS only), family history of diabetes, history of hypercholesterolemia or hypertension, and the Alternative Healthy Eating Index score as a marker of overall diet quality (21). P values for linear trend were calculated by entering an ordinal score based on the median value in each quartile of metabolite levels into the multivariate models.
We used restricted cubic spline regressions with 3 knots to model potential dose-response relations (22). Tests for nonlinearity were based on the likelihood ratio test, comparing the model with only the linear term to the model with the linear and the cubic spline terms. The low ICCs observed in the pilot study suggested that a single measurement of the metabolites would unlikely reflect long-term cumulative averages of polyphenol intake, which are able to incorporate changes of polyphenol intake over time (23). We, therefore, explored the possibility that the metabolites may be more strongly associated with diabetes in early follow-up rather than in the entire follow-up. We used the likelihood ratio test to evaluate the significance of interaction terms between metabolite excretions and length of follow-up. In this analysis, to maximize statistical power, we chose median follow-up as the cutoff point to define early vs. later follow-up. Lastly, we pooled individual-level data from the 2 cohorts in these analyses while maintaining the matched case-control pairs by using conditional logistic regression to leverage on the full range of metabolites and also to increase the statistical power.
All P values were two-sided, and type I error was set to be 0.05. Data were analyzed with the Statistical Analysis Systems software package, version 9.3 (SAS Institute, Inc.).
Results
Characteristics of study participants assessed with use of baseline questionnaires (NHS, 1998; NHSII, 1995) are shown in Table 1. A notable difference between the 2 cohorts was the older age at urine sample collection in the NHS (mean: 66 y) than in the NHSII (mean: 45 y).
TABLE 1.
Baseline characteristics of diabetes cases and controls in the NHS and NHSII1
| NHS |
NHSII |
|||||
| Characteristic | Cases (n = 456) | Controls (n = 456) | P2 | Cases (n = 655) | Controls (n = 655) | P2 |
| Age at urine sample collection,3 y | 65.5 ± 6.4 | 65.6 ± 6.4 | 0.91 | 45.4 ± 4.3 | 45.4 ± 4.3 | 0.88 |
| BMI, kg/m2 | 29.7 ± 5.6 | 26.1 ± 4.7 | <0.0001 | 33.2 ± 6.8 | 25.6 ± 5.3 | <0.0001 |
| Physical activity, METs-h/wk | 16.6 ± 22.6 | 18.9 ± 20.9 | 0.11 | 16.8 ± 27.5 | 20.2 ± 25.8 | 0.02 |
| Smoking status, % | 0.64 | 0.04 | ||||
| Current | 6.4 | 5.7 | 12.8 | 8.6 | ||
| Former | 47.2 | 44.7 | 25.0 | 27.0 | ||
| Never | 46.5 | 49.6 | 62.1 | 64.4 | ||
| Hypertension, % | 62.1 | 39.9 | <0.0001 | 27.3 | 12.5 | <0.0001 |
| Hypercholesterolemia, % | 72.4 | 57.9 | <0.0001 | 45.7 | 23.8 | <0.0001 |
| White,3 % | 98.0 | 98.5 | 0.61 | 95.1 | 96.3 | 0.27 |
| Family history of diabetes, % | 38.8 | 26.8 | 0.0001 | 34.1 | 15.6 | <0.0001 |
| Fasting status,3 % | 89.0 | 90.4 | 0.51 | 69.0 | 71.9 | 0.27 |
| First morning urine,3 % | 90.4 | 90.8 | 0.82 | 84.0 | 84.9 | 0.65 |
| Menopause,3 % | 100 | 99.3 | 0.25 | 31.6 | 31.6 | >0.99 |
| Postmenopausal hormone use,3,4 % | 61.0 | 62.9 | 0.54 | 80.2 | 80.2 | >0.99 |
| Use of oral contraceptive,5 % | — | 0.009 | ||||
| Current | — | — | 2.3 | 5.0 | ||
| Past | — | — | 83.7 | 84.1 | ||
| Never | — | — | 14.1 | 10.8 | ||
| Diet | ||||||
| Total energy, kcal/d | 1784 ± 413 | 1763 ± 399 | 0.44 | 1874 ± 509 | 1789 ± 489 | 0.002 |
| Alcohol, g/d | 4.8 ± 8.2 | 5.3 ± 7.5 | 0.37 | 2.0 ± 4.5 | 3.3 ± 6.1 | <0.0001 |
| Trans fat, % of energy | 1.62 ± 0.40 | 1.57 ± 0.40 | 0.04 | 1.61 ± 0.52 | 1.51 ± 0.50 | 0.0005 |
| P:S ratio | 0.56 ± 0.12 | 0.58 ± 0.15 | 0.02 | 0.50 ± 0.13 | 0.53 ± 0.15 | 0.004 |
| Coffee,6 cup/d | 2.0 ± 1.4 | 2.2 ± 1.5 | 0.03 | 1.4 ± 1.7 | 1.7 ± 1.7 | 0.004 |
| Whole grains, g/d | 18.7 ± 10.3 | 20.9 ± 10.6 | 0.001 | 19.4 ± 12.7 | 22.9 ± 14.8 | <0.0001 |
| Fruits, servings/d | 2.2 ± 1.0 | 2.3 ± 1.0 | 0.03 | 1.8 ± 1.2 | 1.9 ± 1.3 | 0.01 |
| Vegetables, servings/d | 3.2 ± 1.2 | 3.3 ± 1.4 | 0.34 | 2.8 ± 1.7 | 2.7 ± 1.7 | 0.28 |
| Red meat, servings/d | 0.9 ± 0.4 | 0.8 ± 0.4 | <0.0001 | 0.9 ± 0.6 | 0.8 ± 0.5 | <0.0001 |
| Fish, servings/d | 0.27 ± 0.15 | 0.27 ± 0.16 | 0.92 | 0.23 ± 0.20 | 0.22 ± 0.18 | 0.25 |
| Soft drinks, servings/d | 0.9 ± 0.9 | 0.7 ± 0.7 | <0.0001 | 1.7 ± 1.5 | 1.2 ± 1.2 | <0.0001 |
| Yogurt, servings/d | 0.13 ± 0.16 | 0.16 ± 0.20 | 0.008 | 0.13 ± 0.24 | 0.16 ± 0.24 | 0.07 |
| Alternate Healthy Eating Index score | 51.5 ± 8.7 | 54.6 ± 9.4 | <0.0001 | 46.6 ± 9.4 | 49.4 ± 9.9 | <0.0001 |
| Urinary metabolites7 | ||||||
| Quercetin, nmol/g creatinine | 26.8 (8.7–73.0) | 33.6 (12.0–87.0) | 0.01 | 23.9 (7.7–60.5) | 18.7 (5.3–62.6) | 0.17 |
| Isorhamnetin, nmol/g creatinine | 32.4 (14.0–93.5) | 42.2 (16.3–98.9) | 0.07 | 34.5 (12.9–88.7) | 34.0 (13.2–90.7) | 0.84 |
| Naringenin, nmol/g creatinine | 592 (145–1730) | 691 (189–2440) | 0.03 | 685 (211–2650) | 699 (176–2250) | 0.68 |
| Hesperetin, nmol/g creatinine | 123 (33.4–693) | 148 (41.5–849) | 0.14 | 59.1 (14.3–321) | 84.7 (21.9–569) | 0.002 |
| Catechin, nmol/g creatinine | 17.8 (4.8–77.2) | 19.1 (5.0–77.7) | 0.50 | 12.6 (3.8–39.3) | 15.3 (4.5–43.3) | 0.06 |
| Epicatechin, nmol/g creatinine | 165 (23.9–443) | 138 (24.3–508) | 0.91 | 68.8 (13.9–280) | 69.6 (14.1–238) | 0.52 |
| Caffeic acid, nmol/g creatinine | 232 (115–449) | 269 (157–495) | 0.007 | 152 (81.1–306) | 179 (92.2–356) | 0.01 |
| Ferulic acid, mmol/g creatinine | 3.22 (1.57–5.97) | 3.29 (1.79–6.64) | 0.42 | 2.73 (1.30–5.94) | 3.22 (1.55–6.47) | 0.07 |
Values are means ± SDs unless otherwise indicated. Percentages are based on nonmissing data. MET, metabolic equivalent; NHS, Nurses’ Health Study; P:S, polyunsaturated–to–saturated fat.
P value estimates are based on Student’s t test for variables expressed as mean ± SD, Wilcoxon’s rank-sum test for variables expressed as median (IQR), or Pearson χ2 test for variables expressed as percentages.
Matching factors; menopausal status and hormone replacement therapy were matching factors for NHSII only.
Among postmenopausal women only.
Among premenopausal women only.
One cup equals 237 mL.
Values are medians (IQRs).
We examined the intercorrelation among individual metabolites in controls (Supplemental Table 1). As expected, metabolites of the same class of polyphenols or within the same metabolic pathway were generally correlated with each other, and this pattern was consistent in both cohorts. For example, rs values were 0.72 between the flavonols (quercetin and isorhamnetin) and 0.56 between the flavanones (hesperetin and naringenin) in both cohorts; the rs value was 0.55 (NHS) or 0.56 (NHSII) between the phenolic acids (caffeic acid and ferulic acid). For the flavan-3-ol metabolites catechin and epicatechin, the correlation was 0.54 in the NHS and 0.68 in the NHSII.
We observed modest yet significant correlations between intakes of flavonols, flavanones, and flavan-3-ols and the metabolites of these flavonoid subclasses (Supplemental Table 2). For example, we observed an rs value of 0.21 for hesperetin, 0.11 for naringenin, 0.15 for quercetin, and 0.10 for isorhamnetin. Urinary excretions of catechin and epicatechin were also significantly correlated with various dietary flavan-3-ols, especially dietary epicatechin and proanthocyanidins (rs values of 0.12–0.15). Several significant food predictors were identified for phenolic acids among controls. The strongest relation was identified between coffee consumption and phenolic acid metabolites; the rs value was 0.20 for caffeic acid or 0.24 for ferulic acid. Of note, hesperetin excretion was correlated with citrus fruit/juice (rs value = 0.19); quercetin and isorhamnetin excretions were correlated with kale intake (rs values = 0.12 and 0.10, respectively); and epicatechin excretion was correlated with chocolate intake (rs value = 0.11). Other correlations between metabolites and their main food sources were weaker.
Table 2 presents associations between the metabolites and diabetes risk in the 2 cohorts combined. In the crude model accounting for matching factors, urinary excretions of hesperetin and caffeic acid were associated with a lower T2D risk, whereas other markers were not consistently associated with T2D risk. Further adjustment for diabetes risk factors, especially BMI, attenuated these associations, although the top 2 quartiles of hesperetin remained significantly associated with a lower diabetes risk: ORs (95% CIs) were 0.65 (0.47, 0.90) and 0.68 (0.49, 0.96), respectively, although the linear trend test (P-trend: 0.30) was not significant. Cohort-specific associations between individual metabolites and diabetes risk are presented in Supplemental Table 3; the associations generally did not differ by cohort.
TABLE 2.
ORs (95% CIs) of type 2 diabetes by quartiles of urinary polyphenol metabolites (nmol/g creatinine) in the NHS and NHSII1
| Quartiles of urinary markers |
|||||
| 1 (lowest) | 2 | 3 | 4 (highest) | P-trend | |
| Naringenin | |||||
| Median (range) | 87.6 (0.8–177) | 368 (178–692) | 1275 (694–2310) | 5221 (2305–157,000) | |
| Case/control (n) | 274/277 | 297/278 | 269/278 | 271/278 | |
| Model 12 | 1 | 1.09 (0.86, 1.38) | 0.98 (0.78, 1.24) | 0.99 (0.78, 1.24) | 0.67 |
| Model 23 | 1 | 1.02 (0.74, 1.41) | 0.97 (0.70, 1.34) | 0.87 (0.64, 1.20) | 0.31 |
| Hesperetin | |||||
| Median (range) | 12.1 (0.5–29.3) | 51.6 (29.3–106) | 224 (106–691) | 3135 (694–135,000) | |
| Case/control (n) | 351/277 | 278/278 | 251/278 | 231/278 | |
| Model 12 | 1 | 0.77 (0.61, 0.97) | 0.68 (0.53, 0.87) | 0.63 (0.49, 0.80) | 0.01 |
| Model 23 | 1 | 0.75 (0.55, 1.04) | 0.65 (0.47, 0.90) | 0.68 (0.49, 0.96) | 0.30 |
| Quercetin | |||||
| Median (range) | 3.1 (0.3–7.3) | 14.6 (7.3–25.8) | 42.8 (25.9–73.0) | 145 (73.2–2810) | |
| Case/control (n) | 259/277 | 307/278 | 297/278 | 248/278 | |
| Model 12 | 1 | 1.17 (0.93, 1.48) | 1.13 (0.90, 1.43) | 0.95 (0.74, 1.21) | 0.21 |
| Model 23 | 1 | 1.06 (0.77, 1.46) | 0.93 (0.67, 1.29) | 0.83 (0.59, 1.17) | 0.17 |
| Isorhamnetin | |||||
| Median (range) | 7.2 (0.4–14.3) | 23.6 (14.3–37.3) | 56.9 (37.3–94.8) | 186 (95.9–13,100) | |
| Case/control (n) | 298/277 | 287/278 | 258/278 | 268/278 | |
| Model 12 | 1 | 0.95 (0.75, 1.20) | 0.86 (0.67, 1.09) | 0.88 (0.69, 1.13) | 0.40 |
| Model 23 | 1 | 1.05 (0.76, 1.46) | 0.94 (0.67, 1.32) | 1.08 (0.76, 1.53) | 0.66 |
| Catechin | |||||
| Median (range) | 2.6 (0.1–4.7) | 8.1 (4.8–16.5) | 29.8 (16.5–54.5) | 129 (54.8–10,100) | |
| Case/control (n) | 308/277 | 288/278 | 252/278 | 263/278 | |
| Model 12 | 1 | 0.93 (0.73, 1.17) | 0.80 (0.63, 1.02) | 0.83 (0.64, 1.07) | 0.24 |
| Model 23 | 1 | 1.07 (0.78, 1.48) | 0.77 (0.54, 1.08) | 0.92 (0.65, 1.31) | 0.62 |
| Epicatechin | |||||
| Median (range) | 4.6 (0.7–15.1) | 47.3 (15.1–96.4) | 177 (97.3–338) | 696 (340–5640) | |
| Case/control (n) | 271/277 | 283/278 | 275/278 | 282/278 | |
| Model 12 | 1 | 1.04 (0.82, 1.32) | 1.01 (0.80, 1.29) | 1.04 (0.81, 1.33) | 0.85 |
| Model 23 | 1 | 1.19 (0.86, 1.65) | 0.98 (0.71, 1.36) | 1.01 (0.73, 1.41) | 0.71 |
| Caffeic acid | |||||
| Median (range) | 70.1 (2.0–114) | 162 (114–217) | 300 (218–414) | 662 (416–10,900) | |
| Case/control (n) | 353/277 | 281/278 | 231/278 | 246/278 | |
| Model 12 | 1 | 0.78 (0.62, 0.99) | 0.63 (0.49, 0.81) | 0.67 (0.53, 0.86) | 0.004 |
| Model 23 | 1 | 0.85 (0.62, 1.17) | 0.68 (0.48, 0.95) | 0.73 (0.52, 1.02) | 0.08 |
| Ferulic acid4 | |||||
| Median (range) | 0.97 (0.006–1.65) | 2.34 (1.65–3.26) | 4.52 (3.27–6.51) | 10.28 (6.52–291.9) | |
| Case/control (n) | 321/277 | 276/278 | 266/278 | 248/278 | |
| Model 12 | 1 | 0.85 (0.67, 1.07) | 0.82 (0.64, 1.04) | 0.76 (0.59, 0.96) | 0.04 |
| Model 23 | 1 | 1.07 (0.78, 1.48) | 1.07 (0.77, 1.50) | 1.12 (0.80, 1.58) | 0.57 |
NHS, Nurses’ Health Study.
Model 1 was adjusted for the matching factors: age at urine sample collection (y), race (white or not), fasting status (yes or no), first morning urine sample (yes or no), and date of blood drawing.
Based on model 1, model 2 was further adjusted for BMI (kg/m2), smoking status (current smoker, past smoker, nonsmoker), oral contraceptive use (never used, past user, current user; NHSII only), use of hormone replacement therapy (yes or no; NHS only), physical activity (metabolic equivalents, h/wk), alcohol use (abstainer, <5.0 g/d, 5.0–14.9 g/d, ≥15.0 g/d), family history of diabetes (yes or no), history of hypercholesterolemia or hypertension (yes or no), and Alternative Healthy Eating Index score.
The unit was mmol/g creatinine.
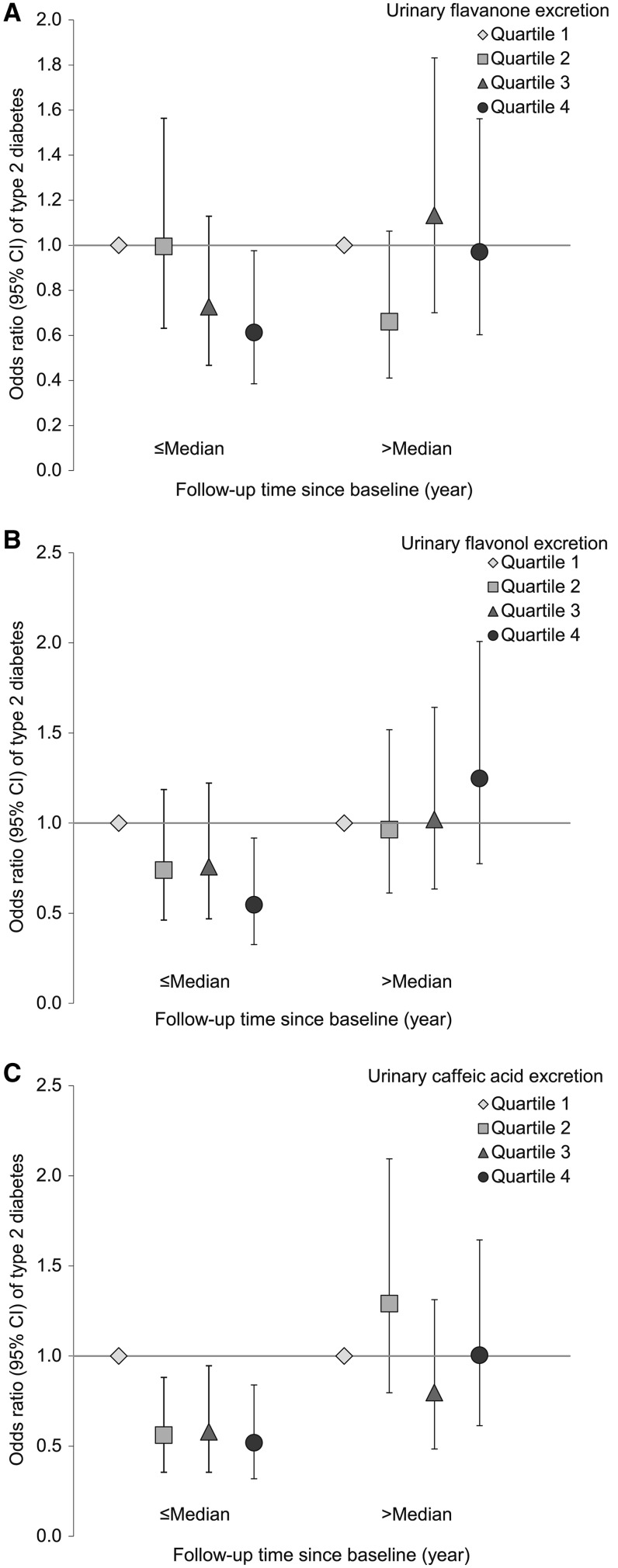
Because of the low within-person stability of the metabolites, we explored whether these markers were associated with diabetes incidence during earlier follow-up rather than longer-term follow-up. Figure 1 demonstrates that, in general, metabolites of flavanones or flavonols tended to be associated with lower diabetes risk in early follow-up, whereas in later follow-up no association was observed. We observed a significant interaction between follow-up time and combined levels of flavanone markers (naringenin and hesperetin) (P-interaction: 0.04) and total flavonol markers (quercetin and isorhamnetin) (P-interaction: 0.03). Comparing extreme quartiles of flavanone metabolites, the ORs (95% CIs) were 0.61 (0.39, 0.98; P-trend: 0.03) during the first half of the follow-up period (≤4.6 y) and 0.97 (0.60, 1.56; P-trend: 0.56) thereafter. The ORs were 0.55 (0.33, 0.92; P-trend: 0.04) and 1.25 (0.78, 2.01; P-trend: 0.27) for flavonol markers during early and later follow-up periods, respectively. Caffeic acid excretion was also associated with significantly lower T2D risk during early follow-up only (OR: 0.52; 95% CI: 0.32, 0.84; P-trend: 0.03 vs. OR: 1.01; 95% CI: 0.61, 1.64; P-trend: 0.64 for later follow-up), although the interaction test was not significant (P-interaction: 0.34). Associations for individual flavanone and flavonol metabolites by follow-up length are presented in Supplemental Figure 1. We did not observe significant associations for the other measured metabolites during either period (data not shown). These associations did not materially change when the analysis was restricted to participants who provided first-morning samples (86.9%; data not shown).
FIGURE 1.
ORs (95% CIs) of type 2 diabetes according to duration of follow-up in the NHS and NHSII. Multivariate conditional logistic regression models were adjusted for the same set of covariates as used for model 2 in Table 2. The median of follow-up length was 4.6 y. (A) Flavanone metabolites (naringenin and hesperetin); (B) flavonol metabolites (quercetin and isorhamnetin); and (C) caffeic acid. The median values (nmol/g creatinine) for quartiles were 138, 563, 1920, and 8850 for flavanone metabolites; 13.1, 45.4, 105, and 341 for flavonol metabolites; and 70.1, 162, 300, and 662 for caffeic acid. The sample sizes for the 8 categories (from left to right) were 292, 275, 294, 259, 281, 284, 275, and 262 for flavanone metabolites; 267, 290, 285, 278, 304, 268, 270, and 260 for flavonol metabolites; and 318, 264, 270, 268, 312, 295, 239, and 256 for caffeic acid. NHS, Nurses’ Health Study.
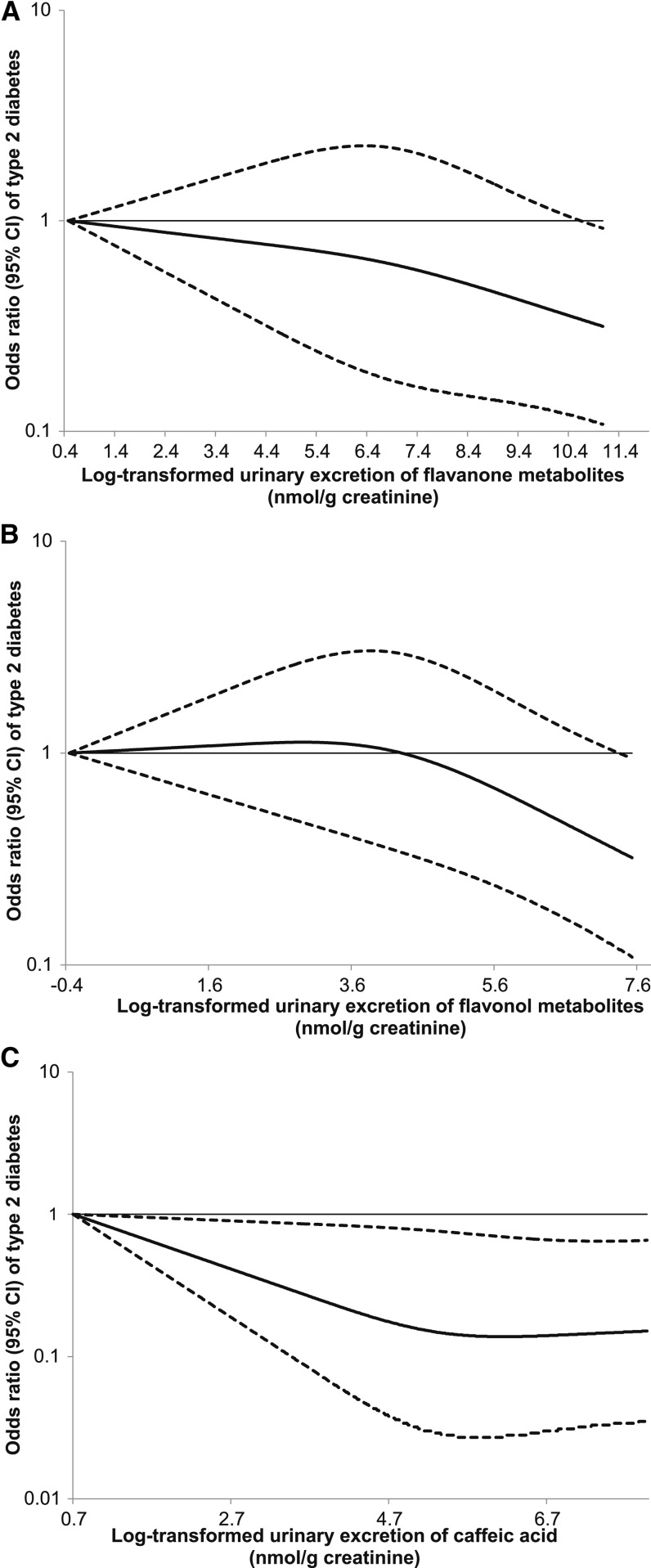
We subsequently explored whether there was a dose-response relation for the association of flavanone and flavonol markers with diabetes risk during early follow-up. We detected a significant linear relation between the metabolites of both subclasses and diabetes risk, with P values for linearity of 0.01 and 0.02, respectively (Figure 2). In addition, we also observed a significant linear relation between caffeic acid and diabetes risk, with a P for linearity of 0.03. We estimated that for each SD increment of log-transformed flavanone metabolites (SD = 1.8), the OR was 0.81 (95% CI: 0.68, 0.95). The corresponding OR was 0.79 (95% CI: 0.66, 0.96) for flavonol markers (SD = 1.4) and 0.82 (95% CI: 0.68, 0.98) for caffeic acid (SD = 1.0). Dose-response relations for individual metabolites of flavanones and flavonols are presented in Supplemental Figure 2.
FIGURE 2.
Dose-response relation between flavanone or flavonol metabolites and risk of type 2 diabetes based on combined data from the NHS and NHSII. Metabolite excretions were log transformed to improve normality. To further minimize the impact of outliers, participants with top 1% of metabolites were excluded. A total of 1109 participants were included in the final analysis. Multivariate conditional logistic regression models were adjusted for the same set of covariates as used for model 2 in Table 2. Solid lines are ORs and dashed lines are 95% CIs. (A) Flavanone metabolites (naringenin and hesperetin); (B) flavonol metabolites (quercetin and isorhamnetin); and (C) caffeic acid. NHS, Nurses’ Health Study.
Discussion
In this large, prospective investigation among 2 cohorts of US women, we observed, for the first time, that several specific flavonoid metabolites, including flavanones (hesperetin and naringenin) and flavonols (quercetin and isorhamnetin), as well as the endogenous phenolic acid metabolite caffeic acid, were associated with a 39–48% lower T2D risk primarily in early follow-up, but not during more distant follow-up. The lack of long-term associations is probably due to the substantial within-person variability of these metabolites in spot urine samples, which renders the urinary concentrations less likely to represent long-term intakes. These associations were independent of established demographic, lifestyle, and dietary risk factors of T2D. Other metabolites, including flavan-3-ols and ferulic acid, were not associated with diabetes risk during either period.
Using a panel of urinary metabolites of polyphenols to examine associations with diabetes risk is a unique strength of our study. Because of the variability in the actual polyphenol contents of a given food (1, 24), questionnaire assessments of polyphenol intake are inevitably subject to measurement errors. The assessments are further complicated by the wide variation in the bioavailability of parent polyphenol compounds (1) and between-individual variation in absorption and metabolism of the polyphenols (25). Overall, these challenges mean that estimated intake levels are not necessarily reflective of the exposure to bioactive compounds, whereas the metabolites may more likely reflect tissue exposure to polyphenols (26).
Our investigation was subject to several limitations. First, the half-lives of most flavonoids are very short, ranging from several hours to less than a day (27). Random errors in terms of reflecting usual metabolite levels are, therefore, substantial in the current investigation, in which we only quantified the metabolites in single spot urine samples. These random errors may subsequently reduce the statistical power for detecting a significant association and may partially explain some of the null associations such as those for flavan-3-ol metabolites. Use of multiple assessments of the metabolites preferably in 24-h urine samples over time would be a more desirable approach. Interestingly, previous studies also documented meaningful correlations between 24-h urine samples and spot urine samples for excretions of certain polyphenol metabolites, e.g., quercetin (rs values = 0.40–0.60) and hesperetin (rs values = 0.50–0.57) (28). In addition, correlations with fruit and vegetable intake were largely similar for these 2 types of urine samples (29). Second, the polyphenol metabolites examined in the current study were limited to selected, well-validated metabolites, and we did not further distinguish the forms of a given polyphenol metabolite. Future studies are warranted to extend this research to identify and examine other polyphenol metabolites. Third, although we controlled for a wide range of established diabetes risk factors, including dietary factors, the possibility of residual confounding still exists. Fourth, we cannot exclude the possibility that some of our findings were due to chance, especially the temporality pattern for the associations of flavanones, flavonols, and caffeic acid. In addition, the choice of median follow-up as the cutoff for defining proximate vs. remote follow-up is arbitrary. Lastly, these findings may not be generalizable to populations with different background polyphenol intake levels or to those with differences in absorption or metabolism.
To our knowledge, the current study is the first investigation that prospectively examined urinary metabolites of flavonoids and phenolic acids in relation to T2D risk. Data for polyphenol intake and long-term risk of cardiovascular disease and cancer are accumulating (30, 31). In contrast, existing evidence for dietary polyphenols and T2D risk is fragmentary and mostly limited to flavonoids. Using data from the NHS and NHSII cohorts and the male Health Professionals Follow-up Study, we observed an inverse association between anthocyanin intake and diabetes risk (8). These findings were in line with an early Finnish study (9) but not with results from the Framingham Offspring cohort, the Iowa Women’s Health Study, or the EPIC-InterAct Study (10, 12, 13). None of these nor other studies (11) documented beneficial effects of other flavonoid subclasses, except that in the Framingham Offspring cohort an inverse association for flavonols was reported (12), and in the EPIC-InterAct Study flavonols and flavan-3-ols were associated with a lower T2D risk (13). The discrepancies could be partially explained by differential measurement error associated with the incomplete representation of food sources on the questionnaire or limitations of food composition databases (8). In contrast, controlled randomized clinical trials, in which the intake levels are more precisely quantified, provide evidence for beneficial effects of flavonoid intakes on diabetes risk factors. For example, in a long-term (1 y) randomized controlled trial, Curtis et al. (32) reported that flavan-3-ol and isoflavone supplementation (950 mg/d) significantly improved insulin resistance among postmenopausal T2D women. These data are consistent with those from short-term trials of flavan-3-ols or their food sources (33). Moreover, 2 crossover controlled trials also demonstrated beneficial effects of flavanones on alleviating endothelial dysfunction and inflammation (34, 35). Until recently, food composition databases included little information on phenolic acids, which may explain the lack of studies in relation to diabetes risk. However, studies have examined some of the major food sources of these compounds. For example, consumption of coffee, the major source of chlorogenic acid, was consistently associated with a lower risk of developing T2D in epidemiologic studies (36). Beneficial effects of coffee consumption on insulin sensitivity and other diabetes risk factors have also been documented in observational and intervention studies (37, 38).
Mechanisms underlying putative beneficial effects of polyphenols are still poorly understood. Antioxidant effects of polyphenols through scavenging free radicals and chelating metal ions is the most elucidated mechanism in in vitro studies, although such effects have not been consistently demonstrated in vivo (39, 40). Emerging evidence suggests that polyphenols may suppress inflammation and oxidative stress through multiple mechanisms, including inhibiting enzymes promoting oxidative stress and modulating transcriptional factors or expression of microRNAs involved in inflammation or apoptosis (4, 41–44). Moreover, results from several animal studies suggest that flavonoids may improve glucose metabolism through multiple pathways, involving promotion of insulin secretion, preservation of β cell function, improvement of glucose metabolism in multiple organs, and other pathways (45). Likewise, chlorogenic acid, and its metabolites, may improve glucose tolerance by delaying glucose absorption, increasing glucose uptake in peripheral tissues, and decreasing hepatic glucose output (27, 37). Further mechanistic studies on the effects of polyphenols, specifically their functional metabolites, on glucose metabolism are needed.
In summary, to our knowledge, this prospective investigation conducted in 2 cohorts of US women demonstrates for the first time that urinary excretions of select polyphenol metabolites, including flavanones, flavonols, and caffeic acid, are associated with lower risk of developing T2D and that the associations are time-dependent, probably owing to the apparent within-person variability of the metabolites in spot urine samples. These novel findings are complementary to the growing knowledge on the effects of polyphenol intake on glucose metabolism and diabetes risk. Further prospective research is warranted to evaluate these and other polyphenol metabolites in relation to diabetes risk.
Acknowledgments
We thank Laurie Custer (University of Hawaii Cancer Center) for the skilled performance of the LC/MS assay. QS, SST, MKT, AC, AAF, EBR, FBH, and RMvD were involved in data collection of dietary flavonoids or urinary metabolites; AAF measured urinary metabolites using LC/MS; QS, SST, MKT, and RMvD conducted pilot studies for the current investigation; QS, NMW, AP, MKT, FBH, and RMvD provided statistical expertise; and QS analyzed the data, wrote the first draft of the manuscript, and is the guarantor of this investigation. All authors contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content and approved the final manuscript.
Footnotes
Abbreviations used: ICC, intraclass correlation coefficient; NHS, Nurses’ Health Study; rs, Spearman partial correlation coefficient; T2D, type 2 diabetes.
References
- 1.Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr 2004;79:727–47. [DOI] [PubMed] [Google Scholar]
- 2.Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr 2003;43:89–143. [DOI] [PubMed] [Google Scholar]
- 3.Del Rio D, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal 2013;18:1818–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milenkovic D, Jude B, Morand C. miRNA as molecular target of polyphenols underlying their biological effects. Free Radic Biol Med 2013;64:40–51. [DOI] [PubMed] [Google Scholar]
- 5.Chen N, Bezzina R, Hinch E, Lewandowski PA, Cameron-Smith D, Mathai ML, Jois M, Sinclair AJ, Begg DP, Wark JD, et al. Green tea, black tea, and epigallocatechin modify body composition, improve glucose tolerance, and differentially alter metabolic gene expression in rats fed a high-fat diet. Nutr Res 2009;29:784–93. [DOI] [PubMed] [Google Scholar]
- 6.Chen YK, Cheung C, Reuhl KR, Liu AB, Lee MJ, Lu YP, Yang CS. Effects of green tea polyphenol (-)-epigallocatechin-3-gallate on newly developed high-fat/Western-style diet-induced obesity and metabolic syndrome in mice. J Agric Food Chem 2011;59:11862–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ortsäter H, Grankvist N, Wolfram S, Kuehn N, Sjoholm A. Diet supplementation with green tea extract epigallocatechin gallate prevents progression to glucose intolerance in db/db mice. Nutr Metab (Lond) 2012;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wedick NM, Pan A, Cassidy A, Rimm EB, Sampson L, Rosner B, Willett W, Hu FB, Sun Q, van Dam RM. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am J Clin Nutr 2012;95:925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knekt P, Kumpulainen J, Jarvinen R, Rissanen H, Heliovaara M, Reunanen A, Hakulinen T, Aromaa A. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr 2002;76:560–8. [DOI] [PubMed] [Google Scholar]
- 10.Nettleton JA, Harnack LJ, Scrafford CG, Mink PJ, Barraj LM, Jacobs DR Jr. Dietary flavonoids and flavonoid-rich foods are not associated with risk of type 2 diabetes in postmenopausal women. J Nutr 2006;136:3039–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song Y, Manson JE, Buring JE, Sesso HD, Liu S. Associations of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflammation in women: a prospective study and cross-sectional analysis. J Am Coll Nutr 2005;24:376–84. [DOI] [PubMed] [Google Scholar]
- 12.Jacques PF, Cassidy A, Rogers G, Peterson JJ, Meigs JB, Dwyer JT. Higher dietary flavonol intake is associated with lower incidence of type 2 diabetes. J Nutr 2013;143:1474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zamora-Ros R, Forouhi NG, Sharp SJ, Gonzalez CA, Buijsse B, Guevara M, van der Schouw YT, Amiano P, Boeing H, Bredsdorff L, et al. The association between dietary flavonoid and lignan intakes and incident type 2 diabetes in European populations: the EPIC-InterAct study. Diabetes Care 2013;36:3961–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willett WC. Nutritional epidemiology. 2nd ed New York: Oxford University Press; 1998. [Google Scholar]
- 15.Spencer JP, Abd El Mohsen MM, Minihane AM, Mathers JC. Biomarkers of the intake of dietary polyphenols: strengths, limitations and application in nutrition research. Br J Nutr 2008;99:12–22. [DOI] [PubMed] [Google Scholar]
- 16.Sun Q, Wedick NM, Pan A, Townsend MK, Cassidy A, Franke AA, Rimm EB, Hu FB, van Dam RM. Gut microbiota metabolites of dietary lignans and risk of type 2 diabetes: a prospective investigation in two cohorts of U.S. women. Diabetes Care 2014;37:1287–95. Erratum in: Diabetes Care 2014;37:3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prentice RL, Breslow NE. Retrospective studies and failure time models. Biometrika 1978;65:153–8. [Google Scholar]
- 18.Manson JE, Rimm EB, Stampfer MJ, Colditz GA, Willett WC, Krolewski AS, Rosner B, Hennekens CH, Speizer FE. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 1991;338:774–8. [DOI] [PubMed] [Google Scholar]
- 19.Cassidy A, O'Reilly EJ, Kay C, Sampson L, Franz M, Forman JP, Curhan G, Rimm EB. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am J Clin Nutr 2011;93:338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franke AA, Custer LJ, Wilkens LR, Le Marchand LL, Nomura AM, Goodman MT, Kolonel LN. Liquid chromatographic-photodiode array mass spectrometric analysis of dietary phytoestrogens from human urine and blood. J Chromatogr B Analyt Technol Biomed Life Sci 2002;777:45–59. [DOI] [PubMed] [Google Scholar]
- 21.McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Hunter DJ, Colditz GA, Willett WC. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr 2002;76:1261–71. [DOI] [PubMed] [Google Scholar]
- 22.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989;8:551–61. [DOI] [PubMed] [Google Scholar]
- 23.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999;149:531–40. [DOI] [PubMed] [Google Scholar]
- 24.Harnly JM, Doherty RF, Beecher GR, Holden JM, Haytowitz DB, Bhagwat S, Gebhardt S. Flavonoid content of U.S. fruits, vegetables, and nuts. J Agric Food Chem 2006;54:9966–77. [DOI] [PubMed] [Google Scholar]
- 25.Erlund I, Silaste ML, Alfthan G, Rantala M, Kesaniemi YA, Aro A. Plasma concentrations of the flavonoids hesperetin, naringenin and quercetin in human subjects following their habitual diets, and diets high or low in fruit and vegetables. Eur J Clin Nutr 2002;56:891–8. [DOI] [PubMed] [Google Scholar]
- 26.Pérez-Jiménez J, Hubert J, Hooper L, Cassidy A, Manach C, Williamson G, Scalbert A. Urinary metabolites as biomarkers of polyphenol intake in humans: a systematic review. Am J Clin Nutr 2010;92:801–9. [DOI] [PubMed] [Google Scholar]
- 27.Scalbert A, Manach C, Morand C, Remesy C, Jimenez L. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr 2005;45:287–306. [DOI] [PubMed] [Google Scholar]
- 28.Mennen LI, Sapinho D, Ito H, Galan P, Hercberg S, Scalbert A. Urinary excretion of 13 dietary flavonoids and phenolic acids in free-living healthy subjects—variability and possible use as biomarkers of polyphenol intake. Eur J Clin Nutr 2008;62:519–25. [DOI] [PubMed] [Google Scholar]
- 29.Mennen LI, Sapinho D, Ito H, Bertrais S, Galan P, Hercberg S, Scalbert A. Urinary flavonoids and phenolic acids as biomarkers of intake for polyphenol-rich foods. Br J Nutr 2006;96:191–8. [DOI] [PubMed] [Google Scholar]
- 30.van Dam RM, Naidoo N, Landberg R. Dietary flavonoids and the development of type 2 diabetes and cardiovascular diseases: review of recent findings. Curr Opin Lipidol 2013;24:25–33. [DOI] [PubMed] [Google Scholar]
- 31.Arts IC, Hollman PC. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr 2005;81:317S–25S. [DOI] [PubMed] [Google Scholar]
- 32.Curtis PJ, Sampson M, Potter J, Dhatariya K, Kroon PA, Cassidy A. Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: a 1-year, double-blind, randomized, controlled trial. Diabetes Care 2012;35:226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hooper L, Kay C, Abdelhamid A, Kroon PA, Cohn JS, Rimm EB, Cassidy A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr 2012;95:740–51. [DOI] [PubMed] [Google Scholar]
- 34.Rizza S, Muniyappa R, Iantorno M, Kim JA, Chen H, Pullikotil P, Senese N, Tesauro M, Lauro D, Cardillo C, et al. Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. J Clin Endocrinol Metab 2011;96:E782–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buscemi S, Rosafio G, Arcoleo G, Mattina A, Canino B, Montana M, Verga S, Rini G. Effects of red orange juice intake on endothelial function and inflammatory markers in adult subjects with increased cardiovascular risk. Am J Clin Nutr 2012;95:1089–95. [DOI] [PubMed] [Google Scholar]
- 36.van Dam RM, Hu FB. Coffee consumption and risk of type 2 diabetes: a systematic review. JAMA 2005;294:97–104. [DOI] [PubMed] [Google Scholar]
- 37.van Dam RM. Coffee and type 2 diabetes: from beans to beta-cells. Nutr Metab Cardiovasc Dis 2006;16:69–77. [DOI] [PubMed] [Google Scholar]
- 38.Wedick NM, Brennan AM, Sun Q, Hu FB, Mantzoros CS, van Dam RM. Effects of caffeinated and decaffeinated coffee on biological risk factors for type 2 diabetes: a randomized controlled trial. Nutr J 2011;10:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halliwell B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch Biochem Biophys 2008;476:107–12. [DOI] [PubMed] [Google Scholar]
- 40.Hollman PC, Cassidy A, Comte B, Heinonen M, Richelle M, Richling E, Serafini M, Scalbert A, Sies H, Vidry S. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J Nutr 2011;141:989S–1009S. [DOI] [PubMed] [Google Scholar]
- 41.Leiherer A, Mundlein A, Drexel H. Phytochemicals and their impact on adipose tissue inflammation and diabetes. Vascul Pharmacol 2013;58:3–20. [DOI] [PubMed] [Google Scholar]
- 42.Du Y, Guo H, Lou H. Grape seed polyphenols protect cardiac cells from apoptosis via induction of endogenous antioxidant enzymes. J Agric Food Chem 2007;55:1695–701. [DOI] [PubMed] [Google Scholar]
- 43.Takikawa M, Inoue S, Horio F, Tsuda T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J Nutr 2010;140:527–33. [DOI] [PubMed] [Google Scholar]
- 44.Fraga CG, Galleano M, Verstraeten SV, Oteiza PI. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol Aspects Med 2010;31:435–45. [DOI] [PubMed] [Google Scholar]
- 45.Babu PV, Liu D, Gilbert ER. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J Nutr Biochem 2013;24:1777–89. [DOI] [PMC free article] [PubMed] [Google Scholar]