Abstract
Study Objectives:
To assess whether daytime naps negatively impact nocturnal sleep.
Design:
Longitudinal, prospective cohort design.
Participants:
161 pregnant women recruited and evaluated in early gestation (10–20 weeks).
Measurements and Results:
Daily sleep information was collected in three 2-week periods (10–12, 14–16, and 18–20 weeks gestation) with a daily sleep diary and an actigraph. The average number of naps, as well as the average length of each nap, were calculated from sleep diaries. Women were categorized first as non nappers (0 naps/2-week period), moderate nappers (1–3 naps/2-week period), or frequent nappers (≥ 4 naps/2-week period). Then, based on the average nap length, they were categorized as short (< 90 min) or long (≥ 90 min) nappers. Nocturnal sleep parameters included SOL, WASO, SE, and TST. SAS procedure MIXED was used for modeling the main effects of nap group and time, and time by nap group interactions. Women who took naps had a decrease in diary-assessed nocturnal TST, but not actigraphy-assessed TST. This observation was group- and time-specific. There were no other group differences. Women who napped ≥ 90 min had poorer diary-assessed SE and lower diary-assessed TST than those who took shorter naps. Length of nap was not associated with any other sleep measures.
Conclusions:
The number of daytime naps have minimal impact on nocturnal sleep parameters; however, long nappers did exhibit modestly impaired sleep continuity and sleep quality. Overall, we propose that daytime naps provide a beneficial countermeasure to the sleep disruption commonly reported by pregnant women. This may be clinically beneficial given that sleep continuity and quality are important correlates of pregnancy outcomes.
Commentary:
A commentary on this article appears in this issue on page 593.
Citation:
Ebert RM, Wood A, Okun ML. Minimal effect of daytime napping behavior on nocturnal sleep in pregnant women. J Clin Sleep Med 2015;11(6):635–643.
Keywords: naps, pregnancy, sleep, women, frequency
The majority of expectant women experience progressively disturbed, fragmented, and shortened nocturnal sleep as a result of normal physiological adaptations to the progressing pregnancy.1–3 Emerging evidence indicates that insufficient quantity and poor sleep quality are associated with increased risk of mood dysregulation,4,5 as well as adverse pregnancy outcomes, such as preterm birth and gestational diabete s.6–8 When sufficient nighttime sleep is not possible or unattainable, certain strategies can be implemented to counteract the negative effects of disturbed nocturnal sleep. One promising approach is to include daytime naps as part of a healthy sleep hygiene progra m.9–11
There is limited information examining the effects of daytime naps on nocturnal sleep outcomes in non-pregnant populations. The majority of evidence stems from examination of daytime naps in elderly or middle-aged individuals or children. The bulk, however, consider daytime naps as a positive health behavior (a “countermeasure”) in that they can effectively combat subjectively reported poor sleep quality and decrease subjective daytime sleepiness by augmenting shortened nocturnal sleep durat ion.11–14 However, there is also a small literature that suggests that naps can be detrimental to nocturnal sleep and next-day daytime alert ness.15–17 Research on varied nap lengths shows that the greatest increase in aler tness,10,18 mood,19 or sleep efficiency20 occurs following a short nap, as opposed to a longer nap (> 30 min). Others have reported contrasting evidence showing no difference between short and long naps on sleep quality or quantity.21 Variations in nap length have been found to be quite common within cohorts and among individuals. Dautovich et al. found a correlation between increased variation in nap duration and a higher number of health issues among an elderl y sample.22 Others have found that those who napped regularly (compared to those who rarely napped) had increased benefits from self-repo rted naps.19,23 Benefits included improved cognitive functioning, short-term memory and mood, and beneficial changes in immune parameters.10,11,19,23
BRIEF SUMMARY
Current Knowledge/Study Rationale: Pregnant women report significantly more nocturnal sleep disruption than non-pregnant women. We wanted to evaluate whether daytime naps either hindered nocturnal sleep or might be a “countermeasure” to offset poor nocturnal sleep.
Study Impact: Naps do not appear to significantly hinder nocturnal sleep in early gestation. Incorporating naps into one's sleep hygiene routine may improve quality of life as well as lessen the risk of adverse pregnancy outcomes.
Presently, pregnant women have received little attention with regard to daytime naps and their possible consequences or benefits. Okun and Coussons-Read reported that women in the first trimester self-reported an average of 1.8 ± 1.4 naps/week, which was statistically more than a comparable non-pregnant cohort who had 0.94 ± 1.0 naps/week.24 Tsai and colleagues recently reported that among 80 upper socioeconomic status (SES) women in the third trimester, 51.3% napped at least 4 times per week. Cross-sectional analyses indicate that women who work longer during the day are more likely to nap since they report shorter nocturnal sleep duration and increased daytime fatigue.25 In a retrospective account of napping behavior, Balserak and colleagues26 found that nap duration was modestly associated with a high glucose challenge test (GCT), suggesting that self-reported long nap duration contributes to hyperglycemia. Women with high GCT napped about 2.27 h, whereas those with normal GCT napped about 1.49 hours. The literature on napping behavior in pregnant women is limited to description. There has been no examination as to whether the incorporation of daytime naps impairs nocturnal sleep during early pregnancy. As previously noted, this is an important association given the links between total sleep duration, sleep fragmentation, and sleep quality with maternal and birth outcomes.5,8,27–29 Given that women of childbearing age comprise about 32% of the world's population,30 additional evidence concerning the length and frequency of naps is necessary to fully comprehend the impact of daytime naps on nocturnal sleep and pregnancy outcomes.
In the current study, we examined napping behavior collected longitudinally via sleep diaries and actigraphy from women in early gestation (10–20 weeks). We identified the frequency and length of naps and whether there were any associations with both diary- and actigraphy-assessed nocturnal sleep parameters. We hypothesized that women who took ≥ 4 naps/2-week period (frequent nappers) would exhibit longer SOL and WASO, poorer SE, and shorter TST than women who took 1–3 naps/2-week period (moderate nappers) and non nappers. We further speculated that women who took shorter naps (< 90 min) would have shorter SOL, WASO, and higher SE and TST than women who took longer naps (≥ 90 min).
METHODS
Participants
This is a secondary analysis of available data from pregnant women (n = 161) between 18 and 45 years old, who were recruited from the greater Pittsburgh area from October 2008 through December 2012 as part of a study evaluating pregnancy-related sleep disturbances in relation to perinatal outcomes. The women were found through self-referral, physician referral, local advertising, or enrollment in University research registries. All participants provided written consent. All intended to keep the pregnancy. Exclusion criteria were self-reported psychopathological diagnosis, sleep disorder, chronic diseases, including diabetes, HIV, or uterine abnormalities, or current use of antidepressants or other treatment such as psychotherapy. Women were not clinically tested for sleep disturbances, such as obstructive sleep apnea/sleep disordered breathing, or restless legs. However, they were assessed for sleep disorders in the initial phone screening. Approval was received from the University of Pittsburgh Institutional Review Board.
Procedures
The research design was a prospective observational study. Daily sleep information was collected in three 2-week periods: 10–12, 14–16, and 18–20 weeks gestation, for a total of roughly 42 days. The specific time period was part of the parent study design to examine the associations between sleep in early gestation (< 20 weeks) and maternal and delivery outcomes. It was intended that all participants would be recruited prior to 10–12 weeks gestation; however, in order to increase enrollment, enrollment time was flexible to allow women up to 14 weeks to enroll. The women recorded subjective sleep and daily activity in the Pittsburgh sleep daily diary31 and wore an actigraph (Mini-Mitter, Phillips/ Respironics) on their nondominant wrist for the full 2 weeks of each collection period. Participants were asked to press the marker button when they “tried to go to sleep” and when they “got out of bed” to begin their day. Sensitivity was set at 0.05 g for 3 to 11 Hz, and the analog signal was digitized using the digital integration method. Sampling was in 1-min epochs and analyzed using the sleep detection algorithm in the Actiware software (Philips Respironics) with a wake threshold of 40 counts. The assessment periods enabled collection of all sleep patterns for the entire time period, often including irregularities due to illness, travel, “on call” nights, etc. All available days with data were included in the descriptive analyses.
Subsequent to each assessment period, the women completed a series of online questionnaires. The Inventory for Depressive Symptoms–16 item (IDS)32 is a commonly used questionnaire for measuring the criterion for DSM-IV diagnosis of a major depressive episode. It has a Cronbach α ranging from 0.92–0.94 in depressed and euthymic patients and 0.79 in the current sample. Sleep items were removed for analyses. The Perceived Stress Scale–10 item (PSS)33 is commonly used to rate subjective stress from the previous month, on a scale of 0 (no stress) to 40 (highest stress) range. It has a Cronbach α ranging from 0.78–0.91 in healthy adult cohorts, and 0.89 in the current sample.
Sleep Variables
Daytime naps were identified and quantified from sleep diaries and actigraphy. We calculated the average number of naps across each 2-week period as well as the average length of each nap. We first determined whether a woman napped or not, and then categorized the women into 3 groups: non nappers (0 naps/2-week period); moderate nappers (1–3 naps/2-week period); and frequent nappers (≥ 4 naps/2-week period). The average length of each nap was further dichotomized into short (< 90 min) or long (≥ 90 min). This cutoff was chosen for 2 reasons: (1) the average length of each nap was 89 min, and (2) this reflects an approximate complete sleep cycle.34 The outcome variables were nocturnal sleep measures derived from sleep diaries and actigraphy. They included (a) sleep onset latency (SOL), the amount of time to fall asleep; (b) wake after sleep onset (WASO), the amount of time awake after the onset of sleep; (c) sleep efficiency (SE), the amount of time spent asleep divided by the amount of time spent in bed; and (d) total sleep time (TST), the total amount of sleep achieved.
Statistical Approach
Descriptive statistics were examined to characterize the demographics for the total cohort and by nap group. Means and standard deviations of all continuous measures and counts and percentages for categorical data are reported for the total sample and by napping group. Since the nap data were similar from diary and actigraphy, we only analyzed and present information in the tables based on diary-assessed napping behavior. Examination of normal distribution assumption for continuous data was determined by q-q plots, histograms, and Shapiro-Wilk test. Diary and actigraphy TST followed a normal distribution. For SE, SOL and WASO (both diary and actigraphy), logarithm base 10 transformations were used. Mixed modeling techniques were used to examine whether daytime napping (average number of naps and average length of naps) was associated with changes in nocturnal sleep parameters. SAS procedure MIXED was used for modeling the main effects of nap group and time, and time by nap group interactions, and to account for within subject correlation. For these models, logarithm base 10 transformations were used for BMI, SOL (both diary and actigraphy), WASO (both diary and actigraphy) and SE (diary only). These models were also adjusted for potential confounders including age, race, marital status, whether there was a child at home, IDS with sleep item removed, PSS, caffeine use, and exercise. All analyses were conducted using SAS, version 9.3 statistical software (SAS Institute Inc., Cary, NC).
RESULTS
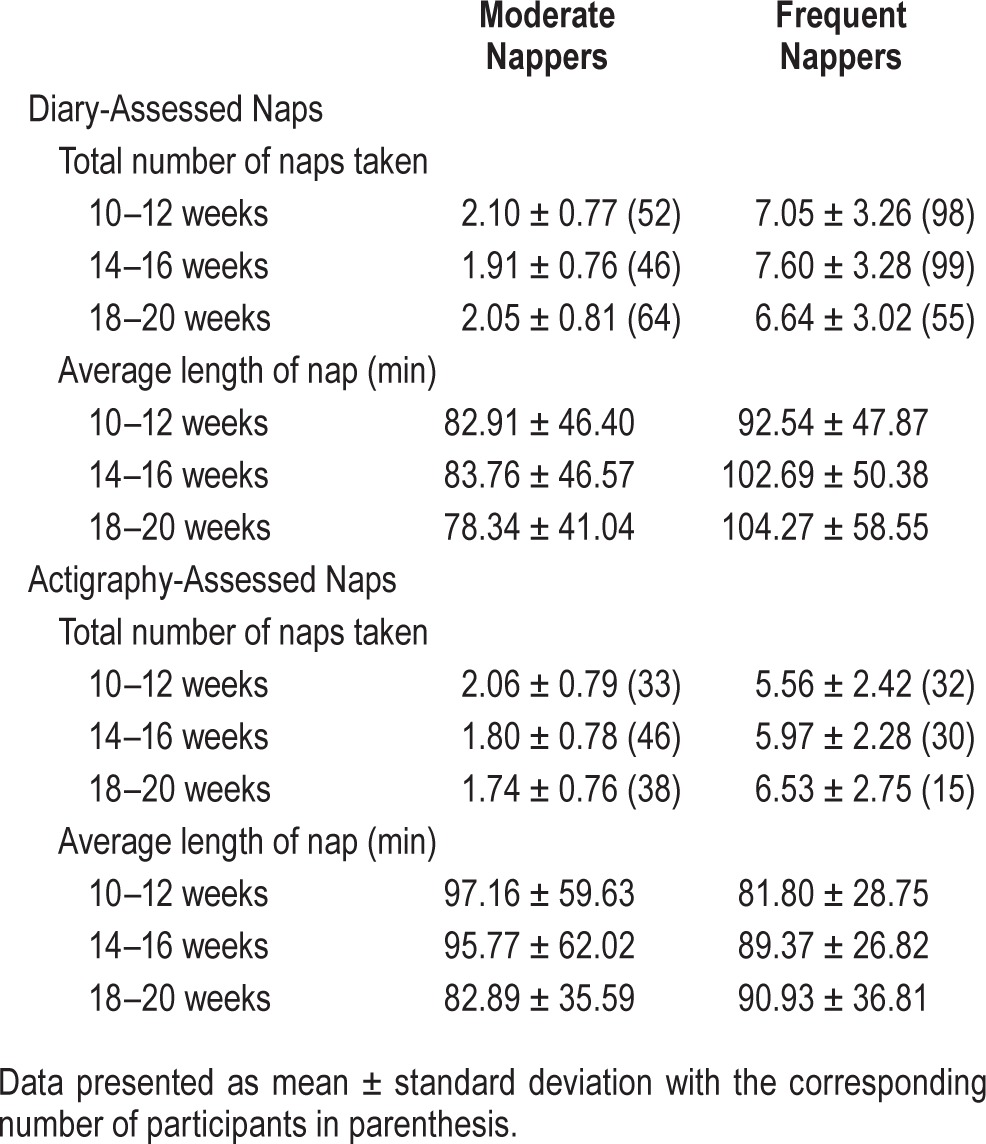
Participant characteristics for the entire cohort as well as by diary-nap group are shown in Table 1. The three groups were demographically similar except for marital status. More married women reported taking more frequent naps (p = 0.012). Interestingly, frequent nappers (≥ 4 naps/2-week period) had slightly higher IDS scores (p = 0.068) and significantly higher PSS scores (p = 0.024). Information about the total number and average length of each nap from diary and actigraphy are shown in Table 2.
Table 1.
Participant characteristics for total cohort and diary naps at 10–12 weeks.
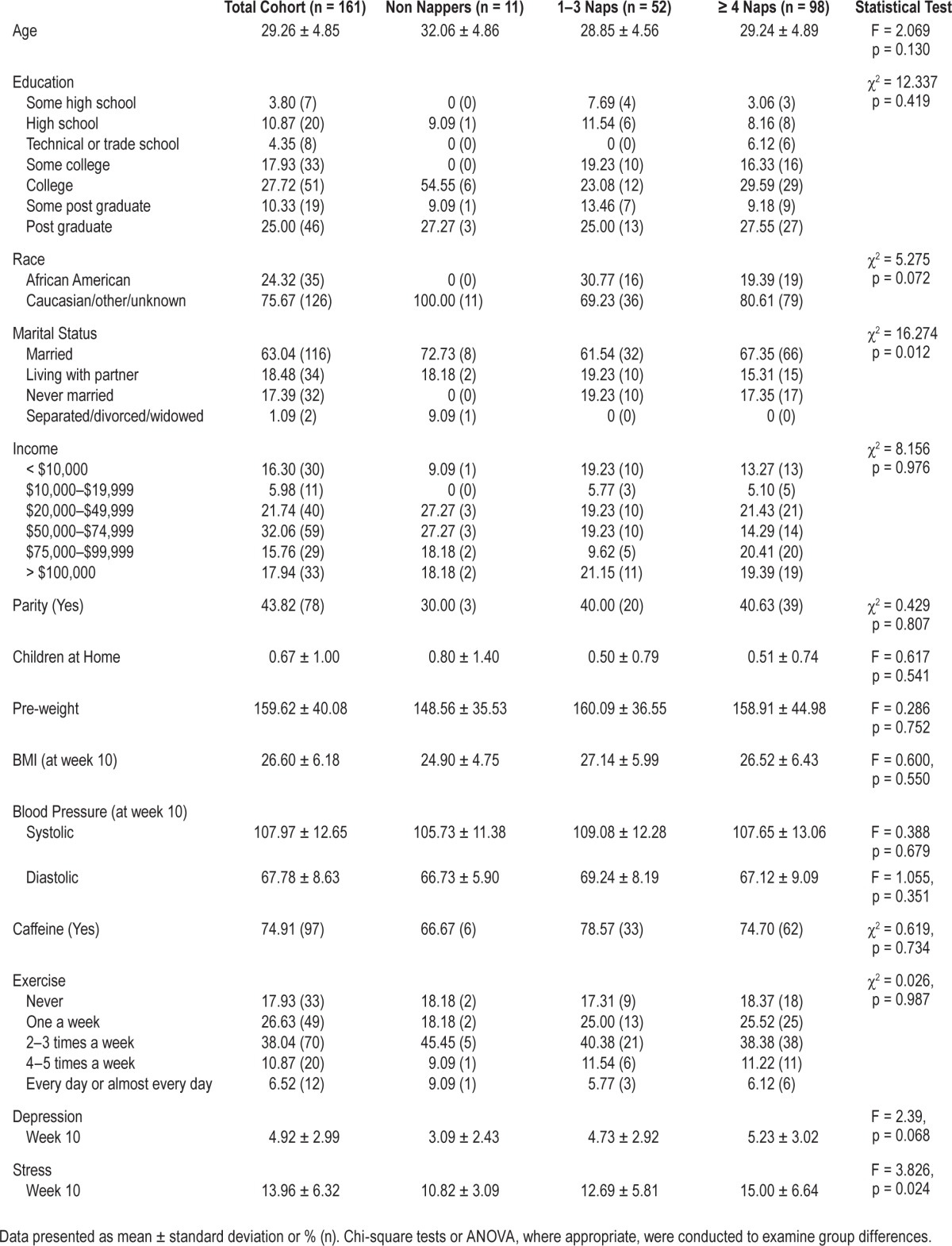
Table 2.
Daytime nap characteristics for each group.
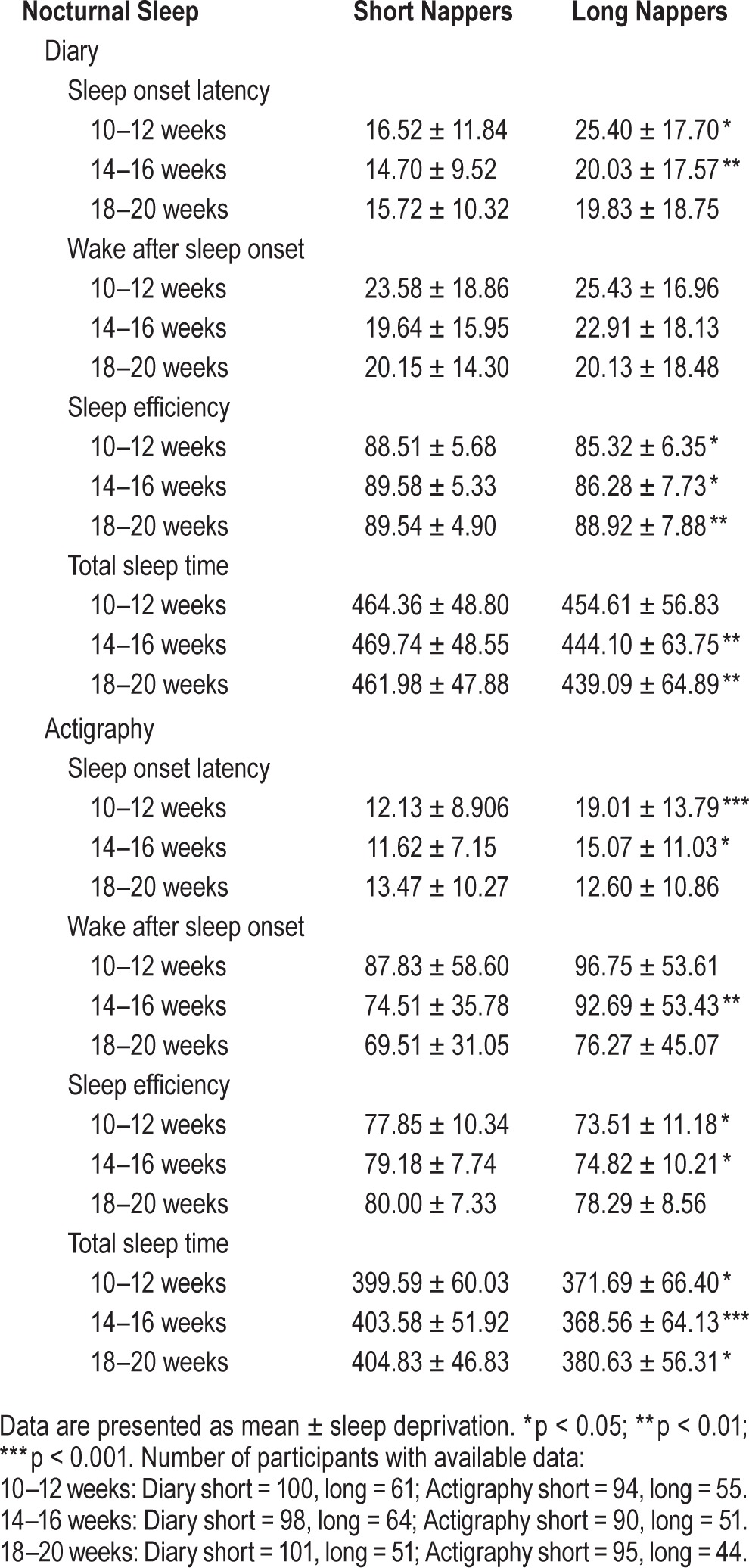
For simplicity, Table 3 presents the nocturnal sleep characteristics for the diary-derived nap groups only. The only significant differences noted were in TST at 10–12 weeks and 18–20 weeks. All other sleep parameters were similar among the three groups. Table 4 presents similar data but separated by short and long nappers. Several sleep parameters were different between the 2 groups, including SOL, SE, and TST. These differences were noted for both sleep diary and actigraphy-assessed sleep.
Table 3.
Nocturnal sleep characteristics by nap group and time.
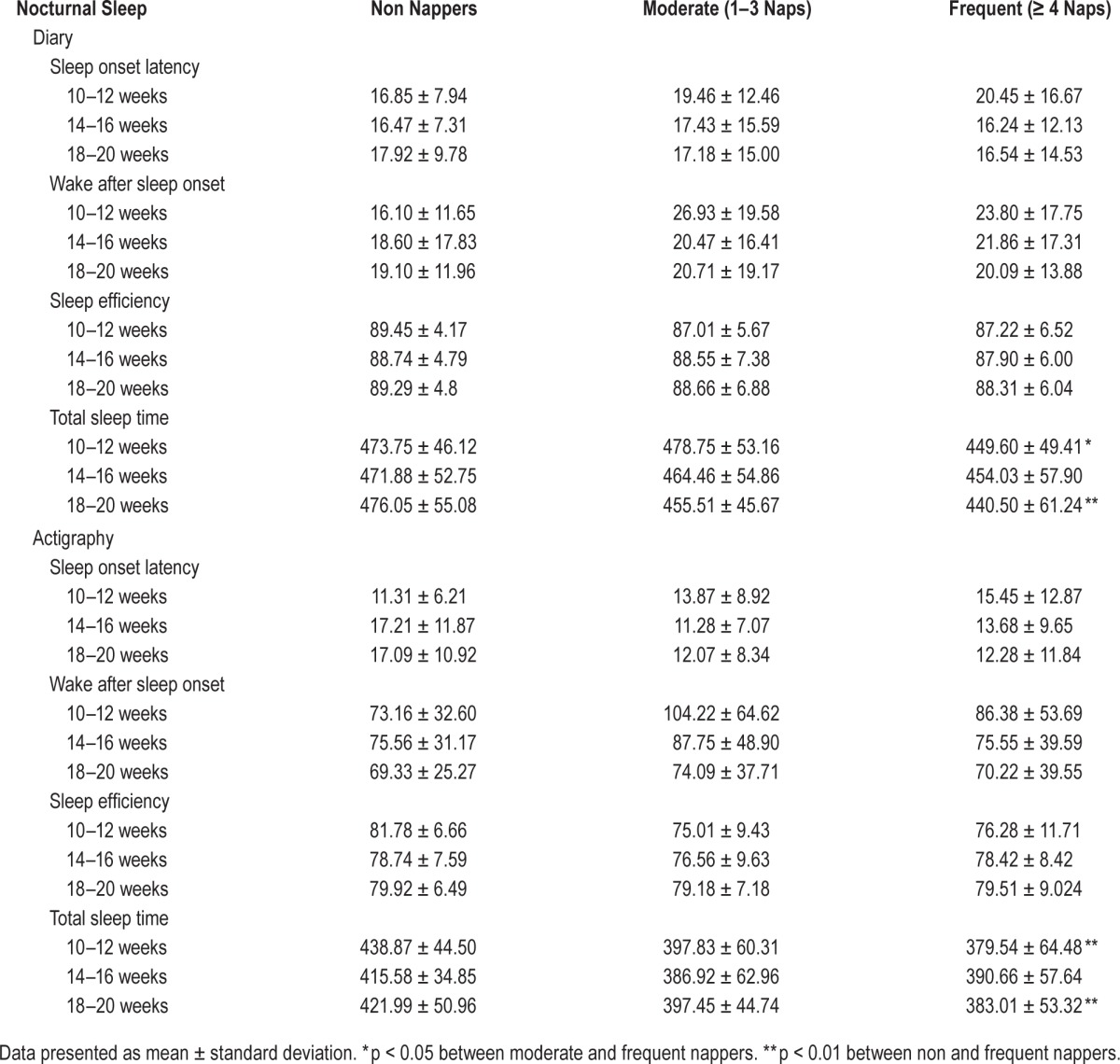
Table 4.
Nocturnal sleep characteristics by nap length group and time.
We conducted mixed models analyses to assess whether frequency of self-reported napping behavior was associated with nocturnal sleep parameters. After adjusting for relevant covariates (age, race, marital status, children, exercise habits, caffeine use, BMI, depression, and stress), we observed a main effect for nap group on subjective total sleep time (TST), but no group × time interaction (Figure 1). At 10–12 weeks, women who napped with moderate regularity (1–3 naps/2-week period) had more self-reported total sleep time than frequent nappers (≥ 4 naps/2-week period; t = 3.00, p = 0.0088). There were no other differences between the non nappers and the other 2 groups at this time point. There were no differences among the groups at 14–16 weeks for subjective or actigraphic TST. However, at 18–20 weeks, women who did not nap had more self-reported total sleep time than frequent nappers (t = 2.83, p = 0.015). Prior to adjustment there was a modest difference between the moderate and frequent nappers in regards to TST (t = 1.80, p = 0.072), but after adjustment, the moderate nappers were similar in TST to those who took ≥ 4 naps/2-week period (p = 0.201). There were no other significant associations observed between nap group and the nocturnal sleep parameters of SOL, WASO, or SE derived from sleep diary or actigraphy.
Figure 1. Average minutes of diary-assessed total sleep time by nap group and time.
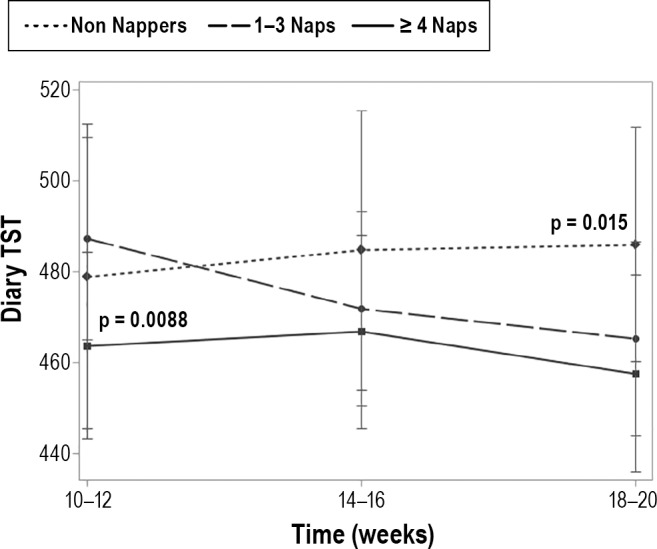
After adjusting for relevant covariates (age, race, marital status, children, exercise habits, caffeine use, BMI, depression, and stress), we observed a main effect for nap group on subjective total sleep time (TST), but no group × time interaction. At 10–12 weeks, women who napped with moderate regularity (1–3 naps/2-week period) had more self-reported total sleep time compared to frequent nappers (≥ 4 naps/2-week period) [t = 3.00, p = 0.0088]. There were no differences among the groups at 14–16 weeks. However, at 18–20 weeks, women who did not nap had more self-reported total sleep time than frequent nappers (t = 2.83, p = 0.015). Prior to adjustment there was a modest difference between the moderate and frequent nappers in TST (t = 1.80, p = 0.072), but after adjustment, the moderate nappers were similar in TST to frequent nappers (p = 0.201).
We were also interested in whether the average length of a nap was associated with nocturnal sleep parameters. We observed that women who took naps with an average duration ≥ 90 min had less diary-assessed TST (p = 0.002; Figure 2) and had poorer diary-assessed SE (p = 0.005) compared to women whose average nap was < 90 min. When evaluating time differences, there was no difference between the groups at 10–12 weeks with regard to diary-assessed TST, but there was a significant difference at 14–16 weeks (t = 3.79, p = 0.0002) and 18–20 weeks (t = 2.97, p = 0.0032). Likewise, there was a modest difference in diary-assessed SE between the women who took longer naps and those who took shorter naps at 10–12 weeks (t = 1.89, p = 0.059), and a significant difference at 14–16 weeks (t = 2.52, p = 0.012). The groups were similar at 18–20 weeks (Figure 3). No association was identified between duration of nap and diary- or actigraphy-assessed SOL and WASO or actigraphy-assessed TST or actigraphy-assessed SE.
Figure 2. Diary-assessed total sleep time by nap length group and time.
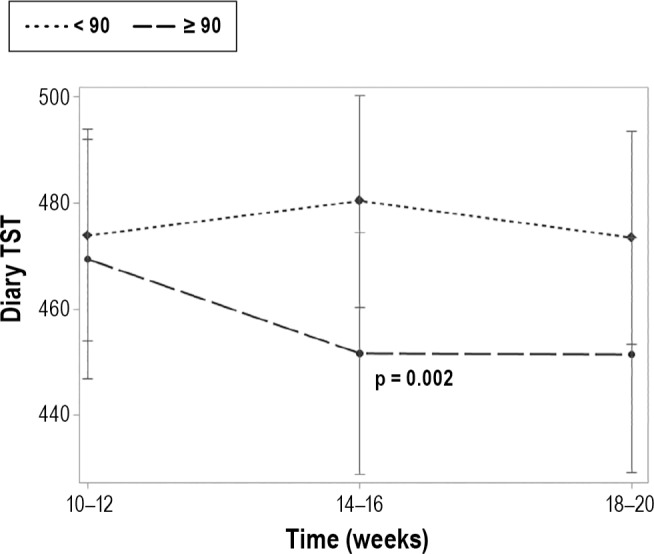
After adjusting for relevant covariates (age, race, marital status, children, exercise habits, caffeine use, BMI, depression, and stress), we observed that long nappers (≥ 90 min) had significantly shorter total sleep time (TST) than short nappers (< 90 min) with a modest group × time interaction (p = 0.062).
Figure 3. Diary-assessed sleep efficiency by nap length group and time.
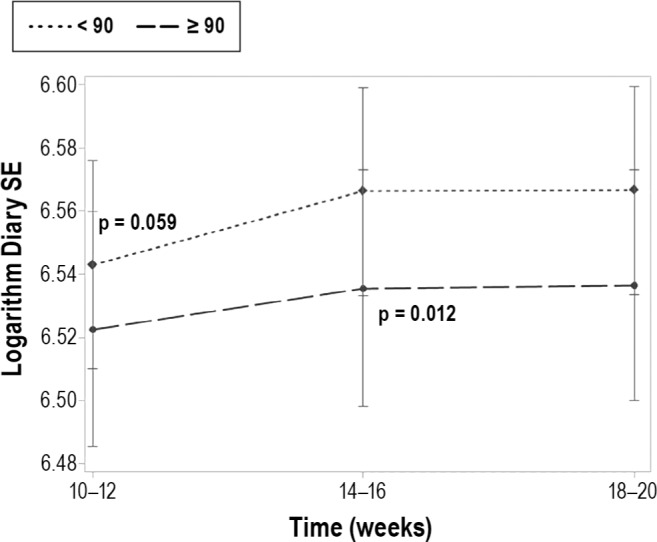
After adjusting for relevant covariates (age, race, marital status, children, exercise habits, caffeine use, BMI, depression, and stress), we observed a modest difference in diary-assessed sleep efficiency (SE) between the women who took longer naps (≥ 90 min) and those who took shorter naps (< 90 min) at 10–12 weeks (t = 1.89, p = 0.059), and a significant difference at 14–16 weeks (t = 2.52, p = 0.012). The groups were similar at 18–20 weeks.
DISCUSSION
In the current study, we describe self-reported napping behavior across an average of 42 days in pregnant women and examine whether moderate and frequent napping is associated with poor nocturnal sleep. We hypothesized that women who napped more frequently would have greater disturbed nocturnal sleep as measured from both sleep diary and actigraphy. As predicted, napping was associated with a decrease in diary-assessed nocturnal TST, but not actigraphy-assessed TST. However, we only observed a significant difference between women who reported frequent napping (≥ 4 naps/2-week period) and moderate napping (1–3 naps/2-week period) at 10–12 weeks, and between frequent nappers and non nappers at 18–20 weeks. Contrary to our predictions, we observed no difference by group on any other nocturnal sleep parameter.
We were also interested in whether the length of a nap significantly affected nocturnal sleep. We noted that women who took longer naps on average had poorer diary-assessed SE and lower diary-assessed TST than those who took shorter naps. There was no adverse effect on SOL or WASO. One takeaway message from these results is that among pregnant women who are significantly sleep disrupted/deprived,4,35 the incorporation of naps into one's sleep hygiene routine does not appear to impair nocturnal sleep.
The mixed findings with regards to the nap groups suggests several and longer naps may be warranted to offset the substantial degree of sleep deprivation/disruption reported in early pregnancy, and that minimal negative impact is observed on nocturnal sleep measures. Our findings both corroborate and contradict other reported findings. In a recent report by Coo and colleagues,36 actigraphy-assessed naps were collected in 29 third-trimester women. They report that the average number of naps taken in a 7-day period was 1.89 ± 1.22, which is very similar to our findings of approximately 3–5 over each 2-week period (data not shown). Interestingly, these values are not dramatically different from what others have reported in mid-life women37 or college students.38 What is starkly different is the average nap length. They report an average nap length of 24.59 ± 9.49 min, whereas we report an average of about 90 min. Our average nap time resembles previous work by Mindell and colleagues who reported that in the first 22 weeks, women napped about 3.5 times/week with duration ∼55 min. An explanation for the discrepancy is the method used. Coo and colleagues used actigraphy without corroborating self-report. They defined naps as “independent sleep periods of more than 10 min within the circadian-defined wake time.”36 While it is plausible to identify naps without a self-report, the interpretive value has been heavily cautioned.39 Our work is most similar to a recent report by Tsai et al.25 with regard to the average number of self-reported naps observed in 41 third-trimester frequent nappers (6.80 ± 2.97) as well as the reduction in TST. We somewhat agree with their suggestion that napping might interfere with subsequent nighttime sleep quantity, and that it does not compromise subsequent nighttime sleep continuity.25 However all our participants, regardless of group, obtained 7–8 hours during 6 weeks of data collection. Thus, it appears as if daytime naps are not detrimental to nocturnal sleep duration, at least not in early pregnancy. Indeed, there were almost no differences in most of the nocturnal sleep measures among the three groups. So it may be that Tsai and colleagues did not ascertain enough data or participants to determine whether naps are detrimental to overall sleep quantity. One concern with the extrapolations from Tsai is that all of their participants napped during the 7-day collection period. As our data show, there are some modest but important differences with regard to sleep between non nappers and nappers. Moreover, we cannot make accurate comparisons, as they only report average nap duration from actigraphy and not diary.
In regards to the impact of length of naps, we observed minimal associations with nocturnal sleep measures. The women whose average nap was ≥ 90 min had significantly shorter TST, and modestly lower SE. So, similar to the number of naps, a longer nap does not appear to differentially impact nocturnal sleep compared to shorter naps. The available literature on appropriate length of naps is quite variable. Most of the research has focused on the critical length that a nap needs to be to observe improvement in cognitive function, performance, or memory.23,40 Essentially all studies indicate positive outcomes on daytime sleepiness following a nap.10,23,40 The consensus here is that healthy young adults should nap 10–30 min; naps > 30 min are thought to produce sleep inertia such that performance is improved only after a period of time has elapsed.10,40,41 This, however, does not adequately address the notion that naps of greater length might be beneficial for augmenting short/disturbed nocturnal sleep,10 particularly among pregnant women. Our findings, along with those of Tsai,25 support the notion that daytime naps only modestly impact nocturnal sleep duration, and do not impair sleep continuity or sleep quality. Given that poor sleep continuity and quality are important correlates of adverse pregnancy outcomes,5,8,27,42–44 we propose that a beneficial countermeasure to sleep disruption is daytime naps, which is in line with several other reports in non-pregnant cohorts.9–11,41 Additional research is still needed to determine the effect of naps > 30 min on pregnant women.
There is no “ideal” nap length. In fact, there is mixed support for naps of varying length. Among elderly cohorts, longer naps are often associated with morbidity.13,20,22,45,46 However, recent data indicate that longer naps are health beneficial, particularly among adults without clinical sleep disorders.10 Among children longer naps are considered beneficial and are encouraged.47–49 Older studies consistently report that the average nap duration for adults is between 30 and 90 min.50,51 We found, similar to other reports,25,26,52,53 that pregnant women take longer naps on average than other cohorts. While we were unable to denote why women took naps (e.g., appetitive or compensatory reasons) or what the ideal nap length for the pregnant woman is, our results support the hypothesis that pregnant women on the whole engage in napping behavior (93%). Future studies should evaluate the reasons as to why people choose to take naps. The impact on nocturnal sleep and health outcomes may indeed vary whether they nap in response to sleep loss (i.e., replacement napping) or in preparation for sleep loss (i.e., prophylactic napping), or simply for the enjoyment of napping (i.e., appetitive napping).50
This study has several strengths including a large prospectively assessed cohort of pregnant women, multiple collection methods (diary and actigraphy), and several weeks of data. This is important to acknowledge since length of data collection correlates with degree of intra-individual variability.54 The concomitant use of diaries and actigraphy affords the opportunity to comment on the use of actigraphy in pregnant women. Although it is well accepted that the use of actigraphy is best validated in normal sleepers,54 we contend that it is a preferable tool to use in pregnant women. Based on our data, it appears that pregnant women fall into the category of “normal sleepers” rather than “abnormal sleepers,” i.e. insomnia.
We also note that there are some limitations to the current study. We did not clinically assess sleep disordered breathing or periodic limb movements by polysomnography (PSG); however, we did exclude women who self-reported any sleep disorder. It was not feasible to clinically evaluate women for sleep disorders given the large number of participants. There was also not a comparable non-pregnant cohort. This is an important limitation, as we cannot determine whether napping in the context of pregnancy has differential effects on nocturnal sleep in similarly aged women. Lastly, while we controlled for a variety of possible confounders including age, race, marital status, whether there was a child at home, IDS with sleep item removed, PSS, caffeine use, and exercise, we did not include employment status in the models. There were no significant differences among the groups so we excluded it from analyses. This has been noted to be an important correlate of nap frequency and length,37 and may depend on the cohort being evaluated. Future studies also need to take into account the timing of a nap. There are differential effects depending on whether the nap is taken in the morning, afternoon, or evening.40,55 These data are not currently available.
In summary, our results do not support the majority of the literature that daytime naps, as well as longer naps, impair nocturnal sleep parameters. In fact, it appears as if the majority of our cohort of women in the early gestational period incorporated naps to offset poor sleep continuity and fragmentation, but not short sleep duration. While this is an initial examination, we purport that it is equally important to denote why naps were taken (e.g., planned or unplanned), as well to appreciate the myriad factors that will determine the degree of benefit derived from napping.10 Future studies need to include homeo-static and circadian processes, subject characteristics, and psychological interpretations gleaned by the subject40 in order to fully comprehend whether naps are beneficial or unfavorable.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest. All aspects of the study protocol were conducted at the University of Pittsburgh
ACKNOWLEDGMENTS
The authors thank Bedda Rivera-Rosario, PhD, for her assistance with the statistical analyses.
ABBREVIATIONS
- PSG
polysomnography
- SE
sleep efficiency
- SOL
sleep onset latency
- TST
total sleep time
- WASO
wake after sleep onset
REFERENCES
- 1.Agatisa PK, Ness RB, Roberts JM, Costantino JP, Kuller LH, McLaughlin MK. Impairment of endothelial function in women with a history of preeclampsia: an indicator of cardiovascular risk. Am J Physiol Heart Circ Physiol. 2004;286:H1389–93. doi: 10.1152/ajpheart.00298.2003. [DOI] [PubMed] [Google Scholar]
- 2.Burton GJ. Oxygen, the Janus gas; its effects on human placental development and function. J Anat. 2009;215:27–35. doi: 10.1111/j.1469-7580.2008.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Challis JRG, Lye SJ. Physiology and endocrinology of term and preterm labor. In: Gabbe SG, Niebyl JR, Simpson JL, editors. Obstetrics: normal and problem pregnancies. New York: Churchill Livingstone; 2003. pp. 93–106. [Google Scholar]
- 4.Okun ML, Kiewra K, Luther JF, Wisniewski SR, Wisner KL. Sleep disturbances in depressed and nondepressed pregnant women. Depress Anxiety. 2011;28:676–85. doi: 10.1002/da.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okun ML, Hanusa BH, Hall M, Wisner KL. Sleep complaints in late pregnancy and the recurrence of postpartum depression. Behav Sleep Med. 2009;7:106–17. doi: 10.1080/15402000902762394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beebe KR, Lee KA. Sleep disturbance in late pregnancy and early labor. J Perinat Neonat Nurs. 2007;21:103–8. doi: 10.1097/01.JPN.0000270626.66369.26. [DOI] [PubMed] [Google Scholar]
- 7.Facco FL, Kramer J, Ho KH, Zee PC, Grobman WA. Sleep disturbances in pregnancy. Obstetr Gynecol. 2010;115:77–83. doi: 10.1097/AOG.0b013e3181c4f8ec. [DOI] [PubMed] [Google Scholar]
- 8.Okun ML, Schetter CD, Glynn LM. Poor sleep quality is associated with preterm birth. Sleep. 2011;34:1493–8. doi: 10.5665/sleep.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faraut B, Boudjeltia KZ, Dyzma M, et al. Benefits of napping and an extended duration of recovery sleep on alertness and immune cells after acute sleep restriction. Brain Behav Immun. 2011;25:16–24. doi: 10.1016/j.bbi.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi M. The role of prescribed napping in sleep medicine. Sleep Med Rev. 2003;7:227–35. doi: 10.1053/smrv.2002.0241. [DOI] [PubMed] [Google Scholar]
- 11.Vgontzas AN, Pejovic S, Zoumakis E, et al. Daytime napping after a night of sleep loss decreases sleepiness, improves performance, and causes beneficial changes in cortisol and interleukin-6 secretion. Am J Physiol Endocrinol Metab. 2007;292:E253–61. doi: 10.1152/ajpendo.00651.2005. [DOI] [PubMed] [Google Scholar]
- 12.Ancoli-Israel S, Martin JL. Insomnia and daytime napping in older adults. J Clin Sleep Med. 2006;2:333–42. [PubMed] [Google Scholar]
- 13.Campbell SS, Murphy PJ, Stauble TN. Effects of a nap on nighttime sleep and waking function in older subjects. J Am Geriatr Soc. 2005;53:48–53. doi: 10.1111/j.1532-5415.2005.53009.x. [DOI] [PubMed] [Google Scholar]
- 14.Dautovich ND, McCrae CS, Rowe M. Subjective and objective napping and sleep in older adults: are evening naps “bad” for nighttime sleep? J Am Geriatr Soc. 2008;56:1681–6. doi: 10.1111/j.1532-5415.2008.01822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owens JF, Matthews KA. Sleep disturbance in healthy middle-aged women. Maturitas. 1998;30:41–50. doi: 10.1016/s0378-5122(98)00039-5. [DOI] [PubMed] [Google Scholar]
- 16.McDevitt EA, Alaynick WA, Mednick SC. The effect of nap frequency on daytime sleep architecture. Physiol Behav. 2012;107:40–4. doi: 10.1016/j.physbeh.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monk TH, Buysse DJ, Carrier J, Billy BD, Rose LR. Effects of afternoon “siesta” naps on sleep, alertness, performance, and circadian rhythms in the elderly. Sleep. 2001;24:680–7. doi: 10.1093/sleep/24.6.680. [DOI] [PubMed] [Google Scholar]
- 18.Tietzel AJ, Lack LC. The recuperative value of brief and ultra-brief naps on alertness and cognitive performance. J Sleep Res. 2002;11:213–8. doi: 10.1046/j.1365-2869.2002.00299.x. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi M, Motoyoshi N, Hori T. Recuperative power of a short daytime nap with or without stage 2 sleep. Sleep. 2005;28:829–36. [PubMed] [Google Scholar]
- 20.Tanaka H, Taira K, Arakawa M, et al. Effects of short nap and exercise on elderly people having difficulty in sleeping. Psychiatry Clin Neurosci. 2001;55:173–4. doi: 10.1046/j.1440-1819.2001.00813.x. [DOI] [PubMed] [Google Scholar]
- 21.Pilcher JJ, Michalowski KR, Carrigan RD. The prevalence of daytime napping and its relationship to nighttime sleep. Behav Med. 2001;27:71–6. doi: 10.1080/08964280109595773. [DOI] [PubMed] [Google Scholar]
- 22.Dautovich ND, Kay DB, Perlis ML, Dzierzewski JM, Rowe MA, McCrae CS. Day-to-day variability in nap duration predicts medical morbidity in older adults. Health Psychol. 2012;31:671–6. doi: 10.1037/a0027374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lovato N, Lack L. The effects of napping on cognitive functioning. Progr Brain Res. 2010;185:155–66. doi: 10.1016/B978-0-444-53702-7.00009-9. [DOI] [PubMed] [Google Scholar]
- 24.Okun ML, Coussons-Read ME. Sleep disruption during pregnancy: how does it influence serum cytokines? J Reproductive Immunol. 2007;73:158–65. doi: 10.1016/j.jri.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Tsai SY, Kuo LT, Lee CN, Lee YL, Landis CA. Reduced sleep duration and daytime naps in pregnant women in Taiwan. Nurs Res. 2013;62:99–105. doi: 10.1097/NNR.0b013e3182830d87. [DOI] [PubMed] [Google Scholar]
- 26.Balserak BI, Jackson N, Ratcliffe SA, Pack AI, Pien GW. Sleep-disordered breathing and daytime napping are associated with maternal hyperglycemia. Sleep Breath. 2013;17:1093–102. doi: 10.1007/s11325-013-0809-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dorheim SK, Bjorvatn B, Eberhard-Gran M. Insomnia and depressive symptoms in late pregnancy: a population-based study. Behav Sleep Med. 2012;10:152–66. doi: 10.1080/15402002.2012.660588. [DOI] [PubMed] [Google Scholar]
- 28.Facco FL, Grobman WA, Kramer J, Ho KH, Zee PC. Self-reported short sleep duration and frequent snoring in pregnancy: impact on glucose metabolism. Am J Obstetr Gynecol. 2010;203:142–5. doi: 10.1016/j.ajog.2010.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goyal D, Gay C, Lee K. Fragmented maternal sleep is more strongly correlated with depressive symptoms than infant temperament at three months postpartum. Arch Womens Mental Health. 2009;12:229–37. doi: 10.1007/s00737-009-0070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.CIA World FactBook. What percentage of the female population is pregnant at any one time? 2014. Available at https://www.cia.gov/library/publications/the-world-factbook/geos/us.html.
- 31.Monk TH, Reynolds CF, Kupfer DJ, et al. The Pittsburgh Sleep Diary. J Sleep Res. 1994;3:111–20. [PubMed] [Google Scholar]
- 32.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–86. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 33.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 34.Hall M, Okun ML, Atwood CW, Buysse DJ, Strollo PJ. Measurement of sleep by polysomnography. In: Luecken LL, Gallo LC, editors. Handbook of physiological research methods in health psychology. Sage Publications; 2008. [Google Scholar]
- 35.Okun ML. Sleep in pregnancy and the postpartum. In: Kushida CA, editor. Encyclopedia of sleep. Academic Press; 2013. [Google Scholar]
- 36.Coo S, Milgrom J, Trinder J. Mood and objective and subjective measures of sleep during late pregnancy and the postpartum period. Behav Sleep Med. 2014;12:317–30. doi: 10.1080/15402002.2013.801348. [DOI] [PubMed] [Google Scholar]
- 37.Johnston SK, Landis CA, Lentz MJ, Shaver JL. Self-reported nap behavior and polysomnography at home in midlife women with and without insomnia. Sleep. 2001;24:913–9. doi: 10.1093/sleep/24.8.913. [DOI] [PubMed] [Google Scholar]
- 38.Vela-Bueno A, Fernandez-Mendoza J, Olavarrieta-Bernardino S, et al. Sleep and behavioral correlates of napping among young adults: a survey of first-year university students in Madrid, Spain. J Am Coll Health. 2008;57:150–8. doi: 10.3200/JACH.57.2.150-158. [DOI] [PubMed] [Google Scholar]
- 39.Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139:1514–27. doi: 10.1378/chest.10-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milner CE, Cote KA. Benefits of napping in healthy adults: impact of nap length, time of day, age, and experience with napping. J Sleep Res. 2009;18:272–81. doi: 10.1111/j.1365-2869.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 41.Tietzel AJ, Lack LC. The short-term benefits of brief and long naps following nocturnal sleep restriction. Sleep. 2001;24:293–300. doi: 10.1093/sleep/24.3.293. [DOI] [PubMed] [Google Scholar]
- 42.Althuizen E, van Poppel MN, de Vries JH, Seidell JC, van Mechelen W. Postpartum behaviour as predictor of weight change from before pregnancy to one year postpartum BMC Public Health. 2011;11:165. doi: 10.1186/1471-2458-11-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bei B, Milgrom J, Ericksen J, Trinder J. Subjective perception of sleep, but not its objective quality, is associated with immediate postpartum mood disturbances in healthy women. Sleep. 2010;33:531–8. doi: 10.1093/sleep/33.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evans ML, Dick MJ, Clark AS. Sleep during the week before labor: relationships to labor outcomes. Clin Nurs Res. 1995;4:238–49. doi: 10.1177/105477389500400302. [DOI] [PubMed] [Google Scholar]
- 45.Hays JC, Blazer DG, Foley DJ. Risk of napping: excessive daytime sleepiness and mortality in an older community population. J Am Geriatr Soc. 1996;44:693–8. doi: 10.1111/j.1532-5415.1996.tb01834.x. [DOI] [PubMed] [Google Scholar]
- 46.Xu Q, Song Y, Hollenbeck A, Blair A, Schatzkin A, Chen H. Day napping and short night sleeping are associated with higher risk of diabetes in older adults. Diabetes Care. 2010;33:78–83. doi: 10.2337/dc09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fukuda K, Sakashita Y. Sleeping pattern of kindergartners and nursery school children: function of daytime nap. Percept Mot Skills. 2002;94:219–28. doi: 10.2466/pms.2002.94.1.219. [DOI] [PubMed] [Google Scholar]
- 48.Komada Y, Asaoka S, Abe T, et al. Relationship between napping pattern and nocturnal sleep among Japanese nursery school children. Sleep Med. 2012;13:107–10. doi: 10.1016/j.sleep.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 49.Kurdziel L, Duclos K, Spencer RM. Sleep spindles in midday naps enhance learning in preschool children. Proc Natl Acad Sci U S A. 2013;110:17267–72. doi: 10.1073/pnas.1306418110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Broughton RJ, Dinges DF. Napping: a ubiquitous enigma. In: Dinges DF, Broughton RJ, editors. Sleep and alertness: chronobiological, behavioral, and medical aspects of napping. New York: Raven Press; 1989. [Google Scholar]
- 51.Dinges DF. Adult napping and its effects on ability to function. In: Stampi C, editor. Why we nap: evolution, chronobiology and functions of polyphasic and ultrashort sleep. Boston, MA: Birkhauser; 1992. [Google Scholar]
- 52.Mindell JA, Jacobson BJ. Sleep disturbances during pregnancy. J Obstet Gynecol Neonatal Nurs. 2000;29:590–7. doi: 10.1111/j.1552-6909.2000.tb02072.x. [DOI] [PubMed] [Google Scholar]
- 53.Okun ML, Luther JF, Wisniewski SR, Wisner KL. Disturbed sleep and inflammatory cytokines in depressed and nondepressed pregnant women: an exploratory analysis of pregnancy outcomes. Psychosom Med. 2013;75:670–81. doi: 10.1097/PSY.0b013e31829cc3e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ustinov Y, Lichstein KL. Actigraphy reliability with normal sleepers. Behav Sleep Med. 2013;11:313–20. doi: 10.1080/15402002.2012.688779. [DOI] [PubMed] [Google Scholar]
- 55.Kubo T, Takeyama H, Matsumoto S, et al. Impact of nap length, nap timing and sleep quality on sustaining early morning performance. Industrial Health. 2007;45:552–63. doi: 10.2486/indhealth.45.552. [DOI] [PubMed] [Google Scholar]


