Abstract
Study Objectives:
Studies examining the longitudinal association of untreated obstructive sleep apnea (OSA) with diabetes in population samples are limited. This study therefore examined the relationship between previously undiagnosed OSA with incident type 2 diabetes in community-dwelling men aged ≥ 40 y.
Methods:
The Men Androgen Inflammation Lifestyle Environment and Stress (MAILES) Study is a longitudinal population-based cohort in Adelaide, South Australia. Clinic assessments at baseline and follow-up identified diabetes (self-reported doctor diagnosed, fasting plasma glucose ≥ 7.0 mmol/L, glycated hemoglobin ≥ 6.5% or diabetes medication use) and included anthropometry. At cohort follow-up (2010–2012), n = 837 underwent full in-home unattended polysomnography (PSG, Embletta X100, Broomfield, CO).
Results:
Of 736 men free of diabetes at baseline, incident diabetes occurred in 66 (9.0%) over a mean follow-up time of 56 mo (standard deviation = 5, range: 48–74 mo). Incident diabetes was associated with current oxygen desaturation index (3%) ≥ 16 events/h (odds ratio [OR]: 1.85 [1.06–3.21]), and severe OSA [OR: 2.6 (1.1–6.1)], in adjusted models including age, percentage total body fat, and weight gain (> 5 cm waist circumference). An age-adjusted association of incident diabetes with percentage of total sleep time with oxygen saturation < 90% did not persist after adjustment for percentage of body fat. No modification of these relationships by excessive daytime sleepiness was observed.
Conclusions:
Severe undiagnosed OSA and nocturnal hypoxemia were independently associated with the development of diabetes. A reduction in the burden of undiagnosed OSA and undiagnosed diabetes is likely to occur if patients presenting with one disorder are assessed for the other.
Citation:
Appleton SL, Vakulin A, McEvoy RD, Wittert GA, Martin SA, Grant JF, Taylor AW, Antic NA, Catcheside PG, Adams RJ. Nocturnal hypoxemia and severe obstructive sleep apnea are associated with incident type 2 diabetes in a population cohort of men. J Clin Sleep Med 2015;11(6):609–614.
Keywords: cohort study, epidemiology, men, nocturnal hypoxemia, obstructive sleep apnea, polysomnography, type 2 diabetes
Obstructive sleep apnea (OSA),1 type 2 diabetes,2 and obesity3 have been increasing in prevalence over the past two decades. Although studies suggest that more than half of people with diabetes also have OSA and diabetes is present in 15–30% of patients with OSA,4 their coexistence may be strongly related to the presence of obesity, a key risk factor for both conditions.
BRIEF SUMMARY
Current Knowledge/Study Rationale: The generalizability of findings from the few studies that identify OSA as a risk factor for the development of diabetes may be limited by small samples, few incident cases, and possible referral bias that may apply to clinical samples. The importance of other polysomnographic measures (e.g., oxygen desaturation) and sleepiness in the development of diabetes is also unclear.
Study Impact: The current study has identified that severe undiagnosed OSA and nocturnal intermittent hypoxemia were independently associated with the development of diabetes in a large population cohort of middle-aged and older men. Given the scale of the problem of undiagnosed OSA, improved management is required to ensure that a patient presenting with one condition is screened and treated for the other with a strategy that does not rely on the presence of excessive daytime sleepiness.
Cross-sectional associations between OSA and diabetes have been reported in clinic5 and population-based samples4,6,7; however, the temporal relationship of OSA to diabetes is unclear.4,8 There is significant heterogeneity across seven published longitudinal studies4,9–15 of OSA-related diabetes risk in terms of sample sizes, numbers of incident cases, follow-up periods, and referral bias associated with selected samples. In the Wisconsin cohort, no independent association of moderate-severe OSA with incident diabetes was observed,9 and other studies have demonstrated only modest associations.10–15 Consequently, a recent systematic review found “little published evidence of a longitudinal association between OSA and diabetes.”8
The importance of polysomnography (PSG) indices other than the apnea-hypopnea index (AHI), such as hypoxia, for incident diabetes is unclear.8 Oxygen desaturation was a predictor of diabetes in men,12 and Kendzerska et al. have recently reported that incident diabetes was associated with time spent with oxygen saturation less than 90%, (in addition to severe OSA) in a large sleep clinic cohort of 8,678 patients.15 Similarly, the role of OSA-related excessive daytime sleepiness (EDS) in the development of diabetes is also unclear.4
Our objectives, using data from a population-based cohort of men aged 40 y and older without a prior diagnosis of OSA, were to examine the longitudinal associations between diabetes that had developed over the previous 4 to 6 years and (1) current undiagnosed OSA and (2) other PSG characteristics (oxygen desaturation and arousals), and any modification by EDS.
METHODS
Study Participants
The Men Androgen Inflammation Lifestyle Environment and Stress (MAILES) Study is composed of randomly selected community dwelling men aged at least 40 y residing in Adelaide, South Australia, and has been described previously.16 Initial random recruitment from electronic white pages occurred in 2000–2002. Clinic biomedical assessment, computer-assisted telephone interviews (CATI), and self-completed questionnaires followed standardized and reproducible study protocols. Data for the current analyses were derived from assessments in 2002–2006 (MAILES 1), 2007– 2010 (MAILES 2) and in 2010–2012 (MAILES, CATI, and PSG studies). The obesity prevalence in the MAILES sleep study sample was identical to the 2012 Australian Health Survey findings.3 The study was approved by the North West Adelaide Health Service and the Royal Adelaide Hospital institutional ethics committee. All subjects gave written informed consent for all study stages, and additional consent was sought to enable the reporting of clinic findings to primary care providers.
The study flow is outlined in Figure 1.
Figure 1. The MAILES study flow diagram: polysomnography recruitment, successful studies, and diabetes status.
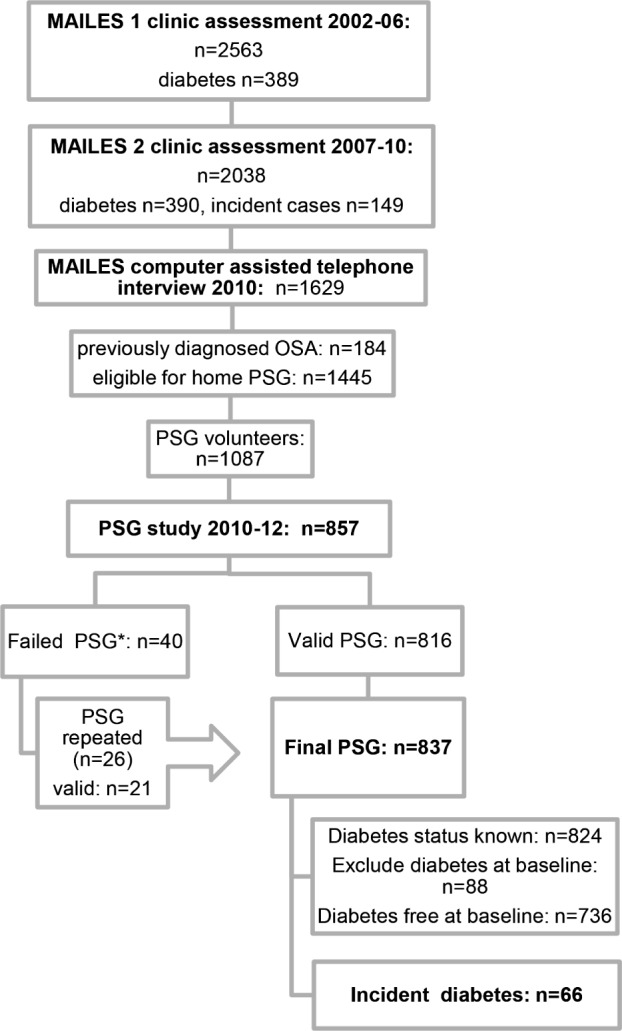
*n = 21 total sleep time (TST) not ≥ 3.5 hours from ≥ 5 hours recording; n = 3 poor respiratory signals; n = 2 poor EEG; n = 14 no oxygen saturation (SaO2); n = 3 all traces/recording failed.
Sleep Data
MAILES participants completed a CATI survey in 2010 (n = 1,629). Of these participants, 184 responded ‘yes” to “Have you ever been diagnosed with obstructive sleep apnea with a sleep study?” and 1,445 men responding “no” were invited to undergo a sleep study, with 75.2% agreeing. Of these individuals, a random sample of 1,000 men was chosen for inclusion, and in 2010–2012, trained staff visited study participants in their homes to set up eight-channel in-home unattended PSG (Embletta X100, Embla Systems, Broomfield, CO). Anthropometry, current medications, and EDS (Epworth Sleepiness Scale [ESS]) were recorded. A physician investigator coordinated any necessary clinical follow-up of participants identified with an AHI ≥ 30/h.
An experienced sleep technician performed manual scoring of all PSGs according to American Academy of Sleep Medicine (AASM) 2007 (alternate) criteria.17 Studies were considered acceptable with 3.5 h of sleep and 5.5 h of total recorded study time. Apneas were defined as cessations of nasal flow lasting ≥ 10 sec and hypopneas as a > 50% decrease in nasal flow (or in both thoracic and abdominal excursions) and associated ≥ 3% oxygen desaturation or an electroencephalographic arousal. OSA was categorized from the AHI as mild: AHI of 10–19/h, moderate: 20–29/h, and severe: ≥ 30/h.18 Previous work has shown that an AHI of 10/h scored with the alternate AASM criteria is equivalent to 5/h with “recommended” AASM criteria.18 In order to maintain comparability with previous studies, a cutoff of 10/h was chosen. Oxygen desaturations ≥ 3% occurring/h of sleep (3% oxygen desaturation index [ODI 3%]) and percent of sleep time with oxygen saturation < 90% (TST90) are also reported.
Clinic Assessment
Clinic assessment at baseline (MAILES 1: 2002–06) and follow-up (MAILES 2: 2007–10) measured blood pressure, height, weight, waist circumference, and fasting glucose, glycated hemoglobin (HbA1c), and lipid levels. Total fat mass at baseline was determined by dual x-ray absorptiometry using a Lunar PRODIGY scanner (GE Medical Systems, Madison, WI, US) in conjunction with Encore 2002 software or a DPX+ scanner (GE Medical Systems), in conjunction with Lunar software version 4.7e. No significant differences between scanners were observed through cross-calibration analysis.16
Incident type 2 diabetes was identified by the presence of at least one diabetes criterion at follow-up in those without any of these abnormalities at baseline: fasting plasma glucose (FPG) ≥ 7.0 mmol/L; HbA1c of ≥ 6.5% (≥ 48 mmol/mol); self-reported physician diagnosis of diabetes; and treatment for diabetes using data linkage to national Pharmaceutical Benefits Scheme data recorded in the 6 mo prior to clinic visits. Smoking status, recreational physical activity levels, usual sleep hours, and shift work were self-reported. Waist circumference (cm) and body mass index (kg/m2) were categorized according to usual international criteria.
Statistical Analysis
Data were analyzed using the Statistical Package for the Social Sciences version 19.0 (SPSS Inc, Chicago, IL, USA). Univariate associations of incident diabetes with current PSG indices of OSA including AHI, ODI 3% ≥ 16/h, quartiles of total arousal index (ArI), oxygen desaturation < 90% (TST90) ≥ 4% of total sleep time (the 75% percentile of TST90), and severity of OSA were determined (overall and stratified by daytime sleepiness) with Chi-square tests, including linear by linear association for OSA severity categories and ArI quartiles. Multivariable logistic regression analysis adjusted the relationship between PSG indices and incident diabetes for baseline confounders of age, education, income, total percentage of body fat, smoking, physical activity, weight gain over the follow-up period, and follow-up measures including ESS scores, sleep hours, and shift work. Modification of these relationships by EDS was assessed by the inclusion of an interaction term.
RESULTS
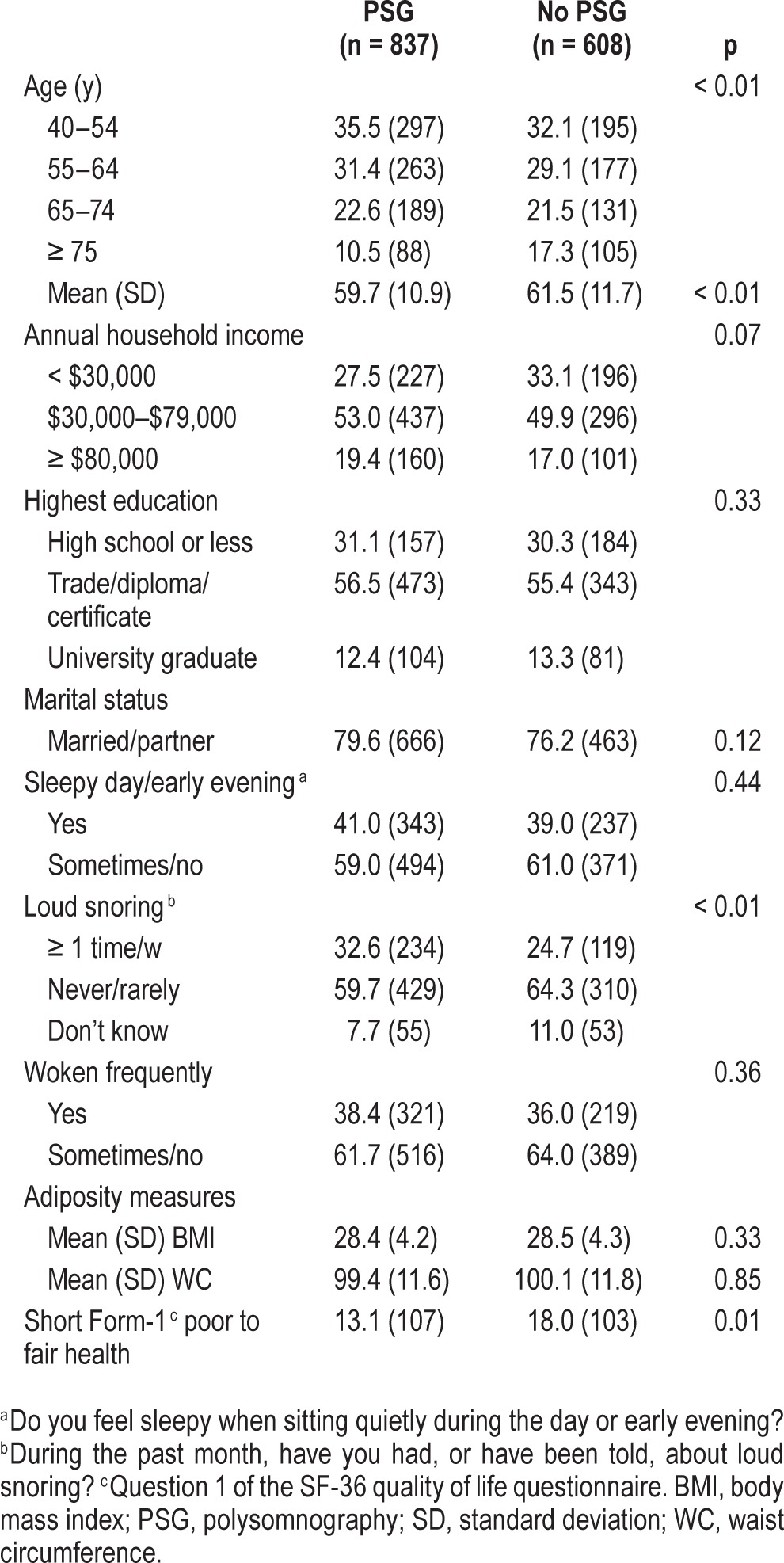
The characteristics of sleep study participants are shown in Table 1. Self-selection bias was also examined and men who underwent a sleep study had similar levels of obesity, daytime sleepiness, and waking frequently overnight compared to those who did not; however, PSG uptake was significantly more common in men who were younger, and those reporting frequent loud snoring, and better self-rated general health (Table 1).
Table 1.
Comparison of subjects [% (n)] who did and did not undertake polysomnography in MAILES participants without a previous diagnosis of sleep apnea (n = 1,445).
Of 736 men without diabetes at baseline, diabetes was identified in 66 men (9.0%) over a mean follow-up time of 56 mo (standard deviation = 5, range: 48–74 mo). Moderate to severe undiagnosed OSA (AHI ≥ 20/h) was present in 35% (n = 23) of those with incident diabetes [and in 32%, (n = 46) of those with prevalent diabetes]. Incident diabetes [% (n)] in relation to participant baseline characteristics is shown in Table 2. Incident diabetes was significantly associated with older age, and significant age-adjusted associations were seen with lower levels of income and education and central and generalized obesity. No association was seen with EDS (ESS ≥ 11).
Table 2.
Incident diabetes [% (n)] in relation to baselinea characteristics of 736 participants who were free of diabetes at baseline and undertook polysomnography.

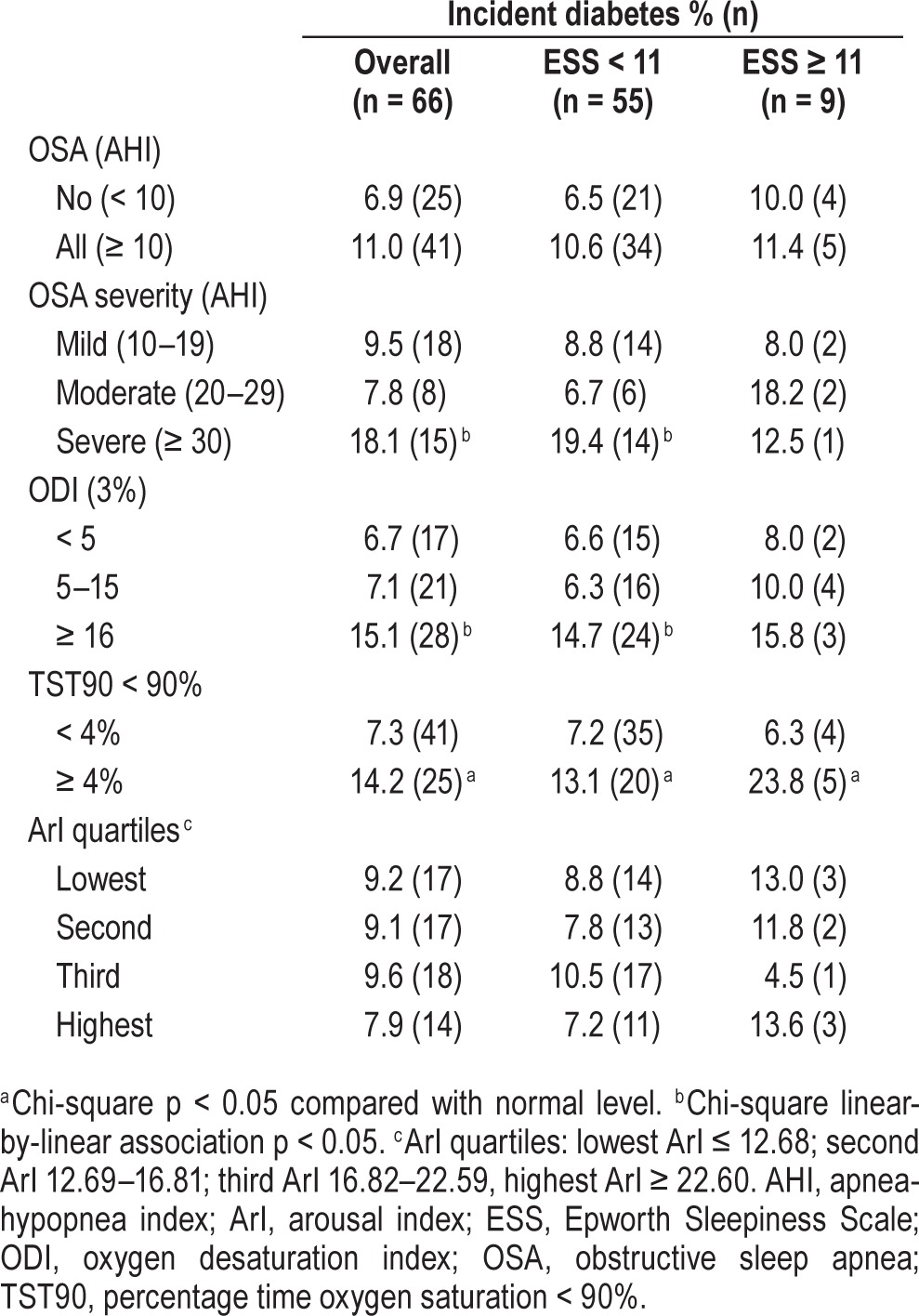
Table 3 shows univariate associations between PSG indices and incident diabetes, and in relation to EDS. Few new diabetes cases occurred in men with EDS (n = 9). Incident diabetes was significantly associated with severe OSA, ODI 3% ≥ 16/h and TST90 ≥ 4%. Daytime sleepiness did not alter these associations.
Table 3.
Incident diabetes [% (n)] in relation to sleepiness and polysomnographic indices of apnea-hypopnea index, oxygen desaturation (≥ 3%) index, percentage time oxygen saturation < 90%, and arousal index quartiles.
In multivariable logistic regression analyses (Table 4), after adjusting for confounders including age, percentage of body fat, and weight gain since baseline, incident diabetes was significantly associated with ODI 3% ≥ 16/h and severe OSA (AHI ≥ 30/h). A significant age-adjusted association of incident diabetes with TST90 ≥ 4% did not persist after additional adjustment for obesity. Effect modification by daytime sleepiness was not seen for any of the associations.
Table 4.
Odds ratio (95% confidence interval) for incident diabetes associated with obstructive sleep apnea severity, oxygen desaturation index (3%) ≥ 16/h and percentage of time oxygen saturation < 90% ≥ 4%.a

DISCUSSION
In an unselected cohort sample of community-dwelling men at least 40 y of age, the development of diabetes over the preceding 4 to 6 y was significantly associated with undiagnosed (and untreated) current severe OSA and ODI 3% ≥ 16/h after adjusting for important baseline confounders including age, total body fat and weight gain. The association of TST90 ≥ 4% was mediated by obesity, and no effects of arousals were observed. Significant-effect modification by daytime sleepiness was not seen for any of the associations.
Our findings are consistent with putative pathophysiological mechanisms linking OSA to diabetes, including oxidative stress caused by chronic intermittent hypoxemia, sleep deprivation, sleep fragmentation, hemodynamic disturbances, and alterations in sympathetic activity.19 In a Japanese community sample aged 40–69 y, ODI 3% > 15 was associated with increased likelihood of incident diabetes over 3 y [odds ratio, OR (95% confidence interval): 1.69 (1.04–2.76), but this study measured only oximetry and not other PSG parameters.14 In a retrospective analysis from a clinical cohort, severe OSA (AHI ≥ 30/h) and oxygen saturation less than 90% for greater than 6.4 min were independently associated with the development of diabetes.15 However, clinic samples may be subject to referral bias, particularly with regard to the development or severity of comorbidities. Obesity causes a decline in oxygen saturation,20 which may partly account for attenuation seen of the association of TST90 with developing diabetes. Consistent with previous work, we found no relationship with arousal frequency.6 Older studies in sleep clinic populations have suggested that impaired glucose metabolism was more prominent in patients with OSA with excessive daytime sleepiness4,21,22 and constant positive airway pressure therapy-related improvements in insulin sensitivity have also been reported in patients with EDS.4,22,23 In contrast, we have shown that in a population sample, sleepiness does not modify the association of OSA with diabetes that has developed over the previous 5 y. Our data are consistent with recent work that has not found sleepiness to be a modifying influence on HbA1c levels.24
Sleep studies were only available at follow-up, which limited us to examining retrospective associations of diabetes development. Longitudinal cohort data has shown weight gain over a 4- to 6-y period is strongly associated with the development of OSA,25 particularly among older men who are already obese.25 We controlled for central weight gain over follow-up and baseline body fat, increasing the likelihood that OSA was an independent contributing factor to the development of diabetes, although our study design means this cannot be certain. The generalizability of these findings to women is uncertain.
In conclusion, severe undiagnosed OSA and nocturnal intermittent hypoxemia were independently associated with the recent development of diabetes in a community sample of middle-aged and older men. Weight loss can produce clinically relevant improvements in OSA among obese patients with diabetes that are sustained at 4 y.26 The low reported rate of diagnosed OSA in a large primary care diabetes population of 18%27 emphasizes the importance of recommendations28 that clinicians managing OSA or diabetes should incorporate screening methods to ensure that a patient presenting with one disorder is assessed for the other.
DISCLOSURE STATEMENT
This study was funded by the National Health and Medical Research Council of Australia Project Grant number 627227. Financial support for the conduct of sleep studies was also obtained from the ResMed Foundation, La Jolla, CA. Dr. Adams has received research funding from the National Health and Medical Research Council of Australia, and the ResMed Foundation, and nonfinancial support from Embla Systems, Broomfield, CO. Dr. Vakulin has received research funding from the National Health and Medical Research Council of Australia. Dr. McEvoy has received research funding from the National Health and Medical Research Council of Australia, the ResMed Foundation, Philips Respironics, and Fisher and Paykel; equipment donations from ResMed, Philips Respironics and SomnoMed; and lecture fees from Philips Respironics. Dr. Antic has received research funding from the National Health and Medical Research Council of Australia, Philips Respironics, and Fisher and Paykel; equipment donations from ResMed, Philips Respironics and SomnoMed; and lecture fees and payment for development of educational presentations from ResMed. Dr. Catcheside has received research funding from the National Health and Medical Research Council of Australia, and the Australian Research Council, and equipment support from Philips Respironics and AirLiquide Healthcare. Dr. Taylor has received research funding from the National Health and Medical Research Council of Australia. Dr. Wittert has received research funding from the National Health and Medical Research Council of Australia and the ResMed Foundation; non-financial support from Embla Systems, Broomfield, CO; and personal fees from Eli Lilly, Bayer Schering, Sanofi, Novo Nordisk, AstraZeneca, I-Nova, and Elsevier. The other authors have indicated no other financial conflicts of interest.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- ArI
total arousal index
- CATI
computer assisted telephone interviews
- EDS
excessive daytime sleepiness
- ESS
Epworth Sleepiness Scale
- MAILES
Men Androgen Inflammation Lifestyle Environment and Stress
- ODI 3%
3% oxygen desaturation index
- PSG
polysomnography
- TST90
percentage of total sleep time with oxygen saturation < 90%
REFERENCES
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Australian Institute of Health and Welfare. Canberra: AIHW; Australia's Health 2012. Australia's health series no.13. Cat. no. AUS 156. [Google Scholar]
- 3.Australian Bureau of Statistics. Australian Health Survey: First Results, 2011-12. Cat 4364.0.55.001. Canberra. 2012 [Google Scholar]
- 4.Pamidi S, Tasali E. Obstructive sleep apnea and type 2 diabetes: is there a link? Front Neurol. 2012;3 doi: 10.3389/fneur.2012.00126. Article 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–6. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 6.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–30. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 7.Seicean S, Kirchner HL, Gottlieb DJ, et al. Sleep-disordered breathing and impaired glucose metabolism in normal-weight and overweight/obese individuals: the Sleep Heart Health Study. Diabetes Care. 2008;31:1001–6. doi: 10.2337/dc07-2003. [DOI] [PubMed] [Google Scholar]
- 8.Kendzerska T, Mollayeva T, Gershon AS, Leung RS, Hawker G, Tomlinson G. Untreated obstructive sleep apnea and the risk for serious long-term adverse outcomes: a systematic review. Sleep Med Rev. 2014;18:49–59. doi: 10.1016/j.smrv.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172:1590–5. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall NS, Wong KK, Phillips CL, Liu PY, Knuiman MW, Grunstein RR. Is sleep apnea an independent risk factor for prevalent and incident diabetes in the Busselton Health Study? J Clin Sleep Med. 2009;5:15–20. [PMC free article] [PubMed] [Google Scholar]
- 11.Botros N, Concato J, Mohsenin V, Selim B, Doctor K, Yaggi HK. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am J Med. 2009;122:1122–7. doi: 10.1016/j.amjmed.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindberg E, Theorell-Haglöw J, Svensson M, Gislason T, Berne C, Janson C. Sleep apnea and glucose metabolism: a long-term follow-up in a community-based sample. Chest. 2012;142:935–42. doi: 10.1378/chest.11-1844. [DOI] [PubMed] [Google Scholar]
- 13.Celen YT, Hedner J, Carlson J, Peker Y. Impact of gender on incident diabetes mellitus in obstructive sleep apnea: a 16-year follow-up. J Clin Sleep Med. 2010;6:244–50. [PMC free article] [PubMed] [Google Scholar]
- 14.Muraki I, Tanigawa T, Yamagishi K, et al. Nocturnal intermittent hypoxia and the development of type 2 diabetes: the Circulatory Risk in Communities Study (CIRCS) Diabetologia. 2010;53:481–8. doi: 10.1007/s00125-009-1616-0. [DOI] [PubMed] [Google Scholar]
- 15.Kendzerska T, Gershon AS, Hawker G, Tomlinson G, Leung RS. Obstructive sleep apnea and incident diabetes. A historical cohort study. Am J Respir Crit Care Med. 2014;190:218–25. doi: 10.1164/rccm.201312-2209OC. [DOI] [PubMed] [Google Scholar]
- 16.Grant JF, Martin SA, Taylor AW, et al. Cohort profile: The Men Androgen Inflammation Lifestyle Environment and Stress (MAILES) Study. Int J Epidemiol. 2014;43:1040–53. doi: 10.1093/ije/dyt064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iber C, Ancoli Israel S, Chesson A, Jr, Quan S. The AASM manual for the scoring of sleep and associated events. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 18.Ruehland WR, Rochford PD, O'Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep. 2009;32:150–7. doi: 10.1093/sleep/32.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy P, Bonsignore MR, Eckel J. Sleep, sleep-disordered breathing and metabolic consequences. Eur Respir J. 2009;34:243–60. doi: 10.1183/09031936.00166808. [DOI] [PubMed] [Google Scholar]
- 20.Vold M, Aasebø U, Melbye H. Low FEV1 smoking history and obesity are factors associated with oxygen saturation decrease in an adult population cohort. Int J Chron Obstruct Pulmon Dis. 2014;9:1225–33. doi: 10.2147/COPD.S69438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ronksley PE, Hemmelgarn BR, Heitman SJ, et al. Obstructive sleep apnoea is associated with diabetes in sleepy subjects. Thorax. 2009;64:834–9. doi: 10.1136/thx.2009.115105. [DOI] [PubMed] [Google Scholar]
- 22.Barcelo A, Barbe F, de la Pena M, et al. Insulin resistance and daytime sleepiness in patients with sleep apnoea. Thorax. 2008;63:946–50. doi: 10.1136/thx.2007.093740. [DOI] [PubMed] [Google Scholar]
- 23.Weinstock TG, Wang X, Rueschman M, et al. A controlled trial of CPAP therapy on metabolic control in individuals with impaired glucose tolerance and sleep apnea. Sleep. 2012;35:617–25B. doi: 10.5665/sleep.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kent BD, Grote L, M RB, et al. Sleep apnoea severity independently predicts glycaemic health in nondiabetic subjects: the ESADA study. Eur Respir J. 2014;44:130–9. doi: 10.1183/09031936.00162713. [DOI] [PubMed] [Google Scholar]
- 25.Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med. 2005;165:2408–13. doi: 10.1001/archinte.165.20.2408. [DOI] [PubMed] [Google Scholar]
- 26.Kuna ST, Reboussin DM, Borradaile KE, et al. Long-term effect of weight loss on obstructive sleep apnea severity in obese patients with type 2 diabetes. Sleep. 2013;36:641–9A. doi: 10.5665/sleep.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heffner JE, Rozenfeld Y, Kai M, Stephens EA, Brown LK. Prevalence of Diagnosed Sleep Apnea Among Patients With Type 2 Diabetes in Primary Care. Chest. 2012;141:1414–21. doi: 10.1378/chest.11-1945. [DOI] [PubMed] [Google Scholar]
- 28.Shaw JE, Punjabi NM, Wilding JP, Alberti KG, Zimmet PZ. Sleep-disordered breathing and type 2 diabetes: a report from the International Diabetes Federation Taskforce on Epidemiology and Prevention. Diabetes Res Clin Pract. 2008;81:2–12. doi: 10.1016/j.diabres.2008.04.025. [DOI] [PubMed] [Google Scholar]


