Abstract
A 41-year-old male-to-female (MtF) transgender patient presented with a symptomatic tender lump in the left breast. There was no family history of breast cancer. She had been receiving estrogen therapy for 14 years to maintain her secondary sexual characteristics. Triple assessment revealed a 13 mm triple-negative grade 3 invasive ductal carcinoma. The tumour was completely excised following a left wide local excision and sentinel lymph node biopsy. There was no regional lymph node involvement. She was referred to the oncologist for adjuvant chemotherapy and radiotherapy.
Background
The incidence of male breast cancer is less than 1%.1 The incidence, however, in male-to-female (MtF) transgender is unknown. Speculations have been made about the risk of long-term use of hormonal treatment following transgender surgery but have not yet been proven.
In the literature, only 15 cases have ever been reported (table 1).
Table 1.
Breast cancer in MtF transgender patients receiving estrogen, in the literature
| Study | Tumour type | Age | Duration of estrogen therapy | Receptor status |
|---|---|---|---|---|
| Symmers2 | Primary mammary adenocarcinoma | 30 | 5 years | NA |
| Symmers2 | Intraductal adenocarcinoma | 30 | Unknown | NA |
| Pritchard et al3 | Invasive ductal carcinoma | 35 | 10 years | ER−, PR + |
| Ganly and Taylor4 | Invasive ductal carcinoma | 36 | 14 years | ER−, PR NA |
| Grabellus et al5 | Secretory breast carcinoma, ETV6-NTRK3 gene fusion | 46 | 8 years | ER−, PR−, HER2− |
| Kelley6 | Phyllodes* | 53 | Unknown but ≥8 years | ER−, PR−, HER2− |
| Dhand and Dhaliwal 7 | Needle aspiration | 58 | 11 years | ER+, PR+, HER2 NA |
| Pattison and McLaren8 | Invasive ductal carcinoma | 43 | 13 years | ER−, PR−, HER2− |
| Gooren et al9 | Ductal carcinoma | 57 | 36 years | ER+, PR−, HER2− |
| Gooren et al9 | Poor differentiated carcinoma in lymph nodes† | 56 | Unknown but ≥8 years | NA |
| Maglione et al10 | Ductal carcinoma in situ* | 65 | 13 years | ER+, PR+, HER2 NA |
| Maglione et al10 | Invasive ductal carcinoma | 55 | 30 years | ER−, PR−, HER2+ |
| Sattari11 | Infiltrating ductal carcinoma | 60 | 8 years | ER+, PR+, HER2− |
| Gooren et al12 | Invasive ductal carcinoma* | 46 | Unknown but ≥6 years | ER+, PR+, HER2+ |
| Gooren et al12 | Adenocarcinoma | 52 | 30 years | ER+, PR−, HER2 NA |
| Teoh et al | Invasive ductal carcinoma | 41 | 14 years | ER−, PR−, HER2− |
*Family history of breast cancer.
†Primary origin most likely from breast tumour removed 10 years earlier but not proven histologically.
ER, estrogen receptor; ETV6, ETS variant gene; HER2, human epidermal growth factor receptor 2; MtF, male-to-female; NA, not applicable; NTRK3, neurotrophic tyrosine kinase receptor type 3; PR, progesterone receptor.
However, in the few reported cases of breast cancer in MtF transgender patients, there appears to be a higher prevalence of hormone receptor-negative (table 1) and triple-negative tumours.5–6 8
Triple-negative breast cancer is biologically aggressive, has a worse prognosis and lacks a therapeutic target in contrast with hormone receptor-positive and HER2+ breast cancers.13 As a result, they pose a challenge in clinical practice.
We present a case of an MtF transgender patient diagnosed with a triple-negative invasive ductal carcinoma following long-term estrogen therapy. This is a contribution to the growing literature questioning the role of long-term estrogen therapy as a risk factor for hormone receptor-negative breast cancer in MtF transgender patients.
Case presentation
A 41-year-old MtF transgender patient with a tender lump in the upper pole of the left breast was referred urgently to the breast clinic by her general practitioner. She had undergone staged gender reassignment surgery, which included bilateral orchiectomy, penectomy and bilateral breast augmentation 4 years later. Subsequently, the patient received both oral estrogen and antiandrogen therapy for 14 years. There was no apparent family history of breast cancer and no other significant risk factors for breast cancer.
Clinical examination revealed a 13 mm hard, tender mass in the upper inner quadrant of the left breast, highly suspicious of a malignancy. There was no clinical evidence of regional or distant disease.
Investigations
Triple assessment confirmed a triple-negative invasive ductal carcinoma.
Treatment
After a long discussion between doctor and patient, the patient underwent a wide local excision, sentinel lymph node biopsy (SLNB) and removal of bilateral implants.
The histology from the excised specimen confirmed a 22 mm grade 3 triple-negative invasive ductal carcinoma (figure 1), surrounded by an area of high-grade ductal carcinoma in situ, 60 mm in diameter. The SLNB was negative for metastases.
Figure 1.
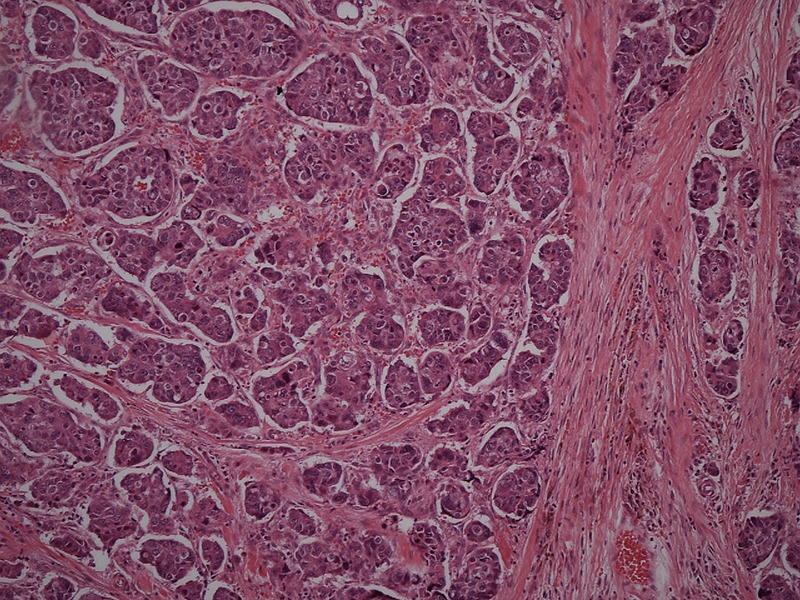
Grade 3 invasive ductal carcinoma (H&E stain ×100).
Outcome and follow-up
Following her surgery, the patient was referred to the oncologist for adjuvant chemotherapy and radiotherapy. She continues taking conjugated estrogen and antiandrogens.
Prior to her operation, the patient received a staging CT of the thorax, abdomen and pelvis. This showed a subtle 13 mm hypoattenuated lesion in the liver. On further characterisation with a liver MRI, these were thought to be incidentalomas. It was decided in the breast multidisciplinary team that these lesions will be monitored closely with serial imaging.
Discussion
The jury is still out on the long-term safety of administering estrogen therapy to MtF transgender patients, particularly with regard to the development of hormone-dependent cancers.
The total cancer mortality in MtF transgender patients remains comparable to the general population.14
Only 15 cases of breast cancer in MtF transgender patients have been reported in the current literature (table 1). These reports include four cases from an Amsterdam gender clinic following 2307 MtF transgender patients since 1975.9 12 This led the authors of the Amsterdam follow-up cohort study to conclude that there is insufficient evidence to suggest an increased risk of breast cancer in MtF transgender individuals receiving estrogen therapy.9
However, the authors were cautious about their findings, which were based on relatively few incident cases.9 12 They also point out that the study's follow-up period is still relatively short and it remains to be seen whether more patients will present over the next decade, with prolonged exposure to estrogen and from ageing.
Theoretically, long-term estrogen therapy could increase the risk of breast cancer. In female patients, the use of estrogen-only therapy has been shown to increase the risk of breast cancer.15 In men, high estrogen levels associated with Klinefelter's syndrome, testicular dysfunction and obesity16 are recognised risk factors for male breast cancer.
However, should breast cancer in MtF transgender patients be considered as male or female breast cancer?
There is some evidence from these reports, albeit weak, to infer that breast cancer in MtF transgender patients may differ from male breast cancers, in terms of hormonal status and age of onset.
Male breast cancers have a peak age of onset at 71 years,16 in contrast to MtF transgender patients, who appear to have an earlier onset (median age of 49 years, see table 1).
Only 10% of male breast cancers are reported as estrogen receptor (ER) negative.17 In contrast, of the 13 cases (including this one) that reported on ER status, 7 (54%) were ER negative. These biological differences could potentially impact on clinical prognosis. A recent study by Chen et al18 stated that male breast cancers had poorer survival outcomes when compared to female breast cancers, which may be attributed to their biological differences.
Invasive ductal carcinoma is the most common histological subtype described from the case reports.
In females, the cessation of estrogen therapy is generally advocated on the development of breast cancer irrespective of the hormone receptor status. However, in transgender patients, discontinuation of such therapy will result in the return of unwanted secondary sexual characteristics with grave psychological consequences.
Historically, high-dose estrogen therapy has been used for the treatment of advanced breast cancer.19 Recently, a clinical trial of low-dose estradiol in women with advanced aromatase inhibitor-resistant breast cancer reported stability of disease, although no objective response was demonstrated.20 It is, therefore, not unreasonable to continue estrogen therapy in the context of MtF transgender patients following treatment of breast cancer.
In this case, after careful deliberation, the patient came to a decision to remain on estrogen therapy, fully aware of the potential risks involved.
We report this case to highlight the frequent occurrence of hormone receptor negative breast cancer in the few reported cases in MtF transgender patients and the implications in practice. In view of the increasing number of patients undergoing transgender surgery,21 patients need to be fully aware of the risk of malignant breast disease, however small.
There might even be an argument to enrol these patients in suitable screening programmes. Weyers et al22 showed that mammography and breast sonography are technically feasible and well accepted among MtF transgender patients. Currently, at least two professional bodies have recommended that MtF transgender patients be enrolled in breast screening programmes similar to their biological female counterparts.23 24 Having said that, further research is warranted to aid quantify this risk to inform the debate on breast cancer surveillance in this patient group.
Learning points.
Male-to-female (MtF) transgender patients tend to have hormone receptor-negative breast cancer.
Primary health practitioners should have a low threshold for suspecting breast cancer in MtF transgender patients on long-term estrogen therapy.
Continuation of estrogen therapy after treatment for breast cancer must be balanced with the potential medical risks, but not ignoring patient's psychosocial needs.
Enrolment in breast screening programmes should be considered for MtF transgender patients.
Footnotes
Contributors: ZHT and DA were involved in the writing and analysis of this manuscript. TG, who oversaw the management of this patient, gave the final approval of the version published.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cancer Research UK. Breast cancer incidence in males. http://www.cancerresearchuk.org/cancer-info/cancerstats/types/breast/incidence/uk-breast-cancer-incidence-statistics#males (accessed 12 Dec 2014).
- 2.Symmers WS. Carcinoma of breast in trans-sexual individuals after surgical and hormonal interference with the primary and secondary sex characteristics. BMJ 1968;2:83–5. 10.1136/bmj.2.5597.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pritchard TJ, Pankowsky DA, Crowe JP et al. . Breast cancer in a male-to-female transsexual: a case report. JAMA 1988;259:2278–80. 10.1001/jama.1988.03720150054036 [DOI] [PubMed] [Google Scholar]
- 4.Ganly I, Taylor EW. Breast cancer in a trans-sexual man receiving hormone replacement therapy. Br J Surg 1995;82:341 10.1002/bjs.1800820319 [DOI] [PubMed] [Google Scholar]
- 5.Grabellus F, Worm K, Willruth A et al. . ETV6-NTRK3 gene fusion in a secretory carcinoma of the breast of a male-to-female transsexual. Breast 2005;14:71–4. 10.1016/j.breast.2004.04.005 [DOI] [PubMed] [Google Scholar]
- 6.Kelley K. Breast cancer in a transgender patient and role for screening mammography. Society of General Internal Medicine, Clinical Vignetter. 1/06 2006. http://www.apconline.org (accessed 24 Oct 2014).
- 7.Dhand D, Dhaliwal G. Examining patient conceptions: a case of metastatic breast cancer in an African American male to female transgender patient. J Gen Intern Med 2010;25:158–61. 10.1007/s11606-009-1159-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pattison ST, McLaren BR. Triple negative breast cancer in a male-to-female transsexual. Intern Med J 2010;25:158–61. [DOI] [PubMed] [Google Scholar]
- 9.Gooren LJ, Trotsenburg MA, Giltay E et al. . Breast cancer development in transsexual subjects receiving cross-sex hormone treatment. J Sex Med 2013;10:3129–34. 10.1111/jsm.12319 [DOI] [PubMed] [Google Scholar]
- 10.Maglione KD, Margolies L, Jaffer S et al. . Breast cancer in male-to-female transsexuals: use of breast imaging for detection. Am J Roentgenol 2014;203:w735–40. 10.2214/AJR.14.12723 [DOI] [PubMed] [Google Scholar]
- 11.Sattari M. Breast cancer in male-to-female transgender patients: a case for caution. Clin Breast Cancer 2015;15:e67–9. 10.1016/j.clbc.2014.08.004 [DOI] [PubMed] [Google Scholar]
- 12.Gooren L, Bowers M, Lips P et al. . Five new cases of breast cancer in transsexual persons. Andrologia 2015. Published Online First: 22 Jan 2015. doi:10.1111/and.12399 [DOI] [PubMed] [Google Scholar]
- 13.Bauer KR, Brown M, Cress RD et al. . Descriptive analysis of oestrogen receptor(ER)-negative, progesterone negative(PR)-negative and HER2-negative invasive breast cancer, the so-called triple negative phenotype. Cancer 2007;109:1721–8. 10.1002/cncr.22618 [DOI] [PubMed] [Google Scholar]
- 14.Asscheman H, Giltay EJ, Megens JAJ et al. . A long term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol 2011;164:635–42. 10.1530/EJE-10-1038 [DOI] [PubMed] [Google Scholar]
- 15.Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million-Women Study. Lancet 2003;362:419–27. 10.1016/S0140-6736(03)14065-2 [DOI] [PubMed] [Google Scholar]
- 16.Fentiman IS, Fourquet A, Hortobagyi GN. Male breast cancer. Lancet 2006;367:595–604. 10.1016/S0140-6736(06)68226-3 [DOI] [PubMed] [Google Scholar]
- 17.Giordano SH, Cohen DS, Buzdar AU et al. . Breast carcinoma in men: a population-based study. Cancer 2004;101:51–7. 10.1002/cncr.20312 [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Liu X, Zhang L et al. . Poorer survival of male breast cancer compared with female breast cancer patients may be due to biological differences. Jpn J Clin Oncol 2013;43:954–63. 10.1093/jjco/hyt116 [DOI] [PubMed] [Google Scholar]
- 19.Lonning PE, Taylor PD, Anker G. High-dose estrogen treatment in postmenopausal breast cancer patients heavily exposed to endocrine therapy. Breast Cancer Res Treat 2001;67:111–16. 10.1023/A:1010619225209 [DOI] [PubMed] [Google Scholar]
- 20.Ellis MJ, Gao F, Dehdashti F et al. . Lower-dose (6 mg daily) versus higher-dose (30 mg daily) oral estradiol therapy of hormone-receptor-positive, aromatase-inhibitor-resistant advanced breast cancer: a randomized phase 2 study. JAMA 2009;302:774–80. 10.1001/jama.2009.1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reed B, Rhodes S, Schofield P et al. . Gender variance in the UK: prevalence, incidence, growth and geographic distribution. Gender Identity Research and Education Society, 2009. [Google Scholar]
- 22.Weyers S, Villeirs G, Vanherreweghe E et al. . Mammography and breast sonography in transsexual women. Eur J Radiol 2010;74:508–13. 10.1016/j.ejrad.2009.03.018 [DOI] [PubMed] [Google Scholar]
- 23.Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA et al. . Endocrine treatment of transsexual persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2009;94:3132–54. 10.1210/jc.2009-0345 [DOI] [PubMed] [Google Scholar]
- 24.Centre of Excellence for Transgender Health. Primary care protocol for transgender patient care. Centre of Excellence for Transgender Health website. Published 2011. http://transhealth.ucsf.edu/trans?page=protocol-screening#S2X (accessed 11 Mar 2015).


