Abstract
Aluminum ions are especially toxic to plants in acidic soils. Here we present evidences that SO2 protects germinating wheat grains against aluminum stress. SO2 donor (NaHSO3/Na2SO3) pretreatment at 1.2 mM reduced the accumulation of superoxide anion, hydrogen peroxide, and malondialdehyde, enhanced the activities of guaiacol peroxidase, catalase, and ascorbate peroxidase, and decreased the activity of lipoxygenase in germinating wheat grains exposed to Al stress. We also observed higher accumulation of hydrogen sulfide (H2S) in SO2-pretreated grain, suggesting the tight relation between sulfite and sulfide. Wheat grains geminated in water for 36 h were pretreated with or without 1 mM SO2 donor for 12 h prior to exposure to Al stress for 48 h and the ameliorating effects of SO2 on wheat radicles were studied. SO2 donor pretreatment reduced the content of reactive oxygen species, protected membrane integrity, and reduced Al accumulation in wheat radicles. Gene expression analysis showed that SO2 donor pretreatment decreased the expression of Al-responsive genes TaWali1, TaWali2, TaWali3, TaWali5, TaWali6, and TaALMT1 in radicles exposed to Al stress. These results suggested that SO2 could increase endogenous H2S accumulation and the antioxidant capability and decrease endogenous Al content in wheat grains to alleviate Al stress.
1. Introduction
Aluminum ions (Al3+) together with silicon and iron are the three most abundant mineral elements in soil. Whereas silicon and iron are required for plant growth, Al is toxic. Many different mechanisms have been advanced to explain Al toxicity in plants [1, 2]. One of the primary causes of Al toxicity is oxidative stress due to accumulation of reactive oxygen species (ROS), such as the superoxide anion (O2 •−) and hydrogen peroxide (H2O2), bringing about lipid peroxidation in plant cells [3–5]. Plants have developed several strategies to counteract oxidative stress caused by Al, such as activation of antioxidants, and exudation of organic acids as a mechanism for Al exclusion [6]. Recently, a range of signaling molecules, such as inositol 1,4,5-triphosphate (IP3), salicylic acid, hydrogen peroxide (H2O2) and nitric oxide (NO), carbon monoxide (CO), and hydrogen sulfide (H2S), were found to participate in plant's resistance to Al-induced oxidative stress [4, 7–10].
Sulfur dioxide (SO2) is a colorless, nonflammable gas with a penetrating odor. Low concentrations of SO2 have been found to play a physiological role in vivo in animal models, participating in various biological processes [11]. The physiological processes regulated by SO2 in animals include cardiac function [11], inhibition of L-calcium channels in cardiomyocytes [12], and improvement in pulmonary vascular structural remodeling [13]. In plants, the toxic effects of SO2 on growth and development have been extensively studied [14, 15]. Exposure to high concentrations of SO2 can cause visible foliar damage, a decline in photosynthesis, an inhibition of plant growth, and structural disorganization and cell death [16–19]. On the other hand, many reports show that low levels of atmospheric SO2 might be beneficial to plants [20]. SO2 can be metabolized and used as a sulfur source for plant growth, especially when the sulfur supply in soil is insufficient for normal growth [20]. Recently, low concentrations of SO2 were found to induce transcriptome reprogramming associated with oxidative signaling and biotic defence responses in plants, suggesting a physiological role of SO2 in plant [21].
In plants, sulfate is taken up from soil by high-affinity transporters. Sulfate is largely transported to shoots where it can be activated by ATP via ATP sulfurylase in the leaves. The product is reduced by 5′-adenylylsulfate (APS) reductase to sulfite which can be reduced to H2S by sulfite reductase [22]. SO2 can also be produced endogenously from sulfur-containing amino acids [23]. The endogenous production of SO2 also suggests that it has a physiological role in plants.
In order to establish the role of SO2 in alleviating Al stress, we investigated the effects of SO2 pretreatment on H2S and ROS accumulation and the antioxidant system in whole wheat grains and in wheat radicles. We also analyzed endogenous H2S and Al content as a means of understanding the mechanism of the role of SO2. We speculated that SO2 might act as an antioxidant molecule to alleviate Al toxicity during wheat grain germination.
2. Materials and Methods
2.1. Materials and Treatments
Wheat (Triticum aestivum L.) grains were supplied by the Anhui Aidi Agricultural Technology Co., Ltd., Anhui Province, China. Sodium bisulfite (NaHSO3) and anhydrous sodium sulfite (Na2SO3) were used as sulfur dioxide (SO2) donors according to Laisk et al. [24]. Wheat grains were sterilized by 0.1% HgCl2 for 3 min and washed extensively with H2O and then dried with filter papers. Wheat grains of similar size were selected and allocated randomly in Petri dish (9 cm diameter × 1.2 cm depth, 50 grains per dish). Wheat grains were germinated in H2O or aqueous solutions of AlCl3 at 5, 10, 15, 20, 25, 30, 60, and 90 mM for 48 h at 25°C and the length of coleoptiles and radicles and radicle number were recorded. To test the protective role of SO2 on germination and seedling growth of wheat grains under Al stress, grains were pretreated with H2O or 0.4, 0.8, 1.2, 1.6, or 2.0 mM SO2 donor for 12 h and subsequently subjected to a semi-inhibitory AlCl3 concentration (15 mM). AlCl3 solutions were renewed every 12 h and geminating grains were sampled every 12 h for further analysis.
2.2. Determination of MDA, O2 •−, and H2O2
The contents of MDA, O2 •−, and H2O2 were determined by the method of Zhang et al. [25].
2.3. Assays of LOX, CAT, APX, and POD Activities
Activity of lipoxygenase (LOX, EC 1.13.11.12) was determined following the description by Surrey [26] and those of catalase (CAT, EC1.11.1.6), ascorbate peroxidase (APX, EC 1.11.1.11), and guaiacol peroxidase (POD, EC 1.11.1.7) were assayed according to Hu et al. [27]. Wheat grains were homogenized in ice-cold 50 mM phosphate buffer (pH 7.8) containing 1.0 mM EDTA. The homogenate was centrifuged at 15,000 g at 4°C for 10 min. The supernatant was used for activity determination.
2.4. Assays of Reducing Sugars and Soluble Protein
Wheat grains (0.5 ± 0.05 g) were ground in 5 mL of phosphate buffer (pH 7.0, 200 mM), the homogenate was centrifuged at 10,000 g for 30 min, and the supernatant was used for detection of reducing sugars and soluble protein content. Reducing sugar content was measured according to Miller [28].
For detection of soluble protein, 0.1 mL supernatant was mixed with 0.9 mL H2O and 5 mL Coomassie brilliant blue for 5 min and the absorbance recorded at 595 nm using the method described by Bradford [29].
2.5. Preparation of Wheat Radicles
Wheat grains were geminated in H2O for 36 h in the dark at 25°C and the average of radicle length was approximately 1.0 cm. The geminated wheat grains were pretreated with or without 1 mM SO2 donor for 12 h and then exposed to 0 or 400 μM AlCl3 for 48 h.
2.6. Detection of Plasma Membrane Integrity, Al Accumulation, and ROS Production in Radicles
Plasma membrane integrity of wheat radicles was detected following the method of Yamamoto et al. [30]. Radicles were stained with Evans blue solution (0.025% [w/v] Evans blue in 100 μM CaCl2, pH 5.6) for 10 min, then washed three times with 100 μM CaCl2 solutions, and photographed. Staining intensity of Evans blue is positively correlated with more damaged plasma membrane.
Al content in radicles was visualized by staining tissues with hematoxylin. Hematoxylin stain was prepared as described by Polle et al. [31]. Wheat radicles were washed with H2O for 30 min and then stained with solution of 0.2% hematoxylin and 0.02% NaIO3 for 30 min at room temperature. Radicles were then immersed in H2O for 30 min to remove excess stain and photographed. Staining intensity of hematoxylin is positively correlated with Al uptake.
ROS distribution in radicle tips was detected by 2′,7′-dichlorofluorescin diacetate (DCFH-DA) following the method of LeBel et al. [32]. Radicle tips were incubated in a solution containing 100 μM CaCl2 and 10 μM DCFH-DA for 20 min and then washed three times with H2O. The fluorescence was detected with a Nikon 80i microscope (excitation at 488 nm and emission at 525 nm). For each treatment, ten individual roots from ten seedlings were examined and similar results were obtained.
2.7. Real-Time Quantitative RT-PCR Analysis in Wheat Radicles
Radicle tips were prepared for RNA extraction according to Li et al. [33]. Total RNA was isolated by grinding with liquid nitrogen according to the manufacturer's instructions (CWBIO, Beijing, China). cDNA was generated from total RNA with a reverse transcription kit (Prime Script RT Master Mix, Takara, Kyoto, Japan). Quantitative PCR was performed using a StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) with SYBR Premix Ex Taq (TaKaRa Bio Inc., China) according to the manufacturer's instructions. cDNA was amplified by PCR using the following primers: Taβ-actin forward (5′-CTATCCTTCGTTTGGACCTT-3′) and reserve (5′-AGCGAGCTTCTCCTTTATGT-3′); TaWali1 forward (5′-CTGATGGAGTCGAGCAAGG-3′) and reserve (5′-CCGAAGTAGCGATTTAGGAGT-3′); TaWali2 forward (5′-AGCCTACTGCTCCGCCTTGT-3′) and reserve (5′-CGTTTCGTCGGCATCTCC-3′); TaWali3 forward (5′-GACGAGCCCTAAGAAGACG-3′) and reserve (5′-CACGGAGCAATGACAACAG-3′); TaWali5 forward (5′-TGGACCCTGCAAGAAGTAC-3′) and reserve (5′-GCTGAACAACAAGCAACACC-3′); TaWali6 forward (5′-TACGGAATAGACAGGACAAGG-3′) and reserve (5′-CAGCATTTCGGGAACTCG-3′); TaALMT1 forward (5′-TGCCACGCTGAGTAAAGG-3′) and reserve (5′-CGCTGACGCTACGAAGAA-3′). Relative gene expression was presented as values relative to the corresponding gene expression in control, after normalization to the control Taβ-actin transcript levels.
2.8. Statistical Analysis
Statistical significance was tested by one-way ANOVA, and the results are expressed as the mean values ± SD (standard deviation) of three independent experiments. Each experiment was repeated three times.
3. Results
3.1. Inhibitory Effect of Al on Wheat Grain Germination
The effect of Al stress on wheat seedling growth and development was examined following incubation of grain in AlCl3 with concentrations ranging from 5 mM to 90 mM (Table 1). At concentrations of 5 mM or below, germination percentage, coleoptile length, and radicle number are almost unaffected, but radicle length was reduced by 13%, suggesting that the radicle is the primary target of Al toxicity. At 15 mM Al, germination percentage was almost halved compared with that of control and this concentration was selected for further experiments. At 90 mM Al, radicle growth was completely inhibited, but very stunted coleoptile growth was still observed.
Table 1.
Inhibitory effect of Al stress on the germination of wheat grains. Wheat grains were exposed to 0, 5, 10, 15, 20, 25, 30, 60, or 90 mM AlCl3 for 48 h.
| Al3+ concentration (mM) |
Germination percentage (%) | Radicle length (cm) |
Coleoptile length (cm) |
Radicle number (50 grains) |
|---|---|---|---|---|
| 0 | 64 ± 1.2a | 3.1 ± 0.8a | 1.5 ± 0.3ab | 178 ± 7.8a |
| 5 | 66 ± 1.1a | 2.7 ± 0.5ab | 1.6 ± 0.2a | 168 ± 8.9a |
| 10 | 51 ± 2.3b | 1.9 ± 0.3b | 1.3 ± 0.3ab | 162 ± 7.6a |
| 15 | 35 ± 3.8c | 1.1 ± 0.2c | 1.1 ± 0.2bc | 142 ± 6.3b |
| 20 | 28 ± 4.2cd | 0.7 ± 0.3cd | 0.8 ± 0.2cd | 80 ± 5.6c |
| 25 | 21 ± 5.1de | 0.4 ± 0.3d | 0.6 ± 0.2de | 68 ± 6.1c |
| 30 | 15 ± 4.7ef | 0.2 ± 0.2d | 0.3 ± 0.3e | 45 ± 5.3d |
| 60 | 8 ± 5.2f | 0.1 ± 0.1d | 0.3 ± 0.2e | 22 ± 3.5e |
| 90 | 7 ± 6.3f | 0 ± 0e | 0.2 ± 0.2e | 0 ± 0f |
Values are the means ± SD (n = 6). Values are the means ± SD (n = 6). Different letters mean significance of difference between different treatments (P < 0.05).
3.2. SO2 Donor Ameliorates Al Stress in Germinating Wheat Grain
To establish whether the SO2 donor Na2SO3/NaHSO3 had a toxic effect on wheat grain germination, grains were germinated in different SO2 donor concentrations ranging from 0.4 to 2.0 mM for 36 h (see Table S1 in Supplementary Material available online at http://dx.doi.org/10.1155/2015/612363). Table S1 shows there was no significant change in germination percentage, coleoptile length, radicle length, or radicle number between water control and SO2 donor treatment, establishing that the concentrations of SO2 donor used in this work exhibited no visible toxic effects. To test the ability of SO2 donor to alleviate Al stress, wheat grains were pretreated with SO2 donor concentrations ranging from 0.4 to 2.0 mM for 12 h prior to incubation with 15 mM Al (Table 2 and Figure 1). At all SO2 donor concentrations used, SO2 pretreatment was effective in alleviating the toxic effects of Al in a dose dependent manner. The optimal SO2 donor concentration for alleviating Al stress was 1.2 mM, a concentration where the germination percentage was increased by 51%, radicle and coleoptile length by 28% and 26%, respectively, compared with those exposed to Al. This result clearly shows that SO2 alleviates Al-induced inhibition of wheat grain germination and seedling growth.
Table 2.
Effects of SO2 donor pretreatment on wheat grain germination under 15 mM Al3+ stress. Wheat grains were pretreated with 0, 0.4, 0.8, 1.2, 1.6, and 2.0 mM SO2 for 12 h and subsequently subjected to 15 mM AlCl3 for further 48 h, and then germination was investigated.
| SO2 donor concentration (mM) |
0.0 | 0.4 | 0.8 | 1.2 | 1.6 | 2.0 |
|---|---|---|---|---|---|---|
| Germination percentage (%) | 37 ± 3.3a | 42 ± 2.7a | 44 ± 3.5a | 56 ± 3.8a | 48 ± 2.7a | 47 ± 3.1a |
| Length of radicle (cm) | 1.42 ± 0.6a | 1.72 ± 0.4a | 1.80 ± 0.7a | 1.82 ± 0.7a | 1.78 ± 0.8a | 1.62 ± 0.4a |
| Length of coleoptile (cm) | 4.64 ± 0.4a | 4.70 ± 0.6a | 5.20 ± 0.5a | 5.83 ± 0.8a | 5.40 ± 0.8 | 5.23 ± 0.6a |
| Radicle number (50 grains) | 127 ± 7.3a | 135 ± 8.1a | 139 ± 8.1a | 148 ± 7.9a | 130 ± 6.7a | 119 ± 7.1a |
Values are the means ± SD (n = 6). Different letters mean significance of difference between different treatments (P < 0.05).
Figure 1.

Effects of SO2 pretreatment on wheat grain germination under 15 mM Al stress. Wheat grains were pretreated with 0, 0.4, 0.8, 1.2, 1.6, and 2.0 mM SO2 for 12 h, subsequently subjected to 15 mM Al for further 48 h, and then photographed.
3.3. Effect of SO2 Donor on the Contents of Reducing Sugars and Soluble Protein in Al-Stressed Wheat Grain
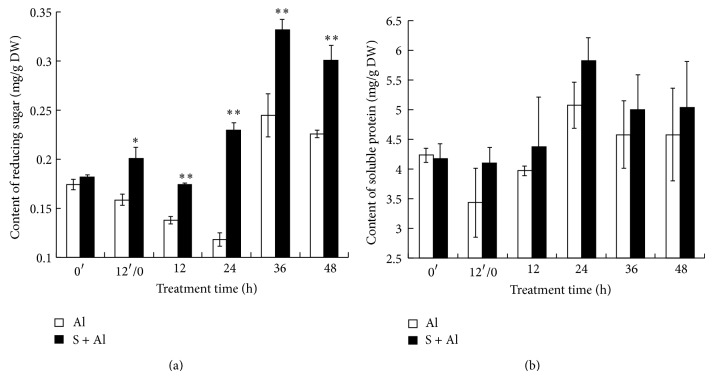
Figure 2(a) shows the changes in reducing sugars in germinating wheat grains preincubated in SO2 donor or H2O for 12 h followed by incubation in Al for 48 h. Within 12 h pretreatment in H2O and 24 h of Al treatment, the content of reducing sugar decreased gradually, whereas reducing sugar in the SO2 donor pretreatment remained stable and slightly increased at 24 h. Thereafter reducing sugar content increased steadily in both treatments followed by a slight decrease at 48 h. The content of reducing sugars in SO2 donor pretreated grain was always significantly higher than the counterpart of only Al treatment.
Figure 2.
Effect of SO2 pretreatment on the contents of reducing sugar and soluble protein in Al-treated grain as shown in (a) and (b), respectively. Wheat grains were pretreated with water (Al) or 1.2 mM SO2 donor (S + Al) for 12 h (shown from 0′ to 12′/0 h of pretreatment time) and then exposed to 15 mM Al for further 48 h (shown as 12′/0, 12, 24, 36, and 48 h). The symbols ∗ and ∗∗ in this figure and following ones stand for significant difference between Al-treated grains with and without SO2 pretreatment at P < 0.05 and P < 0.01, respectively.
The content of soluble protein increased gradually and peaked on 24 h of Al stress followed by a slight decrease (Figure 2(b)). Though the mean values of soluble protein in SO2 donor pretreatment were higher than those pretreated in H2O, they are not significantly different.
3.4. Effect of SO2 Donor Pretreatment on Contents of Endogenous H2S, O2 •−, H2O2, and MDA
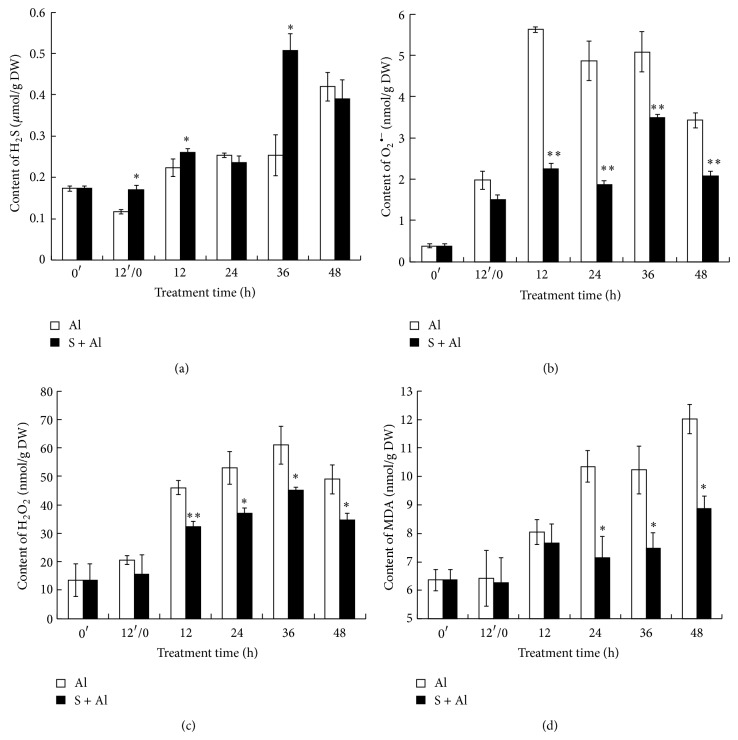
H2S, which can be produced from sulfite, is involved in plant growth regulation including various abiotic stresses [8, 22]. To investigate whether exogenous SO2 application can induce endogenous H2S production, we measured the concentration of H2S in Al-stressed wheat grain. Generally, H2S accumulated during wheat grain germination following pretreatment with water or SO2, but SO2 donor pretreatment significantly enhanced H2S concentration at 12 h of pretreatment and 12 h, 36 h of Al stress (Figure 3(a)).
Figure 3.
Effects of SO2 pretreatment on the accumulation of endogenous H2S (a), superoxide anion (O2 •−) (b), hydrogen peroxide (H2O2) (c), and malondialdehyde (MDA) (d) in germinating wheat grains under Al stress. The numbers (0′, 12′/0, 12, 24, 36, and 48) or letters (CK or SO2) presented are the same as mentioned in Figure 2. Al: Al stress without SO2 pretreatment; S + Al: Al stress with SO2 pretreatment.
To study the protective role of SO2 in the Al-stressed wheat grain, reactive oxygen species O2 •−, H2O2, and malondialdehyde (MDA) were determined with time. As shown in Figure 3(b), a rapid accumulation of O2 •− was observed when H2O-pretreated grains were exposed to Al. During the first 12 h of Al exposure, the increase in O2 •− content was very rapid, but this was followed by a slow decrease. In contrast, the content of O2 •− in SO2 pretreatment increased slowly till 36 h of Al stress followed by a decrease. SO2 pretreatment maintained significantly lower level of O2 •− in Al-stressed wheat grains compared with grains incubated in H2O and exposed to Al.
H2O2 in both treatments increased gradually during pretreatment time and 36 h of Al stress and decreased at 48 h (Figure 3(c)). However, H2O2 content in SO2 pretreatment was significantly lower than that in water pretreatment when exposed to Al stress.
During the 12 h pretreatment time, no significant difference was observed in MDA content in wheat grains whether pretreated with SO2 donor or H2O (Figure 3(d)). After exposure to Al, the content of MDA in water pretreated grains increased rapidly till 48 h of Al stress. An increase of MDA content was also observed in SO2 pretreatment at 12 h of Al stress, but thereafter MDA content remained stable until 36 h. SO2 pretreatment dramatically reduced the amount of MDA from 24 h to 48 h of Al stress in comparison with grains pretreated in water.
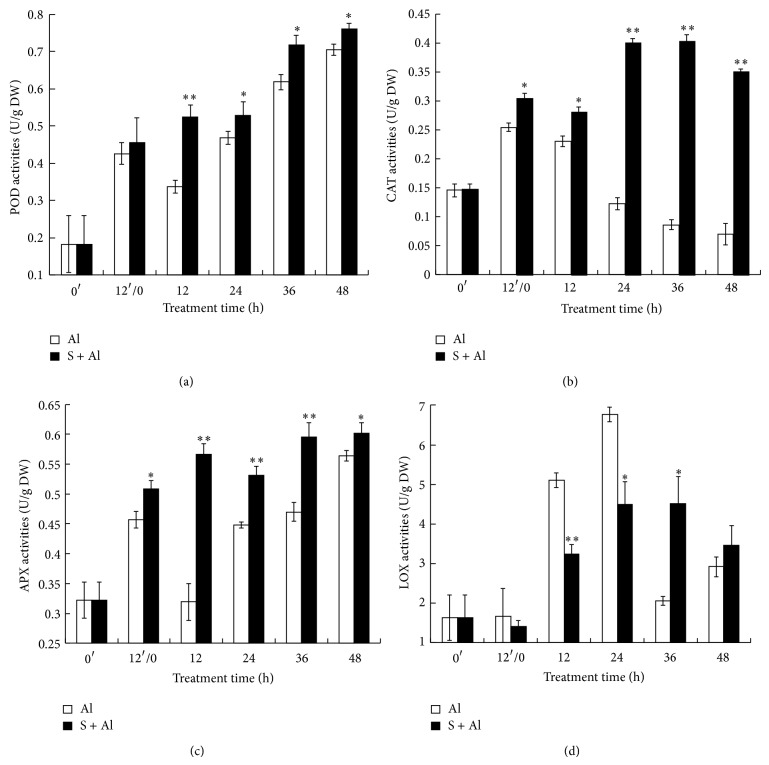
3.5. Effects of SO2 Donor Pretreatment on POD, CAT, APX, and LOX Activities
Activities of POD, CAT, APX, and LOX were determined with time in SO2 donor and H2O-pretreated grains exposed to Al (Figure 4). Figure 4(a) shows the time course of POD activity following pretreatment in SO2 donor or H2O for 12 h when POD activity showed almost a twofold increase. During Al stress, POD activity exhibited a gradual increase in both treatments, but SO2 pretreatment maintained significantly higher level of POD activity during Al stress.
Figure 4.
Effect of SO2 donor pretreatment on the activities of POD (a), CAT (b), APX (c), and LOX (d) in germinating wheat grains under 15 mM Al stress. Grains were treated and the number or letters presented are the same as mentioned in Figure 2. Al: Al stress without SO2 pretreatment; S + Al: Al stress with SO2 pretreatment.
The activity of CAT increased almost twofold during 12 h pretreatment with H2O or SO2 donor (Figure 4(b)). After exposure to Al, CAT activity in water pretreatment decreased gradually till 48 h of Al stress, suggesting that CAT activity is very sensitive to Al stress. In contrast, CAT activity in SO2 pretreatment increased steadily and decreased only slightly at 48 h of Al stress.
As shown in Figure 4(c), SO2 pretreatment enhanced APX activity in Al-stressed wheat grain. A rapid increase in APX activity occurred during the pretreatment time in H2O and SO2. Within the first 12 h of Al stress, APX activity in H2O-pretreated grains decreased sharply, whereas SO2 donor pretreatment enhanced APX activity slightly. Thereafter APX activity increased steadily in water pretreated grain, whereas its activity in SO2 donor pretreatment fluctuated slightly. The APX activity in SO2 donor pretreated grains was always significantly higher than the counterpart of water pretreatment.
An increase in LOX activity was observed during the first 24 h of Al stress in SO2 and H2O-pretreated grains (Figure 4(d)). However, the increase of LOX activity in water pretreatment was more rapid than after SO2 pretreatment. Thereafter LOX activity in water pretreatment showed a sharp decrease at 36 h of Al stress, while its activity in SO2 pretreatment decreased at 48 h. At 12 and 24 h of Al stress, SO2 pretreatment maintained significantly lower level of LOX, while at 36 h LOX activity in SO2 pretreatment was higher than that of water pretreatment.
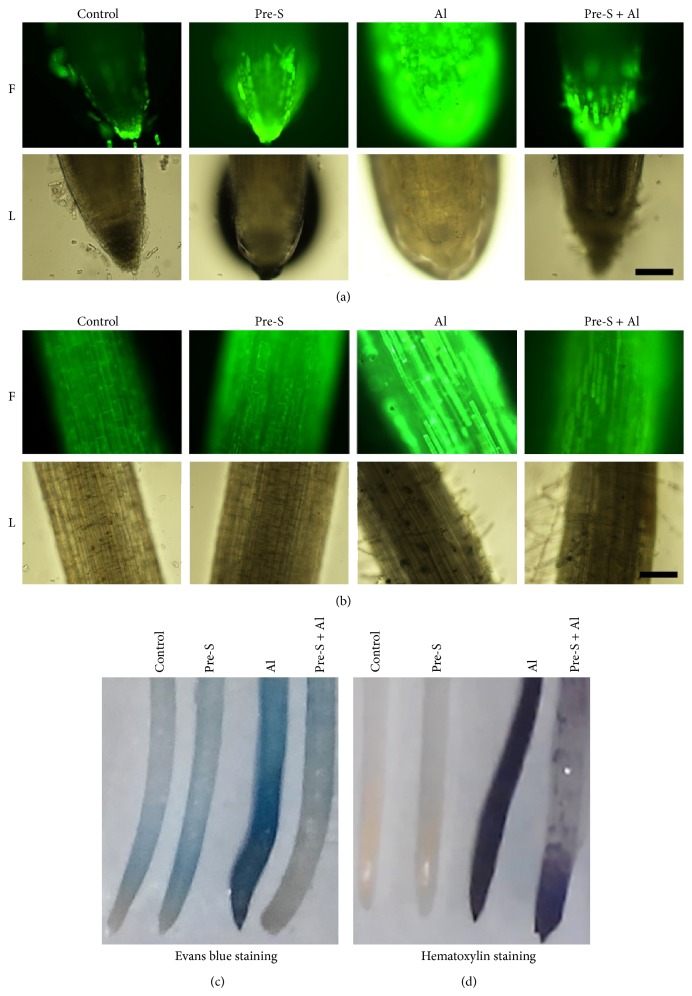
3.6. Effects of SO2 Donor Pretreatment on Localization of Al, Lipid Peroxidation, and ROS Production
To detect ROS production in the radicle tips, we used DCFH-DA fluorescence to indicate ROS accumulation. As shown in Figure 5(a), Al treatment induced higher level of ROS in radicle as intense DCFH-DA fluorescence, while SO2 donor pretreatment for 12 h followed by Al stress significantly reduced fluorescence. Figure 5(b) shows DCFH-DA fluorescence in maturation zone in radicles. Similarly, intense fluorescence in SO2 donor pretreatment followed by Al stress was much weaker than that in water pretreated plus Al-stressed radicles, suggesting that SO2 donor was effective in alleviating oxidative stress in radicles. SO2 donor treatment alone showed comparable fluorescence intensity as observed in water control.
Figure 5.
ROS staining ((a) on radicle tips; (b) on maturation zone; bar: 200 μm), Evans blue staining (c), and hematoxylin staining (d) in wheat radicles. Initially, wheat grains were geminated in water for 36 h. Then four treatment groups were done as follows, control, 60 h in H2O; Pre-S, pretreatment with 1 mM SO2 donor for 12 h, and then exposed to H2O for 48 h; Al, 12 h in H2O prior to exposure to 400 μM AlCl3 for 48 h; Pre-S + Al, 12 h in 1 mM SO2 donor pretreatment followed by 400 μM AlCl3 for 48 h.
The radicles were stained with Evans blue to show membrane integrity. The radicles treated with Al alone were stained extensively with Evans blue, while Al-stressed radicles pretreated with SO2 donor for 12 h were less stained (Figure 5(c)), suggesting SO2 donor serves to protect cell membrane from Al-induced damage. SO2 donor treatment alone showed similar Evans blue staining to water control, suggesting no visible damaging effect of SO2 on radicles.
The hematoxylin staining was used to detect Al accumulation in radicles. As shown in Figure 5(d), the radicles of water control and SO2 treatment incubated with hematoxylin showed no dark staining but wheat radicles treated with Al alone were stained intensively. In contrast, radicles pretreated with SO2 donor for 12 h and then exposed to Al for 48 h showed much weaker staining compared with Al stress, especially in the elongation zone.
3.7. Effect of SO2 Donor Pretreatment on the Relative Expressions of Aluminum Stress Related Genes
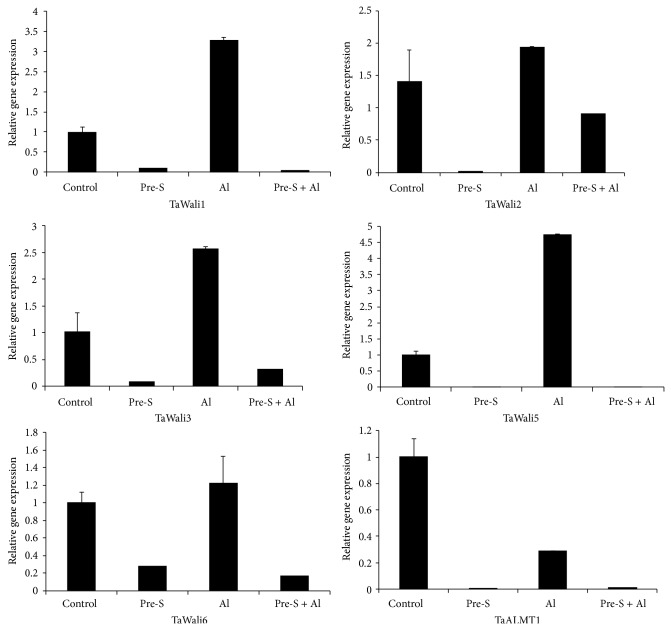
We determined the changes in gene expression of aluminum stress related genes in wheat radicles. Radicles were pretreated with or without 1 mM SO2 donors for 12 h and then exposed to Al for 48 h. As shown in Figure 6, Al stress induced higher expression of TaWali1, TaWali2, TaWali3, TaWali5, and TaWali6 (wheat aluminum induced) in radicles, while pretreatment with SO2 donor for 12 h followed by Al stress alleviated such expression increase. Besides, the gene expression of TaALMT1 (Al-activated malate transporter) was also attenuated by SO2 pretreatment.
Figure 6.
Effect of SO2 donor pretreatment on relative gene expression of TaWali1, TaWali2, TaWali3, TaWali5, TaWali6, and TaALMT1 in wheat radicals exposed to Al stress. Initially, wheat grains were geminated in water for 36 h. Then four treatment groups were done as follows, control, 60 h in H2O; Pre-S, pretreatment with 1 mM SO2 donor for 12 h, and then exposed to H2O for 48 h; Al, 12 h in H2O prior to exposure to 400 μM AlCl3 for 48 h; Pre-S + Al, 12 h in 1 mM SO2 donor pretreatment followed by 400 μM AlCl3 for 48 h.
4. Discussion
In solution, SO2 is dissociated from its sulfite derivatives (NaHSO3/Na2SO3 1 : 3 M/M) [34]. Thus NaHSO3/Na2SO3 (1 : 3 M/M) was chosen as an SO2 donor in our study. Similar to the observation that H2S could promote wheat grain germination and alleviate oxidative damage against Al stress [8], our results show that SO2 donor pretreatment alleviates Al stress in germinating wheat seedlings. Wheat grains pretreated for 12 h with the SO2 donor show an increase in germination percentage, coleoptile length, radicle length, and radicle numbers of wheat. The increase in the contents of reducing sugars and soluble protein suggests that nutrients in wheat grains pretreated with SO2 donor are rapidly mobilized to provide energy to grain germination. SO2 donor maintained lower level of H2O2, O2 •−, and MDA probably by activation of the antioxidant system. These results suggest that SO2 acts as an antioxidant and may function in a way that is similar to what the effects of H2S, CO, and NO do in plants exposed to heavy metal stress [10, 35].
Sulfite can be reduced by sulfite reductase to H2S, which is incorporated into O-acetylserine via O-acetyl(thiol)lyase to form cysteine [22]. In RNA interfered mutant of sulfite reductase (SiR), sulfide synthesis in younger leaves was decreased by the impaired SiR activity [36]. In the present study, exogenous SO2 application can induce endogenous H2S production in Al-stressed wheat grains (Figure 3(a)), suggesting the interplay between sulfite and the formation of H2S.
Consistent with previous observations [7], our results show that Al stress caused overproduction of ROS in wheat. To mitigate and repair oxidative damage, plants have evolved an efficient antioxidant system that includes enzymes such as SOD, CAT, and APX that function to scavenge ROS [37]. SOD catalyzes the dismutation of the superoxide radical O2 •− and H+ into H2O2. CAT, APX, and POD are responsible for the elimination of H2O2 generated by SOD. Al stress brings about a dramatic increase in H2O2 and O2 •−. The elevated levels of H2O2 and O2 •− suggest that antioxidant enzymes in Al-stressed wheat do not efficiently scavenge the overproduction of ROS, and this can result in lipid peroxidation or plasma membrane inhibiting grain germination and seedling growth [8]. Our data show that pretreatment of wheat with SO2 donor activates antioxidant enzymes including POD, CAT, and APX.
LOX, which catalyzes oxygenation of polyunsaturated fatty acids into lipid hydroperoxides, is considered an indicator of oxidative stress during responses to various environmental stresses [9]. Pretreatment with SO2 donor lowers LOX activity in Al-stressed wheat radicles compared to seedlings pretreated with H2O and exposed to Al. The lowering of LOX by SO2 pretreatment also helps to explain the lower MDA content of Al-stressed grain. Taken together, these data suggest that SO2 donor reduced oxidative stress by modulation of the antioxidant system.
Our data indicate that the radicle is the primary target for Al toxicity. DCFH-DA fluorescence assay shows that Al incubation induces higher accumulation of ROS in radicle tips and maturation zone. SO2 donor pretreatment effectively reduces ROS content in subsequent Al stress, suggesting the role of SO2 in alleviating oxidative stress. Correspondently, Al stress causes membrane injury to radicles, while SO2 donor effectively alleviates such injury. To understand whether SO2 donor helps to reduce Al accumulation in radicles, hematoxylin staining was used to indicate Al and the results show that SO2 donor obviously reduces Al content in radicles, implying a potential role of SO2 donor treatment as a strategy to reduce Al uptake.
In response to Al stress, many gene expressions are activated, for instance, TaWali (wheat aluminum induced), aluminum-activated malate transporter (TaALMT1) [38–41]. Relative gene expression analysis shows that Al treatment induces higher expression of TaWali, while these gene expression levels are reduced by SO2 donor pretreatment, suggesting the response to Al stress is attenuated in SO2 donor pretreatment.
5. Conclusion
In the present study, SO2 acts as an antioxidant signal to reduce ROS damage in wheat grains and radicles caused by Al stress. Besides, SO2 also decreases Al uptake. The induced higher level of H2S suggests an intricate interplay of SO2 and H2S in plants. Exogenous application of SO2 may be reduced to H2S by sulfite reductase, thus contributing to H2S production. H2S in itself acts as an antioxidant signaling molecule in plants' response to abiotic stress. Thus the nature of SO2/sulfite functions in alleviating Al stress still needs further research.
Supplementary Material
Table S1 shows the germination percentages of wheat grains under SO2 donor treatment. Wheat grains were germinated in 0.0, 0.4, 0.8, 1.2, 1.6 or 2.0 mM SO2 donor for 36 h, and then the germination percentages are counted.
Acknowledgments
This work was supported by the Natural Science Foundation of China (31271803, 31301820, 31300133, and 31470013), the Scientific Research Foundation for Returned Overseas Chinese Scholars (SRF for ROCS, MOE), the Natural Science Foundations of Anhui Province (11040606M85), and the Anhui Provincial Education Department (2012AJZR0028, ZD200910).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Dong-Bo Zhu, Kang-Di Hu, and Xi-Kai Guo contributed equally to this work.
References
- 1.Ryan P. R., Tyerman S. D., Sasaki T., et al. The identification of aluminium-resistance genes provides opportunities for enhancing crop production on acid soils. Journal of Experimental Botany. 2011;62(1):9–20. doi: 10.1093/jxb/erq272. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z. Q., Xu X. Y., Gong Q. Q., et al. Root proteome of rice studied by iTRAQ provides integrated insight into aluminum stress tolerance mechanisms in plants. Journal of Proteomics. 2014;98(26):189–205. doi: 10.1016/j.jprot.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 3.Huang W., Yang X., Yao S., et al. Reactive oxygen species burst induced by aluminum stress triggers mitochondria-dependent programmed cell death in peanut root tip cells. Plant Physiology and Biochemistry. 2014;82(12):76–84. doi: 10.1016/j.plaphy.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y.-S., Yang Z.-M. Nitric oxide reduces aluminum toxicity by preventing oxidative stress in the roots of Cassia tora L. Plant and Cell Physiology. 2005;46(12):1915–1923. doi: 10.1093/pcp/pci202. [DOI] [PubMed] [Google Scholar]
- 5.Tahara K., Yamanoshita T., Norisada M., et al. Aluminum distribution and reactive oxygen species accumulation in root tips of two Melaleuca trees differing in aluminum resistance. Plant and Soil. 2008;307(1-2):167–178. doi: 10.1007/s11104-008-9593-5. [DOI] [Google Scholar]
- 6.Ding Z. J., Yan J. Y., Xu X. Y., Li G. X., Zheng S. J. WRKY46 functions as a transcriptional repressor of ALMT1, regulating aluminum-induced malate secretion in Arabidopsis. The Plant Journal. 2013;76(5):825–835. doi: 10.1111/tpj.12337. [DOI] [PubMed] [Google Scholar]
- 7.Zheng S. J., Yang J. L. Target sites of aluminum phytotoxicity. Biologia Plantarum. 2005;49(3):321–331. doi: 10.1007/s10535-005-0001-1. [DOI] [Google Scholar]
- 8.Zhang H., Li Y. H., Hu L. Y., Wang S. H., Zhang F. Q., Hu K. D. Effects of exogenous nitric oxide donor on antioxidant metabolism in wheat leaves under aluminum stress. Russian Journal of Plant Physiology. 2008;55(4):469–474. doi: 10.1134/S1021443708040067. [DOI] [Google Scholar]
- 9.Zhang H., Tan Z.-Q., Hu L.-Y., Wang S.-H., Luo J.-P., Jones R. L. Hydrogen sulfide alleviates aluminum toxicity in germinating wheat seedlings. Journal of Integrative Plant Biology. 2010;52(6):556–567. doi: 10.1111/j.1744-7909.2010.00946.x. [DOI] [PubMed] [Google Scholar]
- 10.Xu J., Xuan W., Huang B., et al. Carbon monoxide-induced adventitious rooting of hypocotyl cuttings from mung bean seedling. Chinese Science Bulletin. 2006;51(6):668–674. doi: 10.1007/s11434-006-0668-5. [DOI] [Google Scholar]
- 11.Zhang S. Q., Du J. B., Jin H. F., et al. Endogenous sulfur dioxide aggravates myocardial injury in isolated rat heart with ischemia and reperfusion. Transplantation. 2009;87(4):517–524. doi: 10.1097/TP.0b013e318195fe82. [DOI] [PubMed] [Google Scholar]
- 12.Zhang R. Y., Du J. B., Sun Y., et al. Sulfur dioxide derivatives depress L-type calcium channel in rat cardiomyocytle. Clinical and Experimental Pharmacology and Physiology. 2011;38(7):416–422. doi: 10.1111/j.1440-1681.2011.05528.x. [DOI] [PubMed] [Google Scholar]
- 13.Jin H.-F., Du S.-X., Zhao X., et al. Effects of endogenous sulfur dioxide on monocrotaline-induced pulmonary hypertension in rats. Acta Pharmacologica Sinica. 2008;29(10):1157–1166. doi: 10.1111/j.1745-7254.2008.00864.x. [DOI] [PubMed] [Google Scholar]
- 14.Ziegler I. The effect of SO2 pollution on plant metabolism. Residue Reviews. 1975;56(1):79–105. [Google Scholar]
- 15.Sandhu R., Li Y., Gupta G. Sulphur dioxide and carbon dioxide induced changes in soybean physiology. Plant Science. 1992;83(1):31–34. doi: 10.1016/0168-9452(92)90059-U. [DOI] [Google Scholar]
- 16.Yarmolinsky D., Brychkova G., Fluhr R., Sagi M. Sulfite reductase protects plants against sulfite toxicity. Plant Physiology. 2013;161(2):725–743. doi: 10.1104/pp.112.207712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noji M., Saito M., Nakamura M., Aono M., Saji H., Saito K. Cysteine synthase overexpression in tobacco confers tolerance to sulfur-containing environmental pollutants. Plant Physiology. 2001;126(3):973–980. doi: 10.1104/pp.126.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rakwal R., Agrawal G. K., Kubo A., et al. Defense/stress responses elicited in rice seedlings exposed to the gaseous air pollutant sulfur dioxide. Environmental and Experimental Botany. 2003;49(3):223–235. doi: 10.1016/S0098-8472(02)00072-2. [DOI] [Google Scholar]
- 19.Yin J. J., Liu X., Yi H. L., Yang M. L. Sulfur dioxide induces guard cell death in Vicia faba . Acta Scientiae Circumstantiae. 2010;30(12):2512–2517. [Google Scholar]
- 20.Rennenberg H. The fate of excess sulfur in higher plants. Annual Review of Plant Physiology. 1984;35(4):121–153. [Google Scholar]
- 21.Giraud E., Ivanova A., Gordon C. S., Whelan J., Considine M. J. Sulphur dioxide evokes a large scale reprogramming of the grape berry transcriptome associated with oxidative signalling and biotic defence responses. Plant, Cell and Environment. 2012;35(2):405–417. doi: 10.1111/j.1365-3040.2011.02379.x. [DOI] [PubMed] [Google Scholar]
- 22.Rausch T., Wachter A. Sulfur metabolism: a versatile platform for launching defence operations. Trends in Plant Science. 2005;10(10):503–509. doi: 10.1016/j.tplants.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Stipanuk M. H., Dominy J. E., Jr., Lee J.-I., Coloso R. M. Mammalian cysteine metabolism: new insights into regulation of cysteine metabolism. Journal of Nutrition. 2006;136(6):1652S–1659S. doi: 10.1093/jn/136.6.1652S. [DOI] [PubMed] [Google Scholar]
- 24.Laisk A., Pfanz H., Heber U. Sulfur-dioxide fluxes into different cellular compartments of leaves photosynthesizing in a polluted atmosphere: II. Consequences of SO2 uptake as revealed by computer analysis. Planta. 1988;173(2):241–252. doi: 10.1007/BF00403016. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H., Hu S.-L., Zhang Z.-J., et al. Hydrogen sulfide acts as a regulator of flower senescence in plants. Postharvest Biology and Technology. 2011;60(3):251–257. doi: 10.1016/j.postharvbio.2011.01.006. [DOI] [Google Scholar]
- 26.Surrey K. Spectrophotometric method for determination of lipoxidase activity. Plant Physiology. 1964;39(1):65–70. doi: 10.1104/pp.39.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu L.-Y., Hu S.-L., Wu J., et al. Hydrogen sulfide prolongs postharvest shelf life of strawberry and plays an antioxidative role in fruits. Journal of Agricultural and Food Chemistry. 2012;60(35):8684–8693. doi: 10.1021/jf300728h. [DOI] [PubMed] [Google Scholar]
- 28.Miller G. L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry. 1959;31(3):426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 29.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto Y., Kobayashi Y., Devi S. R., Rikiishi S., Matsumoto H. Aluminum toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cells. Plant Physiology. 2002;128(1):63–72. doi: 10.1104/pp.128.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polle E., Konzak C. F., Kittrick J. A. Visual detection of aluminum tolerance levels in wheat by hematoxylin staining of seeding roots. Crop Science. 1978;18(5):823–827. [Google Scholar]
- 32.LeBel C. P., Ischiropoulos H., Bondy S. C. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chemical Research in Toxicology. 1992;5(2):227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- 33.Li S.-P., Hu K.-D., Hu L.-Y., et al. Hydrogen sulfide alleviates postharvest senescence of broccoli by modulating antioxidant defense and senescence-related gene expression. Journal of Agricultural and Food Chemistry. 2014;62(5):1119–1129. doi: 10.1021/jf4047122. [DOI] [PubMed] [Google Scholar]
- 34.Shapiro R. Genetic effects of bisulfite (sulfur dioxide) Mutation Research. 1977;39(2):149–175. doi: 10.1016/0165-1110(77)90020-3. [DOI] [PubMed] [Google Scholar]
- 35.Hu K.-D., Hu L.-Y., Li Y.-H., Zhang F.-Q., Zhang H. Protective roles of nitric oxide on germination and antioxidant metabolism in wheat seeds under copper stress. Plant Growth Regulation. 2007;53(3):173–183. doi: 10.1007/s10725-007-9216-9. [DOI] [Google Scholar]
- 36.Yarmolinsky D., Brychkova G., Kurmanbayeva A., et al. Impairment in sulfite reductase leads to early leaf senescence in tomato plants. Plant Physiology. 2014;165(4):1505–1520. doi: 10.1104/pp.114.241356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma P., Dubey R. S. Involvement of oxidative stress and role of antioxidative defense system in growing rice seedlings exposed to toxic concentrations of aluminum. Plant Cell Reports. 2007;26(11):2027–2038. doi: 10.1007/s00299-007-0416-6. [DOI] [PubMed] [Google Scholar]
- 38.Snowden K. C., Gardner R. C. Five genes induced by aluminum in wheat (Triticum aestivum L.) roots. Plant Physiology. 1993;103(3):855–861. doi: 10.1104/pp.103.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ligaba A., Dreyer I., Margaryan A., Schneider D. J., Kochian L., Piñeros M. Functional, structural and phylogenetic analysis of domains underlying the Al sensitivity of the aluminum-activated malate/anion transporter, TaALMT1. The Plant Journal. 2013;76(5):766–780. doi: 10.1111/tpj.12332. [DOI] [PubMed] [Google Scholar]
- 40.Yoshimoto N., Takahashi H., Smith F. W., Yamaya T., Saito K. Two distinct high-affinity sulfate transporters with different inducibilities mediate uptake of sulfate in Arabidopsis roots. The Plant Journal. 2002;29(4):465–473. doi: 10.1046/j.0960-7412.2001.01231.x. [DOI] [PubMed] [Google Scholar]
- 41.Kataoka T., Watanabe-Takahashi A., Hayashi N., et al. Vacuolar sulfate transporters are essential determinants controlling internal distribution of sulfate in Arabidopsis . The Plant Cell. 2004;16(10):2693–2704. doi: 10.1105/tpc.104.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 shows the germination percentages of wheat grains under SO2 donor treatment. Wheat grains were germinated in 0.0, 0.4, 0.8, 1.2, 1.6 or 2.0 mM SO2 donor for 36 h, and then the germination percentages are counted.