Abstract
Study Objective:
Esophageal manometry (Pes) is the gold standard to detect repetitive episodes of increased respiratory effort followed by arousal (RERAs). Because RERAs are not included in the apnea-hypopnea index (AHI), we often refer patients with symptoms of sleep disordered breathing (SDB) and AHI < 5 for a second polysomnogram (PSG) with Pes. Often, the second PSG will demonstrate AHI > 5, confirming a diagnosis of OSA. We speculate that in most cases of suspected SDB, Pes does not add further diagnostic data and that night-to-night variability in OSA severity results in a first false-negative study.
Methods:
We conducted a retrospective review of PSGs between 2008 and 2012 in adults with initial PSG negative for OSA followed by a second study (with or without Pes) within 6 mo.
Results:
Of 125 studies that met inclusion criteria, a second study was completed with Pes in 105 subjects. SDB was diagnosed in 73 subjects (68.5%) completing a second PSG with Pes: 49 (46.7%) received a diagnosis based on AHI, and 24 (22.8%) received a diagnosis based on Pes (p = 0.003). There were no statistically significant differences in the mean AHI change between the two PSGs in subjects who completed the second study with or without Pes.
Conclusions:
In patients with symptoms of SDB and initial PSG with AHI < 5, the majority met criteria for OSA on second PSG by AHI without additional information added by Pes. Because Pes is not widely available and is somewhat invasive, a repeat study without Pes may be sufficient to diagnose SDB.
Citation:
Skiba V, Goldstein C, Schotland H. Night-to-night variability in sleep disordered breathing and the utility of esophageal pressure monitoring in suspected obstructive sleep apnea. J Clin Sleep Med 2015;11(6):597–602.
Keywords: obstructive sleep apnea, polysomnography, sleep apnea/hypopnea syndrome, sleep disordered breathing, upper airway resistance sleep apnea syndrome
Obstructive sleep apnea (OSA), the most common form of sleep disordered breathing (SDB), has a prevalence of 10– 17%.1 It is characterized by frequent breathing pauses related to partial (hypopnea) or complete (apnea) airway collapse. Untreated OSA not only causes excessive daytime sleepiness, but it is also known to be a risk factor for hypertension, stroke, cardiac disease, depression, cognitive impairment and increased mortality.2–5
Upper airway resistance syndrome (UARS) is a proposed diagnostic classification for patients who have respiratory events that would not meet diagnostic criteria for hypopneas or apneas. Individuals with UARS may have an apnea-hypopnea index (AHI) less than 5 but may have frequent respiratory effort-related arousals (RERAs). Because patients with UARS are presumed to have the same pathophysiology as those with OSA, the International Classiification of Sleep Disorders, Third Edition (ICSD-3) recommends that UARS be considered a variant of OSA.6
BRIEF SUMMARY
Current Knowledge/Study Rationale: In certain patients with symptoms suggestive of obstructive sleep apnea (OSA), a single polysomnogram (PSG) may not be diagnostic for sleep disordered breathing. Esophageal manometry (Pes) is considered the gold standard to measure upper airway resistance and when added to PSG may increase sensitivity to detect OSA; however, since the introduction of the nasal pressure transducer, a repeat PSG without Pes may be sufficient to confirm a diagnosis of OSA in most patients.
Study Impact: In patients with symptoms of sleep disordered breathing and an initial PSG with AHI < 5, the majority met diagnostic criteria for OSA on second PSG by AHI alone without additional information added by Pes. Because Pes is not widely available and is somewhat invasive, a repeat study without the use of Pes may be sufficient to diagnose sleep disordered breathing.
Esophageal pressure monitoring (Pes) uses a fluid- or airfilled catheter inserted into the esophagus to measure variations in transmitted intrathoracic pressure with respiration. It is considered the reference standard for measurement of respiratory effort and, as such, can accurately detect increased respiratory effort.7 However, in 1999 when the American Academy of Sleep Medicine (AASM) recommended that Pes be considered the reference standard, there were no other studies that compared alternative techniques of measuring airflow or flow limitation with the reference standard. Prior to the addition of nasal pressure transducers to polysomnography, thermal airflow sensors were used. Thermistors and thermo-couples measure respiratory airflow based on measuring relative temperature during expiration and inspiration and on the heat content of the air passing over the device. Thermistors work well for detecting absence of airflow and thus indicating apneas. However, temperature may not be related to volume when used to assess reduced airflow as occurs during a hypopnea. Norman et al. showed that using nasal pressure monitoring helps to identify 21–61% more events that are missed by thermistor.8 The use of a nasal pressure transducer, in addition to an oronasal thermal airflow device, is now standard of care when performing diagnostic polysomnography.9 Although Pes is generally well tolerated by patients, it is an invasive procedure that requires a trained technician and interpreting physician and is not widely available or used. There are limited studies evaluating the utility of the addition of esophageal pressure monitoring to diagnose SDB, and many did not use nasal pressure monitoring.10–13
A second night of polysomnographic recording may demonstrate OSA when the first did not due to a first-night effect and night-to-night variability. The first-night effect includes shorter total sleep time (TST), decreased sleep efficiency (SE), increased sleep onset latency, greater wakefulness after sleep onset, increased number of awakenings, and decreased rapid eye movement (REM) sleep.14,15 Because SDB is often worse during REM sleep, decreased duration of REM sleep seen on a first night in the sleep laboratory may contribute to a lower AHI on the first night. There is significant variability in the AHI between 2 nights of polysomnographic recording, with 25–45% of patients having a difference in AHI of greater than five to 20 events per hour. The second night of recording often has a higher AHI and is diagnostic of OSA in 15–25% of patients who had a negative initial polysomnogram.14–17
In our sleep laboratory, we often see patients who present with symptoms suggestive of OSA. If the first PSG, using a thermal sensor and a nasal pressure transducer to measure airflow, does not show OSA, these patients may be referred for a second night of recording with Pes. We have found, anecdotally, that these patients often meet the diagnostic criteria for OSA on the second night of recording based solely on AHI. We speculate that in most cases of suspected SDB, Pes does not add further diagnostic data after an initial negative polysomnogram and that first-night effect and night-to-night variability result in the second study being diagnostic of OSA.
METHODS
Subjects
We conducted a retrospective review of our computerized polysomnogram database and identified all subjects who had two baseline polysomnograms performed between June 2008 and June 2012; polysomnograms were completed for clinical purposes. We chose this time frame to ensure all studies were recorded and scored using the AASM Manual for the Scoring of Sleep and Associated Events, 1st edition.18 We included adult patients 18 y and older with an initial baseline PSG without Pes that did not meet diagnostic criteria for OSA (AHI < 5) and who underwent a second study with or without the use of Pes. We excluded studies completed greater than 6 mo apart to minimize significant weight or health changes that could change the severity of SDB. We also excluded pregnant women due to the dynamic changes in weight and SDB that can occur during pregnancy. The decision to obtain a second polysomnogram was made by the sleep medicine physician responsible for the patient. Typically, in our clinic, if the physician has a high clinical suspicion that a patient has SDB but the first PSG does not show OSA, a second PSG is often obtained. Because Pes is available and commonly used in our sleep laboratory, the second PSG is often completed with Pes with the assumption that this will increase the diagnostic yield.
Procedures
Nocturnal polysomnography included six electroencephalographic (EEG) leads (C3-M2, C4-M1, O1-M2, O2-M1, F3-M2, F4-M1 of the 10-20 international electrode placement system), two electrooculographic (EOG) leads (right and left outer canthi), chin and bilateral anterior tibialis surface electromyograms (EMGs), two electrocardiographic (ECG) leads, nasal pressure signal, oronasal thermocouple, thoracic and abdominal inductance plethysmography, and pulse oximetry. Pes monitoring was completed with an air-filled catheter, which was inserted into one nostril with or without use of a topical anesthetic.
Sleep-stage scoring and identification of respiratory events was performed according to the AASM Manual for the Scoring of Sleep and Associated Events, 1st edition.18 All studies were scored by technologists who had undergone an extensive training program including reliability scoring, and were certified by the AASM as a Polysomnographic Technician or Polysomnographic Technologist. The raw data were then reviewed by the interpreting physician board certified in Sleep Medicine. An apnea was scored when there was a drop in the peak thermal sensor excursion by ≥ 90% of the baseline, lasting at least 10 sec, with at least 90% of the event's duration meeting the amplitude reduction criteria for apnea. A hypopnea was scored if all the following criteria were met: (1) The nasal pressure signal excursions drop by ≥ 50% of baseline and there is a ≥ 3% desaturation from pre-event baseline or the event is associated with an arousal; (2) The duration of this drop occurs for a period lasting at least 10 sec; (3) At least 90% of this event's duration must meet the amplitude reduction of criteria for hypopnea. Arousals were scored if there was an abrupt shift of EEG frequency that lasted at least 3 sec. Continuous Pes monitoring was reviewed by the interpreting sleep physician. Peak-to-trough differences in Pes values larger than 10 cm of water and nadirs less than −10 cm water (absolute value of nadir greater than 10 cm of water, |Pes nadir| > 10 cm of water) are considered abnormal.19 Episodes of increased respiratory effort may terminate in an arousal (RERA) or reverse spontaneously. In our laboratory we do not use Pes values to score RERAs for generation of a respiratory disturbance index (RDI), but rather use them to detect the presence or absence of increased respiratory effort.
The following data was extracted from the study reports: age, sex, body mass index (BMI), AHI, REM AHI, RDI, TST, time (min) and percent of N1, N2, N3, and REM sleep, SE, time (min) in supine sleep, arousal index, minimum oxygen saturation, and study interpretation. By criteria for inclusion, all of the first-night studies were negative for OSA with OSA defined as AHI less than 5. A second-night AHI positive study was defined as PSG positive for OSA if AHI was greater than 5. A second-night Pes-positive, AHI-negative study was defined as AHI less than 5 but physician's interpretation stating that Pes values were abnormal indicating increased upper airway resistance. A second-night negative study was defined as AHI was less than 5 and the interpretation stated that the Pes values were normal.
Analysis
All data were analyzed with the SPSS Statistics Software (Version 21, IBM, Armonk, NY, 2012). Descriptive statistics were calculated for the demographics and statistical differences between groups were calculated with analysis of variance.
The primary aim of the study was to determine if the addition of Pes monitoring on second-night PSG aids in the diagnosis of OSA in patients with symptoms suspicious for OSA but with first-night negative PSG. Among the subjects who completed a second night with Pes, we compared the proportion of second-night AHI positive studies to the proportion of second-night Pes-positive, AHI-negative studies. Proportion data were compared with chi-square testing.
The secondary aim was to determine if the esophageal catheter used for Pes monitoring is associated with changes in AHI and sleep architecture. We calculated the differences in the AHI and other sleep architecture parameters between the first and second night recording for each subject, calculated the means for each group (second night with Pes and second night without Pes), and compared for statistical differences with t test. Because of the known first-night effect on sleep architecture and AHI, we chose to compare the changes in these parameters between the 2 nights in the groups who completed a second night with Pes to the group who completed a second night without Pes.
RESULTS
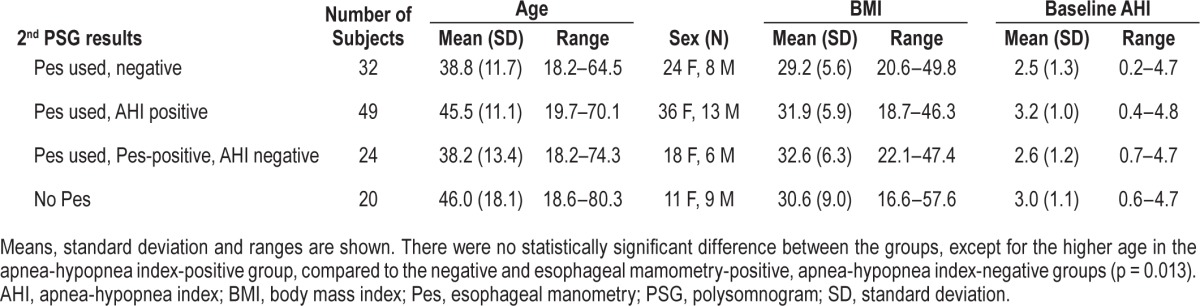
One hundred twenty-five subjects met inclusion criteria; 105 PSGs were completed with use of Pes on the second night, and 20 PSGs were completed without use of Pes on the second night. Results of PSG are shown in Figure 1 and subject characteristics are presented in Table 1. The subjects in whom OSA was diagnosed based on a second-night AHI-positive study tended to be older (45.5 y) compared to the subjects who had negative studies (38.8 y) or who had second-night Pes-positive, AHI-negative studies (38.2 y), p = 0.013. Otherwise, there were no statistically significant differences in sex, BMI, and baseline AHI between the groups.
Figure 1. Polysomnogram results.
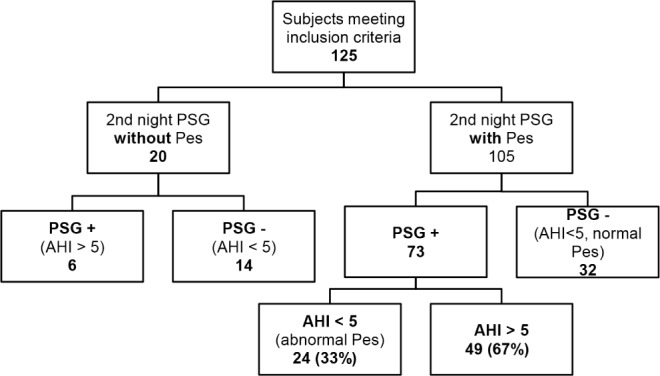
AHI, apnea-hypopnea index; Pes, esophageal manometry.
Table 1.
Subject characteristics.
Of the 105 subjects who completed the second night with Pes, PSG was positive (either by AHI or Pes) in 73 (69.5%). Of those 73 subjects, a significantly greater proportion (67.1%) were diagnosed by AHI > 5 than by abnormal Pes values (32.9%) (p = 0.003) (Figure 1).
Twenty subjects completed the second night without the use of Pes. The second-night polysomnogram was ordered with Pes in four of these 20 subjects: one subject did not tolerate the catheter and requested to have it removed, the catheter could not be placed in two of the subjects, and one subject declined the placement of the catheter. OSA was diagnosed in three of these four subjects based on the AHI. Five of the 20 subjects who completed a second-night polysomnogram without Pes completed a multiple sleep latency test the following day; the PSG was negative for OSA in all of the five subjects.
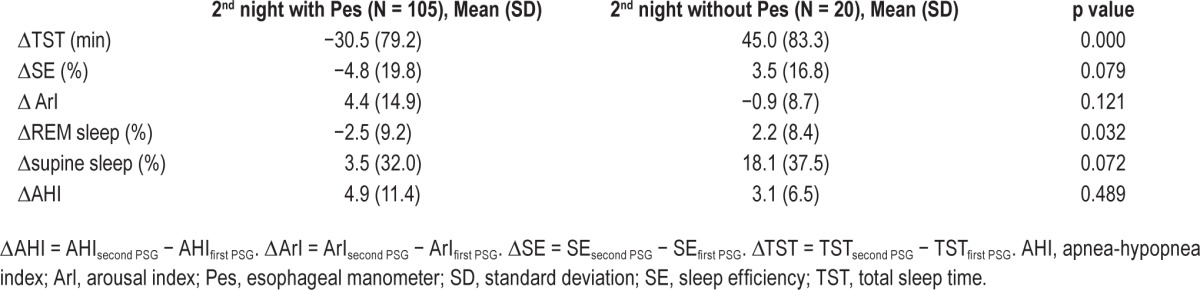
To determine if use of the Pes catheter changes AHI and sleep architecture, we compared the following parameters in subjects who completed the second PSG with Pes to those who completed the second PSG without Pes: AHI, arousal index (ArI), SE, TST, %REM sleep, and %supine sleep. Both groups demonstrated a similar increase in AHI from the first to second PSG, ΔAHI 4.9 (standard deviation [SD] 11.4) in the group where Pes was used and ΔAHI 3.1 (SD 6.5) in the group without use of Pes (p = 0.489).
Use of the Pes catheter on the second night PSG was associated with a trend toward an increase in ArI (Δ ArI = 4.4, SD 14.9) and decrease in SE (ΔSE = −4.8%, SD 19.8) compared to a decrease in ArI (Δ ArI = −0.9, SD 8.7) and increase in SE (ΔSE = 3.5%, SD 16.8) on the second night of recording in the group without Pes (p = 0.121, 0.079). TST also decreased on the second night in the group with Pes (ΔTST = −30.5 min, SD 79.2) compared to a mean increase of 45 min (SD 83.3) in the group without Pes (p = 0.000). The percent REM sleep decreased by 2.5% (SD 9.2) in the group with Pes and increased by 8.4% (SD 8.4) in the group without Pes (p = 0.032). On the second night of recording, the subjects without Pes tended to sleep supine more (18.1% more, SD 37.5) than the group with Pes (3.5%, SD 32) (p = 0.072) (Table 2).
Table 2.
Mean difference and standard deviation for total sleep time, sleep efficiency, arousal index, %rapid eye movement, %supine sleep, and apnea-hypopnea index between the second and first night recording.
DISCUSSION
Pes in the Detection of the UARS
The clinical suspicion for OSA often remains high despite initial PSG that demonstrates an AHI less than 5. A single PSG recording may not be sufficient to diagnose obstructive SDB and a repeat study with Pes can be beneficial to evaluate for obstructive SDB. The ICSD 2 and ICSD 3 include UARS as part of OSA. UARS is subsumed under the diagnosis of OSA by the inclusion of RERAs in the definition of OSA. However, this definition does not take into account long durations of continuously increased inspiratory effort that ultimately terminate in arousal or episodes of increased inspiratory effort that resolve without scorable cortical arousal (Pes reversal).20 Therefore, apart from the ability to accurately score RERAs, Pes monitoring may be beneficial to detect these breathing patterns.
There are limited data evaluating the utility of the addition of Pes monitoring to diagnose SDB. Hutter et al. re-tested 28 patients who were clinically suspected to have OSA and had a single PSG negative for OSA. Repeat PSG used Pes and diagnosed OSA in 18 patients.12 Nearly 70% of those 18 individuals received a diagnosis of OSA by meeting AHI criteria on the second study without additional information added by Pes monitoring. Notably, this study preceded the 2007 AASM rules for the scoring of respiratory events and used a thermistor for the detection of apneas and hypopneas. We studied a larger sample of 105 subjects and used nasal pressure in addition to a nasal thermistor, which may allow for improved detection of hypopneas. Our findings were similar as we demonstrated that 67% of the 73 individuals found to have obstructive SDB on second-night PSG received a diagnosis based on AHI alone.
Subjects found to have OSA based on Pes values alone tended to be younger than those in whom a diagnosis was made based on the AHI (38.3 versus 45.5 years old, p = 0.013); other baseline characteristics such as BMI and baseline AHI were not statistically different. More research with a larger sample is needed to determine what characteristics (such as symptoms of OSA, Epworth Sleepiness Scale, or physical examination findings) may increase the likelihood of detecting SDB based on Pes values alone. This information may assist in selecting patients with a first-night negative PSG based on AHI who should undergo a second PSG with esophageal manometry. This information would be especially relevant in sleep laboratories that do not offer Pes and patients could potentially be referred to another sleep laboratory.
It is worth mentioning that scoring RERAs on the first PSG may identify subjects whose AHI is less than 5 but in whom SDB can be diagnosed on the first study if the RDI is greater than 5. The current version of the scoring manual states that scoring RERAs is optional.9 A RERA is scored if “there is a sequence of breaths lasting ≥ 10 seconds characterized by increasing respiratory effort or by flattening of the inspiratory portion of the nasal pressure…waveform leading to an arousal from sleep“. RDI combines apneas, hypopneas and RERAs. Therefore, scoring RERAs may be sufficient to diagnose SDB if RDI is greater than 5; this approach may decrease the need for a second PSG or use of Pes. More research is needed to determine the diagnostic yield of this approach.
Effect of Pes on Sleep and AHI
We were also interested in examining the effects of the Pes catheter on various sleep characteristics and whether the catheter itself may affect the AHI. We compared the mean differences in AHI and other sleep characteristics between second and first recordings for each patient, then calculated the means for each group. Skatvedt et al. tested 28 subjects and did not find any significant differences for respiratory parameters between PSGs completed with Pes and those without Pes, but only thermistors were used to measure respiratory flow.13 Our data add to this limited literature, as we did not find a statistically significant difference in the AHI change between the studies with and without Pes. This suggests that the use of the catheter does not change the upper airway characteristics sufficiently to predispose to increased upper airway resistance. Use of Pes was also associated with a trend toward lower SE and higher arousal index, and a statistically significant decrease in TST. Perhaps somewhat surprisingly, use of Pes was associated with a smaller increase in the percent of supine sleep compared to the subjects who did not have Pes monitoring. If a second PSG is requested, patients are often encouraged to sleep supine in order to improve the likelihood of observing subtle forms of SDB. Subjects who completed a second night without Pes slept 37.5% longer in the supine position, compared to only 3.5% more time in the supine position among subjects with Pes.
Night-to-Night Variability
In our sample of 125 subjects with PSGs negative for OSA, the second recording was positive for obstructive SDB in 63% of the subjects. When OSA is defined as an AHI greater than 5, 44% of patients were negative for OSA based on a single-night PSG and in whom OSA was diagnosed on repeat testing consistent with other findings on night-to-night variability in SDB.14–17,21 Night-to-night variability may be especially significant on patients with overall mild OSA, when even small changes in the number of respiratory events may increase AHI above the diagnostic threshold of 5. However, our sample was not a random sample, but rather a sample of subjects whose treating provider had a high suspicion for OSA, therefore increasing the pretest probability of a positive study. Further research is needed to determine if the various subgroups of patients have different responses to treatment of SDB, and if suboptimal response is the result of misdiagnosis of SDB.
CONCLUSIONS
A weakness of our study is the small sample size of subjects who completed a second recording without Pes. Only 20 subjects who met our inclusion criteria completed the second night without Pes, limiting our power to detect differences in the ΔAHI between groups. Furthermore, most of the studies conducted without Pes were ordered without Pes; therefore, these two populations (second-night study with Pes and second-night study without Pes) may have differed regarding indication for repeat PSG (the ordering clinician may have had a lower suspicion for moderate or severe OSA).
Our study contributes to the limited literature on the utility of esophageal pressure monitoring during PSGs and is relevant because PSGs were conducted with use of the current technical specifications outlined by AASM. Although the majority of our subjects received a diagnosis based on the AHI from the second study, the diagnosis of obstructive SDB would be missed in almost a quarter patients (22.8%) if Pes were not used. We propose that if obstructive SDB is highly suspected based on clinical evaluation, a repeat PSG has a high yield of diagnosing OSA. However, a small subgroup of patients (potentially those of younger age) may remain undiagnosed without the additional information gained for esophageal pressure monitoring. Esophageal pressure monitoring remains a safe and generally well-tolerated procedure, although expertise in patient selection, placement, and interpretation are needed. Furthermore, this investigation underlines the importance of repeat evaluation for OSA in individuals with clinical symptoms of the disorder.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Judy Fetterolf for her assistance with study design and data collection.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- ECG
electrocardiograph
- EEG
electroencephalograph
- EMGs
electromyograms
- EOG
electrooculograph
- OSA
obstructive sleep apnea
- Pes
esophageal manometry
- PSG
polysomnogram
- REM
rapid eye movement
- RERAs
respiratory effort followed by arousal
- SBD
sleep disordered breathing
- SD
standard deviation
- UARS
upper airway resistance syndrome
REFERENCES
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Mae Hla K. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest. 1993;103:30–6. doi: 10.1378/chest.103.1.30. [DOI] [PubMed] [Google Scholar]
- 3.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease. J Am Coll Cardiol. 2008;52:686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–9. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Mae Hla K. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort Study. WMJ. 2009;108:246–9. [PMC free article] [PubMed] [Google Scholar]
- 6.American Academy of Sleep Medicine. International classification of sleep disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 7.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 8.Norman RG, Ahmed MM, Walsleben JA, Rapoport DM. Detection of respiratory events during NPSG: nasal cannula/pressure sensor versus thermistor. Sleep. 1997;20:1175–84. [PubMed] [Google Scholar]
- 9.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayappa I, Norman RG, Krieger AC, Rosen A, O'Malley RL, Rapoport DM. Noninvasive detection of respiratory effort-related arousals (RERAs) by a nasal cannula/pressure transducer system. Sleep. 2000;23:763–71. doi: 10.1093/sleep/23.6.763. [DOI] [PubMed] [Google Scholar]
- 11.Chervin RD, Aldrich MS. Effects of esophageal pressure monitoring on sleep architecture. Am J Respir Crit Care Med. 1997;156:881–5. doi: 10.1164/ajrccm.156.3.9701021. [DOI] [PubMed] [Google Scholar]
- 12.Hutter DA, Holland BK, Ashtyani H. Occult sleep apnea: the dilemma of negative polysomnography in symptomatic patients. Sleep Med. 2004;5:501–6. doi: 10.1016/j.sleep.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Skatvedt O, Akre H, Godtlibsen OB. Nocturnal polysomnography with and without continuous pharyngeal and esophageal pressure measurements. Sleep. 1996;19:485–90. doi: 10.1093/sleep/19.6.485. [DOI] [PubMed] [Google Scholar]
- 14.Bon OL, Hoffmann G, Tecco J, et al. Mild to moderate sleep respiratory events, one night may not be enough. Chest. 2000;118:353–9. doi: 10.1378/chest.118.2.353. [DOI] [PubMed] [Google Scholar]
- 15.Newell J, Mairesse O, Verbank P, Neu D. Is a one-night stay in the lab really enough to conclude? First-night effect and night-to-night variability is polysomnographic recordings among different clinical population samples. Psychiatry Res. 2012;200:795–801. doi: 10.1016/j.psychres.2012.07.045. [DOI] [PubMed] [Google Scholar]
- 16.Ahmadi N, Shapiro GK, Chung SA, Shapiro CM. Clinical diagnosis of sleep apnea based on single night of polysomnography vs. two nights of polysomnography. Sleep Breath. 2009;13:221–6. doi: 10.1007/s11325-008-0234-2. [DOI] [PubMed] [Google Scholar]
- 17.Levendowski DJ, Zack N, Rao S, et al. Assessment of the test-retest reliability of laboratory polysomnography. Sleep Breath. 2009;13:163–7. doi: 10.1007/s11325-008-0214-6. [DOI] [PubMed] [Google Scholar]
- 18.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st edition. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 19.Kushida CA, Giacomini A, Lee MK, Guilleminault C, Dement WC. Technical protocol for the use of esophageal manometry in the diagnosis of sleep-related breathing disorders. Sleep Med. 2002;3:163–73. doi: 10.1016/s1389-9457(01)00143-5. [DOI] [PubMed] [Google Scholar]
- 20.Guilleminault C, Stoohs R, Clerk A, Cetel M, Maistros P. A cause of excessive daytime sleepiness. The upper airway resistance syndrome. Chest. 1993;104:781–7. doi: 10.1378/chest.104.3.781. [DOI] [PubMed] [Google Scholar]
- 21.Meyer TJ, Eveloff SE, Kline LR, Millman RP. One negative polysomnogram does not exclude obstructive sleep apnea. Chest. 1993;103:756–60. doi: 10.1378/chest.103.3.756. [DOI] [PubMed] [Google Scholar]


