Abstract
Study Objectives:
One-third of deployed military personnel will be diagnosed with insomnia, placing them at high risk for comorbid depression, posttraumatic stress disorder (PTSD), and medical conditions. The disruption of trophic factors has been implicated in these comorbid conditions, which can impede postdeployment recovery. This study determined if improved sleep quality is associated with (1) reductions in depression and posttraumatic symptoms, as well as enrichments in health-related quality of life (HRQOL), and (2) changes in plasma concentrations of brain derived neurotrophic factor (BDNF) and insulin-like growth factor-1 (IGF-1).
Methods:
Forty-four military personnel diagnosed with insomnia underwent clinical evaluations and blood draws at pretreatment and at posttreatment following cognitive behavioral therapy for insomnia and automatic positive airway pressure treatment. Participants were classified as sleep improved (n = 28) or sleep declined (n = 16) based on their change in pretreatment to posttreatment Pittsburgh Sleep Quality Index (PSQI) score. Both groups were compared on outcomes of depression, PTSD, HRQOL, BDNF, and IGF-1.
Results:
Paired t-tests of the sleep improved group revealed significant declines in depression (p = 0.005) and posttraumatic arousal (p = 0.006) symptoms, and a significant increase in concentrations of IGF-1 (p = 0.009). The sleep declined group had no relevant change in psychiatric symptoms or trophic factors, and had further declines on five of eight dimensions of HRQOL. Between-group change score differences were significant at p < 0.05.
Conclusions:
These findings suggest that interventions, which successfully improve sleep quality, are an effective means to reduce the depression and posttraumatic arousal symptoms common to military personnel, as well as increase protective trophic factors implicated in these conditions.
Citation:
Rusch HL, Guardado P, Baxter T, Mysliwiec V, Gill JM. Improved sleep quality is associated with reductions in depression and PTSD arousal symptoms and increases in IGF-1 concentrations. J Clin Sleep Med 2015;11(6):615–623.
Keywords: BDNF, depression, IGF-1, insomnia, military, PTSD, sleep quality, mTBI, trauma
The prolonged stress of deployment and erratic sleep schedules imposed by mission requirements contributes to the onset of sleep disturbances in up to one-third of military personnel.1 In military personnel with mild traumatic brain injury (mTBI), this risk is elevated, which results in steep declines in health-related quality of life (HRQOL).2 Prior research has suggested a bidirectional relationship between sleep quality and psychological health, in which sleep disturbances triggered, or alternatively, developed as a result of depression and posttraumatic stress disorder (PTSD).3 However, recently there has been a shift towards conceptualizing sleep disturbance as a marker of underlying processes involved in the development of, as well as the delayed recovery from psychopathology following a stressful experience including military deployment.4 For example, predeployment daytime and nighttime sleep complaints predicted the onset of depression and PTSD up to two years postdeployment.5 General nightmares at postdeployment were also predictive of trauma reexperiencing six months after combat exposure.6 Although sleep disturbances may improve with depression and PTSD remission, they often do not abate entirely. Research indicates that residual insomnia can predict depression reoccurrence,7 which highlights the critical importance of treating underlying sleep disturbances in patients with mood and anxiety disorders to maintain psychiatric recovery, especially in the often stressful period of deployment readjustment. Although sleep disturbance represents a strong risk factor for depression and PTSD, less is known regarding the impact of targeting sleep disturbances directly to improve sleep in mitigating these psychiatric conditions and enriching HRQOL.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Previous studies recognized a relationship between sleep disturbance and psychiatric morbidity; however, limited work has examined this link in military personnel, a cohort at elevated risk for both insomnia and psychopathology. The current study examines the relation between improved sleep and attenuation of depression and posttraumatic symptoms in military personnel, as well as changes in trophic factors that likely modulate these conditions.
Study Impact: Results indicate a strong association between improved sleep and reduced psychiatric symptoms, as well as enriched health-related quality of life. Sleep-focused treatments may be an effective means to facilitate psychiatric recovery. Findings highlight the importance of conducting sleep assessments in military personnel with mood and anxiety disorders.
Sleep disruptions are also associated with impaired secretion of trophic factors, including brain-derived neurotrophic factor (BDNF) and insulin-like growth factor-1 (IGF-1).8,9 BDNF and IGF-1 interact synergistically in the same cascade of transmission to modulate learning, memory processes, neuronal plasticity, and tissue repair.10,11 In military personnel, the stress of combat training (i.e., deficiencies in sleep, energy, and water) significantly reduced plasma BDNF and IGF-1, which resumed to baseline levels following the cessation of training.12,13 This finding illustrates the relationship between chronic stress and the restoration of physiological equilibrium upon stressor elimination. Evidence also suggests that the BDNF/IGF-1 system contributes to sleep homeostasis, including the regulation of restorative slow wave sleep oscillations.14 This relationship may be attributed to BDNF/IGF-1 regulated neuronal plasticity changes, which are hypothesized to increase slow wave sleep activity.15 Chronic deregulation of the BDNF/IGF-1 system has consequences for the brain as well as for many body systems that may decrease physical health. Studies have also found that increased concentrations of BDNF and IGF-1 facilitate cognitive and physical rehabilitation following TBI.16 However, the extent to which these trophic factors can be modified through treatment intervention is less clear.
Optimal functioning of the BDNF/IGF-1 system may also play a protective role in mitigating psychiatric morbidity following stressors such as combat exposure and mTBI. Alternately, deviations in this system may be a susceptibility factor for psychiatric disorder development and relapse. BDNF is notably reduced in patients with PTSD and depression and is increased following antidepressant treatment.17,18 In recent years, numerous studies have identified the development of IGF-1 deficiencies following mTBI, which often manifests in physical, emotional, behavioral, cognitive, and social deficits with varying degrees of severity.16 However, studies investigating IGF-1 concentrations in patients with depression and PTSD are less prevalent, and these limited findings are discordant. In currently depressed patients, IGF-1 is increased compared with non-depressed controls,19 yet antidepressant treatments have also been shown to up regulate IGF-1.20 In the sole study of IGF-1 in patients with PTSD, pretreatment IGF-1 concentrations did not differ between PTSD patients and trauma-exposed controls; moreover, there was not a significant pre-post treatment change in PTSD patients whose symptoms remitted with paroxetine.21 Nevertheless, IGF-1 increases the synthesis and activity of BDNF to modulate antidepressive effects in the brain and enhances the expression of tropomyosin related kinase B (TrkB) receptors, which is required for the consolidation of hippocampal-dependent learning, a deficit implicated in PTSD.22 Although most studies focus on identifying the role of trophic factors in depression and PTSD diagnoses, the mediating role of sleep disruption on the BDNF/IGF-1 system and its subsequent effects on psychiatric morbidity have not been a focus of recent research.
Whether sleep is maintained or disturbed might predict an individual's ability to handle a certain stress load in lieu of developing a psychiatric disorder. It may also predict an individual's ability to recover from a preexisting psychiatric condition. Restorative sleep is necessary to maintain adequate concentrations of BDNF and IGF-1; therefore, sleep may be a key mediator at the connection between trauma exposure and the BDNF/IGF-1 system, for which deregulation can exacerbate the mental and physical comorbidity commonly observed in service members. We therefore designed an observational study of military personnel with insomnia to determine (1) if improved sleep quality, following sleep-focused standard of care, is associated with reductions in depression and posttraumatic symptoms as well as enrichments in HRQOL, and (2) if these symptom improvements are associated with plasma concentration changes of BDNF and IGF-1. These findings will ultimately inform interventions to address the complexity of comorbid symptoms in military personnel and attenuate the risk of psychiatric development.
METHODS
Study Design
This study was part of a larger observational study of US military personnel presenting for an initial evaluation of sleep disturbance.23 Potential participants were recruited through clinic referral and flyers posted in the sleep medicine clinic at the Madigan Army Medical Center, Tacoma, WA. The institutional review board approved the study, and informed consent was obtained from each participant. All participants underwent a sleep medicine evaluation and overnight polysomnogram using standardized techniques previously described23; they were then prescribed standard of care treatment based on their sleep disorder diagnosis. Participants diagnosed with insomnia received 4–8 biweekly sessions of cognitive behavioral therapy for insomnia (CBT-i). This included treatment components of cognitive therapy, stimulus control, sleep restriction, sleep practice (hygiene) education, and time monitoring behavior.24,25 Participants diagnosed with comorbid insomnia with obstructive sleep apnea (OSA) received either sleep education or 4–8 biweekly sessions of CBT-i for their insomnia, and automatic positive airway pressure (APAP) for their OSA. All treatments were administered and supervised by either a psychologist certified in Behavioral Sleep Medicine or a sleep medicine physician with expertise in CBT-i. Diagnoses of mTBI, as well as symptoms related to sleep quality, depression, PTSD, and HRQOL were evaluated through validated instruments at pretreatment and at 12-week posttreatment. Plasma concentrations of BDNF and IGF-1 were also assayed at pretreatment and at 12-week posttreatment.
Participants
A total of 145 military personnel were screened; 111 participants who met preliminary eligibility criteria were consented for enrollment. Inclusion criteria included (1) active duty military status with redeployment within the past 18 months, (2) a current diagnosis of insomnia or comorbid insomnia with OSA, (3) no recent history of drug or alcohol abuse, and (4) no current diagnosis of bipolar disorder, schizophrenia, or other psychotic disorder. Of the participants who consented, 67 were excluded from the analysis. Twenty-seven participants were diagnosed with OSA without insomnia, and another 14 participants were diagnosed with behaviorally induced insufficient sleep syndrome (BIISS) with an otherwise normal polysomnogram and did not receive treatment. Twelve participants did not return for posttreatment assessment, 11 had missing data, and 3 did not report a change in sleep outcome, which was required for our analysis. Thus, the final analysis included 44 military personnel with a current diagnosis of insomnia or comorbid insomnia with OSA. The study flow chart is depicted in Figure 1.
Figure 1. Study flow chart.
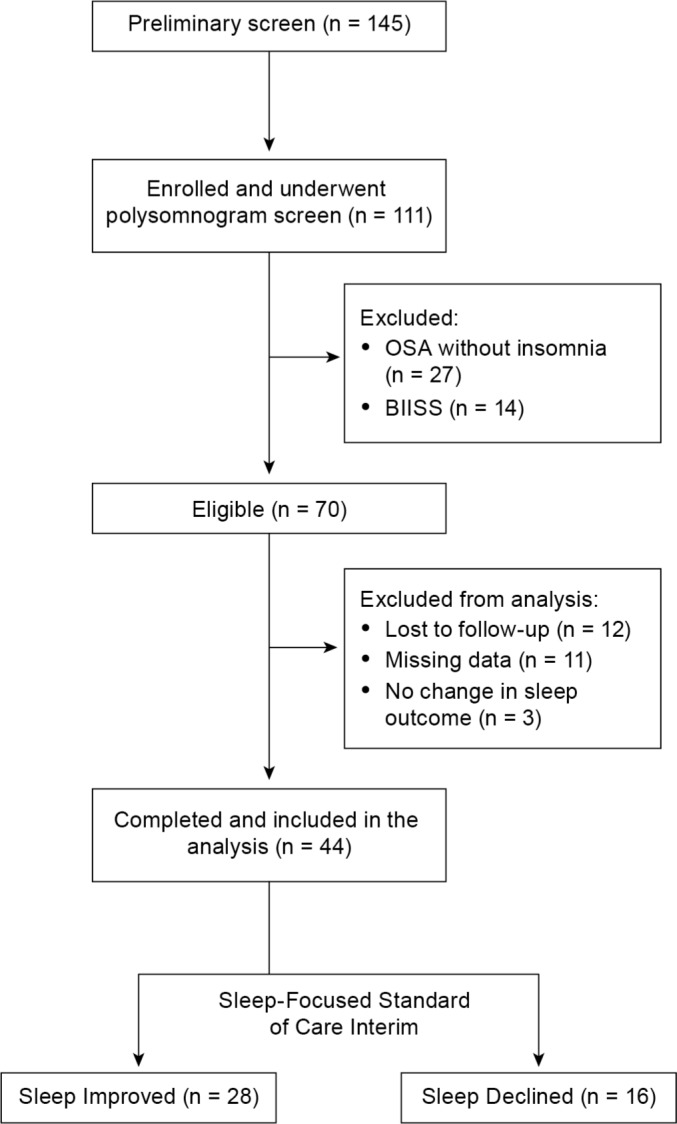
BIISS, behaviorally-induced insufficient sleep syndrome; OSA, obstructive sleep apnea.
Diagnostic Assessment
Insomnia/Obstructive Sleep Apnea
Sleep disorder diagnoses were classified in accordance with the International Classification of Sleep Disorders, 2nd edition.26 For the diagnosis of insomnia, participants were required to have one or more chronic sleep-related complaints of initiating sleep, maintaining sleep, or waking too early, with at least one accompanying symptom of daytime impairment assessed by the Epworth Sleepiness Scale (ESS).27 The comorbid diagnosis of insomnia with OSA was rendered when the participant's polysomnogram demonstrated apneas, hypopneas, or respiratory event related arousals resulting in an apnea-hypopnea index > 5/hour. A board-certified sleep medicine physician adjudicated all diagnoses, and our prior research validated these diagnostic criteria acceptable for military personnel.23
Mild Traumatic Brain Injury
Diagnosis of mTBI was determined using the Warrior Administered Retrospective Casualty Assessment Tool (WARCAT).28 This tool obtains data on possible TBI-related war injuries and assesses alterations in consciousness and the presence of postconcussional symptoms. A positive mTBI diagnosis was made if the participant indicated any loss of consciousness, loss of memory, and/or alteration in mental state that was caused by the head being struck or striking an object, or the brain undergoing an acceleration/deceleration movement (to include whiplash effect of blast exposure) without direct external head trauma.
Outcome Measures
The Pittsburgh Sleep Quality Index (PSQI)29 was used to assess self-reported sleep quality and sleep dysfunction over the previous month. It is a 19-item index, with a total possible range from 0 (good sleep quality) to 21 (poor sleep quality). A total score ≥ 6 yields a diagnostic sensitivity of 0.90 and a specificity of 0.87 in distinguishing good and poor sleep.
The Quick Inventory of Depressive Symptomatology (QIDS-SR)30 was used to assess self-reported depressive symptom severity and screen for depression based on the DSM-IV module for diagnosing major depressive episode. It is a 16-item inventory, with a total possible range from 0 (lowest severity) to 27 (highest severity). A total score ≥ 11 resulted in a positive screen for moderate depression.
The PTSD Checklist-Military Version (PCL-M)31 was used to assess self-reported posttraumatic symptom severity and screen for PTSD based on the DSM-IV module for diagnosing PTSD. It is a 17-item inventory, specific for military personnel, with one total score and 3 subscale scores including: intrusion, avoiding/numbing, and arousal. A total score ranges from 17 (lowest severity) to 85 (highest severity). To provide the maximum specificity (0.98), a total score ≥ 50 resulted in a positive PTSD diagnostic screen.
The Short Form Health Survey-36 (SF-36)32 was used to evaluate self-reported HRQOL on 8 outcomes, including bodily pain, emotional wellbeing, energy/fatigue, general health perceptions, physical functioning, role limitations due to emotional difficulties, role limitations due to physical difficulties, and social functioning. Each outcome has a unique score, with a total possible range from 0 to 100; lower scores indicate greater disability.
Biomarker Acquisition
Non-fasting venous blood samples were collected through routine venipuncture into ethylenediaminetetraacetic acid (EDTA) 10 mL tubes (Becton-Dickinson, Franklin Lakes, NJ), which were immediately placed on ice until processing. All samples were then stored in a biorepository at −80°C to prevent sample degradation until batch assayed by a technician who was blinded to the participant group. Plasma BDNF (pg/mL) and IGF-1 (ng/mL) concentrations were measured using an antibody-coated tube radioimmunoassay (R&D Systems, Minneapolis, MN). The inter-assay and intra-assay coefficients of variation were 6.8% and 9.0%, and 3.1% and 7.9%, respectively, with a lower limit detection of 1 pg/mL for BDNF and 12 ng/ mL for IGF-1. Every effort was made to collect blood samples during a midday window (11:00–12:00), although because of variability in subject availability, some collection times ranged from 09:00 to 16:00 (mean 11:36; SD 1 h 54 min).
Statistical Analyses
All variables were first examined for normality and normal distribution was confirmed. Demographics and clinical characteristics were dichotomized to illustrate the homogeneity of the sample. Preliminary analyses were used to describe pretreatment demographics and clinical characteristics of the entire sample, and 2-tailed chi-squared tests and independent t-tests were used to investigate any pretreatment group differences that might affect the main analysis. Fisher exact tests were used when expected cell counts were less than 5. The main analysis investigated the relationship between sleep quality improvements or declines following sleep-focused standard of care on symptoms of depression, PTSD, and HRQOL, as well as concentrations of BDNF and IGF-1. Participants were classified as “sleep improved” or “sleep declined” based on the direction (negative or positive) of their PSQI change score from pretreatment to posttreatment. A one point difference (i.e., 1 SD) was considered a valid change in sleep quality. Separate paired t-tests were used to examine changes in outcome variables from pretreatment to posttreatment for both groups. Independent t-tests were used to examine between group differences on outcome variables. Confidence level was set at p = 0.05 for all analyses.
RESULTS
Pretreatment Analysis
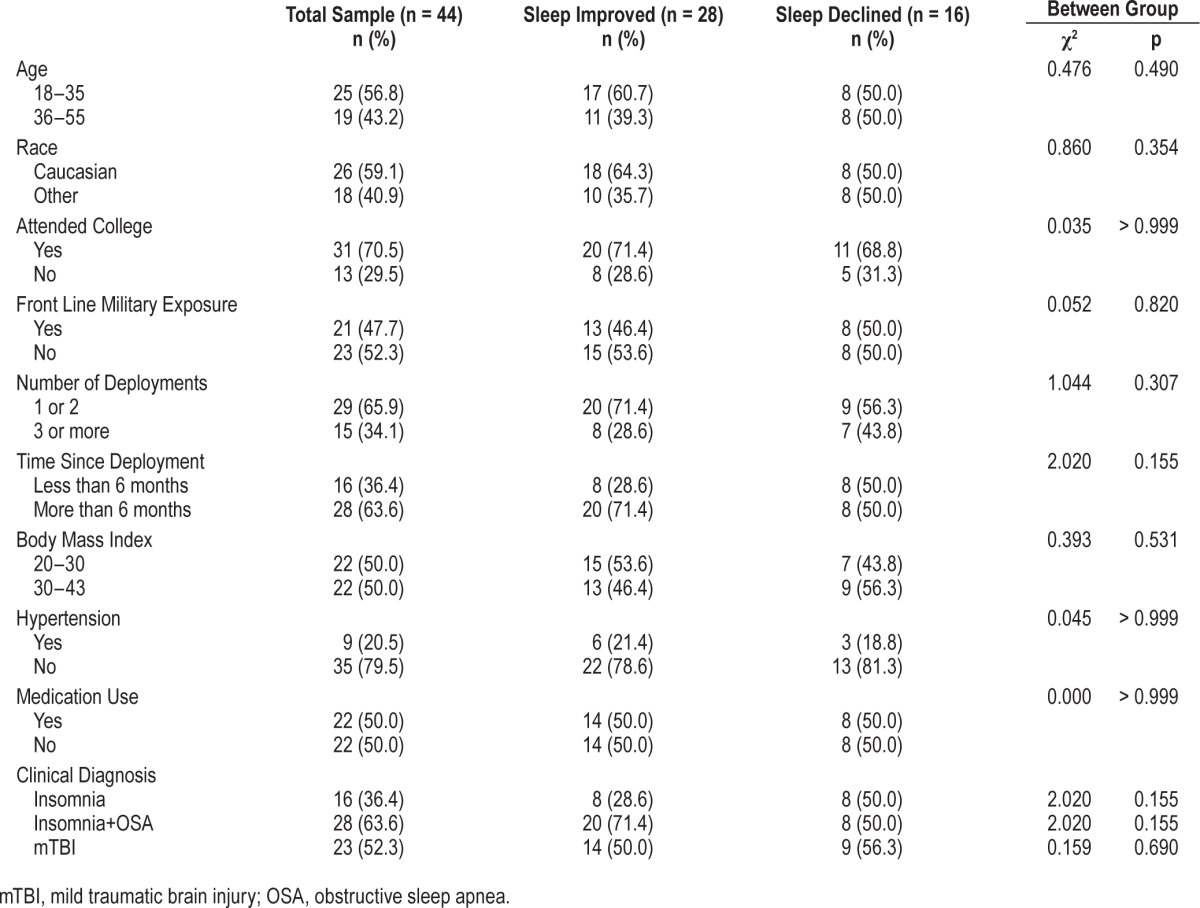
The demographic and clinical characteristics of the 44 participants included in this analysis are illustrated in Table 1. The sample as a whole had a mean age of 33.3 years (SD 8.1) and 13.7 years (SD 1.7) of education. Half of the sample reported current use of relevant medications including, antidepressants (39%), narcotics (23%), benzodiazepines (7%), non-benzodiazepine receptor agonists (7%), and prazosin (7%). The 2 groups did not differ in the number or type of prescribed medication they were taking. All participants had a score ≥ 6 on the PSQI, which is a valid determination of poor sleep. Sixty-four percent (28/44) of participants screened positive for depression, and 41% (18/44) screened positive for PTSD. Compared to the participants who completed the study, the non-completers did not differ on any pretreatment variables, with the exception of having more front-line military exposure (χ2(1) = 4.837, p = 0.028). This may explain the sample attrition, because combat roles can result in abrupt out-of-state deployment.
Table 1.
Pretreatment demographics and clinical characteristics.
Main Analysis
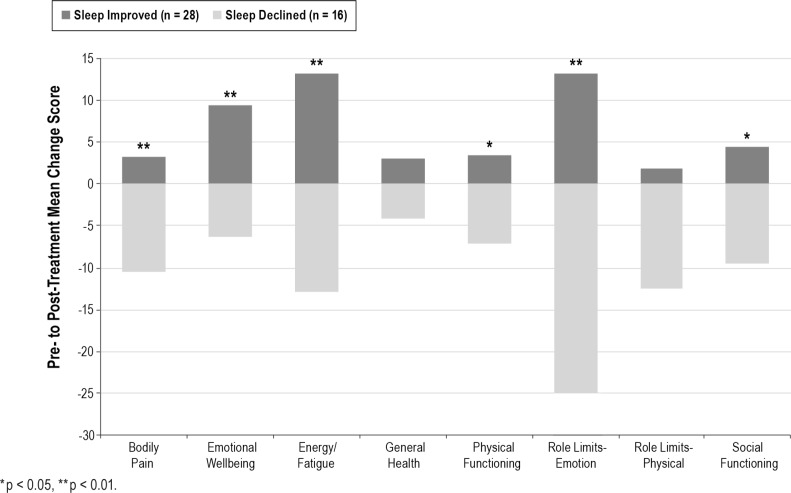
Sleep quality, depression, PTSD, and HRQOL outcomes were assessed before and after the sleep-focused standard of care interim. The type of treatment administered to both groups was comparable. At posttreatment, 64% (28/44) of participants reported improved sleep quality, while 36% (16/44) reported sleep quality declines from pretreatment assessment. Pretreatment demographics, clinical characteristics, and outcome variables were comparable between the sleep improved group (n = 28) and the sleep declined group (n = 16). Table 2 details the pretreatment and posttreatment symptom severity of both groups. Briefly, from pretreatment to posttreatment, the sleep improved group reported significant reductions in depression (t27 = 3.061, p = 0.005) and posttraumatic arousal symptoms (t27 = 2.993, p = 0.006), as well as significant enrichments in emotional wellbeing (t27 = −3.207, p = 0.003) and energy/fatigue (t27 = −3.229, p = 0.003). These improvements were significant between groups (all p values < 0.01). Meanwhile, the sleep declined group reported no significant change in depression (p = 0.164) or posttraumatic symptoms (p = 0.177). In addition, the sleep declined group had significant worsening of bodily pain (t15 = 2.338, p = 0.034), energy/fatigue (t15 = 3.380, p = 0.004), role limitations due to emotional difficulties (t15 = 3.494, p = 0.003), role limitations due to physical difficulties (t15 = 2.236, p = 0.041), and social functioning (t15 = 2.586, p = 0.021). With the exception of role limitations due to physical difficulties, this symptom exacerbation was significant between groups (all p values < 0.02). Figure 2 depicts the inverse relationship of HRQOL changes according to sleep quality improvements or declines.
Table 2.
Pretreatment and posttreatment symptom severity.
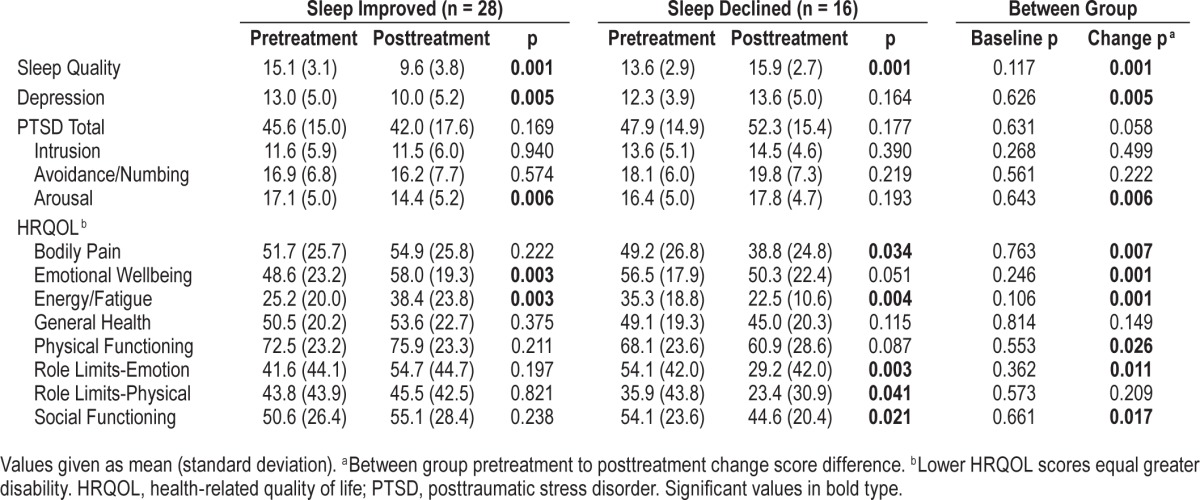
Figure 2. Change in health-related quality of life according to change in sleep quality.
*p < 0.05, **p < 0.01.
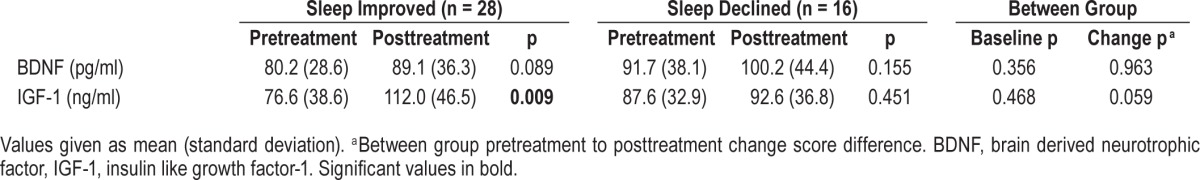
Concentrations of BDNF and IGF-1 were also assayed before and after the sleep-focused standard of care interim. Both groups exhibited comparable pretreatment concentrations (Table 3). From pretreatment to posttreatment, the sleep improved group had a significant increase in concentrations of IGF-1 (t15 = −2.979, p = 0.009) and a nonsignificant increase in BDNF (t17 = −1.822, p = 0.086). In contrast, no significant change in concentrations of BDNF or IGF-1 was observed in the sleep declined group (p = 0.155; p = 0.451).
Table 3.
Pretreatment and posttreatment BDNF and IGF-1 concentrations
Depression Subgroup Analysis
Of the 28 participants who screened positive for depression at pretreatment evaluation, 64% (18/28) reported improved sleep quality, while 36% (10/28) reported sleep quality declines from pretreatment assessment (Table 4). Pretreatment demographics, clinical characteristics, and outcome variables were comparable between the depression-sleep improved subgroup (n = 18) and the depression-sleep declined subgroup (n = 10), with the exception that the former subgroup reported lower emotional wellbeing (t26 = 2.223, p = 0.035), and the latter subgroup had a higher prevalence of comorbid insomnia with OSA (χ2(1) = 5.535, p = 0.035). At posttreatment assessment, 44% (8/18) of the depression-sleep improved subgroup had a clinically relevant reduction in depression symptoms (i.e., 5 points),30 and 50% (9/18) no longer met criteria for depression (i.e., a QIDS score < 11). In the depression-sleep declined subgroup, not one participant had a clinically relevant reduction in depression symptoms. Clinically relevant symptom reduction was significant between groups (χ2(1) = 6.222, p = 0.025); however, depression symptom reduction was not associated with significant concentration changes in BDNF or IGF-1 (p = 0.190; p = 0.059).
Table 4.
Odds ratio of improved depression in the depression-sleep improved subgroup.

When comparing the entire depression subgroup with participants who had a negative pretreatment screen for depression, no between group differences were found on pretreatment concentrations of BDNF and IGF-1 (p = 0.826; p = 0.714). There were also no group differences between current antidepressant users and non users on pretreatment concentrations of BDNF and IGF-1 (p = 0.375; p = 0.209) and on posttreatment concentrations (p = 0.151; p = 0.995).
PTSD Subgroup Analysis
Of the 18 participants who screened positive for PTSD at pretreatment evaluation, 61% (11/18) reported improved sleep quality, while 39% (7/18) reported sleep quality declines from pretreatment assessment. Pretreatment demographics, clinical characteristics, and outcome variables were comparable between the PTSD-sleep improved subgroup (n = 11) and the PTSD-sleep declined subgroup (n = 7), with the exception that the latter subgroup had a slightly greater number of deployments (χ2(1) = 5.657, p = 0.043). At posttreatment assessment, 45% (5/11) of the PTSD-sleep improved subgroup had a clinically relevant reduction in PTSD symptoms,33 and 27% (3/11) no longer met criteria for PTSD (i.e., PCL-M score < 50). In the PTSD-sleep declined subgroup, 14% (1/7) had a clinically relevant reduction in PTSD symptoms; however, none of the 7 participants in this group recovered from PTSD. No significant between group differences were found on clinically relevant symptom reduction or clinically defined recovery (p = 0.600; p = 0.999). PTSD symptom reduction was not associated with significant concentration changes in BDNF or IGF-1 (p = 0.314; p = 0.488), nor did the duration since trauma exposure (i.e., deployment greater or less than 6 months) have an effect on BDNF and IGF-1 concentrations (p = 0.841; p = 0.716).
When comparing the entire PTSD subgroup with participants who had a negative pretreatment screen for PTSD, no between group differences were found on pretreatment concentrations of BDNF and IGF-1 (p = 0.512; p = 0.329). There were also no significant between group pretreatment BDNF and IGF-1 concentration differences when comparing participants with mTBI to those without mTBI (p = 0.933; p = 0.842), as well as when comparing participants with primary insomnia to those with comorbid insomnia with OSA (p = 0.658; p = 0.949).
DISCUSSION
The primary aim of the current study was to examine the relationship between changes in patient-reported sleep quality and symptoms of depression, PTSD, and HRQOL, in military personnel with insomnia and comorbid psychiatric disorders. At posttreatment, participants who reported improved sleep quality had significant reductions in depression and posttraumatic arousal symptoms, as well as enrichments in HRQOL. These outcomes are notable because the treatment was specifically tailored for improving sleep quality without explicitly targeting depression, PTSD, or HRQOL. Alternatively, when sleep quality declines were reported, there were no significant improvements in psychiatric symptoms, and further declines in HRQOL were observed, including impairments in social functioning. This finding has additional ramifications because positive social relations promote psychological resilience against the deleterious effects of stress, including military exposure; and impoverished social support has been linked to depression and PTSD.34
Improving sleep quality in individuals with depression and PTSD using an adjuvant sleep intervention has begun to attract attention because of the known link between sleep disturbance and psychiatric morbidity. For example, sleep focused mindfulness-based cognitive therapy (MBCT) lowered total wake time and increased sleep efficiency in antidepressant users relative to controls.35 In a similar mindfulness intervention, improved sleep quality was associated with reductions in posttraumatic stress symptoms.36 However, unlike other studies that examined whether a sleep intervention could improve psychiatric symptoms as part of a general depression or PTSD treatment regimen, the current study targeted sleep exclusively, given the prospect that improved sleep quality may result in psychiatric recovery.
Theses results provide preliminary support that sleep-focused treatments, such as cognitive behavioral therapy and autotitrating positive airway pressure, are efficacious to address both insomnia and comorbid psychiatric conditions. Such treatment approaches may be preferred to current first-line treatments for depression and PTSD, which have considerable side effects or minimal effectiveness. Antidepressant medications, including selective serotonin reuptake inhibitors, notably disrupt sleep continuity, often resulting in non-adherence.37 Both pharmacologic and nonpharmacologic treatments for PTSD commonly increase sleep disruptions and anxiety, and their efficacy is questionable compared to placebo.38 Moreover, because of the stigma associated with depression and PTSD, military personnel are more likely to seek treatment for sleep disturbances than pursue treatment for a psychiatric condition. As new evidence points to sleep as a mediator at the nexus between stress and psychiatric onset and relapse, treatments that address sleep disturbance foremost, when comorbid with a psychiatric disorder, may provide a viable and global intervention.
The second aim of the current study was to examine the association of symptom improvement to changes in trophic factor concentrations. The current findings indicate that BDNF and IGF-1 increases are associated with improved sleep quality following sleep-focused standard of care. These findings suggest that changes in stress regulating systems may promote recovery from deployment-related psychiatric disorders, even when treatment is provided for a short period of time. These findings have important clinical implications because BDNF and IGF-1 have important roles in promoting neuroplasticity, which is disrupted in depression and preclinical models of stress.22,39 Moreover, BDNF is essential for the consolidation of hippocampal-dependent learning; as such, concentration increases may reduce impairments in contextual fear learning, a deficit central to PTSD.11 Increases in IGF-1 concentrations have additional significance in promoting physical health. Alterations in the IGF-I axis have been associated with a number of pathological conditions such as cancer, obesity, and type II diabetes.40 Because recently deployed military personnel are relatively young and healthy, early intervention to normalize trophic factor concentrations with a known morbidity and mortality risk is critical and may ultimately lead to reductions in medical costs and increases in HRQOL.
In our subgroup analyses, we compared trophic factor concentration levels between positive and negative diagnoses at pretreatment. Prior studies report reduced BDNF concentrations in patients with depression18 and PTSD.17 IGF-1 concentration increases are reported in depression20 and marked decreases have been found in TBI and in military personnel under stress.41 These prior findings were not replicated in the current sample. There were no group differences in pre-treatment BDNF concentrations between participants with depression and/or PTSD diagnoses and participants with negative diagnoses. IGF-1 concentrations were also not associated with depression, PTSD, or TBI diagnoses. The discrepancy between the current findings and past research may be attributed to BDNF and IGF-1 concentration changes following sleep quality declines rather than depression or PTSD symptom increases. Thus, this subgroup analysis suggests that plasma BDNF and IGF-1 levels are not indicative of a specific psychiatric diagnosis, but may be independently associated with the presence or absence of insomnia symptoms. This is supported by prior research where only the subjects who suffered from increased stress with comorbid sleep disturbances had decreased BDNF levels.8 Another study reports that although low concentrations of BDNF were associated with a depression diagnosis, there's suspect of a mediating variable since the concentrations levels were unrelated to the core clinical features of depression.42 Alternatively, IGF-1 concentration increases were associated with successful completion of three months of continuous positive airway pressure therapy in patients with OSA.43 Taken together, this suggests that sleep may be a key mediator in the connection between stress and the BDNF/IGF-1 system. Therefore, addressing sleep disturbances early on may increase protective trophic factor concentrations and reduce the risk for mental and physical morbidity in stress-exposed populations.
Although our investigation has strengths, such as the longitudinal design and simultaneous evaluation of both subjective and objective sleep measures paired with biomarker assay, there are some important limitations. First, because our sample consisted of sleep disturbed military personnel, the range of scores was somewhat restricted, which may have resulted in the underestimation of effect sizes. Second, we obtained only a single measurement of each trophic factor at pretreatment and at posttreatment. Due to the within-person variation of trophic factors, the reliability of our pretreatment and posttreatment estimates would have been enhanced had we obtained and averaged two separate measurements at both time points. Third, we included only two waves of data in our analyses, pre-treatment and 12 weeks posttreatment. While there were significant symptom improvements, had we assessed at 6 months posttreatment, we may have seen additional significance and would be able to map a more accurate course of recovery and validate sustainability of treatment. Finally, our sample size was relatively small, and because our sample consisted of only male military personnel, our findings may not generalize to females or civilians. Future studies with a larger, more heterogeneous population, and a design including posttreatment objective measurements of sleep, such as polysomnography, should be undertaken to verify the results of our exploratory investigation. Since this was an observational study, we cannot determine with certainty that sleep quality improvements and associated psychiatric symptom reduction did not occur via outside treatments for related illnesses. However, these conditions are often chronic, so the expeditious recovery over 12 weeks is more likely attributed to the sleep-focused standard of care. This preliminary/pilot study provides data which can be used to develop further randomized control trials. Specifically, more research is needed to determine the role of sleep and trophic factors on psychiatric disorder onset and recovery.
In conclusion, we report that military personnel with substantial health declines and neurobehavioral symptom burden report reductions in depression and PTSD arousal following improvements in sleep quality through a standard of care sleep treatment. This highlights the clinical importance of conducting sleep assessments in military personnel with mood and anxiety disorders. Furthermore, we report that sleep quality improvements are associated with increases in sleep regulating trophic factors. This is particularly important because BDNF and IGF-1 are implicated in memory processes and neuronal growth. Sleep focused interventions may be an effective treatment to reduce the elevated depressive and excessive arousal symptoms common in military personnel. Therapeutic restoration of trophic factors to improve sleep quality and reduce psychiatric symptom burden warrants further investigation.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest. The work was performed at the Madigan Army Medical Center, Tacoma, WA. Support for this work included funding from Department of Defense in the Center for Neuroscience and Regenerative Medicine.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Dr. Marquisha Lee for providing hours of CBT-i to the participants, Dr. Anlys Olivera and Ms. Whitney Livingston for their editing advice, and the participants for without them this research would not be possible.
ABBREVIATIONS
- APAP
automatic positive airway pressure
- BDNF
brain derived neurotrophic factor
- BIISS
behaviorally induced insufficient sleep syndrome
- EDTA
ethylenediaminetetraacetic acid
- ESS
Epworth Sleepiness Scale
- HRQOL
health-related quality of life
- IGF-1
insulin-like growth factor-1
- MBCT
mindfulness-based cognitive therapy
- mTBI
mild traumatic brain injury
- OSA
obstructive sleep apnea
- PCL-M
PTSD Checklist-Military Version
- PSQI
Pittsburgh Sleep Quality Index
- PTSD
posttraumatic stress disorder
- QIDS
Quick Inventory of Depressive Symptomatology
- QOIDS-SR
Quick Inventory of Depressive Symptomatology
- SD
standard deviation
- SF-36
Short Form Health Survey-36
- WARCAT
Warrior Administered Retrospective Casualty Assessment Tool
REFERENCES
- 1.Seelig AD, Jacobson IG, Smith B, et al. Sleep patterns before, during, and after deployment to Iraq and Afghanistan. Sleep. 2010;33:1615–22. doi: 10.1093/sleep/33.12.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med. 2008;358:453–63. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- 3.Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411–8. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 4.Gehrman P, Seelig AD, Jacobson IG, et al. Predeployment sleep duration and insomnia symptoms as risk factors for new-onset mental health disorders following military deployment. Sleep. 2013;36:1009–18. doi: 10.5665/sleep.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koffel E, Polusny MA, Arbisi PA, Erbes CR. Pre-deployment daytime and nighttime sleep complaints as predictors of post-deployment PTSD and depression in National Guard troops. J Anxiety Disord. 2013;27:512–9. doi: 10.1016/j.janxdis.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Pigeon WR, Campbell CE, Possemato K, Ouimette P. Longitudinal relationships of insomnia, nightmares, and PTSD severity in recent combat veterans. J Psychosom Res. 2013;75:546–50. doi: 10.1016/j.jpsychores.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Cho HJ, Lavretsky H, Olmstead R, Levin MJ, Oxman MN, Irwin MR. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165:1543–50. doi: 10.1176/appi.ajp.2008.07121882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giese M, Unternaehrer E, Brand S, Calabrese P, Holsboer-Trachsler E, Eckert A. The interplay of stress and sleep impacts BDNF level. PLoS One. 2013;8:e76050. doi: 10.1371/journal.pone.0076050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jarrett DB, Greenhouse JB, Miewald JM, Fedorka IB, Kupfer DJ. A reexamination of the relationship between growth hormone secretion and slow wave sleep using delta wave analysis. Biol Psychiatry. 1990;27:497–509. doi: 10.1016/0006-3223(90)90441-4. [DOI] [PubMed] [Google Scholar]
- 10.Johnson-Farley NN, Travkina T, Cowen DS. Cumulative activation of akt and consequent inhibition of glycogen synthase kinase-3 by brain-derived neurotrophic factor and insulin-like growth factor-1 in cultured hippocampal neurons. J Pharmacol Exp Ther. 2006;316:1062–9. doi: 10.1124/jpet.105.094433. [DOI] [PubMed] [Google Scholar]
- 11.Andero R, Choi DC, Ressler KJ. BDNF-TrkB receptor regulation of distributed adult neural plasticity, memory formation, and psychiatric disorders. Prog Mol Biol Transl Sci. 2014;122:169–92. doi: 10.1016/B978-0-12-420170-5.00006-4. [DOI] [PubMed] [Google Scholar]
- 12.Gomez-Merino D, Drogou C, Chennaoui M, Tiollier E, Mathieu J, Guezennec CY. Effects of combined stress during intense training on cellular immunity, hormones and respiratory infections. Neuroimmunomodulation. 2005;12:164–72. doi: 10.1159/000084849. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki G, Tokuno S, Nibuya M, et al. Decreased plasma brain-derived neurotrophic factor and vascular endothelial growth factor concentrations during military training. PLoS One. 2014;9:e89455. doi: 10.1371/journal.pone.0089455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bachmann V, Klein C, Bodenmann S, et al. The BDNF Val66Met polymorphism modulates sleep intensity: EEG frequency- and state-specificity. Sleep. 2012;35:335–44. doi: 10.5665/sleep.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Rosario ER, Aqeel R, Brown MA, Sanchez G, Moore C, Patterson D. Hypothalamic-pituitary dysfunction following traumatic brain injury affects functional improvement during acute inpatient rehabilitation. J Head Trauma Rehabil. 2013;28:390–6. doi: 10.1097/HTR.0b013e318250eac6. [DOI] [PubMed] [Google Scholar]
- 17.Angelucci F, Ricci V, Gelfo F, et al. BDNF serum levels in subjects developing or not post-traumatic stress disorder after trauma exposure. Brain Cogn. 2014;84:118–22. doi: 10.1016/j.bandc.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64:527–32. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weber-Hamann B, Blum WF, Kratzsch J, Gilles M, Heuser I, Deuschle M. Insulin-like growth factor-I (IGF-I) serum concentrations in depressed patients: relationship to saliva cortisol and changes during antidepressant treatment. Pharmacopsychiatry. 2009;42:23–8. doi: 10.1055/s-0028-1085442. [DOI] [PubMed] [Google Scholar]
- 20.Schilling C, Blum WF, Heuser I, Paslakis G, Wudy SA, Deuschle M. Treatment with antidepressants increases insulin-like growth factor-I in cerebrospinal fluid. J Clin Psychopharmacol. 2011;31:390–2. doi: 10.1097/JCP.0b013e3182189d86. [DOI] [PubMed] [Google Scholar]
- 21.Bonne O, Gill JM, Luckenbaugh DA, et al. Corticotropin-releasing factor, interleukin-6, brain-derived neurotrophic factor, insulin-like growth factor-1, and substance P in the cerebrospinal fluid of civilians with posttraumatic stress disorder before and after treatment with paroxetine. J Clin Psychiatry. 2011;72:1124–8. doi: 10.4088/JCP.09m05106blu. [DOI] [PubMed] [Google Scholar]
- 22.Paslakis G, Blum WF, Deuschle M. Intranasal insulin-like growth factor I (IGF-I) as a plausible future treatment of depression. Med Hypotheses. 2012;79:222–5. doi: 10.1016/j.mehy.2012.04.045. [DOI] [PubMed] [Google Scholar]
- 23.Mysliwiec V, Gill J, Lee H, et al. Sleep disorders in US military personnel: a high rate of comorbid insomnia and obstructive sleep apnea. Chest. 2013;144:549–57. doi: 10.1378/chest.13-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siebern AT, Manber R. New developments in cognitive behavioral therapy as the first-line treatment of insomnia. Psychol Res Behav Manag. 2011;4:21–8. doi: 10.2147/PRBM.S10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams J, Roth A, Vatthauer K, McCrae CS. Cognitive behavioral treatment of insomnia. Chest. 2013;143:554–65. doi: 10.1378/chest.12-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Academy of Sleep Medicine. International classification of sleep disorders: diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 27.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 28.Terrio H, Brenner LA, Ivins BJ, et al. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J Head Trauma Rehabil. 2009;24:14–23. doi: 10.1097/HTR.0b013e31819581d8. [DOI] [PubMed] [Google Scholar]
- 29.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 30.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 31.Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety. 2001;13:132–56. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- 32.Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 33.Monson CM, Gradus JL, Young-Xu Y, Schnurr PP, Price JL, Schumm JA. Change in posttraumatic stress disorder symptoms: do clinicians and patients agree? Psychol Assess. 2008;20:131–8. doi: 10.1037/1040-3590.20.2.131. [DOI] [PubMed] [Google Scholar]
- 34.Pietrzak RH, Johnson DC, Goldstein MB, Malley JC, Southwick SM. Psychological resilience and postdeployment social support protect against traumatic stress and depressive symptoms in soldiers returning from Operations Enduring Freedom and Iraqi Freedom. Depress Anxiety. 2009;26:745–51. doi: 10.1002/da.20558. [DOI] [PubMed] [Google Scholar]
- 35.Britton WB, Haynes PL, Fridel KW, Bootzin RR. Mindfulness-based cognitive therapy improves polysomnographic and subjective sleep profiles in antidepressant users with sleep complaints. Psychother Psychosom. 2012;81:296–304. doi: 10.1159/000332755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura Y, Lipschitz DL, Landward R, Kuhn R, West G. Two sessions of sleep-focused mind-body bridging improve self-reported symptoms of sleep and PTSD in veterans: a pilot randomized controlled trial. J Psychosom Res. 2011;70:335–45. doi: 10.1016/j.jpsychores.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 37.Rush AJ, Armitage R, Gillin JC, et al. Comparative effects of nefazodone and fluoxetine on sleep in outpatients with major depressive disorder. Biol Psychiatry. 1998;44:3–14. doi: 10.1016/s0006-3223(98)00092-4. [DOI] [PubMed] [Google Scholar]
- 38.Escamilla M, LaVoy M, Moore BA, Krakow B. Management of post-traumatic nightmares: a review of pharmacologic and nonpharmacologic treatments since 2010. Curr Psychiatry Rep. 2012;14:529–35. doi: 10.1007/s11920-012-0306-7. [DOI] [PubMed] [Google Scholar]
- 39.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 40.Rajpathak SN, Gunter MJ, Wylie-Rosett J, et al. The role of insulin-like growth factor-I and its binding proteins in glucose homeostasis and type 2 diabetes. Diabetes Metab Res Rev. 2009;25:3–12. doi: 10.1002/dmrr.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Creyghton WM, van Dam PS, Koppeschaar HP. The role of the somatotropic system in cognition and other cerebral functions. Semin Vasc Med. 2004;4:167–72. doi: 10.1055/s-2004-835375. [DOI] [PubMed] [Google Scholar]
- 42.Molendijk ML, Bus BA, Spinhoven P, et al. Serum levels of brain-derived neurotrophic factor in major depressive disorder: state-trait issues, clinical features and pharmacological treatment. Mol Psychiatry. 2011;16:1088–95. doi: 10.1038/mp.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoyos CM, Killick R, Keenan DM, Baxter RC, Veldhuis JD, Liu PY. Continuous positive airway pressure increases pulsatile growth hormone secretion and circulating insulin-like growth factor-1 in a time-dependent manner in men with obstructive sleep apnea: a randomized sham-controlled study. Sleep. 2014;37:733–41. doi: 10.5665/sleep.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]