Abstract
Background: In many species, including humans, arginine is considered a semiessential amino acid because under certain conditions endogenous synthesis cannot meet its demand. The requirements of arginine for growth in mice are ill defined and seem to vary depending on the genetic background of the mice.
Objective: The objective of this study was to determine the metabolic and molecular basis for the requirement of arginine in 2 mouse strains.
Methods: Institute of Cancer Research (ICR) and C57BL/6 (BL6) male mice were fed arginine-free or arginine-sufficient diets (Expt. 1) or 1 of 7 diets with increasing arginine concentration (from 0- to 8-g/kg diet, Expt. 2) between day 24 and 42 of life to determine the arginine requirements for growth. Citrulline production and “de novo” arginine synthesis were measured with use of stable isotopes, and arginine requirements were determined by breakpoint analysis and enzyme expression by reverse transcriptase-polymerase chain reaction.
Results: In Expt. 1, ICR mice grew at the same rate regardless of the arginine concentration of the diet (mean ± SE: 0.66 ± 0.04 g/d, P = 0.80), but BL6 mice had a reduced growth rate when fed the arginine-free diet (0.25 ± 0.02 g/d, P < 0.001) compared to the 8-g arginine/kg diet (0.46 ± 0.03 g/d). ICR mice showed at least a 2-fold greater expression (P < 0.001) of ornithine transcarbamylase (OTC) than BL6 mice, which translated into a greater rate of citrulline (25%) and arginine synthesis (49%, P < 0.002). In Expt. 2, breakpoint analysis showed that the requirement for growth of BL6 mice was met with 2.32 ± 0.39 g arginine/kg diet; for ICR mice, however, no breakpoint was found.
Conclusion: Our data indicate that a reduced expression of OTC in BL6 mice translates into a reduced production of citrulline and arginine compared with ICR mice, which results in a dietary arginine requirement for growth in BL6 mice, but not in ICR mice.
Keywords: amino acid, arginine, citrulline, growth, requirements
Introduction
Arginine is considered a semiessential amino acid because, during certain physiologic and pathophysiologic conditions, endogenous synthesis cannot meet the demand for this amino acid. The endogenous synthesis of arginine is a multiorgan process that involves the synthesis of citrulline by the gut and further conversion into arginine by the kidney (1), where argininosuccinate synthase and lyase (enzymes that catalyze the conversion of citrulline into arginine) are highly expressed. In addition, these 2 enzymes are expressed in multiple tissues, and it is likely that a fraction of the citrulline produced is used directly by the tissues to meet, at least partially, their arginine needs (2, 3).
The synthesis of arginine shows marked species differences [for a review see Ball et al. (4)]. Arginine is an essential amino acid in birds because of the lack of carbamoyl phosphate synthase I (CPS I)6 (5), the enzyme that catalyzes the synthesis of carbamoyl phosphate, which becomes the ureido group of citrulline. In felines, it seems that the inability to synthesize citrulline is due to reduced ornithine aminotransferase activity, and thus, to the low availability of ornithine, the immediate citrulline precursor (6). In other species, such as the rat and the pig, citrulline production is limited and endogenous arginine synthesis is not sufficient to allow for maximal growth (7, 8). In fact, the rate-limiting step in the endogenous synthesis of arginine is citrulline availability, and for this reason citrulline supplementation has been shown to successfully address arginine deficiency in multiple species [birds (9, 10), cats (11), pigs (12), rats (13)].
In mice, early observations indicated that this species was able to synthesize arginine (14), but it was not clear if this endogenous source of arginine was sufficient to meet the demand for this amino acid. Current dietary arginine recommendations [3-g/kg diet (15)] are based on the work of John and Bell (16, 17). However, these researchers could not establish arginine requirements because growth was not affected, even at the lowest arginine concentrations tested [1 g/kg (17) and 3 g/kg (16)]. In their work, John and Bell used Swiss × Carworth Farms No. 1 crossbred mice (both outbred lines) that have a high postweaning growth rate (∼0.8 g/d). Our work with Institute of Cancer Research (ICR) mice, an outbred line also derived from Swiss mice, agrees with these previous observations and indicates that these mice may not have an arginine requirement. This contrasts with our observations in C57BL6/J mice, a widely used inbred strain and the most common genetic background for genetically modified mice, in which growth was compromised when arginine-free diets were fed.
The objective of the present work was to elucidate the basis of arginine requirements in 2 mouse lines and to determine their arginine requirements for growth after weaning.
Methods
Mice and housing.
Twenty-one-day-old male C57BL/6 (BL6) JOlaHsd mice and ICR [Hsd:ICR (CD-1)] mice were purchased from Harlan Laboratories. Upon arrival (day 21) mice were weighed, caged in pairs, and fed an irradiated 18% crude protein feed (Rodent Diet 2920× Harlan Teklad). Dietary proximate analysis was as follows: protein (185 g/kg), arginine (8 g/kg), gross energy (14.1 MJ/kg), fat (60 g/kg), fiber (28 g/kg), and ash (46 g/kg). This pelleted diet was ground using a blender (Waring), and mice consumed the diet ad libitum using jar feeders for 3 d. After this acclimatization period (day 24), mice were fed the different diets described below until week 6 of life (day 42). Mice were weighed twice weekly between 1000 and 1200. Mice were under a 12-h light cycle (0600 to 1800) in a temperature-controlled (22 ± 2°C) and humidity-controlled (55 ± 5%) environment. Autoclaved reverse osmosis water was available at all times and the bedding used was corn cobs. All animal procedures were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee.
Expt. 1.
BL6 and ICR mice (n = 10) were fed an arginine-sufficient (8-g/kg diet) or arginine-free purified diet based on the Rodent Diet 2920× (Table 1) from day 24 to day 42. At the end of feeding trial, after 4 h of feed deprivation, mice were infused with stable isotopes to determine the rate of appearance of arginine, citrulline, and the contribution of citrulline to plasma arginine (“de novo” arginine synthesis). Phenylalanine and tyrosine tracers were also used to determine whole-body protein turnover. Methods and calculations have been described in detail elsewhere (18, 19). The plasma concentrations of arginine and citrulline were determined by isotopic dilution with use of an LC-MS/MS (TSQ Vantage; Thermo Scientific).
TABLE 1.
Fluxes and amino acid concentrations of BL6 and ICR mice fed arginine-sufficient (8-g arginine/kg diet) and arginine-free diets1
| BL6 |
ICR |
|||||||
| Arg− | Arg+ | Arg− | Arg+ | Gen | TRT | Gen × TRT | ||
| Fluxes, μmol ⋅ kg−1 ⋅ h−1 | ||||||||
| RaPhe2 | 330 ± 10 | 336 ± 16 | 326 ± 14 | 320 ± 17 | 0.52 | 0.99 | 0.71 | |
| RaTyr2 | 298 ± 10 | 294 ± 14 | 276 ± 10 | 303 ± 12 | 0.62 | 0.43 | 0.29 | |
| RcPhe→Tyr2 | 58 ± 5 | 55 ± 5 | 47 ± 3 | 68 ± 11 | 0.86 | 0.17 | 0.09 | |
| Plasma concentrations, μmol/L | ||||||||
| Citrulline | 124 ± 5 | 126 ± 11 | 170 ± 5 | 146 ± 7* | 0.001 | 0.23 | 0.16 | |
| Arginine | 124 ± 8 | 165 ± 18 | 150 ± 16 | 147 ± 10 | 0.83 | 0.29 | 0.23 | |
| Ornithine | 103 ± 11 | 109 ± 12 | 162 ± 4 | 131 ± 5* | 0.002 | 0.31 | 0.14 | |
Values are means ± SEMs, n = 10. *Different from corresponding Arg−, P < 0.05. BL6, C57BL/6; Gen, genotype; ICR, Institute of Cancer Research; TRT, treatment.
RaPhe, RaTyr, and RcPhe→Tyr: rate of appearance of phenylalanine and tyrosine, and rate of conversion of phenylalanine into tyrosine.
Expt. 2.
To determine the arginine requirements of BL6 and ICR mice for growth (n = 10) between day 24 and day 42, diets with different arginine concentrations were fed. By mixing the arginine-free diet and arginine-sufficient diet (Supplemental Table 1), diets containing 0-, 0.6-, 1-, 1.7-, 3-, 5-, and 8-g arginine/kg diet were obtained.
Expt. 3.
To determine the expression of CPS I and ornithine transcarbamylase (OTC), enzymes that catalyze the synthesis of citrulline in the small intestine, day 24, day 32, and day 42 mice (n = 5) were killed. Mice were fed Rodent Diet 2920×. Gut tissue was snap frozen immediately. Tissue was homogenized in guanidinium thiocyanate-phenol-chloroform extraction (TRIzol; Life Technologies), and RNA was isolated with use of Qiagen RNeasy Mini elute columns (Qiagen). After cDNA synthesis, PCR amplification and gene differentiation expression (primers are shown in Supplemental Table 2) were performed with SYBR Green1on a multicolor real-time PCR detection system (CFX96; BioRad Laboratories).
Data analysis.
Data were analyzed statistically with use of the proc mixed procedure of SAS (v. 9.2; SAS Institute) with arginine concentration, genetic background, and their interaction as the fixed effect of the model (20). mRNA expression data were log transformed before analysis; genotype and age were the fixed effects of the model. Preplanned orthogonal contrasts were used to determine the effect of dietary arginine within genotype. Arginine requirements were determined by broken line regression with use of the proc NLIN statement of SAS (21). Values presented in the text are means ± SEs and were tested for significance at the 5% level.
Results
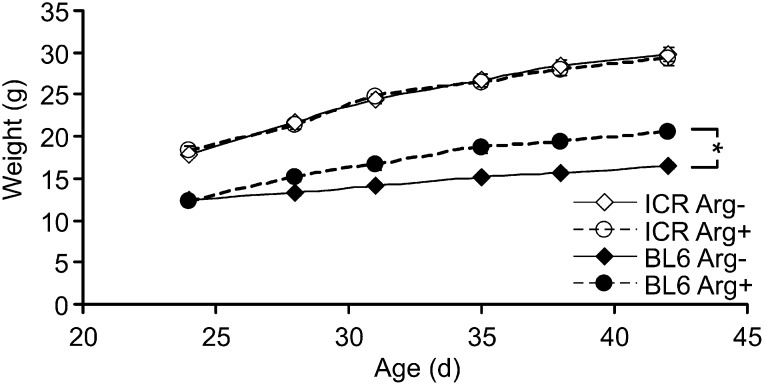
ICR mice were heavier than BL6 mice (12.1 ± 0.20 g vs. 8.7 ± 0.11 g, P < 0.001) at weaning (day 21) and displayed a greater growth rate between day 24 and day 42 when fed the arginine-sufficient diet (0.66 ± 0.04 g/d and 0.46 ± 0.03 g/d, P < 0.001). Although ICR mice grew at the same rate regardless of the arginine concentration of the diet (P = 0.80), BL6 mice showed a reduction in growth rate when fed the diet devoid of arginine (0.46 ± 0.03 g/d vs. 0.25 ± 0.02 g/d, P < 0.001; Figure 1).
FIGURE 1.
Growth curves of BL6 and ICR mice fed arginine-free or arginine-sufficient diets (8-g/kg diet). Symbols are means ± SEs, n = 10. *Difference in growth rate within genotype, P < 0.05. BL6, C57BL/6; ICR, Institute of Cancer Research.
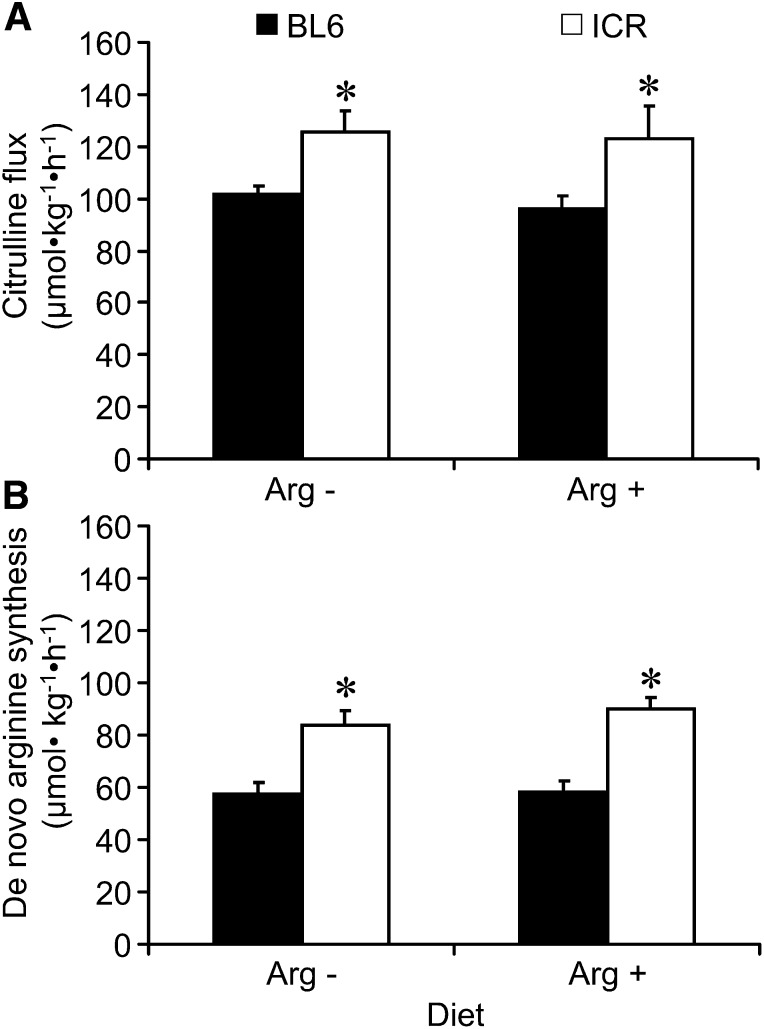
ICR mice had a faster rate of citrulline production (P < 0.002; Figure 2A) than BL6 mice, which translated into a greater “de novo” arginine synthesis (P < 0.001; Figure 2B). This increase, however, did not result into a higher flux of arginine (370 ± 18 μmol ⋅ kg−1 ⋅ h−1, P > 0.40). Likewise, no differences in the appearance rate of phenylalanine and tyrosine or in the rate of conversion of phenylalanine into tyrosine were observed for genotype (P > 0.17) or arginine concentration of the diet (P > 0.52; Table 1). ICR mice had higher plasma concentrations of ornithine and citrulline than their BL6 counterparts (P ≤ 0.002; Table 1), and plasma arginine concentration was increased (P < 0.05) in BL6 mice consuming the arginine-sufficient diet (Table 1).
FIGURE 2.
Citrulline flux (A) and de novo arginine synthesis (B) of BL6 and ICR mice fed arginine-free or arginine-sufficient diets (8-g/kg diet). BL6 mice had a reduced citrulline flux and de novo arginine synthesis than ICR mice (P < 0.002). Diet had no effect on these variables. Bars are means ± SEs, n = 10. *Different from BL6 mice fed the same diet, P < 0.01. BL6, C57BL/6; ICR, Institute of Cancer Research.
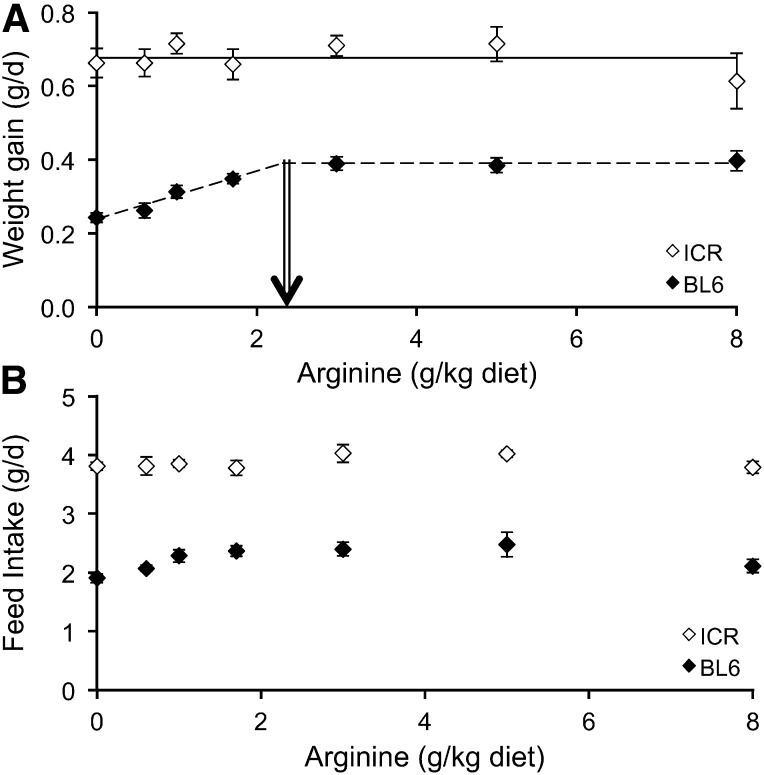
Arginine concentration of the diet had no effect (P = 0.53) on the growth curve of ICR mice (Supplemental Figure 1), but low concentrations of arginine reduced growth in BL6 mice (P < 0.008; Supplemental Figure 2). For this reason no breakpoint for weight gain was found for ICR mice, which indicates that this strain does not require dietary arginine for growth (Figure 3A). BL6 mice, however, showed a clear breakpoint and thus a dietary requirement (2.32 ± 0.39 g arginine/kg) to maximize weight gain (Figure 3A). Thus, the dietary arginine needed to meet the requirements of 95% of the population was calculated as 3.1-g arginine/kg.
FIGURE 3.
Daily weight gain (A) and feed intake (B) of BL6 and ICR mice fed diets containing different amounts of arginine. No breakpoint was identified for ICR mice. The arrow denotes the breakpoint for the BL6 mice (2.32 ± 0.39 g arginine/kg). Symbols are means ± SEs, n = 10. BL6, C57BL/6; ICR, Institute of Cancer Research.
Feed intake for the 18-d period studied was greater for ICR mice than for the BL6 mice (P < 0.001; Figure 3B). Although, we were not able to detect differences in feed intake for the mice fed the different diets, a trend (P = 0.06) for a reduced intake was observed for the BL6 mice consuming diets with <1 g arginine/kg (Figure 3B).
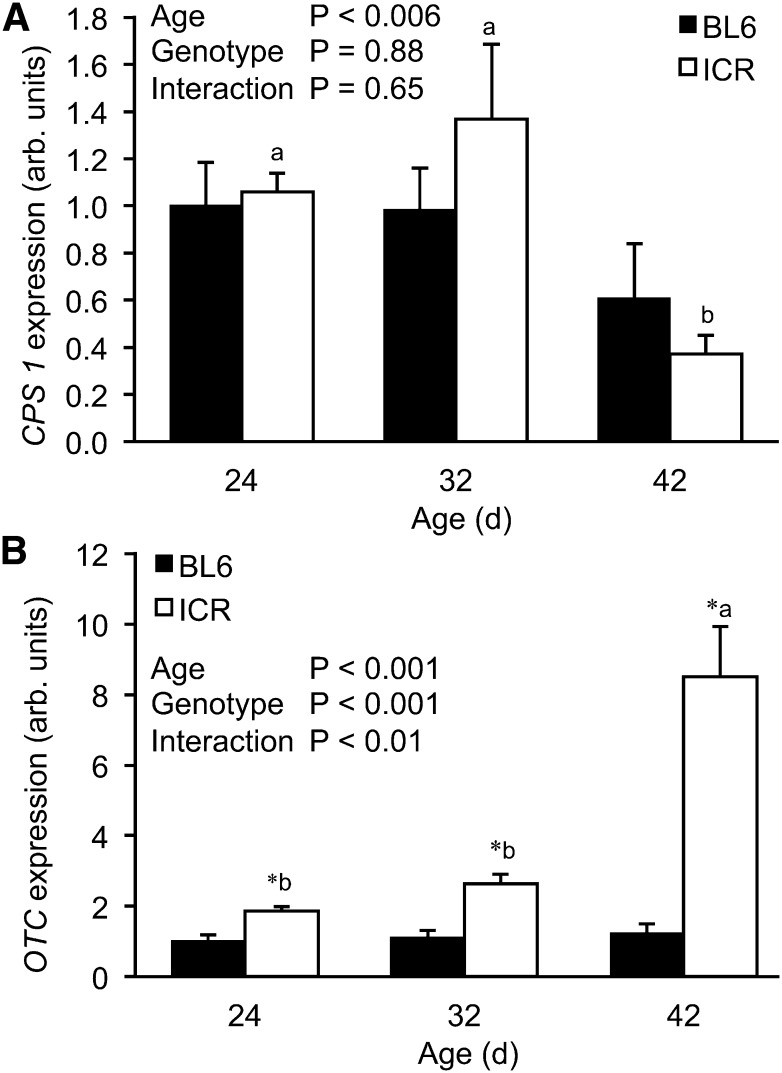
The expression of CPS I in the small intestine was not different (P = 0.88) between BL6 and ICR mice; however, there was an age effect (P < 0.006) with a reduction by 42 d in the ICR mice (Figure 4A). A greater expression of OTC in ICR mice (P < 0.001) was observed at all ages; the 2-fold increased expression of OTC measured in ICR mice on 24 and 32 d increased (P < 0.001) to a 7-fold difference by 42 d (Figure 4B).
FIGURE 4.
CPS I (A) and OTC (B) mRNA expression in the small intestine of BL6 and ICR mice fed regular chow at different ages. *Different from BL6 mice at that age, P < 0.05. Within a genotype, labeled means without a common letter differ, P < 0.05. arb., arbitrary; BL6, C57BL/6; CPS I, carbamoyl phosphate synthase I; ICR, Institute of Cancer Research; OTC, ornithine transcarbamylase.
Discussion
Arginine is an amino acid used not only for protein synthesis, but also for the synthesis of creatine, polyamines, and nitric oxide and thus has a central role in energy metabolism, cell proliferation, and the regulation of blood pressure and the immune response. We chose to determine arginine requirements for growth because of the high demand of this amino acid for tissue deposition that takes place after weaning. Additionally, the difficulties and variability involved in determining the response of other physiologic and pathophysiologic endpoints to relatively small changes in dietary arginine make the task of estimating requirements for these additional endpoints daunting. Therefore, the implicit assumption in the determination of arginine requirements is that, under normal conditions, once the requirements for growth are met, the requirements for the multiple functional roles of this amino acid are also met.
Previous attempts to determine requirements in mice indicated that this species had no arginine requirements for growth or that they were below the lowest arginine concentration fed (16, 17). Milner et al. (22), however, showed that mice fed an arginine-free diet resulted in feed intake depression and a reduction in body weight gain. These observations can be reinterpreted in light of the results we obtained. Although John and Bell (16, 17) used mice resulting from a cross between 2 outbred Swiss-derived mouse lines, Milner et al. (22) used mice derived from a cross between 2 inbred lines (BDF mice, C57BL/6 × DBA/2). In our studies, we used outbred ICR mice (a line derived from Swiss strains) and inbred C57BL/6 mice. Paradoxically, the Swiss-derived lines with a higher growth potential (∼0.8 g/d) and presumably a higher need for arginine did not show growth depression when fed arginine-free diets, whereas BL6 mice or their crosses, with a more modest growth rate (∼0.4–0.5 g/d), suffered an ∼50% reduction in their growth rate. The difference in the production of citrulline by the 2 genotypes may explain these findings because it can account for enough arginine to support ∼0.20 g weight gain/d (see Appendix A). We previously reported similar genetic background differences in the production of citrulline between ICR and mixed-background mice (B6EiC3sn, the product of a C57BL/6J subline, and C3H/HeSnJX) (23). A similar difference in plasma citrulline and citrulline production between C57BL/6J and FVB mice (an inbreed Swiss-derived strain) has been reported by others (24).
Not surprisingly, we did not find a growth rate breakpoint in ICR mice because these mice performed similarly at all the arginine concentrations fed. BL6 mice, however, showed a breakpoint at 2.3-g arginine/kg diet, which indicates the arginine requirement for this strain. Thus, our present results confirm published observations that suggested that C57BL/6 mice and their crosses, but not mice from Swiss lineage, require arginine for growth. It seems that in the process of inbreeding to establish the C57BL/6 strain a reduced capacity for citrulline production was established. This is supported by the OTC expression data that indicates that this key enzyme for the synthesis of citrulline has a lower expression in BL6 mice than in ICR mice.
In conclusion, the variability in the expression of OTC resulted in a reduction in the production of citrulline and endogenous synthesis of arginine, which translated in the need for dietary arginine to meet the requirements for growth. Variability in the processes that result in the disposal of arginine (e.g., arginase) can further modify the requirement of a particular strain for arginine. Thus, these observations within a particular species described in this article recapitulate to a certain extent what has been known regarding the dietary need for arginine of different species (4). The first implication of our study is for those species in which arginine is required for maximal growth (e.g., neonatal pig). In these species, it is possible that enough genetic variability for citrulline production may be present and selecting for this trait may lead to an increase in endogenous arginine synthesis and a reduced need for dietary arginine. The second implication is that arginine is an essential amino acid for growth at least in some strains of mice. Thus, the requirements determined in a strain may not be valid for mice with a different genetic background. Current recommendations [3-g arginine/kg diet (15)] are able to meet the requirements for growth of C57BL/6 mice. Commonly fed diets, however, contain at least 8 g arginine/kg and are likely to meet the requirements under most, if not all, physiologic conditions.
Acknowledgments
JCM designed and conducted the research, analyzed the data, wrote the paper, and has primary responsibility for final content; UA analyzed samples and reviewed the article; and ICD conducted the research and prepared samples for analysis. All authors read and approved the final manuscript.
Appendix A
Here, we attempt to quantify the contribution of endogenous and dietary arginine to protein deposition and growth of BL6 mice.
Contribution of endogenous arginine. The measured production of citrulline in ICR and BL6 mice was 124 and 98 μmol ⋅ kg−1 ⋅ h−1, respectively. If BL6 mice were able to sustain the same level of citrulline production as their ICR counterparts, it would amount to an extra 624-μmol citrulline ⋅ kg−1 ⋅ d−1 (26 μmol ⋅ kg−1 ⋅ h−1 × 24 h/d). Because the only known fate for citrulline is its conversion into arginine, we expect this difference to correspond to an endogenous production of 109-mg arginine ⋅ kg−1 ⋅ d−1 (624 μmol ⋅ kg−1 ⋅ d−1 × 174 mg/mmol). Therefore, for a 17-g mouse, this represents ∼1.9-mg arginine/d.
The arginine concentration of protein is 4.4-g arginine/100-g protein (1), and thus, 1.9-mg arginine/d if used for protein synthesis represents 42-mg protein/d. Finally, protein is ∼19% of weight gain (2), and thus, it translates into 0.22-g weight gain/d.
Contribution of dietary arginine. The calculated arginine requirement (2.3-g arginine/kg diet) at the observed feed intake (2.2 g/d) provides 5.1-mg arginine/d. Arginine undergoes a high first pass extraction [75% (3)], which reduces the amount of arginine available to the peripheral tissues (1.3-mg arginine/d). Performing similar calculations as above, this represent a 0.15-g weight gain/d.
Although the implicit assumption of 100% efficiency in the use of arginine for protein synthesis is unlikely, a very high efficiency in the present scenarios (in which arginine is limiting) is reasonable. The first pass extraction rate used for these calculations was determined in mice fed an arginine-sufficient diet, and it is likely that in conditions of arginine deficiency the actual value is lower. This also assumes that first pass extraction implies a loss of arginine and ignores the use of arginine for protein synthesis (and other functions). Thus, the 0.15-g weight gain/d calculated is likely to be an underestimation. However, both calculations (contribution of endogenous and dietary arginine) give similar values that are remarkably close to the reduction in weight gain observed in the mice fed the arginine-free diet (0.21 g/d).
Footnotes
Abbreviations used: BL6, C57BL/6; CPS I, carbamoyl phosphate synthase I; ICR, Institute of Cancer Research; OTC, ornithine transcarbamylase.
References
- 1.Brosnan ME, Brosnan JT. Renal arginine metabolism. J Nutr 2004;134:2791S–5S. [DOI] [PubMed] [Google Scholar]
- 2.Featherston WR, Rogers QR, Freedland RA. Relative importance of kidney and liver in synthesis of arginine by the rat. Am J Physiol 1973;224:127–9. [DOI] [PubMed] [Google Scholar]
- 3.Marini JC, Didelija IC, Fiorotto ML. Extrarenal citrulline disposal in mice with impaired renal function. Am J Physiol Renal Physiol 2014;307:F660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ball RO, Urschel KL, Pencharz PB. Nutritional consequences of interspecies differences in arginine and lysine metabolism. J Nutr 2007;137:1626S–41S. [DOI] [PubMed] [Google Scholar]
- 5.Tamir H, Ratner S. Enzymes of arginine metabolism in chicks. Arch Biochem Biophys 1963;102:249–58. [DOI] [PubMed] [Google Scholar]
- 6.Morris JG, Rogers QR. Arginine: an essential amino acid for the cat. J Nutr 1978;108:1944–53. [DOI] [PubMed] [Google Scholar]
- 7.Hepburn FN, Bradley WB. The glutamic acid and arginine requirement for high growth rate of rats. J Nutr 1964;84:305–12. [DOI] [PubMed] [Google Scholar]
- 8.Kim SW, McPherson RL, Wu G. Dietary arginine supplementation enhances the growth of milk-fed young pigs. J Nutr 2004;134:625–30. Corrected in: J Nutr 2007;137:2168. [DOI] [PubMed] [Google Scholar]
- 9.Tamir H, Ratner S. A study of ornithine, citrulline and arginine synthesis in growing chicks. Arch Biochem Biophys 1963;102:259–69. [DOI] [PubMed] [Google Scholar]
- 10.Klose AA, Almquist HJ. The ability of citrulline to replace arginine in the diet of the chick. J Biol Chem 1940;1940:153–55. [Google Scholar]
- 11.Morris JG, Rogers QR, Winterrowd DL, Kamikawa EM. The utilization of ornithine and citrulline by the growing kitten. J Nutr 1979;109:724–9. [DOI] [PubMed] [Google Scholar]
- 12.Urschel KL, Shoveller AK, Uwiera RRE, Pencharz PB, Ball RO. Citrulline is an effective arginine precursor in enterally fed neonatal piglets. J Nutr 2006;136:1806–13. [DOI] [PubMed] [Google Scholar]
- 13.Hoogenraad N, Totino N, Elmer H, Wraight C, Alewood P, Johns RB. Inhibition of intestinal citrulline synthesis causes severe growth-retardation in rats. Am J Physiol 1985;249:G792–9. [DOI] [PubMed] [Google Scholar]
- 14.Bauer CD, Berg CP. The amino acids required for growth in mice and the availability of their optical isomers. J Nutr 1943;26:51–64. [Google Scholar]
- 15.National Research Council, Subcommittee on Laboratory Animal Nutrition, Committee on Animal Nutrition, Board on Agriculture. Nutrient requirements of laboratory animals. 4th revised ed Washington (DC): The National Academies Press; 1995. [Google Scholar]
- 16.John AM, Bell JM. Amino-acid requirements of growing mouse. J Nutr 1976;106:1361–7. [DOI] [PubMed] [Google Scholar]
- 17.Bell JM, John AM. Amino-acid-requirements of growing mice—arginine, lysine, tryptophan and phenylalanine. J Nutr 1981;111:525–30. [DOI] [PubMed] [Google Scholar]
- 18.Marini JC. Arginine and ornithine are the main precursors for citrulline synthesis in mice. J Nutr 2012;142:572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marini JC, Keller B, Didelija IC, Castillo L, Lee B. Enteral arginase II provides ornithine for citrulline synthesis. Am J Physiol Endocrinol Metab 2011;300:E188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat 1998;23:323–55. [Google Scholar]
- 21.Robbins KR, Saxton AM, Southern LL. Estimation of nutrient requirements using broken-line regression analysis. J Anim Sci 2006;84(Suppl):E155–65. [DOI] [PubMed] [Google Scholar]
- 22.Milner JA, Prior RL, Visek WJ. Arginine deficiency and orotic aciduria in mammals. Proc Soc Exp Biol Med 1975;150:282–8. [DOI] [PubMed] [Google Scholar]
- 23.Marini JC, Erez A, Castillo L, Lee B. Interaction between murine spf-ash mutation and genetic background yields different metabolic phenotypes. Am J Physiol Endocrinol Metab 2007;293:E1764–71. [DOI] [PubMed] [Google Scholar]
- 24.Luiking YC, Hallemeesch MM, Vissers YLJ, Lamers WH, Deutz NEP. In vivo whole body and organ arginine metabolism during endotoxemia (sepsis) is dependent on mouse strain and gender. J Nutr 2004;134:2768S–74S. [DOI] [PubMed] [Google Scholar]
- 25.Eisen EJ. Results of growth curve analyses in mice and rats. J Anim Sci 1976;42:1008–23. [DOI] [PubMed] [Google Scholar]
References (for Appendix A)
- 1.John AM, Bell JM. Amino-acid requirements of growing mouse. J Nutr 1976;106:1361–7. [DOI] [PubMed] [Google Scholar]
- 2.Eisen EJ. Results of growth curve analyses in mice and rats. J Anim Sci 1976;42:1008–23. [DOI] [PubMed] [Google Scholar]
- 3.Marini JC, Keller B, Didelija IC, Castillo L, Lee B. Enteral arginase II provides ornithine for citrulline synthesis. Am J Physiol Endocrinol Metab 2011;300:E188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]