Abstract
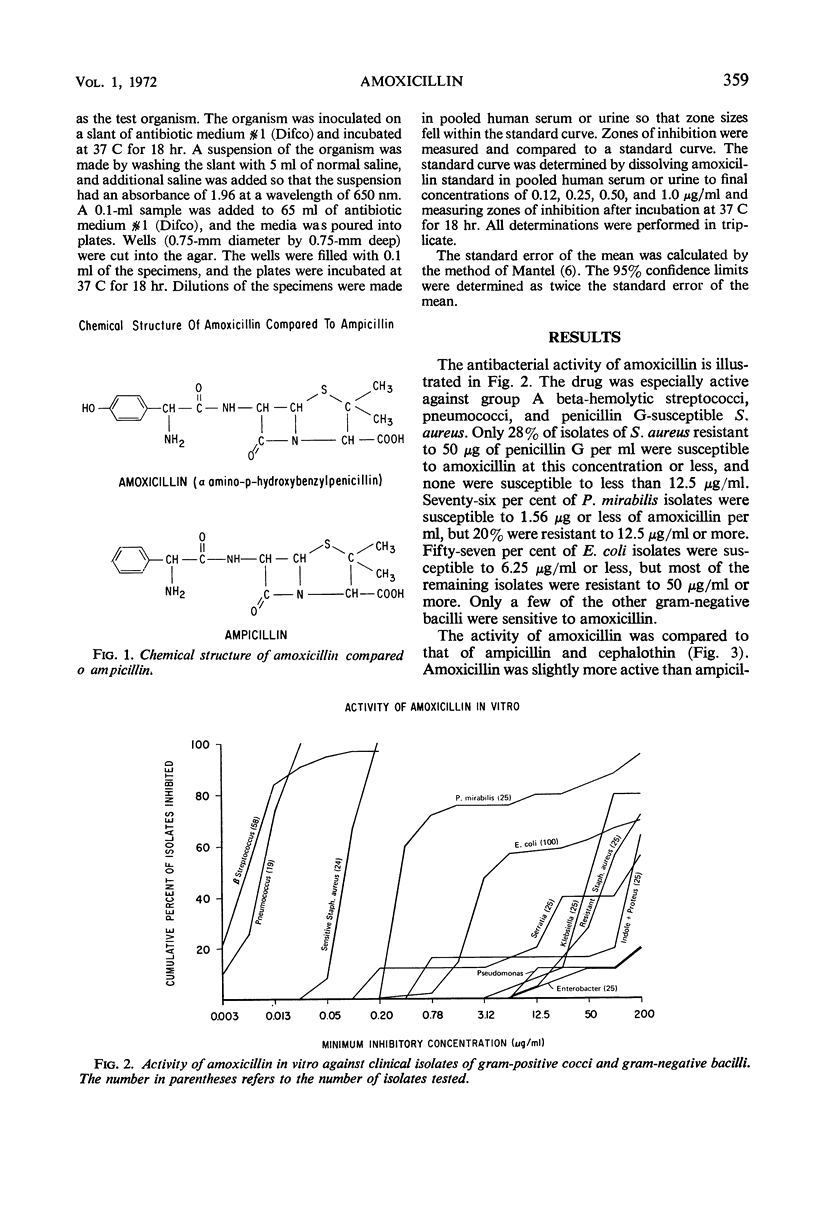
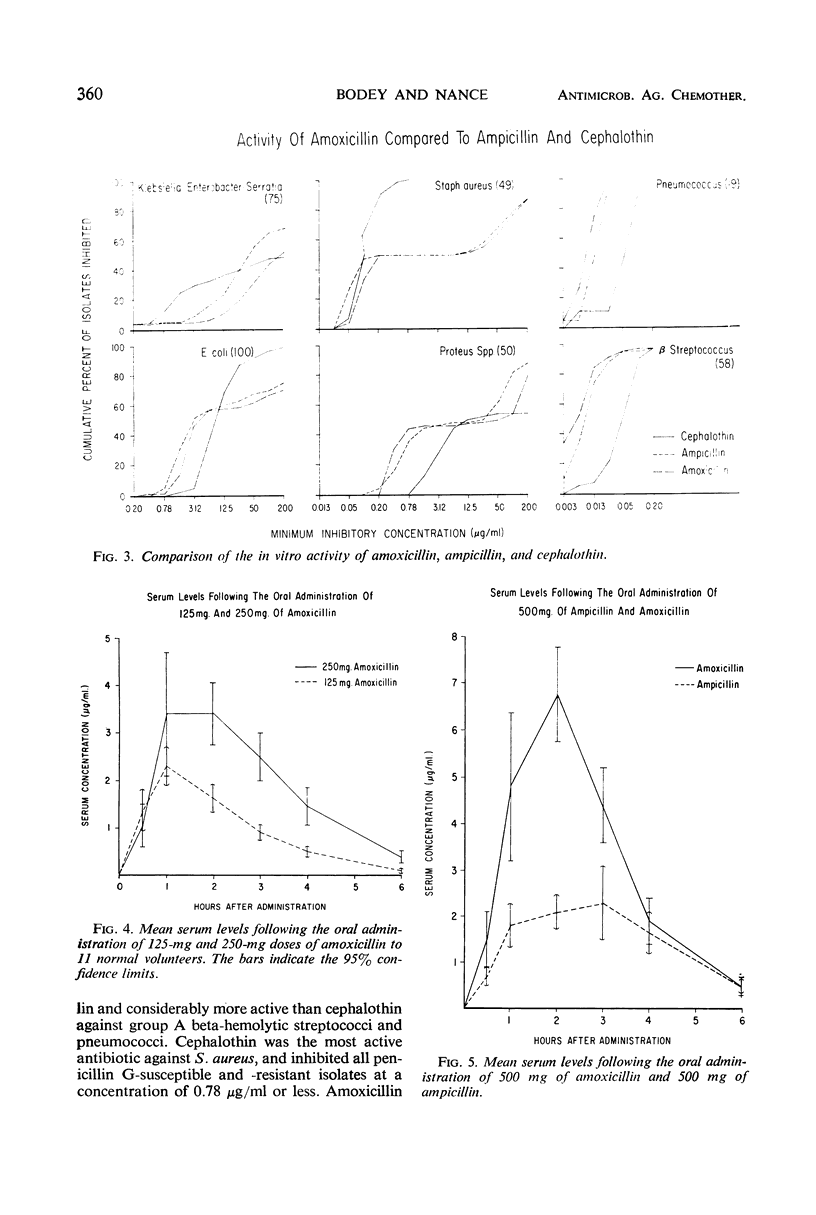
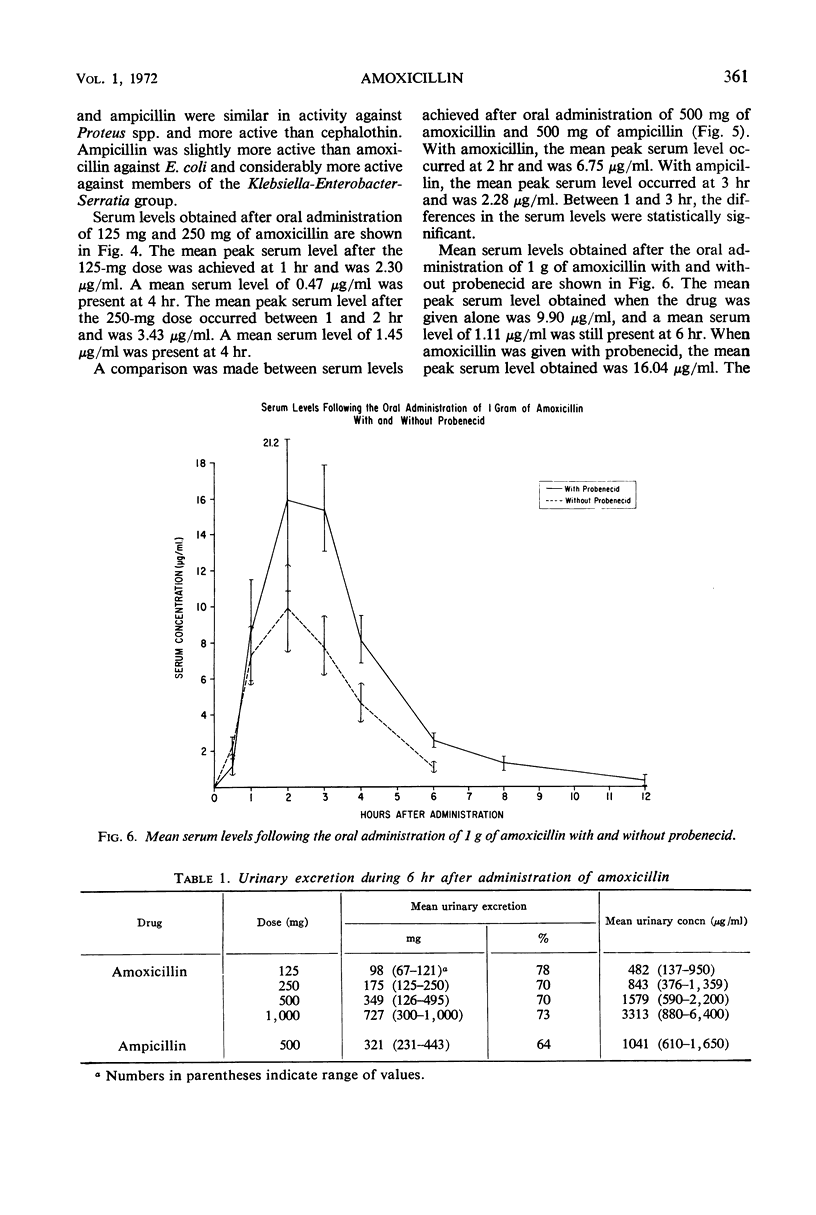
Amoxicillin is a new semisynthetic penicillin which is active in vitro against gram-positive cocci (except penicillin G-resistant Staphylococcus aureus) and most isolates of Proteus mirabilis and Escherichia coli. Its in vitro activity is quite similar to ampicillin, but it produces higher serum levels after oral administration. The mean peak serum levels of amoxicillin in 11 normal volunteers were 2.30 μg/ml after 125 mg, 3.43 μg/ml after 250 mg, 6.75 μg/ml after 500 mg, and 9.90 μg/ml after 1 g. About 70% of the drug was excreted in the urine during the first 6 hr.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acred P., Hunter P. A., Mizen L., Rolinson G. N. -amino-p-hydroxybenzylpenicillin (BRL 2333), a new broad-spectrum semisynthetic penicillin: in vivo evaluation. Antimicrob Agents Chemother (Bethesda) 1970;10:416–422. [PubMed] [Google Scholar]
- Croydon E. A., Sutherland R. -amino-p-hydroxybenzylpenicillin (BRL 2333), a new semisynthetic penicillin: absorption and excretion in man. Antimicrob Agents Chemother (Bethesda) 1970;10:427–430. [PubMed] [Google Scholar]
- KLEIN J. O., FINLAND M. Ampicillin activity in vitro and absorption and excretion in normal young men. Am J Med Sci. 1963 May;245:544–555. [PubMed] [Google Scholar]
- Kvale P. A., Keys T. F., Johnson D. W., Holmes K. K. Single oral dose ampicillin-probenecid treatment of gnorrhea in the male. JAMA. 1971 Mar 1;215(9):1449–1453. [PubMed] [Google Scholar]
- MacLowry J. D., Marsh H. H. Semiautomatic microtechnique for serial dilution-antibiotic sensitivity testing in the clinical laboratory. J Lab Clin Med. 1968 Oct;72(4):685–687. [PubMed] [Google Scholar]
- Neu H. C., Winshell E. B. In vitro antimicrobial activity of 6(D(-) -amino-p-hydroxyphenylacetamido) penicillanic acid, a new semisynthetic penicillin. Antimicrob Agents Chemother (Bethesda) 1970;10:407–410. [PubMed] [Google Scholar]
- Neu H. C., Winshell E. B. Pharmacological studies of 6 (D(-) -amino-p-hydroxyphenylacetamido) penicillanic acid in humans. Antimicrob Agents Chemother (Bethesda) 1970;10:423–426. [PubMed] [Google Scholar]
- SIDELL S., BURDICK R. E., BRODIE J., BULGER R. J., KIRBY W. M. New antistaphylococcal antibiotics. Comparative in vitro and in vivo activity of cephalothin, nafcillin, cloxacillin, oxacillin, and methicillin. Arch Intern Med. 1963 Jul;112:21–28. doi: 10.1001/archinte.1963.03860010067005. [DOI] [PubMed] [Google Scholar]
- Sutherland R., Rolinson G. N. -amino-p-hydroxybenzylpenicillin (BRL 2333), a new semisynthetic penicillin: in vitro evaluation. Antimicrob Agents Chemother (Bethesda) 1970;10:411–415. doi: 10.1128/AAC.10.3.411. [DOI] [PubMed] [Google Scholar]


