Abstract
Background: An objective measure of added sugar (AS) and sugar-sweetened beverage (SSB) intake is needed. The δ13C value of finger-stick blood is a novel validated biomarker of AS/SSB intake; however, nonsweetener corn products and animal protein also carry a δ13C value similar to AS sources, which may affect blood δ13C values. The δ15N value of blood has been proposed as a “correction factor” for animal protein intake.
Objectives: The objectives were to 1) identify foods associated with δ13C and δ15N blood values, 2) determine the contribution of nonsweetener corn to the diet relative to AS intake, and 3) determine if the dual-isotope model (δ13C and δ15N) is a better predictor of AS/SSB intake than δ13C alone.
Methods: A cross-sectional sample of southwest Virginian adults (n = 257; aged 42 ± 15 y; 74% overweight/obese) underwent dietary intake assessments and provided finger-stick blood samples, which were analyzed for δ13C and δ15N values by using natural abundance stable isotope mass spectrometry. Statistical analyses included ANOVAs, paired-samples t tests, and multiple linear regressions.
Results: The mean ± SD daily AS intake was 88 ± 59 g and nonsweetener corn intake was 13 ± 13 g. The mean δ13C value was −19.1 ± 0.9‰, which was significantly correlated with AS and SSB intakes (r = 0.32 and 0.39, respectively; P ≤ 0.01). The δ13C value and nonsweetener corn intake and the δ15N value and animal protein intake were not correlated. AS intake was significantly greater than nonsweetener corn intake (mean difference = 76.2 ± 57.2 g; P ≤ 0.001). The δ13C value was predictive of AS/SSB intake (β range: 0.28–0.35; P ≤ 0.01); however, δ15N was not predictive and minimal increases in R2 values were observed when the δ15N value was added to the model.
Conclusions: The data do not provide evidence that the dual-isotope method is superior for predicting AS/SSB intakes within a southwest Virginian population. Our results support the potential of the δ13C value of finger-stick blood to serve as an objective measure of AS/SSB intake. This trial was registered at clinicaltrials.gov as NCT02193009.
Keywords: added sugars, biomarker validation, dietary assessment, obesity, sugar-sweetened beverages
Introduction
The consumption of added sugars (ASs)5 and sugar-sweetened beverages (SSBs) provides 16% and 7%, respectively, of total energy intake in US adults (1, 2), and excessive intake of ASs and SSBs has been identified as a contributor to the increased prevalence of obesity and related comorbidities (3–5). ASs are sugars that are added to foods before or after processing and preparation as well as sugars added at the table (6). SSBs include regular sodas, fruit drinks, energy drinks, and tea or coffee sweetened with a caloric sweetener (5). Despite the implementation of many public policies (taxation, AS/SSB recommendations) (7–9), existing research on AS and health outcomes is often limited by its reliance on self-reported dietary intake, which makes it increasingly difficult to evaluate the effectiveness of AS/SSB reduction interventions. Although dietary records and recalls are considered the “gold standards” of dietary assessment (10–12), because of the self-reported subjective nature of these methods, under- or overreporting of certain dietary items may occur (e.g., underreporting of socially undesirable high fat and/or AS foods such as SSBs) (11, 13). The ability to objectively measure the effectiveness of AS/SSB policies on dietary consumption patterns is limited, as evidenced by a recent review acknowledging the need for objective measures of SSB intake (14). Thus, an objective measure, such as a dietary biomarker (15–19), could facilitate a more accurate determination of the impact of ASs and SSBs on the health status of the US population (20).
The δ13C value of blood as a novel biomarker of AS and SSB intake (20) has shown preliminary validity in several clinical laboratory–based investigations (i.e., data were collected in a clinical research laboratory) (21–24) and within community-based settings (i.e., data were collected in a field setting) (25–27). Corn [e.g., high-fructose corn syrup (HFCS)] and cane sugars, which are derived from C4 plants, exhibit high δ13C values relative to sugars derived from more common C3 plants because of enzymatic differences in the biosynthetic pathways of sugar fixation between the 2 types of plants (22, 28, 29). Because the δ13C value of living tissues is considered first and foremost to reflect the ultimate carbon source for metabolism (30), it may be possible to recognize the isotopic signature of AS consumption via tissue isotopic analysis (29). Although this approach has shown promise as an intake biomarker of AS/SSB intake (21, 31), several limitations need to be addressed before its validation for use in a clinical research setting.
First, although the previously mentioned C4 sugars comprise a majority of AS intake among Americans (20, 32), the consumption of C3 sugars (e.g., beet sugar, honey, and maple syrup) would not contribute to a high δ13C value, the mechanism upon which the biomarker is premised (21). However, C3 plants represent only 22% of ASs consumed in America compared with C4 sources of ASs (see Table 1). Second, because nonsweetener corn products also exhibit high δ13C values, the consumption of these items may affect δ13C values (20). Furthermore, the consumption of nonsweetener corn products (per capita availability = 26 kg) is much lower than sweetener corn and cane sugar consumption (per capita availability = 91 kg) based on USDA data (33). Third, animal protein consumption may affect δ13C values because corn is a primary food source of milk and meat animals in the United States (28); thus, corn-fed meat possesses a δ13C signature intermediate between C3 and C4 plants (20, 28). It has been suggested that the δ15N value may be used as a “correction factor” to account for animal protein consumption because of elevated δ15N values of animal products relative to plant products (20, 26, 35). This may be accomplished by using a dual-isotope model to explain AS intake by using δ13C as a predictor and δ15N as a covariate, which may increase the biomarker’s sensitivity for AS intake (20, 26). Nash et al. (26, 27, 35) showed the utility of this dual-isotope model in the RBC fraction from a Yup’ik population with a high marine animal intake. To date, no investigations have examined the ability of a dual-isotope method to predict AS intake or the impact of nonsweetener corn and terrestrial animal protein consumption on δ13C blood values within a US population.
TABLE 1.
Total energy contribution of added sugar to US diet, by type1
| Sweetener | Total energy consumed, % | Relative contributions of added sugar sources, % |
| C4 plant sources | 78 | |
| High-fructose corn syrup | 4.6 | |
| Other corn syrups | 1.5 | |
| Cane sugar | 4.0 | |
| C3 plant and other sources | 22 | |
| Beet sugar | 2.7 | |
| Other sweeteners (e.g., honey, maple syrup) | 0.2 | |
| Total added sugars | 13.0 |
The objective of this investigation was to assess the ability of a dual-isotope model (δ13C and δ15N) to predict AS/SSB intake compared with a single-isotope model (δ13C) within a southwest Virginian adult population while concurrently exploring potential confounds to the biomarker. The specific aims of this investigation were as follows: 1) to identify which food sources (ASs, SSBs, nonsweetener corn products, and animal protein) are associated with δ13C values; 2) to determine if δ15N blood values are associated with animal protein intake within this population; 3) to assess the relative amounts of nonsweetener corn products (e.g., corn oil, corn starch), sweetener corn products, and AS intake to determine the magnitude of nonsweetener corn’s potential to confound interpretation of the AS biomarker; and 4) to determine if the dual-isotope model is a better predictor of AS/SSB intake than a model with only the δ13C value. Age is controlled for in the models, because previous investigations identified an effect of age on the model when predicting the δ13C value (25, 26, 36). We hypothesized that the δ13C value is correlated with AS/SSB and with nonsweetener corn and animal protein intakes and that the δ15N value is correlated with animal protein intake. We also hypothesized that the intake of nonsweetener corn is minimal relative to the consumption of sweetener corn and that using the dual-isotope method of δ13C and δ15N values to predict AS /SSB intake, while controlling for age, will increase the model’s explained variance.
Methods
Subjects and design.
Two hundred fifty-seven participants were included in this investigation, which used baseline data from 2 investigations. The first investigation (n = 60) included data from a previous cross-sectional trial that aimed to validate a 15-item beverage intake questionnaire (BEVQ-15) (21, 37). Participants (adults aged >21 y) were recruited from a local university community from June 2008 to June 2009. The second investigation (n = 197) is an ongoing trial [“Talking Health” (38)], which is a 6-mo, community-based, randomized controlled trial that targets SSB consumption behaviors among low-socioeconomic adult (>18 y) residents in rural southwest Virginia. Participants were recruited from April 2012 to March 2014. All participants from the validation study (n = 60) had δ15N values analyzed; however, only a subset of the Talking Health participants had δ15N values analyzed because the decision to measure both δ15N and δ13C was made after the start of data collection. Thus, a δ15N value sample size of 115 participants was used for data analyses. These studies were conducted according to the guidelines laid down in the Declaration of Helsinki. The Virginia Tech Institutional Review Board approved the study protocols, and participants provided written informed consent before enrollment.
Methods.
Participants underwent assessments of height, which was measured in meters without shoes by using a portable stadiometer, and weight, which was measured wearing light clothing without shoes to the nearest 0.1 kg by using a digital scale (model 310GS; Tanita). BMI was calculated, and dietary intake assessed by using three 24-h dietary recalls [Talking Health (38)] or one 4-d food intake record [BEVQ-15 validation study (37)]. Records and recalls were collected by trained research technicians who were supervised by a registered dietitian. The dietary intake records and recalls were analyzed by using Nutrition Data System for Research (NDSR) nutritional analysis software (39). Participants also provided demographic information (age, gender, race/ethnicity) and completed the BEVQ-15 (37, 40–42), which is a validated measure of habitual SSB consumption. All participants completed both a three-day 24-h recall or a 4-d food record as well as the BEVQ-15 (n = 257).
Fasting whole-blood samples were provided via a routine finger-stick and blotted onto sterilized Whatman spun-glass filters (type GF/D, 2.5 cm; GE Healthcare). Punches, 3.1 mm in diameter, were collected from air-dried samples, loaded into high-purity tin capsules, and quantitatively combusted to carbon dioxide and nitrogen in a Costech ECS 4010 Elemental Analyzer (Costech Analytical) coupled to a Delta V Advantage Isotope Ratio Mass Spectrometer (Thermo Fisher). Stable isotope values are reported in standard δ-notation relative to international standards (Vienna Pee Dee Belemnite for carbon and atmospheric AIR for nitrogen] by using the following equation:
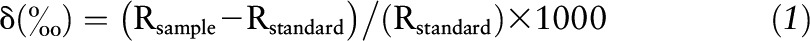 |
where R is the ratio of heavy:light isotope (13C:12C or 15N:14N). Reported values represent the mean of 3 analyses (SD of the 3 measurements never exceeded 0.05‰).
Data analysis.
Average grams of ASs were calculated by NDSR, and average grams of nonsweetener corn (whole corn, corn chips and snacks, corn tortillas, corn-based cereal grains, corn-based prepared cereals, processed meat and dairy products, and condiments) in food products were extracted from the component/ingredient level of dietary intake by using NDSR (39). Because grams of ASs are extracted from items containing ASs (e.g., gelatin), we used a similar approach to extract grams of nonsweetener corn. For whole-corn food items (corn kernels, popcorn), the weight of the product (g; excluding water weight) was considered the total nonsweetener corn product amount. Similarly, for processed food items containing corn (corn tortillas, chips, cereal), the contribution of nonsweetener corn intake to the product was estimated from the total weight of the product (excluding water weight and grams of ASs). Animal protein intake (g) was extracted from the food group amount of dietary intake by using NDSR (39).
Statistical analyses were performed by using SPSS statistical analysis software (version 21.0 for Windows, 2012; IBM). Descriptive statistics (means ± SDs and frequencies) are reported for demographic characteristics. One-factor ANOVA evaluated differences in BMI and dietary variables between varying age groups (18–24, 25–44, 45–64, and ≥65 y old); differences between groups were assessed by using Tukey’s post hoc tests. Pearson correlations were used to determine associations between δ13C and δ15N values and intakes of ASs, nonsweetener corn products, and animal protein. Paired-samples t tests were used to examine differences in mean AS and nonsweetener corn product intakes. Multiple linear regression (MLR) models using δ13C and δ15N values of finger-stick blood to predict AS and SSB intakes were used. Skewness, kurtosis, and Kolmogorov-Smirnov tests were used to confirm normal data distribution on the following variables: δ13C and δ15N values, age, and intakes of ASs and SSBs. AS and SSB variables were log-transformed due to nonnormal data distribution. Variables were entered into an MLR model by using the “enter” method with either 1 or 2 independent variables (δ13C value or δ13C and δ15N values, with age as a covariate). Tolerance and variance inflation factor statistics were used to determine if multicollinearity issues were present, and both were found to be acceptable (i.e., tolerance >0.10 and variance inflation factor <10). By using the MLR model, prediction equations for AS and SSB intakes, which accounted for animal protein intake (via the δ15N value) and age, were developed. Missing data were addressed by using list-wise deletion methods for MLR and case-by-case deletion for paired t tests and ANOVA. The multiple regression analyses recommended approach (n ≥ 50 + 8m, where m equals the number of predictor variables) to detect a moderate effect size with 80% power and an α of 0.05 was applied (43). The a priori hypothesis included a maximum of 3 predictor variables per model; therefore, ≥74 participants provide sufficient power.
Results
Demographic and dietary characteristics.
Participants (n = 257) were primarily female and white, with a mean age of 42 ± 15 y (range: 18–89 y). Although BMI was widely distributed (in kg/m2; range: 17.3–71.7), 74% of the sample was considered overweight or obese (BMI ≥25) (Table 2). Total intakes of energy, ASs, SSBs, total nonsweetener corn, and animal protein are presented in Table 3. Participants aged 25–44 y consumed significantly more ASs than those who were older than 45 y (mean difference = 24.6 g/d) and more SSBs than those who were older than 65 y (mean difference = 237 kcal/d). No significant differences between age groups were found for total energy, nonsweetener corn, or animal protein intakes.
TABLE 2.
Participant characteristics1
| n (%) | |
| Gender | |
| Male | 60 (23) |
| Female | 197 (77) |
| Race | |
| White | 235 (91) |
| African American | 12 (5) |
| Asian | 4 (1.5) |
| Other | 2 (1) |
| More than 1 race | 4 (1.5) |
| Hispanic/Latino | 2 (1) |
| BMI, kg/m2 | |
| Underweight, ≤18.4 | 3 (1) |
| Normal weight, 18.5–24.9 | 66 (25.5) |
| Overweight, 25–29.9 | 64 (25) |
| Obese, ≥30 | 124 (48.5) |
| Class 1, 30–34.9 | 46 (18) |
| Class 2, 35–39.9 | 32 (12.5) |
| Class 3, ≥40 | 46 (18) |
n = 257.
TABLE 3.
BMI and selected dietary intakes of southwest Virginian adults stratified by age1
| Age |
||||||
| Characteristics | Total | 18–24 y | 25–44 y | 45–64 y | ≥65 y | P (ANOVA) |
| n | 257 | 33 | 117 | 91 | 16 | |
| BMI, kg/m2 | 31.8 ± 9 | 28.3 ± 9a | 33.8 ± 10b | 31.3 ± 8a,b | 27.2 ± 5a | ≤0.01 |
| Total energy,2 kcal/d | 1867 ± 675 | 1869 ± 619 | 1961 ± 727 | 1756 ± 641 | 1804 ± 516 | 0.19 |
| Added sugars,2 g/d | 88.8 ± 58.8 | 84.0 ± 43.0a,b | 102 ± 67.2a | 77.8 ± 51.7b | 61.3 ± 32.8b | ≤0.01 |
| Sugar-sweetened beverages,3 kcal/d | 359 ± 347 | 392 ± 380a | 420 ± 370a | 299 ± 300a,b | 183 ± 264b | ≤0.05 |
| Total nonsweetener corn,2 g/d | 12.6 ± 13.3 | 8.7 ± 8.6 | 14.9 ± 14.6 | 11.4 ± 12.4 | 11.5 ± 14.1 | 0.06 |
| Animal protein,2 g/d | 45.9 ± 21.8 | 49.9 ± 20.2 | 46.2 ± 22.5 | 44.4 ± 22.2 | 44.4 ± 18.4 | 0.50 |
| δ13C, ‰ | −19.1 ± 0.9 | −19.0 ± 0.8a,b | −19.0 ± 0.9a | −19.3 ± 0.7b,c | −19.7 ± 0.5c | ≤0.001 |
| δ15N,4 ‰ | 7.4 ± 0.5 | 7.2 ± 0.5a | 7.5 ± 0.5a | 7.4 ± 0.4a | 7.2 ± 0.3a | ≤0.05 |
Finger-stick δ13C values ranged from −22.3‰ to −17.0‰ and δ15N values ranged from 6.1‰ to 8.6‰ (Table 3). Individuals aged 25–44 y demonstrated significantly higher δ13C values than did those aged 45–64 y (mean difference ± SE = 0.3 ± 0.1, P = 0.02) and those older than 65 y (mean difference ± SE = 0.8 ± 0.2, P ≤ 0.01). The overall association of the δ15N value and age was significant; however, post hoc tests were unable to detect with significant confidence which pairs of means differed between age groups.
Aims 1 and 2: determining associations of dietary variables with δ13C and δ15N.
The δ13C value was significantly correlated with intakes of ASs (r = 0.32, P ≤ 0.01) and SSBs (r = 0.39, P ≤ 0.01) but was not significantly correlated with intake of total nonsweetener corn (r = 0.06, P = 0.31); in addition, no significant correlations of δ13C or δ15N values were found with animal protein intake (r = 0.11, P = 0.08, and r = 0.12, P = 0.22, respectively) in this sample. However, when different sources of animal protein intake were examined, it was found that both intake of fish/shellfish (r = 0.12, P ≤ 0.05) and red meat (r = 0.16, P ≤ 0.05) were significantly correlated with δ13C values. No significant correlations were found for poultry intake and δ13C or for any animal protein sources and δ15N values. Fish/shellfish, poultry, and red meat intake comprised 18%, 53%, and 29%, respectively, of total animal protein consumption.
Aim 3: determining relative dietary contributions of nonsweetener corn and AS.
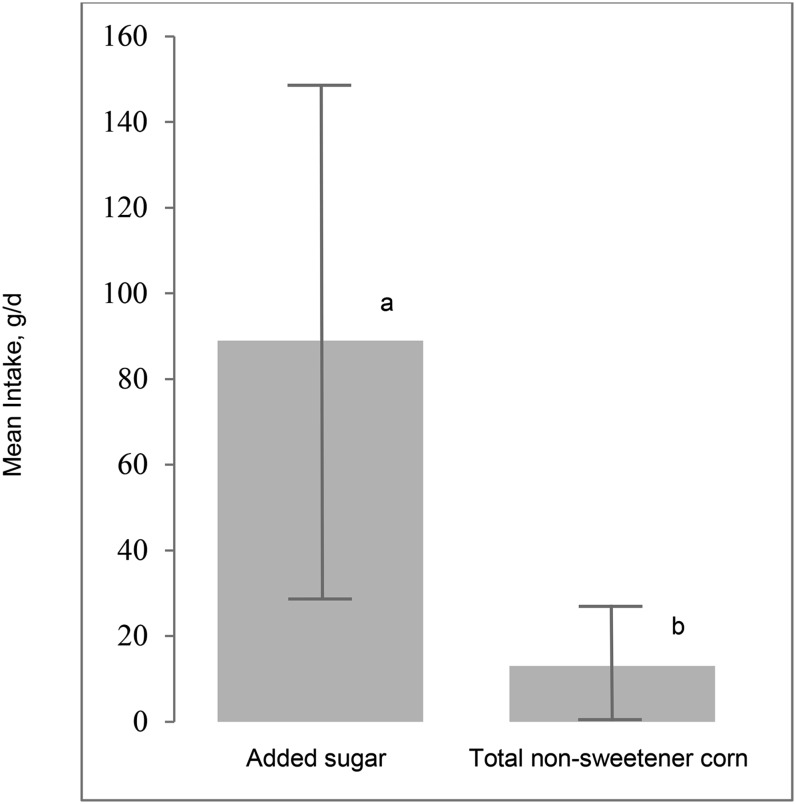
Mean AS intake ranged from 5.4 to 330 g/d, and mean total nonsweetener corn intake ranged from 0 to 86.0 g/d. Intake of ASs was significantly greater than intake of total nonsweetener corn (mean difference = 76.2 ± 57.2 g/d, P ≤ 0.001) (Figure 1).
FIGURE 1.
Contributions of added sugar and nonsweetener corn to the diet of southwest Virginian adults. Values are means ± SDs, n = 257. Means without a common letter differ, P ≤ 0.05.
Aim 4: Predicting AS and SSB intake from δ13C and δ\5N values.
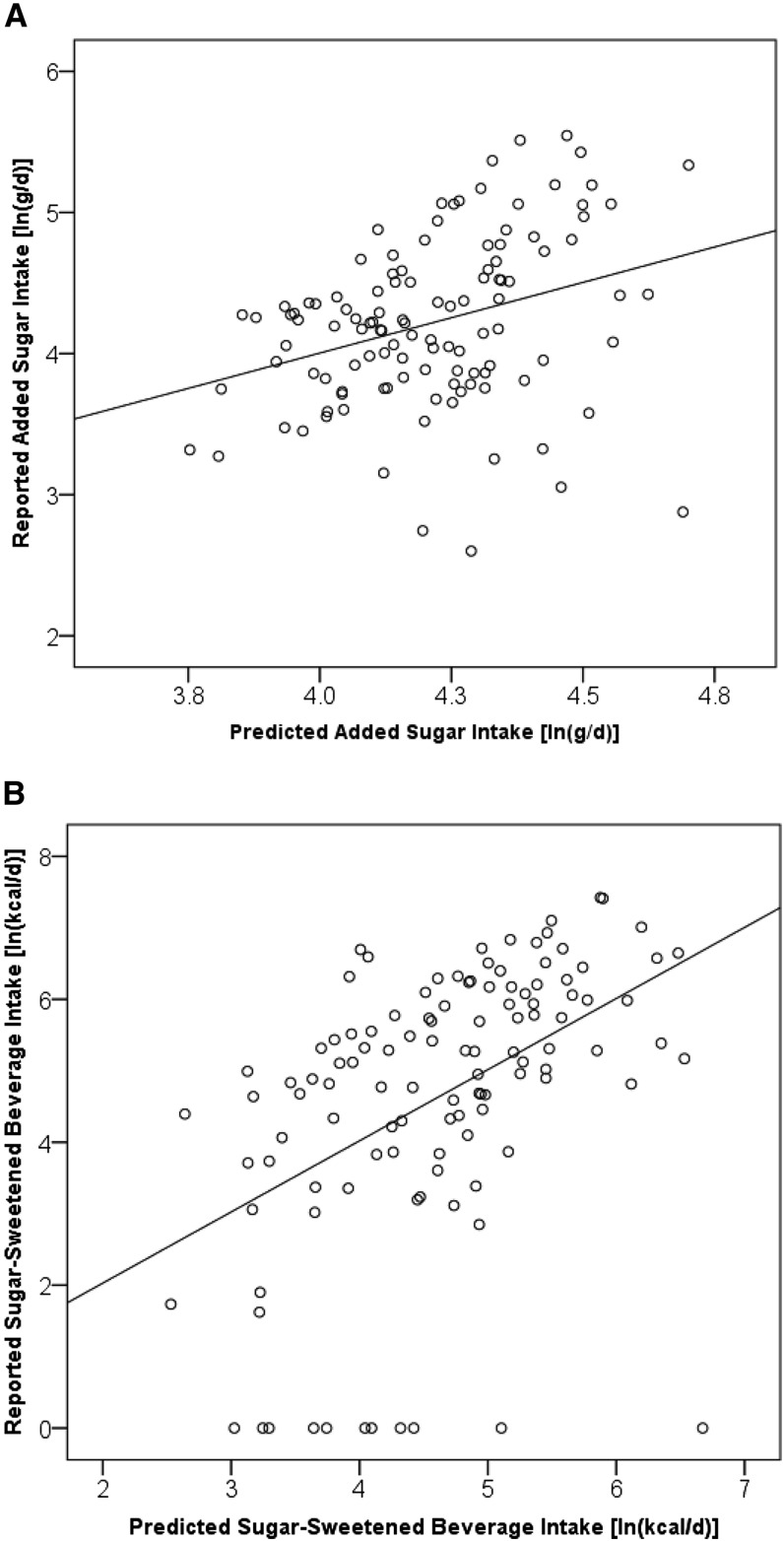
To assess the utility of the dual-isotope method, 4 MLR models (n = 115) were generated (Table 4). In model 1a, only δ13C was a significant predictor of AS intake (P ≤ 0.01); however; in model 2a, both the δ13C value and age were significant predictors of SSB intake (P ≤ 0.001). With the addition of the δ15N value, models 1b and 2b remained significant; however, minimal changes in R2 were observed (i.e., R2 change: 0.21 to 0.22). In model 1b predicting AS intake, the β-weights were no longer significant; however, for model 2b predicting SSB intake, the β-weights for the δ13C value and age remained relatively consistent and significant (P ≤ 0.01). In both models, the δ15N value was not predictive of AS or SSB intake. Figure 2 shows the relation between reported and predicted AS (Figure 2A) and SSB (Figure 2B) intakes using the following equations generated from the MLR models with the use of δ13C and δ15N values and age as predictors (AS R2 = 0.11, SSB R2 = 0.22; both P ≤ 0.001):
TABLE 4.
Predicting added sugar and sugar-sweetened beverage intake in southwest Virginian adults from δ13C and δ15N1
| Model and predictors | β | P | 95% CI | R2 | P |
| Added sugars, g/d | |||||
| Model 1a | |||||
| δ13C | 0.28 | ≤0.01 | 0.07, 0.31 | 0.09 | ≤0.01 |
| Age | −0.11 | 0.23 | −0.01, 0.01 | ||
| Model 1b | |||||
| δ13C | 0.19 | 0.10 | −0.20, 0.28 | 0.11 | ≤0.01 |
| δ15N | 0.15 | 0.18 | −0.09, 0.45 | ||
| Age | −0.11 | 0.23 | −0.09, 0.45 | ||
| Sugar-sweetened beverages, kcal/d | |||||
| Model 2a | |||||
| δ13C | 0.35 | ≤0.001 | 0.40, 1.12 | 0.21 | ≤0.001 |
| Age | −0.28 | ≤0.001 | −0.06, −0.01 | ||
| Model 2b | |||||
| δ13C | 0.29 | ≤0.01 | 0.19, 1.09 | 0.22 | ≤0.001 |
| δ15N | 0.10 | 0.90 | −0.44, 1.18 | ||
| Age | −0.28 | ≤0.01 | −0.06, −0.01 |
n = 115. The dependent variables (added sugar, sugar-sweetened beverages) were log-transformed. β, standardized β-weight.
FIGURE 2.
Associations of reported and predicted added sugar and sugar-sweetened beverage intake of southwest Virginian adults (n = 115). Values are log-transformed.
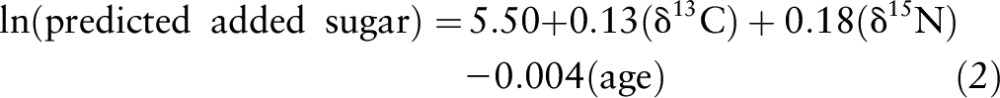 |
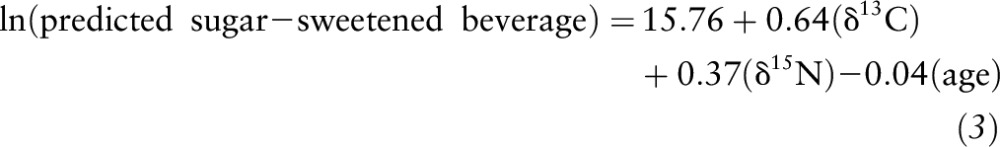 |
Discussion
This investigation contributes new information to a growing body of literature addressing δ13C as a potential biomarker of AS and SSB intakes (20–23, 25–29), by reporting findings from a large sample of community-dwelling US adults using the minimally invasive finger-stick sampling method. We explored potential confounds (age, nonsweetener corn intake, and animal protein intake) on the basis of findings from previous investigations (20, 23, 25–27) and found that age was a significant predictor of SSB but not AS intake, nonsweetener corn intake relative to ASs was significantly lower, nonsweetener corn and total animal protein intakes were not significantly correlated with the δ13C value, and total animal protein intake was not correlated with the δ15N value. We evaluated the ability of a dual-isotope method (δ13C and δ15N values) to predict AS and SSB intakes in a US population using an approach similar to that of Nash et al. (26, 27) in a Yup’ik population. Our findings were not consistent with our hypothesis or with the findings of Nash et al. (26) in that the addition of the δ15N value (dual-isotope method) did not significantly increase the explained variance of the model when predicting AS and SSB intakes. We note that key attributes of diet differed between the Yup’ik population studied by Nash et al. (26) and the population of our study: within our study, AS intake was slightly higher (89 vs. 74 g), SSB intake was substantially higher [2.5 vs. 1.4 servings (240 mL/8 fluid ounce serving size)], and marine and terrestrial animal protein intake was lower (1% vs. 18% and 8% vs. 11% of total energy intake, respectively). In addition, the mean δ13C value for this sample (−19.1‰) was greater than that reported in previous investigations [−19.3‰ (23) and −19.8‰ (26)].
Age.
δ13C values decreased significantly with age, but differences in δ15N values across age groups were not significant. In contrast, Nash et al. (26, 36) reported differences by age in δ13C values as well as in δ15N values, which may be attributed to the relative higher intake of marine animal protein as compared with that of the population in our study. Because age was significantly correlated with the δ13C value and intakes of ASs and SSBs in this investigation, and it was shown to be a significant predictor of the δ13C value in a previous study (25), the present investigation used age as a covariate when predicting AS and SSB intakes. Differences in AS and SSB intakes across age groups were similar to a comparable study sample (26), and others reported that different age groups consume varying amounts of ASs and SSBs (i.e., younger adults consume more ASs and SSBs than do older adults) (2, 44), which is consistent with findings from the present investigation.
Nonsweetener corn.
Nonsweetener corn consumption was comparable to intakes from Nash et al. (26) (11 vs. 12.6 g), and both studies showed no significant correlations of nonsweetener corn intake with δ13C blood values. This may be due in part to the significant differences in consumption of nonsweetener corn compared with other C4 sources of ASs (cane sugar, HFCS). In 2010, via the USDA’s Economic Research Service, the per capita availability of cane/beet sugar was 30 kg, total corn sweeteners was 29 kg, and nonsweetener corn products was 15 kg (relative contributions of cane vs. beet sugar are 45% and 55%, respectively) (32). When taking the relative contribution of beet sugar into consideration, the proportion of per capita availability of nonsweetener corn was less than one-third of the availability of ASs (cane and corn sweeteners). In comparison to this sample, the proportion of nonsweetener corn consumption to ASs was 16%, which was slightly less than US per capita availability. Two additional investigations also explored the impact of nonsweetener corn intake on the δ13C value within US populations. Fakhouri et al. (23) found no significant correlation between the δ13C value and nonsweetener corn intake (56% of participants were African American), which was assessed as a percentage of consumers (e.g., the percentage of people who reported consuming nonsweetener corn on a 24-h dietary recall) as opposed to actual total gram intake. Conversely, Yeung et al. (31) found a significant, but weak, correlation between the δ13C value and whole-corn intake (no race categories provided). Although the present investigation did not show a significant impact of nonsweetener corn consumption on δ13C blood values, due to the majority of participants being white, there are potential implications to these findings because other populations may consume greater amounts of nonsweetener corn products [e.g., Hispanic populations (45)].
Animal protein.
In this sample, no significant correlations between animal protein intake (marine or terrestrial) and δ15N values were found; however, δ13C values were found to be positively associated with both marine and terrestrial animal protein. The contrast between our results and those of previous studies (with other sample substrates not including finger-stick samples), which established significant associations between animal protein intake and both δ13C and δ15N values, may be attributable to multiple geocultural dietary factors (34). Relative to Nash et al. (26), the intake of marine animals by our sample population, which possess higher δ15N values relative to their terrestrial counterparts (34), was significantly lower. Furthermore, terrestrial protein intake, which comprised a majority of animal protein in our sample population, was not associated with δ15N in their sample population (26). Petzke et al. (46) found an association between δ13C and δ15N values and meat intake in a German population consuming primarily terrestrial animal proteins [and higher animal protein intake compared with this investigation (71 vs. 46 g/d)]. An additional investigation, based in the United Kingdom, found positive associations between δ13C and δ15N values and fish protein; however, only δ15N values were associated with terrestrial animal protein intake (47). The differences between our results and those in European populations may be explained by the higher overall meat intake in their population, resulting in larger intake variations between omnivorous and vegetarians in their sample set (47), or by the different feeding practices in German vs. American livestock; grass-fed cattle, which predominate in Europe, may possess higher overall δ15N values than those fed corn-based diets, possibly due to the fact that conventionally grown crops fed to American cattle are depleted in δ15N relative to grass/hay due to low δ15N values of synthetic fertilizers used in their production (48). Further studies within an American population are warranted to determine the association between animal protein intake and tissue isotope values.
Predicting AS and SSB intakes from δ13C and δ\5N values.
By using only the δ13C value and age to predict AS and SSB intakes, this sample produced higher R2 values than a previous study (26) (ASs = 0.09 vs. 0.03; SSBs = 0.21 vs. 0.05) and higher β-weights for the δ13C value (ASs = 0.28 vs. 0.20; SSBs = 0.35 vs. 0.21). Age was not a significant predictor of AS intake, although it was for SSB intake. This provides additional evidence that age should be included in AS/SSB prediction models, because the δ13C value and age were both significant predictors and the δ13C value produced a β-weight comparable to that reported by Nash et al. (26). When the δ15N value was added as a predictor, minimal changes were shown in R2 values and β-weights for the δ13C value and age in the SSB model. In addition, the δ15N value was not considered a significant predictor in either model, which contrasts with previous findings (26).
This investigation extends findings by Nash et al. (26, 27) who investigated a unique population who consumes greater quantities of fish and marine animal protein than the US adult population. The present study inferentially evaluated the effects of animal protein intake in grams [i.e., absolute intake vs. percentage of energy (26)] and nonsweetener corn intake in grams [vs. percentage of consumers (23)]. We nevertheless acknowledge several limitations of this investigation. The consumption of C3 plants (beet sugar and maple syrup) is not reflected by this AS biomarker and this sample’s analysis of AS does not distinguish between C3 and C4 sources. However, because C3 plants provide only ∼22% of ASs in the US diet (which translates to ∼3% of total energy intake) (Table 1) and SSBs are typically sweetened by C4 sweeteners (HFCS), this may account for the lower R2 value when predicting AS intake than when predicting SSB intake. In addition, AS intake was assessed via food intake records and recalls, whereas SSB intake was determined by the BEVQ-15 (37, 40, 41), which possibly explains the higher correlation of the δ13C value to habitual SSB intake rather than recent AS consumption, because the whole-blood δ13C value reflects intake over several months (20). However, it should be noted as a limitation that there is a potential for different levels of misreporting/error between the food records/recalls and the BEVQ-15. Typical correlations of nutritional biomarkers to their respective dietary variables average ∼0.39; however, a wide range of correlations have been reported when evaluating dietary biomarkers (0.03–0.73) (11). Nonetheless, acceptable correlations for this area of research range from 0.5 to 0.7 (11). Although the reported values are below this threshold (∼0.2), as with any study using self-reported dietary intake over-/underreporting errors are possible, thus demonstrating the need for future controlled feeding studies to further refine this AS/SSB biomarker approach.
In conclusion, the analysis of these cross-sectional data does not provide additional evidence that the dual-isotope method (i.e., measurement of both the δ13C and δ15N value) is superior for predicting AS and SSB intakes within a US population. Data do suggest that the δ13C value of finger-stick blood is an objective measure of AS and SSB intake and that age should be considered as a significant predictor of AS and SSB consumption. Future directions should include further assessing the impact of various types of animal proteins (specifically fish) on the dual-isotope method and exploring the potential of this biomarker within racially diverse populations (23), as well as in children and adolescents. The δ13C value of finger-stick blood is a promising biomarker of AS and SSB intake, which could be used in community and field-type settings and in large-scale epidemiologic trials because of the ease of sample collection.
Acknowledgments
We thank Drs. Wen You and Jyoti Tina Savla for providing statistical guidance. VEH, JMZ, AHJ, NAW, JNB, and BMD designed the research, wrote the manuscript, and had primary responsibility for final content; VEH and NAW conducted the research; VEH, NAW, and JNB analyzed the data; VEH performed statistical analysis; and AHJ and JNB provided essential reagents and materials. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AS, added sugar; BEVQ-15, 15-Item Beverage Intake Questionnaire; HFCS, high-fructose corn syrup; MLR, multiple linear regression; NDSR, Nutrition Data System for Research; SSB, sugar-sweetened beverage.
References
- 1.Kit BK, Fakhouri TH, Park S, Nielsen SJ, Ogden CL. Trends in sugar-sweetened beverage consumption among youth and adults in the United States: 1999–2010. Am J Clin Nutr 2013;98:180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marriott BP, Olsho L, Hadden L, Connor P. Intake of added sugars and selected nutrients in the United States, National Health and Nutrition Examination Survey (NHANES) 2003–2006. Crit Rev Food Sci Nutr 2010;50:228–58. [DOI] [PubMed] [Google Scholar]
- 3.Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr 2002;76:911–22. [DOI] [PubMed] [Google Scholar]
- 4.Yang Q, Zhang Z, Gregg EW, Flanders WD, Merritt R, Hu FB. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern Med 2014;174:516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu FB. Resolved: there is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes Rev 2013;14:606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson RK, Appel LJ, Brands M, Howard BV, Lefevre M, Lustig RH, Sacks F, Steffen LM, Wylie-Rosett J. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation 2009;120:1011–20. [DOI] [PubMed] [Google Scholar]
- 7.Farley T, Just DR, Wansink B. Clinical decisions: regulation of sugar-sweetened beverages. N Engl J Med 2012;367:1464–6. [DOI] [PubMed] [Google Scholar]
- 8.Pomeranz JL. The bittersweet truth about sugar labeling regulations: they are achievable and overdue. Am J Public Health 2012;102:e14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sturm R, Powell LM, Chriqui JF, Chaloupka FJ. Soda taxes, soft drink consumption, and children's body mass index. Health Aff (Millwood) 2010;29:1052–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monsen E. Research: successful approaches. 2nd ed Chicago (IL): American Dietetic Association; 2003. [Google Scholar]
- 11.Willett WC, Lenart E. Nutritional epidemiology. 2nd ed New York: Oxford University Press; 1998. [Google Scholar]
- 12.Thompson FE, Subar AF. Nutrition in the prevention and treatment of disease. In: Dietary assessment methodology. 2nd ed Coulston AM, Boushey CJ, editors. San Diego: Academic Press; 2008. p. 3–39. [Google Scholar]
- 13.Schoeller DA, Thomas D, Archer E, Heymsfield SB, Blair SN, Goran MI, Hill JO, Atkinson RL, Corkey BE, Foreyt J, et al. . Self-report-based estimates of energy intake offer an inadequate basis for scientific conclusions. Am J Clin Nutr 2013;97:1413–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Althuis MD, Weed DL. Evidence mapping: methodologic foundations and application to intervention and observational research on sugar-sweetened beverages and health outcomes. Am J Clin Nutr 2013;98:755–68. [DOI] [PubMed] [Google Scholar]
- 15.Institute of Medicine of the National Academies. Dietary Reference Intakes: research synthesis workshop summary. Washington (DC): The National Academies Press; 2007. [Google Scholar]
- 16.Hardin DS. Validating dietary intake with biochemical markers. J Am Diet Assoc 2009;109:1698–9. [DOI] [PubMed] [Google Scholar]
- 17.Hedrick VE, Dietrich AM, Estabrooks PA, Savla J, Serrano E, Davy BM. Dietary biomarkers: advances, limitations and future directions. Nutr J 2012;11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCabe-Sellers B. Advancing the art and science of dietary assessment through technology. J Am Diet Assoc 2010;110:52–4. [DOI] [PubMed] [Google Scholar]
- 19.Kuhnle GG. Nutritional biomarkers for objective dietary assessment. J Sci Food Agric 2012;92:1145–9. [DOI] [PubMed] [Google Scholar]
- 20.Jahren AH, Bostic JN, Davy BM. The potential for a carbon stable isotope biomarker of dietary sugar intake. J Anal At Spectrom 2014;29:795–816. [Google Scholar]
- 21.Davy BM, Jahren AH, Hedrick VE, Comber DL. Association of δ13C in fingerstick blood with added-sugar and sugar-sweetened beverage intake. J Am Diet Assoc 2011;111:874–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraft RA, Jahren AH, Saudek CD. Clinical-scale investigation of stable isotopes in human blood: δ13C and δ15N from 406 patients at the Johns Hopkins Medical Institutions. Rapid Commun Mass Spectrom 2008;22:3683–92. [DOI] [PubMed] [Google Scholar]
- 23.Fakhouri THI, Jahren AH, Appel LJ, Chen L, Alavi R, Anderson CAM. Serum carbon isotope values change in adults in response to changes in sugar-sweetened beverage intake. J Nutr 2014;144:902–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook CM, Alvig AL, Liu YQ, Schoeller DA. The natural 13C abundance of plasma glucose is a useful biomarker of recent dietary caloric sweetener intake. J Nutr 2010;140:333–7. [DOI] [PubMed] [Google Scholar]
- 25.Hedrick V, Davy B, Wilburn G, Jahren AH, Zoellner J. Evaluation of a novel biomarker of added sugar intake (δ13C) compared to self-reported added sugar intake and the healthy eating index in a community-based, rural US sample. Publ Health Nutr. In press. [DOI] [PMC free article] [PubMed]
- 26.Nash SH, Kristal AR, Bersamin A, Hopkins SE, Boyer BB, O'Brien DM. Carbon and nitrogen stable isotope ratios predict intake of sweeteners in a Yup'ik study population. J Nutr 2013;143:161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nash SH, Kristal AR, Hopkins SE, Boyer BB, O'Brien DM. Stable isotope models of sugar intake using hair, red blood cells, and plasma, but not fasting plasma glucose, predict sugar intake in a Yup'ik study population. J Nutr 2014;144:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jahren AH, Kraft RA. Carbon and nitrogen stable isotopes in fast food: signatures of corn and confinement. Proc Natl Acad Sci USA 2008;105:17855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jahren AH, Saudek C, Yeung EH, Kao WL, Kraft RA, Caballero B. An isotopic method for quantifying sweeteners derived from corn and sugar cane. Am J Clin Nutr 2006;84:1380–4. [DOI] [PubMed] [Google Scholar]
- 30.DeNiro M, Epstein S. Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta 1978;42:495–506. [Google Scholar]
- 31.Yeung EH, Saudek CD, Jahren AH, Kao WH, Islas M, Kraft R, Coresh J, Anderson CA. Evaluation of a novel isotope biomarker for dietary consumption of sweets. Am J Epidemiol 2010;172:1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.US Department of Agriculture, Economic Research Service. US per capita food availability: caloric sweeteners by subgroup [cited 2010 Jul 9]. Available from: www.ers.usda.gov/Data/FoodConsumption/app/reports/displayCommodities.
- 33.US Department of Agriculture, Economic Research Service. Sugar and sweeteners [cited 2014 Apr 9]. Available from: http://www.ers.usda.gov/topics/crops/sugar-sweeteners.aspx#.U0WZgVfLLng.
- 34.Huelsemann F, Koehler K, Braun H, Schaenzer W, Flenker U. Human dietary δ15N intake: representative data for principle food items. Am J Phys Anthropol 2013;152:58–66. [DOI] [PubMed] [Google Scholar]
- 35.Nash SH, Kristal AR, Bersamin A, Choy K, Hopkins SE, Stanhope KL, Havel PJ, Boyer BB, O'Brien DM. Isotopic estimates of sugar intake are related to chronic disease risk factors but not obesity in an Alaska native (Yup'ik) study population. Eur J Clin Nutr 2014;68:91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nash SH, Bersamin A, Kristal AR, Hopkins SE, Church RS, Pasker RL, Luick BR, Mohatt GV, Boyer BB, O'Brien DM. Stable nitrogen and carbon isotope ratios indicate traditional and market food intake in an indigenous circumpolar population. J Nutr 2012;142:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hedrick VE, Comber DL, Estabrooks PA, Savla J, Davy BM. The Beverage Intake Questionnaire: determining initial validity and reliability. J Am Diet Assoc 2010;110:1227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zoellner J, Chen Y, Davy B, You W, Hedrick V, Corsi T, Estabrooks P. Talking Health, a pragmatic randomized-controlled health literacy trial targeting sugar-sweetened beverage consumption among adults: rationale, design and methods. Contemp Clin Trials 2014;37:43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nutrition Coordinating Center. Nutrition Data System for Research. Minneapolis (MN): University of Minnesota; 2011. [Google Scholar]
- 40.Hedrick VE, Comber DL, Ferguson KE, Estabrooks PA, Savla J, Dietrich AM, Serrano E, Davy BM. A rapid beverage intake questionnaire can detect changes in beverage intake. Eat Behav 2013;14:90–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hedrick VE, Savla J, Comber DL, Flack KD, Estabrooks PA, Nsiah-Kumi PA, Ortmeier S, Davy BM. Development of a brief questionnaire to assess habitual beverage intake (BEVQ-15): sugar-sweetened beverages and total beverage energy intake. J Acad Nutr Diet 2012;112:840–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riebl SK, Paone AC, Hedrick VE, Zoellner JM, Estabrooks PA, Davy BM. The comparative validity of interactive multimedia questionnaires to paper-administered questionnaires for beverage intake and physical activity: pilot study. JMIR Res Protoc. 2013;2:e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Green S. How many subjects does it take to do a regression analysis. Multivariate Behav Res 1991;26:499–510. [DOI] [PubMed] [Google Scholar]
- 44.Popkin BM. Patterns of beverage use across the lifecycle. Physiol Behav 2010;100:4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bressani R, Rooney L, Serna Saldivar S. Fortification of corn masa flour with iron and/or other nutrients: a literature and industry experience review. Washington (DC): Sharing United States Technology to Aid in the Improvement of Nutrition (SUSTAIN); 1997. p. 103. [Google Scholar]
- 46.Petzke KJ, Boeing H, Klaus S, Metges CC. Carbon and nitrogen stable isotopic composition of hair protein and amino acids can be used as biomarkers for animal-derived dietary protein intake in humans. J Nutr 2005;135:1515–20. [DOI] [PubMed] [Google Scholar]
- 47.Patel PS, Cooper AJ, O'Connell TC, Kuhnle GG, Kneale CK, Mulligan AM, Luben RN, Brage S, Khaw KT, Wareham NJ, et al. . Serum carbon and nitrogen stable isotopes as potential biomarkers of dietary intake and their relation with incident type 2 diabetes: the EPIC-Norfolk study. Am J Clin Nutr 2014;100:708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bateman AS, Kelly S, Woolfe M. Nitrogen isotope composition of organically and conventionally grown crops. J Agric Food Chem 2007;55:2664–70. [DOI] [PubMed] [Google Scholar]