Abstract
Nanotechnology has been integrated into healthcare system in terms of diagnosis as well as therapy. The massive impact of imaging nanotechnology has a deeper intervention in cardiology i.e. as contrast agents, to target vulnerable plaques with site specificity and in a theranostic approach to treat these plaques, stem cell delivery in necrotic myocardium, etc. Thus cardiovascular nanoimaging is not limited to simple diagnosis but also can help real time tracking during therapy as well as surgery. The present review provides a comprehensive description of the molecular imaging techniques for cardiovascular diseases with the help of nanotechnology and the potential clinical implications of nanotechnology for future applications.
Keywords: cardiovascular disease, nanoimaging, nanotoxicity, theranostic, thrombus imaging
Introduction
Nanotechnology is considered as a cutting edge technology in the 21st century. In ancient period also people prepared and used nanoparticles in different fields of art and medicine, without knowing their in-depth physico-chemical properties, but believing their potential to prevent diseases. Window glasses and ceramic containers in the Roman Empire were found to contain gold, copper and silver nanoparticles to give them eternal bright colour1,2. Gold nanoparticles were being used in medicines in China and India. In India, till now gold nanoparticles are used in medicine as ‘Swarno Vasmo’3,4. In 1857, Michael Faradayfirst prepared the pure colloidal gold nanoparticles, and named it as ‘activated gold’5, though the first introduction of the concept of modern nanotechnology was by renowned Noble laureate Physicist, Richard Phillips Feymann in 1959 in his famous talk called “There's Plenty of Room at the Bottom”6. Robert Curl, Harold Kroto, and Richard Smalley were awarded Nobel Prize in Chemistry in 1996 for their roles in the discovery of buckyballs or fullerenes (spherical carbon nanoparticles), the first synthetic nanoform with known characteristics7.
The word ‘Nano’ is derived from the Greek word ‘Nanos’ which means dwarf, denoting a factor of 10ˉ9 (1meter= 1,000,000,00 nano meter). According to National Nanotechnology Initiative “Nanotechnology is the understanding and control of matter at the nanoscale, at dimensions between approximately 1 and 100 nanometers, where unique phenomena enable novel applications”8,9. Nanoscale dimension acquires some special characteristics (e.g. optical, magnetic, electrochemical, etc.) which are neither present in bulk material nor in molecular state8,10,11,12,13. A good example is gold nanoparticles which remain in colloidal phase and are red in colour, though bulk gold is solid in nature and is yellow in colour. This unique colour phenomenon of gold nanoparticles is due to surface plasmon resonance (SPR), found only in nanoscale dimension. Therefore, these exclusive nano-specific properties make them unique entity in classical chemistry. Interestingly, one can easily modify or customize these properties just by modulating shape, size and/or surface topology of nanoforms14. In a nutshell, the maneuverability of designing the materials at the nanoscale and tunability on its surface make this an independent branch of science having wide applicability.
Nanobiotechnology
Nanotechnology is truly an interdisciplinary area of modern science. It involves vast area of chemistry, physics, electronics, material science as well as biology15,16. Among all of these areas, biology is the most recently introduced subject. Application of nanotechnology in biology is thought to be one of the most successful and wide spread areas of utility, which includes basic understanding of biological event as well as medical diagnosis, surgery and therapy17,18,19,20. Biomolecule compatible size distribution of nanoforms along with their tuneable properties (physical, chemical, topological) help nanotechnology to merge with biology to give birth to one of the most advanced fields of science - Nanobiotechnology21,22,23. Depending on the constituting material, mode of synthesis, surface topologies, mode of applications, etc., scientists are classifying different forms of nanomaterials. Some of these basic nanoforms synthesized so far and are proposed to be used in biomedical field are given in Table I.
Table I.
Different types of synthesized nanoforms

The ultra small dimension and uniform size distribution (compared to liposome), specialized optical, physico-chemical, electrical, magnetic properties; high cellular penetration power, tuneable size, shape, texture, unique surface topology and surface chemistry make nanoparticles to play promising role in the biomedical field (e.g. therapy, drug delivery, diagnostics, etc.)40,41,42. The nanoforms that belong to this category (bio-medical) are listed in Table II.
Table II.
Use of nanoparticles in different field of biomedicine.

Nanotechnology and cardiovascular diseases
Biomedical application of primitive era nanotechnology was mostly in the field of cancer43,44,49, though with advanced exploration, it encompasses almost all fields of biomedical research. Cardiovascular nanomedicine is the most recent area. Diagnosis, drug delivery, stem cell therapy, tissue engineering, stent surgery, are the few other areas where nanotechnology imprints its signature. The most promising area however, is the nanomaterial based improved clinical imaging, e.g. nanoimaging of cardiovascular diseases50.
Cardiovascular imaging is one of the most reliable diagnostic tools for cardiovascular diseases51. After extensive research, it was hypothesised that nanoparticles could be unique contributors in the field of the medical imaging, due to their special features, which are as follows:
(i) Biocompatible size distribution: The ultra small nano size helps them to accommodate with different biocomponents even inside the subcellular organelle52.
(ii) High penetration power: This is another aspect fulfilled by nanoparticles for bio-medical imaging53.
(iii) Image contrasting ability: Paramagnetic nanoparticles are magnetic resonance imaging (MRI) contrast agents. Iodinated nanoparticles can be used as computed tomography (CT) contrast agents, whereas quantum dots can act as fluorescent enhancers54.
(iv) Surface tuneable property: Nanosurface can be modified with the molecules of choice. Thus, it is possible to conjugate a nanomaterial with multimodal entity, for example, target specific molecules (targeted delivery), imaging probes and/or therapeutic molecules55,56.
(iv) Stability: Contrast enhancer nanomaterials are much more stable than a chemical image probe57.
(v) Half life: In case of carrier nanoforms, used as image contrast agents, the half life of the chemical image probes is also increased due to their conjugation with nanoparticles.
Thus, atypical size distribution, target specific delivery, high contrast capability, increase lifetime are the key features that make nanomaterials indispensable in the future medical imaging.
Nano based cardiovascular imaging can monitor the live physiological system in a noninvasive manner, with almost no pain. This live imaging is not only important for proper diagnosis or therapy, but is also beneficial for the basic understanding of the pathological conditions, which in turn helps us to develop future advanced techniques. Though majority of the nano-based cardiovascular imaging modules are in the field of diagnosis, but with advancement of this technology, it has entered in the domain of therapy and surgery also.
In most of the cases, the nano based imaging are not discrete, but are inter-connected between the fields of diagnosis, therapy or surgery. Thus for the better understanding, nano-cardiovascular-imaging can be broadly divided into four categories depending on the site of detection and/or mode of action: (i) Thrombus imaging; (ii) Theranostic approach; (iii) Stem cell imaging; and (iv) Graft imaging
(i) Thrombus imaging: Acute coronary syndrome (ACS) is one of the leading causes of death in the world58,59,60,61,62. Atherosclerotic plaques in humans consist of different bio-components which are heterogeneous in nature, i.e. macrophages, smooth muscle, endothelial cells, other undefined mesenchymal cells, etc63. Proper detection of the plaques, in a non-invasive way is crucial and is the most demanding diagnostic procedure for the accurate treatment of the disease. In modern physics different non-invasive imaging techniques have been developed for the detection of plaques which generally require contrast agents64,65. The choice of the contrast agent depends on the type of technique used. Most of the clinically applied contrast agents pose two significant difficulties. First, these show sometimes toxic effects and second, these get non-specifically distributed to the whole body by circulation due to absence of any target specificity. This is where nano-based imaging system can come as a rescuer over the conventional clinical imaging techniques. For example, one of the most reliable imaging methods for the detection of plaques is by cardiac magnetic resonance imaging (CMRI), which requires a contrast agent gadolinium (GD), that often exerts toxic effects66. This toxic effect can be minimized by nanotechnology based approaches. It has been found recently that intracellular self-assembled gadolinium nanoparticles show enhanced MRI contrast ability with reduced toxicity67. The toxicity of GD is due to free ions. One way to reduce this toxicity is by using chelating agents, for example, diethylene triamine pentaacetic (DTPA). It has been shown that GD-DTPA exhibits less toxicity than GD alone68,69, though there are some reports about the possibility of leakage of free ions from GD- chelate complex70. It has also been shown that nano-GD complex exhibits large loading capacity as well as large ionic relaxivity which in turn increases the contrast ability71,72. Anti-fibrin antibody conjugated oleate modified GD-DTPA nanoparticles (microemulsion) can effectively detect fibrin in vulnerable plaques73. Again, GD-chelate conjugation, incorporating nanoparticles reduces the toxicity to a significant extent74. Though nanotechnology based approaches manage some initial success to reduce GD toxicity, search is still on for new nontoxic agents for MRI. In that direction, ultra-small super-paramagnetic iron oxide nanoparticles (SPION) are thought to be an ideal substitution, as in the laboratory conditions these have shown high contrast ability with no toxicity75. One report shows that SPIONs have high efficacy for CMRI without manifesting any toxic effect in humans76. Same as SPION, perfluorocarbon nanoparticles contain fluorine, generate good contrast without any background signal, and can also be used in CMRI45. Another advantage of SPION is that these can be easily conjugated by any surface ligand, therefore, are efficient enough for targeting and therapy, compared to GD-chelate complex. The last and most effective point is that SPION are degraded by lysosomes and free iron from particles is released into the intracellular iron pool; hence, there is no chance of deposition inside the body77.
The atherosclerotic plaques are mainly of two types; stable plaques (fibrous plaque) and unstable plaques (lipid plaque). Unstable plaques are the main culprits for thrombosis, also known as vulnerable plaques78. Vulnerable plaques are made up of large amount of lipids, covered by a thin fibrous cap. Destruction of this fibrous cap makes the plaques unstable and these become detached from the endothelial layer79. This phenomenon activates circulating resting platelets and the consequence is the formation of platelet rich thrombus. Thrombus blocks the artery, inhibits local circulation, resulting in muscle necrosis. Detection of the vulnerable plaques is a crucial step to initiate therapy for this disease.
Macrophages being one of the key components of atherosclerotic plaque play a decisive role in plaque destabilization80. These get attached to the thin fibrous cap of the plaques and secrete proteolytic enzymes which dissolve the fibrous cap81. Therefore, conceptually macrophages can be used as a good identifier of vulnerable plaques82. Phagocytic activity, which is the key feature of macrophages, has been exploited for the identification of the vulnerable plaque. It has been shown that macrophages can effectively take up a wide range of nanomaterials, including contrast enhancing nanoforms83. Therefore, nanoform loaded inflammatory macrophages on the plaque can be easily identified by non-invasive imaging techniques84,85,86,87,88. Now, the choice of imaging techniques will depend on the constituent of nanomaterials, or vice versa. For example, if macrophages are loaded with SPION, then CMRI will be the ideal technique, whereas CT scan can be done if the particles are iodinated. Gold nanoparticles can be used as good CT contrast agents. It has been found that gold nanoparticles have three times greater photon absorption capacity compared to iodinated contrast agent and, therefore, can enhance image contrast ability89,90. In addition, high contrast ability, inert character and surface modification are the other additional advantages of gold nanoparticles (AuNP). It has been found that CNA35 (small peptide which has excellent affinity for collagen) conjugated AuNP effectively identify myocardial scar signature by CT scan91. Au-HDL nanoparticles (gold nanoparticles coated with apolipoprotein A1, phospholipid and rhodamine lipid) specifically target macrophages of plaques and are identified in multicolour CT92. Specific targets of the plaque, choice of nanoforms and corresponding imaging techniques are listed in Table III.
Table III.
Different nano-conjugates and their mode of action
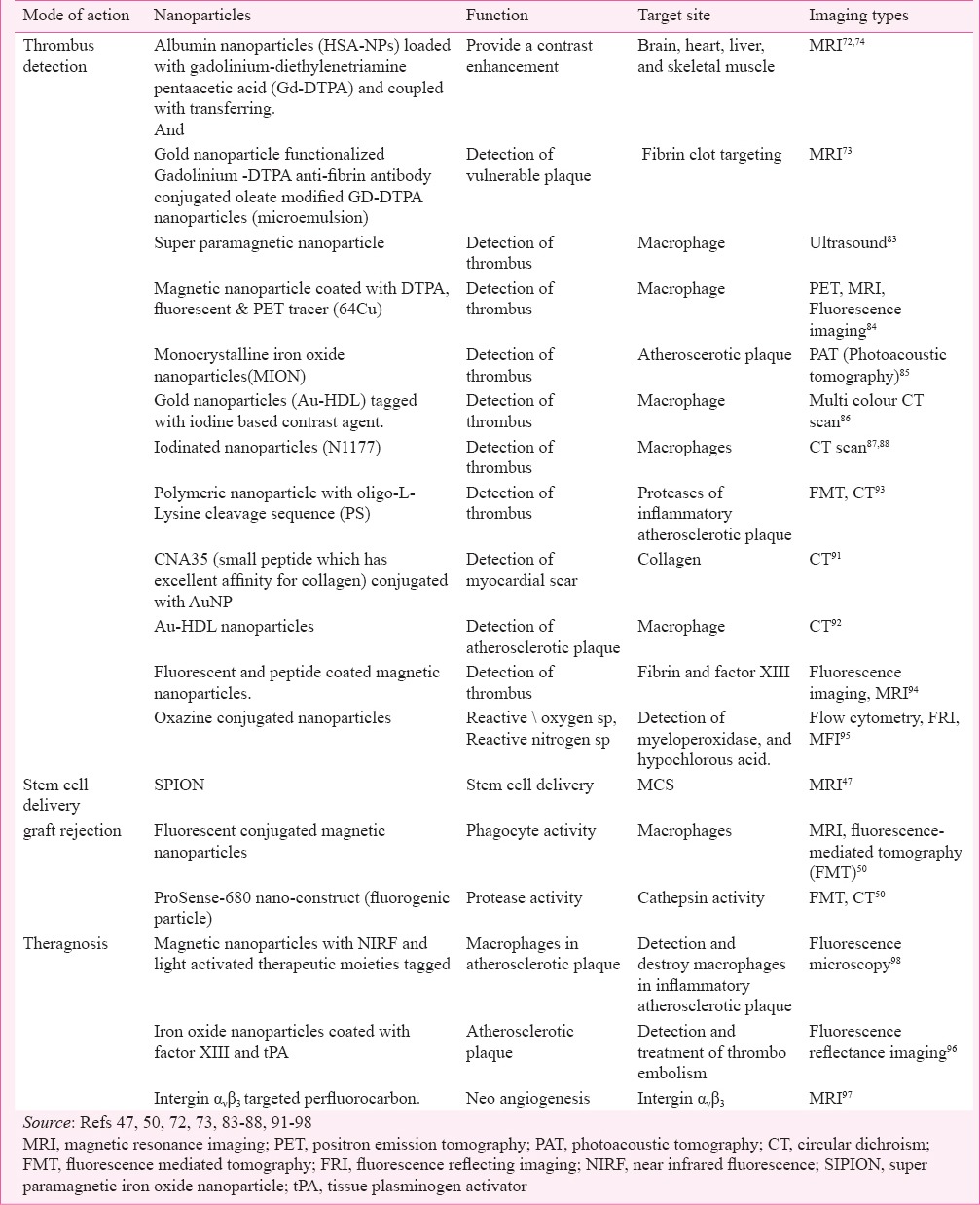
Amino acid sequence specific targeting of plaque destabilize proteases (secreted by the inflammatory macrophages) can also be used as an identifier of atheromata. It was found that polymeric nanoparticles with fluorochrome labelled oligo-L-Lysine cleavage sequence (target for plaque specific proteases) can efficiently detect inflammatory plaque93. Factor XIII is another important constituent of acute thrombus; it converts linear fibrin to crosslink fibrin (fibrin α- and γ-chains), ultimately increases fibrinolytic resistance, and increases the plaque lifetime. Therefore, nano-mediated factor XIII specific targeting and imaging is another approach to detect thrombus. This detection process can also be applicable for other fibrin specific molecules. Magnetic nanoparticles coated with factor XIII, as well as fibrin specific peptide act as effective contrast agents for the detection acute thrombus, especially when thrombus is in its growing phase94.
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are generated by atheroscerosis induced inflammation. Under certain circumstances these oxidizing species can neutralize local antioxidant defences, thus leading to oxidative stress and tissue injury. These oxidation reactions are mainly catalyzed by myeloperoxidase (MPO), a heme protein secreted from activated phagocytes in human atherosclerotic lesions99. Though in vivo imaging of ROS/RNS has significant clinical impact, yet there is no conventional method for their detection. An oxazine nano based imaging method has been developed to monitor hypochlorous acid (HOCl/OCl−) formation by peroxynitrite, a reactive nitrogen species and myeloperoxidase (MPO), thereby identify the oxidative damage by atherosclerosis95,100.
(ii) Theranostic approach: ‘Theranostics’ is a newly established term in clinical medicine which deals with a treatment strategy in combination with therapeutics and diagnostics101. It can be defined as ‘a modified diagnostic procedure equipped with therapeutic molecules/ device’. Theranosis has created a huge expectation in medical sciences because of its multimodal applications. It can reduce the steps and costs of both diagnosis and the therapy. Nanoparticles can themselves act as diagnostic probes (image contrast agent) and get conjugated with therapeutic or diagnostic molecules or vice versa.
Nanoimaging mediated cardiovascular theranosis is a recently introduced area. Simultaneous detection and volume reduction (thrombolysis/fibrinolysis) of thrombus is one such approach. The sole component of the thrombolytic/ fibrinolytic pathway is plasminogen, which gets converted into plasmin (serine proteinase) by plasminogen activators, i.e. tissue-type PA (tPA) and urokinasetype PA (uPA). This plasmin then degrades fibrin and different extracellular matrix proteins (fibronectin, laminin, proteoglycan, and type IV collagen), thus reducing the plaque volume102. Recombinant tissue plasminogen activator (rTPA) is now being recognized as an effective clinically used therapeutic molecule to dissolve plaque103. In this context, a nano-based theranostic approach can be conceptualised to monitor as well as to reduce plaque volume. It has been already found that iron oxide nanoparticles tagged with rTPA can efficiently dissolve clot96. A real time monitoring on thrombolytic effect has been done using nanoparticles coated with fluorophores. Therefore, diagnosis of plaque and reduction in its volume can be carried out simultaneously with the help of nanotechnology.
Another theranostic approach is detection and inhibition of angiogenesis. Angiogenesis is an important phenomenon during development of atherosclerotic plaque104. Neovascularisation is directly associated with plaque progression, risk of plaque rupture; therefore the subsequent consequence is myocardial infarction105. Integrin ανβ3 is only expressed in angiogenic vasculature, not in mature vasculature; hence can act as a marker of active angiogenesis106. To get molecular image (MRI) of angiogenesis, ultra small super paramagnetic iron oxide nanoparticle has been developed to target integrin ανβ3 receptor107. The research in this direction has further led to the detection and quantification of early angiogenesis (through MRI) by integrin ανβ3 targeted perfluorocarbon108. The ultimate nanotechnology based theranostic approach shows that fumagillin (potent angiogenic inhibitor) incorporated with paramagnetic nanoparticle not only detects early angiogenesis, but also effectively inhibits it98.
Magnetic nanoparticles tagged with near infrared fluorophores and light activated therapeutic moieties can be used to detect and destroy inflammatory macrophages in atherosclerotic plaques. Intravenous administration of these nanoparticles in murine system showed that these were readily taken up by the macrophages and killed (phototoxic effect due to activation of therapeutic moiety THPC) them after exposure at 650 nm light. It is thought to be highly effective theranosis for atherosclerosis97. All the imaging based theranostic approaches are listed in Table III.
(iii) Stem cell imaging: The most recent approach though is under in-depth investigation, shows a hope of using stem cell technology in the treatment of cardiovascular diseases109,110. Infarcted myocardium cannot be replaced spontaneously; the reason behind it is that human cardiomyocytes are post-mitotic cells; therefore cannot proliferate after birth111. Recent findings show that mesenchymal stem cells (MSCs) are the bone marrow stromal cells which can differentiate into cardiomyocytes in an appropriate condition112,113. Most excitingly, transplantation of MSCs can improve cardiac activity in patients with myocardial infarction (MI)114,115. But the proper implementation of the MSCs that are going to be transplanted in terms of fraction (%) of cells reached to the infracted myocytes is of great importance in respect to prognosis of the disease. So far, SPION are found effective markers in this regard. Super paramagnetic iron oxide nanoparticle labelled stem cell tracking and targeting has been piloted effectively in animal models with chronic MI46,116. Cellular magnetic resonance imaging is found to be convenient method for the study of SPION guided delivery of MSCs to the infarcted muscle47.
(iv) Graft imaging: Heart transplantation is the only treatment for patients with end-stage heart failure or severe coronary artery disease117. Even after heart transplantation, patients have to undergo repeated endomyocardial biopsies to see transplant graft rejection118. This procedure has significant risk, prone to sampling error and can induce fibrotic tissue build up at the site of biopsies119. A recent nanotechnology based approach has shown that fluorophore tagged iron oxide nanoparticle can efficiently diagnose this pathological condition96,97. Macrophages and cathepsin (protease) play a key role during graft rejection; therefore, these are attractive molecular imaging targets. These fluorescent conjugated magnetic nanoparticles have been used as a marker for macrophages with phagocytic activity and ProSense-680 nano-construct (fluorogenic particle) for determination of cathepsin activity50 (Table III).
Concern
With the increasing demand of nanotechnology in day to day life, one should be concerned about its negative effects also. It is already established that one of the main targets that can be affected by nanotoxicity is cardiovascular system. The general toxicity effect is mainly due to nanoparticles present in atmosphere, in fuel exhausts from car, though in some cases it has also been found that designer nanoparticles (chemically synthesised nanoparticles in laboratory) can also exert toxic effect, if not properly modified.
Peters and colleagues120 have shown that there is a consistent and clear dependence of duration of exposure to traffic with onset of myocardial infarction. The most common and ambient nanoparticles in traffic are carbon nanoparticles generated from diesel exhaust which show toxic effect on vascular cells121. Air borne particulate matter enters into our body through alveolar wall during inhalation. After penetrating the alveolar wall it comes in contact with blood and thus gets access into the cardiovascular system122 and induces cytotoxic injury, inflammation in endothelium, inhibition of celll growth, and cardiovascular toxicity123.
Apart from the carbon black nanoparticles most of the metallic nanoparticles are found to induce platelet activation and aggregation thus increase the cardiac risk124,125,126. Cosmetics with nanoparticles [titanium oxide (TiO2), silicon oxide (SiO2)] also can increase the risk of cardiac arrest by inducing plaque progression, vasodialatory dysfunction, myocardial ischaemic damage, atrio-ventricular blockage, etc.127,128. Copper oxide (CuO) nanoparticle increases the oxidative stress, and ROS generation which ultimately activates plasminogen activator inhibitor-1 expression, and increases the risk of myocardial infarction129. Nickel nanoparticles have been shown to induce atherosclerosis during long term exposure in mice model130. The known toxicity of industrial nanoforms is listed in Table IV.
Table IV.
Nanotoxicity of industrial nanoforms

The toxic effects that seem to be induced by nanoforms might not be due to the nanoscale. The adverse effects are possibly due to the corresponding ions that get adsorbed on the outer surface of bare nano-forms, during the leaching of particles, when they are in solution phase131,134 (Fig. 1). It is well known that metallic ions can induce oxidative stress in biological system135. Compared to the bare nanoparticles, ion coated nanoforms are easily taken up by cells as nanoparticles by virtue of their good penetration power136. Therefore, the effects shown are actually by the ions carried by nanoparticles. This concept is well correlated with some experimental facts, where it has been shown that surface modified nanoparticles do not show any toxicity (as leaching of ions are much less due to surface stability) compared to the bare nanoforms137,138,139,140. The carbon nanoparticle mediated toxic effect is probably due to a different mechanism. In this case, their (carbon nanoparticles) amorphous (nanotubes, or carbon black) and super hydrophobic nature is the major cause of their toxic effect141.
Fig. 1.

Schematic representation of metallic nanotoxicity mechanism. Zero valent metallic nanoparticles rarely show toxicity but the ions leaching from it.often adsorbed onto outer surface and can induce oxidative stress while permeating healthy normal cell (ions themselves cannot penetrate cell membrane). ROS, reactive oxygen species.
Conclusion and future prospects
Nanoparticles along with their own unique properties (image contrast capacity, electromagnetic properties, bio-size compatibility, etc.) can be customised for individual needs. Nano-based imaging is applicable for both diagnosis and therapy. The nano-based diagnosis covers detection of disease condition, appropriate therapy, as well as detection of post- surgery conditions (Fig. 2). Though several techniques have already been developed to make nanoparticles as a potent candidate in the cardiovascular imaging field, yet there is much more to be done (Fig. 2). One important aspect is related to the stent technology. With the advancement of technology drug eluting stents have been developed which are more potent than the bare stent. Nano-mediated drug eluting stents are more efficient than the only drug eluting stent as nanoforms can increase the half life of the drug, by sustained release. Therefore, a new nano-based technique can be conceptualised with SPION, or nanoparticle with imaging probe, in drug eluting stent that will not only slow down the drug release, but also can be monitored in real time, by imaging devices. Another important application can be in the area of stem cell delivery. Gold nanowire is a good scaffold for the delivery of stem cell in the infarcted myocardium as it has the ability to synchronize the electrical signal in the cardiac stem cells. Therefore, one can hypothesize a SPION and gold nanowire based model scaffold system that will not only synchronize the rhythm but also be monitored on a real time basis. The same holds good for the theranosis of urokinase mediated thrombus reduction. In conclusion, nanotechnology imposes a huge scope in future clinical imaging field of cardiovascular diseases.
Fig. 2.

Schematic representation of potential Nano-mediated cardiovascular imaging in diagnosis and therapy (e.g. identification of thrombus, monitoring graft rejection, stem cell tracking, and identification of angiogenesis, etc.)
Acknowledgment
The first author (SD) acknowledges the ICMR for providing ICMR post doctoral Fellowship.
References
- 1.Hardn DB, Toynbee JMC., VII The Rothschild Lycurgus Cup. Archaeologia (Second Series) 1959;97:179–212. [Google Scholar]
- 2.Freestone I, Meeks N, Sax M, Higgitt C. The Lycurgus Cup – a Roman nanotechnology. Gold Bull. 2007;40:270–7. [Google Scholar]
- 3.Bhattacharya R, Mukherjee P. Biological properties of “naked” metal nanoparticles. Ad Drug Deliv Rev. 2008;60:1289–306. doi: 10.1016/j.addr.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Arvizo RR, Bhattacharyya S, Kudgus RA, Giri K, Bhattacharya R, Mukherjee P. Intrinsic therapeutic applications of noble metal nanoparticles: past, present and future. Chem Soc Rev. 2012;41:2943–70. doi: 10.1039/c2cs15355f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faraday M. The Bakerian lecture. Experimental relations of gold (and other metals) to light. Philos Trans R Soc Lond. 1857;147:145–81. [Google Scholar]
- 6.Feynman RP. There's is plenty of room at the bottom. Eng Sci. 1960;23:22–36. [Google Scholar]
- 7.Kroto H. The 2009 Lindau Nobel Laureate Meeting: Sir Harold Kroto, Chemistry 1996. J Vis Exp. 2010;(38) doi: 10.3791/1576. pii.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Washington, DC: National Science and Technology Council; 2007. Dec, The national nanotechnology initiative strategic plan. [Google Scholar]
- 9.Washington, DC: U.S Department of Health and Human Services; 2004. Jul, National Cancer Institute. Cancer nanotechnology plan: a strategic initiative to transform clinical oncology and basic research through the directed application of nanotechnology. [Google Scholar]
- 10.Said K. Nanomedicine: A modern-day miracle. Heart Mirror J. 2012;6:11–4. [Google Scholar]
- 11.Yang F, Jin C, Subedi S, Lee CL, Wang Q, Jiang Y, et al. Emerging inorganic nanomaterials for pancreatic cancer diagnosis and treatment. Cancer Treat Rev. 2012;38:566–79. doi: 10.1016/j.ctrv.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Wilhelm S, Hirsch T, Scheucher E, Mayr T, Wolfbeis OS. Magnetic nanosensor particles in luminescence upconversion capability. Angew Chem Int Ed Engl. 2011;50:A59–62. [PubMed] [Google Scholar]
- 13.Chen H, Wang F, Li K, Woo KC, Wang J, Li Q, et al. Plasmonic percolation: Plasmon-manifested dielectric-to-metal transition. ACS Nano. 2012;6:7162–71. doi: 10.1021/nn302220y. [DOI] [PubMed] [Google Scholar]
- 14.Mokari T. Synthesis and characterization of hybrid nanostructures. Nano Rev. 2011;2 doi: 10.3402/nano.v2i0.5983. doi: 10./nano 2/nanov2i05983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porter AL, Youtie J. How interdisciplinary is nanotechnology? J Nanopart Res. 2009;11:1023–41. doi: 10.1007/s11051-009-9607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fakruddin M, Hossain Z, Afroz H. Prospects and applications of nanobiotechnology: a medical perspective. J Nano- biotechnology. 2012;10:31. doi: 10.1186/1477-3155-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Ambrosi A1, Airò F, Merkoçi A. Enhanced gold nanoparticle based ELISA for a breast cancer biomarker. Anal Chem. 2010;82:1151–6. doi: 10.1021/ac902492c. [DOI] [PubMed] [Google Scholar]
- 18.Wickline SA, Lanza GM. Nanotechnology for molecular imaging and targeted therapy. Circulation. 2003;107:1092–5. doi: 10.1161/01.cir.0000059651.17045.77. [DOI] [PubMed] [Google Scholar]
- 19.Khang D, Carpenter J, Chun YW, Pareta R, Webster TJ. Nanotechnology for regenerative medicine. Biomed Microdevices. 2010;12:575–87. doi: 10.1007/s10544-008-9264-6. [DOI] [PubMed] [Google Scholar]
- 20.Surendiran A, Sandhiya S, Pradhan SC, Adithan C. Novel applications of nanotechnology in medicine. Indian J Med Res. 2009;130:689–701. [PubMed] [Google Scholar]
- 21.Grunwald I, Rischka K, Kast SM, Scheibel T, Bargel H. Mimicking biopolymers on a molecular scale: nano(bio)technology based on engineered proteins. Philos Trans A Math Phys Eng Sci. 2009;367:1727–47. doi: 10.1098/rsta.2009.0012. [DOI] [PubMed] [Google Scholar]
- 22.Carrara S. Nano-bio-technology and sensing chips: new systems for detection in personalized therapies and cell biology. Sensors (Basel) 2010;10:526–43. doi: 10.3390/s100100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho CM, Chen JM. The convergence of bio, nano, and information technology: When Worlds Collide. IEEE Nanotechnol Mag. 2008;1:18–21. doi: 10.1109/MNANO.2007.912099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freitas RA., Jr Current status of nanomedicine and medical nanorobotics. J Compu Theo Nanoscience. 2005;2:1–25. [Google Scholar]
- 25.Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010;75:1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, et al. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano. 2008;2:889–96. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirsch LR, Gobin AM, Lowery AR, Tam F, Drezek RA, Halas NJ, et al. Metal nanoshells. Ann Biomed Eng. 2006;34:15–22. doi: 10.1007/s10439-005-9001-8. [DOI] [PubMed] [Google Scholar]
- 28.Bardhan R, Lal S, Joshi A, Halas NJ. Theranostic nanoshells: from probe design to imaging and treatment of cancer. Acc Chem Res. 2011;44:936–46. doi: 10.1021/ar200023x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lieber CM. Semiconductor nanowires: a platform for nanoscience and nanotechnology. MRS Bull. 2011;36:1052–63. doi: 10.1557/mrs.2011.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patolsky F, Zheng G, Lieber CM. Nanowire sensors for medicine and the life sciences. Nanomedicine (Lond) 2006;1:51–65. doi: 10.2217/17435889.1.1.51. [DOI] [PubMed] [Google Scholar]
- 31.Esteve-Turrillas FA, Abad-Fuentes A. Applications of quantum dots as probes in immunosensing of small-sized analytes. Biosens Bioelectron. 2013;41:12–29. doi: 10.1016/j.bios.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 32.Lu ZS, Li CM. Quantum dot-based nanocomposites for biomedical applications. Curr Med Chem. 2011;18:3516–28. doi: 10.2174/092986711796642634. [DOI] [PubMed] [Google Scholar]
- 33.Goodman G, Gershwin ME, Bercovich D. Fullerene and the origin of life. Isr Med Assoc J. 2012;14:602–6. [PubMed] [Google Scholar]
- 34.Harrison BS, Atala A. Carbon nanotube applications for tissue engineering. Biomaterials. 2007;28:344–53. doi: 10.1016/j.biomaterials.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 35.Pednekar PP, Jadhav KR, Kadam VJ. Aptamer-dendrimer bioconjugate: a nanotool for therapeutics, diagnosis, and imaging. Expert Opin Drug Deliv. 2012;9:1273–88. doi: 10.1517/17425247.2012.716421. [DOI] [PubMed] [Google Scholar]
- 36.da Fonseca Antunes AB, De Geest BG, Vervaet C, Remon JP. Solvent-free drug crystal engineering for drug nano- and micro suspensions. Eur J Pharm Sci. 2013;48:121–9. doi: 10.1016/j.ejps.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 37.Parhi R, Suresh P. Preparation and characterization of solid lipid nanoparticles-a review. Curr Drug Discov Technol. 2012;9:2–16. doi: 10.2174/157016312799304552. [DOI] [PubMed] [Google Scholar]
- 38.Rehman Z, Zuhorn IS, Hoekstra D. How cationic lipids transfer nucleic acids into cells and across cellular membranes: recent advances. J Control Release. 2013;166:46–56. doi: 10.1016/j.jconrel.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 39.Gustafson JA, Price RA, Greish K, Cappello J, Ghandehari H. Silk-elastin-like hydrogel improves the safety of adenovirus-mediated gene-directed enzyme-prodrug therapy. Mol Pharm. 2010;7:1050–6. doi: 10.1021/mp100161u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrow KJ, Jr, Bawa R, Wei C. Recent advances in basic and clinical nanomedicine. Med Clin North Am. 2007;91:805–43. doi: 10.1016/j.mcna.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Salata O. Applications of nanoparticles in biology and medicine. J Nanobiotechnology. 2004;2:3. doi: 10.1186/1477-3155-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azzazy HM, Mansour MM, Kazmierczak SC. Nanodiagnostics: a new frontier for clinical laboratory medicine. Clin Chem. 2006;52:1238–46. doi: 10.1373/clinchem.2006.066654. [DOI] [PubMed] [Google Scholar]
- 43.Patra HK, Banerjee S, Chaudhuri U, Lahiri P, Dasgupta AK. Cell selective response to gold nanoparticles. Nanomedicine. 2007;3:111–9. doi: 10.1016/j.nano.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 44.Patra HK, Dasgupta AK. Cancer cell response to nanoparticles: criticality and optimality. Nanomedicine. 2012;8:842–52. doi: 10.1016/j.nano.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Waters EA, Chen J, Yang X, Zhang H, Neumann R, Santeford A, et al. Detection of targeted perfluorocarbon nanoparticle binding using 19F diffusion weighted MR spectroscopy. Magn Reson Med. 2008;60:1232–6. doi: 10.1002/mrm.21794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jasmin, Torres AL, Jelicks L, de Carvalho AC, Spray DC, Mendez-Otero R. Labeling stem cells with superparamagnetic iron oxide nanoparticles: analysis of the labeling efficacy by microscopy and magnetic resonance imaging. Methods Mol Biol. 2012;906:239–52. doi: 10.1007/978-1-61779-953-2_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Z, Mascheri N, Dharmakumar R, Li D. Cellular magnetic resonance imaging: potential for use in assessing aspects of cardiovascular disease. Cytotherapy. 2008;10:575–86. doi: 10.1080/14653240802165699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodriguez R, Loske AM, Fernàndez F, Estevez M, Vargas S, Fernàndez G, et al. In vivo evaluation of implant-host tissue interaction using morphology-controlledhydroxyapatite-based biomaterials. J Biomater Sci Polym Ed. 2011;22:1799–810. doi: 10.1163/092050610X523674. [DOI] [PubMed] [Google Scholar]
- 49.Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev. 2004;56:1649–59. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 50.McCarthy JR. Nanomedicine and cardiovascular disease. Curr Cardiovasc Imaging Rep. 2010;3:42–9. doi: 10.1007/s12410-009-9002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chopard R, Jehl J, Dutheil J, Genon VD, Seronde MF, Kastler B, Meneveau N, et al. Evolution of acute coronary syndrome with normal coronary arteries and normal cardiac magnetic resonance imaging. Arch Cardiovasc Dis. 2011;104:509–17. doi: 10.1016/j.acvd.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 52.Stelzer R, Hutz RJ. Gold nanoparticles enter rat ovarian granulosa cells and subcellular organelles, and alter in-vitro estrogen accumulation. J Reprod Dev. 2009;55:685–90. doi: 10.1262/jrd.20241. [DOI] [PubMed] [Google Scholar]
- 53.Qi L, Wu L, Zheng S, Wang Y, Fu H, Cui D. Cell-penetrating magnetic nanoparticles for highly efficient delivery and intracellular imaging of siRNA. Biomacromolecules. 2012;13:2723–30. doi: 10.1021/bm3006903. [DOI] [PubMed] [Google Scholar]
- 54.Pouliquen D, Le Jeune JJ, Perdrisot R, Ermias A, Jallet P. Iron oxide nanoparticles for use as an MRI contrast agent: pharmacokinetics and metabolism. Magn Reson Imaging. 1991;9:275–83. doi: 10.1016/0730-725x(91)90412-f. [DOI] [PubMed] [Google Scholar]
- 55.Deb S, Raja SO, Dasgupta AK, Sarkar R, Chattopadhyay AP, Chaudhuri U, et al. Sardar P.Surface tunability of nanoparticles in modulating platelet functions. Blood Cells Mol Dis. 2012;48:36–44. doi: 10.1016/j.bcmd.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 56.Karra N, Benita S. The ligand nanoparticle conjugation approach for targeted cancer therapy. Curr Drug Metab. 2012;13:22–41. doi: 10.2174/138920012798356899. [DOI] [PubMed] [Google Scholar]
- 57.Park MH, Agasti SS, Creran B, Kim C, Rotello VM. Controlled and sustained release of drugs from dendrimer-nanoparticle composite films. Adv Mater. 2011;23:2839–42, 2843. doi: 10.1002/adma.201004409. [DOI] [PubMed] [Google Scholar]
- 58.Deb S, Dasgupta AK. Brizzio ME, editor. Thrombotic inception at nano-scale. Acute coronary Syndromes. Intech, Rijeka, Croatia. 2012. [accessed on March 15, 2015]. Available from: http://www.intechopen.com/books/acute-coronary-syndromes/thrombotic-inception-atnano-scale .
- 59.Guha S, Mookerjee S, Guha P, Sardar P, Deb S, Roy PD, et al. Antiplatelet drug resistance in patients with recurrent acute coronary syndrome undergoing conservative management. Indian Heart J. 2009;61:348–52. [PubMed] [Google Scholar]
- 60.Guha S, Sardar P, Guha P, Deb S, Karmakar R, Chakraborti P, et al. Dual antiplatelet therapy in ACS: time-dependent variability in platelet aggregation during the first week. Indian Heart J. 2009;61:173–7. [PubMed] [Google Scholar]
- 61.Guha S, Sardar P, Guha P, Roy S, Mookerjee S, Chakrabarti P, et al. Dual antiplatelet drug resistance in patients with acute coronary syndrome. Indian Heart J. 2009;61:68–73. [PubMed] [Google Scholar]
- 62.Lahiri P, Roy S, Sardar P, Deb S, Chakrabarti P, Guha P, et al. Platelet responsiveness to yohimbine hydrochloride and MRS2179 in the context of the interaction between collagen and epinephrine in acute coronary syndrome. Blood Cells Mol Dis. 2009;43:105–10. doi: 10.1016/j.bcmd.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 63.Hyafil F, Cornily JC, Rudd JH, Machac J, Feldman LJ, Fayad ZA. Quantification of inflammation within rabbit atherosclerotic plaques using the macrophage-specific CT contrast agent N1177: a comparison with 18F-FDG PET/CT and histology. J Nucl Med. 2009;50:959–65. doi: 10.2967/jnumed.108.060749. [DOI] [PubMed] [Google Scholar]
- 64.Kerwin WS, Naumova A, Storb R, Tapscott SJ, Wang Z. Mapping contrast agent uptake and retention in MRI studies of myocardial perfusion: case control study of dogs with Duchenne muscular dystrophy. Int J Cardiovasc Imaging. 2013;29:819–26. doi: 10.1007/s10554-012-0137-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arifin DR, Kedziorek DA, Fu Y, Chan KW, McMahon MT, Weiss CR, et al. Microencapsulated cell tracking. NMR Biomed. 2013;26:850–9. doi: 10.1002/nbm.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perazella MA. Gadolinium-contrast toxicity in patients with kidney disease: nephrotoxicity and nephrogenic systemic fibrosis. Curr Drug Saf. 2008;3:67–75. doi: 10.2174/157488608783333989. [DOI] [PubMed] [Google Scholar]
- 67.Cao CY, Shen YY, Wang JD, Li L, Liang GL. Controlled intracellular self-assembly of gadolinium nanoparticles as smart molecular MR contrast agents. Sci Rep. 2013;3:1024. doi: 10.1038/srep01024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shellock FG, Kanal E. Safety of magnetic resonance imaging contrast agents. J Magn Reson Imaging. 1999;10:477–84. doi: 10.1002/(sici)1522-2586(199909)10:3<477::aid-jmri33>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 69.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium(III) chelates as MRI contrast agents: Structure, dynamics, and applications. Chem Rev. 1999;99:2293–352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 70.Grobner T, Prischl FC. Gadolinium and nephrogenic systemic fibrosis. Kidney Int. 2007;72:260–4. doi: 10.1038/sj.ki.5002338. [DOI] [PubMed] [Google Scholar]
- 71.Ghaghada KB, Ravoori M, Sabapathy D, Bankson J, Kundra V, Annapragada A. New dual mode gadolinium nanoparticle contrast agent for magnetic resonance imaging. PLoS One. 2009;4:e7628. doi: 10.1371/journal.pone.0007628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Korkusuz H, Ulbrich K, Welzel K, Koeberle V, Watcharin W, Bahr U, et al. Transferrin-coated gadolinium nanoparticles as MRI contrast agent. Mol Imaging Biol. 2013;15:148–54. doi: 10.1007/s11307-012-0579-6. [DOI] [PubMed] [Google Scholar]
- 73.Flacke S, Fischer S, Scott MJ, Fuhrhop RJ, Allen JS, McLean M, et al. Novel MRI contrast agent for molecular imaging of fibrin. 4:1280–5. doi: 10.1161/hc3601.094303. [DOI] [PubMed] [Google Scholar]
- 74.Park JA, Kim HK, Kim JH, Jeong SW, Jung JC, Lee GH, et al. Gold nanoparticles functionalized by gadolinium-DTPA conjugate of cysteine as a multimodal bioimaging agent. Bioorg Med Chem Lett. 2010;20:2287–91. doi: 10.1016/j.bmcl.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 75.Wang YX. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant Imaging Med Surg. 2011;1:35–40. doi: 10.3978/j.issn.2223-4292.2011.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yilmaz A, Rösch S, Yildiz H, Klumpp S, Sechtem U. First multiparametric cardiovascular magnetic resonance study using ultrasmall superparamagnetic iron oxide nanoparticles in a patient with acute myocardial infarction: new vistas for the clinical application of ultrasmall superparamagnetic iron oxide. Circulation. 2012;126:1932–4. doi: 10.1161/CIRCULATIONAHA.112.108167. [DOI] [PubMed] [Google Scholar]
- 77.Gu J, Xu H, Han Y, Dai W, Hao W, Wang C, et al. The internalization pathway, metabolic fate and biological effect of superparamagnetic iron oxide nanoparticles in the macrophage-like RAW264.7 cell. Sci China Life Sci. 2011;54:793–805. doi: 10.1007/s11427-011-4215-5. [DOI] [PubMed] [Google Scholar]
- 78.Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010;30:1282–92. doi: 10.1161/ATVBAHA.108.179739. [DOI] [PubMed] [Google Scholar]
- 79.Waxman S, Ishibashi F, Muller JE. Detection and treatment of vulnerable plaques and vulnerable patients: novel approaches to prevention of coronary events. Circulation. 2006;114:2390–411. doi: 10.1161/CIRCULATIONAHA.105.540013. [DOI] [PubMed] [Google Scholar]
- 80.Van der Wal A C C, Becker A E. Atherosclerotic plaque rupture - pathologic basis of plaque stability and instability. Cardiovasc Res. 1999;44:334–44. doi: 10.1016/s0008-6363(98)00276-4. [DOI] [PubMed] [Google Scholar]
- 81.Martinet W, De Meyer G R Y. Selective depletion of macrophages in atherosclerotic plaques: Myth, hype, or reality? Circ Res. 2007;100:751–3. doi: 10.1161/01.RES.0000263397.14481.96. [DOI] [PubMed] [Google Scholar]
- 82.Jaffer FA, Libby P, Weissleder R. Molecular imaging of cardiovascular disease. Circulation. 2007;116:1052–61. doi: 10.1161/CIRCULATIONAHA.106.647164. [DOI] [PubMed] [Google Scholar]
- 83.Hirota K, Terada H. Ceresa B. Endocytosis of particle formulations by macrophages and its application to clinical treatment, molecular regulation of endocytosis. Biochemistry, genetics and molecular biology. CCBY. 2012. [accessed on March 25, 2015]. Available from: http://www.intechopen.com/books/molecular-regulation-of-endocytosis/endocytosis-of-particleformulations-by-macrophages-and-its-application-to-clinicaltreatment .
- 84.Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Aikawa E, et al. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379–87. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kang H W, Kim J, Yu Y, Oh J. Photoacoustic response of magnetic nanoparticles to pulsed laser irradiation. J Korean Phys Soc. 2009;55:2224–8. [Google Scholar]
- 86.Cormode DP, Roessl E, Thran A, Skajaa T, Gordon RE, Schlomka JP, et al. Atherosclerotic plaque composition: analysis with multicolor CT and targeted gold nanoparticles. Radiology. 2010;256:774–82. doi: 10.1148/radiol.10092473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Van Herck JL, De Meyer GR, Martinet W, Salgado RA, Shivalkar B, De Mondt R, et al. Multi-slice computed tomography with N1177 identifies ruptured atherosclerotic plaques in rabbits. Basic Res Cardiol. 2010;105:51–9. doi: 10.1007/s00395-009-0052-0. [DOI] [PubMed] [Google Scholar]
- 88.Majumudar MD, Yoo J, Keliher EJ, Truelove JJ, Iwamoto Y, Sena B, et al. Polymeric nanoparticle PET/MR imaging allows macrophage detection in atherosclerotic plaques. Circ Res. 2013;112:755–61. doi: 10.1161/CIRCRESAHA.111.300576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Au JT, Craig G, Longo V, Zanzonico P, Mason M, Fong Y, et al. Gold nanoparticles provide bright long-lasting vascular contrast for CT imaging. AJR Am J Roentgenol. 2013;200:1347–51. doi: 10.2214/AJR.12.8933. [DOI] [PubMed] [Google Scholar]
- 90.Kim D, Park S, Lee JH, Jeong YY, Jon S. Antibiofouling polymer-coated gold nanoparticles as a contrast agent for in vivo X-ray computed tomography imaging. J Am Chem Soc. 2007;129:7661–5. doi: 10.1021/ja071471p. [DOI] [PubMed] [Google Scholar]
- 91.Danila D, Johnson E, Kee P. CT imaging of myocardial scars with collagen-targeting gold nanoparticles. Nanomedicine. 2013;9:1067–76. doi: 10.1016/j.nano.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 92.Cormode DP, Naha PC, Fayad ZA. Nanoparticle contrast agents for computed tomography: a focus on micelles. Contrast Media Mol Imaging. 2014;9:37–52. doi: 10.1002/cmmi.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nahrendorf M, Waterman P, Thurber G, Groves K, Rajopadhye M, Panizzi P, et al. R. Hybrid in vivo FMT-CT imaging of protease activity in atherosclerosis with customized nanosensors. Arterioscler Thromb Vasc Biol. 2009;29:1444–51. doi: 10.1161/ATVBAHA.109.193086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McCarthy JR, Patel P, Botnaru I, Haghayeghi P, Weissleder R, Jaffer FA. Multimodal nanoagents for the detection of intravascular thrombi. Bioconjug Chem. 2009;20:1251–5. doi: 10.1021/bc9001163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Panizzi P, Nahrendorf M, Wildgruber M, Waterman P, Figueiredo JL, Aikawa E, et al. Oxazine conjugated nanoparticle detects in vivo hypochlorous acid and peroxynitrite generation. J Am Chem Soc. 2009;131:15739–44. doi: 10.1021/ja903922u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McCarthy JR, Sazonova IY, Erdem SS, Hara T, Thompson BD, Patel P, et al. Multifunctional nanoagent for thrombus-targeted fibrinolytic therapy. Nanomedicine (Lond) 2012;7:1017–28. doi: 10.2217/nnm.11.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Winter PM, Neubauer AM, Caruthers SD, Harris TD, Robertson JD, Williams TA, et al. Endothelial alpha(v)beta3 integrin-targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:2103–9. doi: 10.1161/01.ATV.0000235724.11299.76. [DOI] [PubMed] [Google Scholar]
- 98.McCarthy JR, Korngold E, Weissleder R, Jaffer FA. A light-activated theranostic nanoagent for targeted macrophage ablation in inflammatory atherosclerosis. Small. 2010;6:2041–9. doi: 10.1002/smll.201000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Podrez EA, Abu-Soud HM, Hazen SL. Myeloperoxidase-generated oxidants and atherosclerosis. Free Radic Biol Med. 2000;28:1717–25. doi: 10.1016/s0891-5849(00)00229-x. [DOI] [PubMed] [Google Scholar]
- 100.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xie J, Lee S, Chen X. Nanoparticle-based theranostic agents. Adv Drug Deliv Rev. 2010;62:1064–79. doi: 10.1016/j.addr.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meltzer ME, Doggen CJ, de Groot PG, Rosendaal FR, Lisman T. The impact of the fibrinolytic system on the risk of venous and arterial thrombosis. Semin Thromb Hemost. 2009;35:468–77. doi: 10.1055/s-0029-1234142. [DOI] [PubMed] [Google Scholar]
- 103.Anderson HV, Willerson JT. Thrombolysis in acute myocardial infarction. N Engl J Med. 1993;329:703–9. doi: 10.1056/NEJM199309023291006. [DOI] [PubMed] [Google Scholar]
- 104.O’Brien ER, Garvin MR, Dev R, Stewart DK, Hinohara T, Simpson JB, et al. Angiogenesis in human coronary atherosclerotic plaques. Am J Pathol. 1994;145:883–94. [PMC free article] [PubMed] [Google Scholar]
- 105.Moreno PR, Purushothaman KR, Fuster V, Echeverri D, Truszczynska H, Sharma SK, et al. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation. 2004;110:2032–8. doi: 10.1161/01.CIR.0000143233.87854.23. [DOI] [PubMed] [Google Scholar]
- 106.Sajid M, Stouffer GA. The role of alpha(v)beta3 integrins in vascular healing. Thromb Haemost. 2002;87:187–93. [PubMed] [Google Scholar]
- 107.zang Cl, Jugold M, Woenne EC, Lammers T, Morgenstern B, Mueller MM, et al. Specific targeting of tumor angiogenesis by RGD-conjugated ultrasmall superparamagnetic iron oxide particles using a clinical 1.5-T magnetic resonance scanner. Cancer Res. 2007;67:1555–62. doi: 10.1158/0008-5472.CAN-06-1668. [DOI] [PubMed] [Google Scholar]
- 108.Waters EA, Chen J, Allen JS, Zhang H, Lanza GM, Wickline SA. Detection and quantification of angiogenesis in experimental valve disease with integrin-targeted nanoparticles and 19-fluorine MRI/MRS. J Cardiovasc Magn Reson. 2008;10:43. doi: 10.1186/1532-429X-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mohyeddin-Bonab M, Mohamad-Hassani MR, Alimoghaddam K, Sanatkar M, Gasemi M, Mirkhani H, et al. Autologous in vitro expanded mesenchymal stem cell therapy for human old myocardial infarction. Arch Iran Med. 2007;10:467–73. [PubMed] [Google Scholar]
- 110.Poh KK, Sperry E, Young RG, Freyman T, Barringhaus KG, Thompson CA. Repeated direct endomyocardial transplantation of allogeneic mesenchymal stem cells: safety of a high dose, “off-the-shelf”, cellular cardiomyoplasty strategy. Int J Cardiol. 2007;117:360–4. doi: 10.1016/j.ijcard.2006.04.092. [DOI] [PubMed] [Google Scholar]
- 111.Zhang Y, Li TS, Lee ST, Wawrowsky KA, Cheng K, Galang G, et al. Dedifferentiation and proliferation of mammalian cardiomyocytes. PLoS One. 2010;5:e12559. doi: 10.1371/journal.pone.0012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro . J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–8. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 114.Chen SL, Fang WW, Qian J, Ye F, Liu YH, Shan SJ, et al. Improvement of cardiac function after transplantation of autologous bone marrow mesenchymal stem cells in patients with acute myocardial infarction. Chin Med J (Engl) 2004;117:1443–8. [PubMed] [Google Scholar]
- 115.Lu M, Zhao S, Liu Q, Jiang S, Song P, Qian H, et al. Transplantation with autologous mesenchymal stem cells after acute myocardial infarction evaluated by magnetic resonance imaging: an experimental study. J Thorac Imaging. 2012;27:125–35. doi: 10.1097/RTI.0b013e31820446fa. [DOI] [PubMed] [Google Scholar]
- 116.Kedziorek DA, Kraitchman DL. Superparamagnetic iron oxide labeling of stem cells for MRI tracking and delivery in cardiovascular disease. Methods Mol Biol. 2010;660:171–83. doi: 10.1007/978-1-60761-705-1_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Boilson BA, Raichlin E, Park SJ, Kushwaha SS. Device therapy and cardiac transplantation for end-stage heart failure. Curr Probl Cardiol. 2010;35:8–64. doi: 10.1016/j.cpcardiol.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 118.Bhargava H, Donner RM, Sanchez G, Dunn JM, Zaeri N, Brickley S, Cavarocchi N. Endomyocardial biopsy after heart transplantation in children. J Heart Transplant. 1987;6:298–302. [PubMed] [Google Scholar]
- 119.Calé R, Almeida M, Gonçalves P, Rebocho MJ, Raposo L, Teles R, et al. Complications of endomyocardial biopsy after heart transplantation: a lesser evil. Rev Port Cardiol. 2012;31:159–62. doi: 10.1016/j.repc.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 120.Peters A, von Klot S, Heier M, Trentinaglia I, Hörmann A, Wichmann HE, et al. Cooperative health research in the Region of Augsburg Study Group. Exposure to traffic and the onset of myocardial infarction. N Engl J Med. 2004;351:1721–30. doi: 10.1056/NEJMoa040203. [DOI] [PubMed] [Google Scholar]
- 121.Mills NL, Miller MR, Lucking AJ, Beveridge J, Flint L, Boere AJ, et al. Combustion-derived nanoparticulate induces the adverse vascular effects of diesel exhaust inhalation. Eur Heart J. 2011;32:2660–71. doi: 10.1093/eurheartj/ehr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shimada A, Kawamura N, Okajima M, Kaewamatawong T, Inoue H, Morita T. Translocation pathway of the intratracheally instilled ultrafine particles from the lung into the blood circulation in the mouse. Toxicol Pathol. 2006;34:949–57. doi: 10.1080/01926230601080502. [DOI] [PubMed] [Google Scholar]
- 123.Yamawaki H, Iwai N. Mechanisms underlying nano-sized air-pollution-mediated progression of atherosclerosis: carbon black causes cytotoxic injury/inflammation and inhibits cell growth in vascular endothelial cells. Circ J. 2006;70:129–40. doi: 10.1253/circj.70.129. [DOI] [PubMed] [Google Scholar]
- 124.Deb S, Patra HK, Lahiri P, Dasgupta AK, Chakrabarti K, Chaudhuri U. Multistability in platelets and their response to gold nanoparticles. Nanomedicine. 2011;7:376–84. doi: 10.1016/j.nano.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 125.Deb S, Chatterjee M, Bhattacharya J, Lahiri P, Chaudhuri U, Pal Choudhuri S, et al. Role of purinergic receptors in platelet-nanoparticle interactions. Nanotoxicology. 2007;1:93–103. [Google Scholar]
- 126.Deb S, Raja SO, Dasgupta AK, Sarkar R, Chattopadhyay AP, Chaudhuri U, et al. Sardar P.Surface tunability of nanoparticles in modulating platelet functions. Blood Cells Mol Dis. 2012;48:36–44. doi: 10.1016/j.bcmd.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 127.Mikkelsen L, Sheykhzade M, Jensen KA, Saber AT, Jacobsen NR, Vogel U, et al. Modest effect on plaque progression and vasodilatory function in atherosclerosis-prone mice exposed to nanosized TiO(2) Part Fibre Toxicol. 2011;8:32. doi: 10.1186/1743-8977-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen Z, Meng H, Xing G, Yuan H, Zhao F, Liu R. Age-related differences in pulmonary and cardiovascular responses to SiO2 nanoparticle inhalation: nanotoxicity has susceptible population. Environ Sci Technol. 2008;42:8985–92. doi: 10.1021/es800975u. [DOI] [PubMed] [Google Scholar]
- 129.Yu M, Mo Y, Wan R, Chien S, Zhang X, Zhang Q. Regulation of plasminogen activator inhibitor-1 expression in endothelial cells with exposure to metal nanoparticles. Toxicol Lett. 2010;195:82–9. doi: 10.1016/j.toxlet.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kang GS, Gillespie PA, Gunnison A, Moreira AL, Tchou-Wong KM, Chen LC. Long-term inhalation exposure to nickel nanoparticles exacerbated atherosclerosis in a susceptible mouse model. Environ Health Perspect. 2011;119:176–81. doi: 10.1289/ehp.1002508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jun EA, Lim KM, Kim K, Bae ON, Noh JY, Chung KH, Chung JH. Silver nanoparticles enhance thrombus formation through increased platelet aggregation and procoagulant activity. Nanotoxicology. 2011;5:157–67. doi: 10.3109/17435390.2010.506250. [DOI] [PubMed] [Google Scholar]
- 132.Geys J, Nemmar A, Verbeken E, Smolders E, Ratoi M, Hoylaerts MF, et al. Acute toxicity and prothrombotic effects of quantum dots: impact of surface charge. Environ Health Perspect. 2008;116:1607–13. doi: 10.1289/ehp.11566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Radomski A, Jurasz P, Alonso-Escolano D, Drews M, Morandi M, Malinski T, et al. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br J Pharmacol. 2005;146:882–93. doi: 10.1038/sj.bjp.0706386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Reijnders L. The release of TiO 2 and SiO 2 nanoparticles from nanocomposites. Polym Degrad Stabi. 2009;94:873–6. [Google Scholar]
- 135.Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 136.Baroli B, Ennas MG, Loffredo F, Isola M, Pinna R, López-Quintela MA. Penetration of metallic nanoparticles in human full-thickness skin. J Invest Dermatol. 2007;127:1701–12. doi: 10.1038/sj.jid.5700733. [DOI] [PubMed] [Google Scholar]
- 137.Mano SS, Kanehira K, Sonezaki S, Taniguchi A. Effect of Polyethylene Glycol Modification of TiO2 Nanoparticles on Cytotoxicity and Gene Expressions in Human Cell Lines. Int J Mol Sci. 2012;13:3703–17. doi: 10.3390/ijms13033703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Phenrat T, Long TC, Lowry GV, Veronesi B. Partial oxidation (“aging”) and surface modification decrease the toxicity of nanosized zerovalent iron. Environ Sci Technol. 2009;43:195–200. doi: 10.1021/es801955n. [DOI] [PubMed] [Google Scholar]
- 139.Takahashi H, Niidome Y, Niidome T, Kaneko K, Kawasaki H, Yamada S. Modification of gold nanorods using phosphatidylcholine to reduce cytotoxicity. Langmuir. 2006;22:2–5. doi: 10.1021/la0520029. [DOI] [PubMed] [Google Scholar]
- 140.Iijima M, Kamiya H. Surface modification for improving the stability of nanoparticles in liquid media. KONA Powder Parti J. 2009;27:119–29. [Google Scholar]
- 141.Yan L, Zhao F, Li S, Hu Z, Zhao Y. Low-toxic and safe nanomaterials by surface-chemical design, carbon nanotubes, fullerenes, metallofullerenes, and graphenes. Nanoscale. 2011;3:362–82. doi: 10.1039/c0nr00647e. [DOI] [PubMed] [Google Scholar]


