Abstract
Background & objectives:
Early neurological deterioration (END) occurs in about 20 to 40 per cent of patients with acute ischaemic stroke and results in increased mortality and functional disability. In recent studies relative dehydration has been found to be associated with END in patients with acute ischaemic stroke. This study was conducted to identify factors useful for predicting END and to assess the role of blood urea nitrogen/creatinine ratio (BUN/creatinine) and urine specific gravity (USG) as predictors of END in patients with acute ischaemic stroke.
Methods:
The present study was an observational prospective study. Various parameters comprising demographic, clinical, laboratory and radiological variables along with stroke severity were assessed and studied as predictors of early neurological deterioration in 114 consecutive patients presenting to the Emergency department during 2012. BUN/creatinine >15 and USG >1.010 were studied as markers of relative dehydration contributing to END.
Results:
Of the 114 patients enrolled in the study, END was observed in 25 (21.9%) patients. National Institutes Health Stroke Scale score (NIHSS) ≥ 12 at admission was found to be an independent risk factor for END. Amongst markers of relative dehydration, BUN/creatinine >15 at admission was found to be an independent risk factor for END, as also USG >1.010. Also, cerebral oedema and size of hypodensity >1/3rd of the middle cerebral artery territory on cranial CT were observed to be independent risk factors for END.
Interpretation & conclusions:
Our study findings highlighted a possible association of relative dehydration, as indicated by BUN/creatinine ratio >15, with END along with other parameters like stroke severity at presentation, extent of hypodensity >1/3rd of the middle cerebral artery (MCA) territory and cerebral oedema. Dehydration being a treatable condition, the use of BUN/creatinine >15 as a marker of relative dehydration, can be helpful in detecting patients with dehydration early and thus play a role in preventing END.
Keywords: Blood urea nitrogen/creatinine, early neurological deterioration, ischaemic stroke, predictors, urine specific gravity
Stroke is a major cause of long-term disability among patients and has enormous emotional and socio-economic consequences1,2. In 20-40 per cent of patients with acute ischaemic stroke, neurological symptoms progress during the initial hours, resulting in increased mortality3,4. Factors that have been reported to be predictors of early neurological deterioration (END) include clinical parameters like stroke severity at presentation [high initial National Institutes of Health Stroke Scale (NIHSS) score5,6, low initial Canadian Stroke Severity score]7, medical history of diabetes mellitus8, blood pressure both elevated and decreased9,10,11, body temperature7, and laboratory tests like markers of coagulation7,9,10, markers of inflammation7,12, and serum glucose at admission7,10. Also, early computerized tomography (CT) findings of stroke severity7,8,13 and changes in cerebral blood flow affecting the ischaemic penumbra14,15 have been identified as predictors of early deterioration.
Patients with stroke are often at increased risk of dehydration as they have a reduced level of consciousness, are physically dependent, unable to communicate, have difficulties in swallowing and decreased oral intake. Rodriguez et al16 have demonstrated that elderly patients presenting with transient ischaemic attack or acute ischaemic stroke often demonstrate increased plasma osmolality that likely represents a fluid depleted state, and possibly contributes to cerebral ischaemia and worse neurological outcome in stroke patients. The role of volume depletion or dehydration as a risk factor contributing to END was further studied by Lin et al17,18 who demonstrated that blood urea nitrogen/creatinine (BUN/Cr) ratio higher than 15 and urine specific gravity (USG) > 1.010 were more frequently seen in SIE (Stroke in Evolution) patients.
Early identification of dehydration is essential for timely intervention to improve outcome. Unfortunately, the clinical assessment of dehydration by physicians is not always accurate especially in geriatric patients. Hence, biochemical parameters like plasma osmolality, BUN/creatinine ratio and urine specific gravity have been used by various investigators for assessment of hydration status16,17,18,19,20,21, but the results have been inconsistent.
The present study was designed to determine predictors of END in patients with acute ischaemic stroke with emphasis on blood urea nitrogen/creatinine ratio and urine specific gravity that could be readily assessed in an Emergency department (ED) setting and potentially help physicians identify high risk stroke patients.
Material & Methods
The study was conducted in the Department of Medicine at Vardhman Mahavir Medical College & Safdarjung Hospital, New Delhi, India, from January to December, 2012. It was an observational prospective study in which 114 consecutive patients presenting to the Emergency department and after fulfilling the inclusion and exclusion criteria were includedin the study. Inclusion criteria included patients >18 yr presenting withinfirst 24 h after symptom onset withfirst episode of acute ischaemic stroke. Exclusion criteria included a previous episode of acute ischaemic stroke, time between the onset of neurologic symptoms and emergency department (ED) presentation more than 24 h, evidence of haemorrhagic stroke, patients with transient ischaemic attack and patients with other co-morbid conditions like congestive cardiac failure (CCF), renal failure and decompensated cirrhosis of liver. A written informed consent was obtained from the patients or their relatives and the study protocol was approved by the institution's ethics committee.
A detailed history was taken and a thorough general physical and systemic examination was performed. The following details were collected: age; sex; signs and symptoms; Glasgow coma scale (GCS), history of diabetes, hypertension, coronary artery disease, dyslipidaemia, diuretic use and smoking habits. Laboratory investigations comprising complete blood count with haematocrit, blood urea nitrogen (BUN), serum creatinine, BUN/creatinine ratio, serum electrolytes, blood glucose at admission, plasma osmolality (calculated), serum bilirubin, lipid profile, glycated haemoglobin (HbA1C), d-dimer (qualitative assay), C-reactive protein (CRP, qualitative assay), urine specific gravity, non-contrast CT (NCCT) scan of head and electrocardiogram were performed within 24 h of admission in all the patients. Parameters like urine specific gravity, BUN, creatinine and BUN/creatinine ratio were assessed at the time of admission and then subsequently on days 1, 2 and 3. The remaining parameters were assessed only once within 24 h of admission.
Quantitative determination of BUN was done on SYNCHRON® LX20 Systems (Beckman Coulter, Fullerton, USA) using the BUN reagent kit (Beckman Coulter, Inc., USA). The analytical coefficient of variation (CV) for estimating BUN was 24.8 per cent. Serum creatinine was determined using the Autopak® reagent kit (Siemens Ltd., India) which utilizes the alkaline picrate Jaffe's reaction method. The analytical CV for measuring creatinine was 21.1 per cent.
Urine specific gravity (USG) was determined using SD Urocolor™ 10 Reagent strips (Standard Diagnostics, Inc., Korea). These reagent strips are used for rapid bedside urinalysis. Clean catch and freshly voided urine specimens were collected at admission and subsequently on days 1, 2 and 3 from the patients. The Urocolor 10 reagent strips have specific-gravity scale ranging from 1.000 to 1.030, with colour blocks in intervals of 0.005 units.
All patients underwent a NCCT scan of brain within six hours of admission in the ED. A repeat NCCT head was performed after 24 h of admission in patients with signs and symptoms suggestive of acute stroke but with a normal NCCT of brain on initial assessment. The NCCT findings were interpreted by a radiologist and the following features were recorded: site of the territory (lacunar infarct, anterior cerebral artery, middle cerebral artery, posterior cerebral artery infarct); size of hypodensity [> 1/3rd or <1/3rd of middle cerebral artery (MCA) territory involvement; mid line shift; cerebral oedema; hyperdense MCA (HMCA)] sign. Volume of infarct was measured using the formula ABC/2; where A=longest diameter of the infarct, B=largest diameter perpendicular to the first at the widest dimension, C= number of axial slices the abnormality appears on, multiplied by the slice thickness. A lesion measuring > 100 cm3 roughly correlated with size of hypodensity >1/3rd of the MCA territory involvement.
The neurological status of the patient and the severity of stroke were assessed by using the NIHSS scoring system22. Evaluations were performed immediately at the time of admission, and then subsequently on days 1, 2, 3 and at the time of discharge. Patients for whom the NIHSS score returned to zero within the initial 24 h were classified as having a transient ischaemic attack (TIA) and were excluded from the study.
Early neurological deterioration was defined as worsening of neurological condition as indicated by an increase of three or more points in the NIHSS score or death not attributed to other cause, within the first three days. An increase of three or more points in the NIHSS score was used to diagnose END. Patients were divided into two groups. The first group included patients with END and the second group included those without END. Demographic characteristics, clinical characteristics, NIHSS score (≥12 or <12), GCS score (≤12 or >12), urine specific gravity (>1.010 or ≤1.010), BUN/Cr ratio (>15 or ≤15) and other variables were compared between patients diagnosed as having stroke with END and those without END.
Statistical analysis: The data were compiled and analyzed using standard statistical methods and relevant conclusions were drawn using a computer based software SPSS version 16.0. Continuous data were expressed as mean ± standard deviation (SD) and were compared using Student t test for normally distributed variables and Mann Whitney U test for non-parametric data. Categorical data were expressed as frequencies and percentages, and were compared using chi-square test and Fisher's Exact test, wherever applicable. Bivariate and multivariable analyses were done by logistic regression to determine factors related to END. Variables found associated with END by bivariate logistic regression analysis were entered into multivariable logistic regression models to control for the potential confounding variables. The correlation between variables was ascertained using Pearson's correlation coefficient for normally distributed data and Spearman's coefficient for non-parametric data. P< 0.05 was considered significant.
Results
A total of 114 patients with acute ischaemic stroke participated in the study and END was seen in 25 patients (21.9%). The mean age of the patients with END was 56.7 ± 10.6 yr as compared to 57.6 ± 9.9 yr in patients without END; 64 per cent of the patients with END were males. No significant association was seen between age, sex and END. Detailed history taking revealed the presence of various risk factors like hypertension (n=58, 51%), diabetes (n=33, 29%), smoking (n=19, 16.7%) and past history of transient ischaemic attack (n=4, 3.5%), in the study population. However, no significant association was seen between the various risk factors and END. Similarly, mean systolic and diastolic blood pressures (SBP and DBP) were not different between the two END categories. Table I summarizes the clinical and demographic characteristics of the patients.
Table I.
Clinical and demographic characteristics of patients enrolled in the study
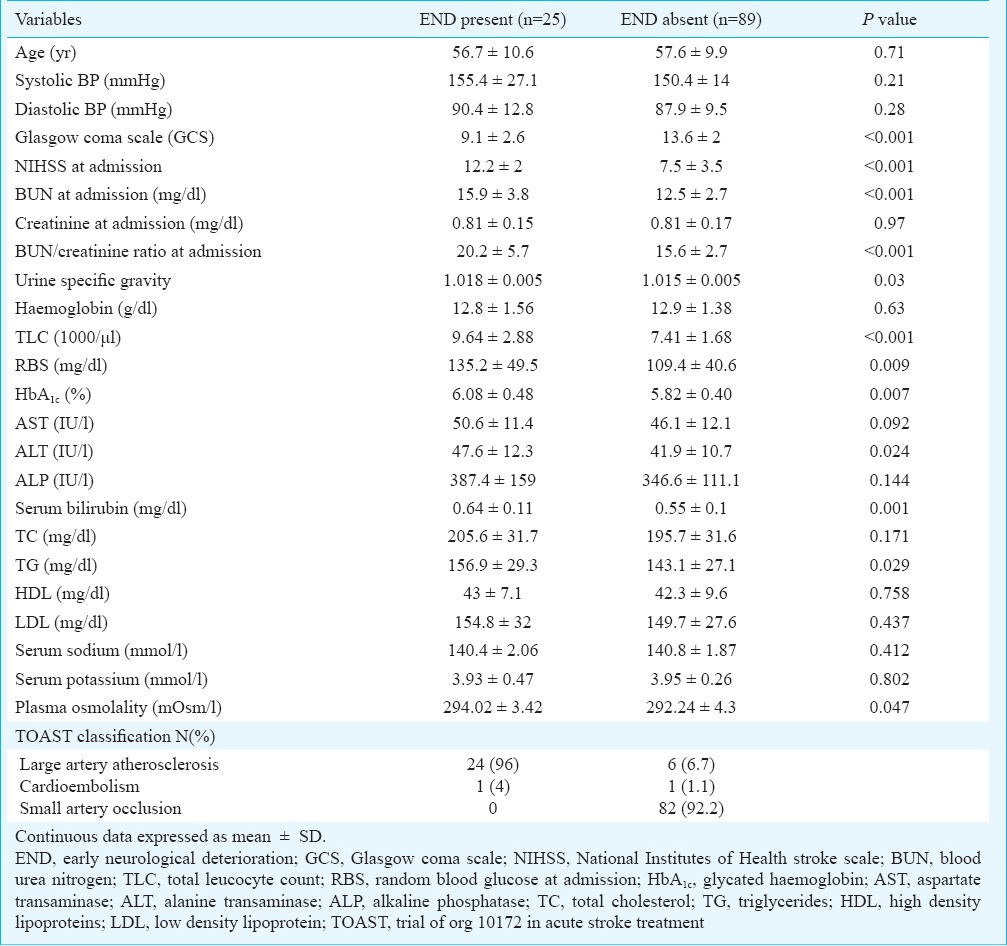
A higher stroke severity at admission, as measured by assessing NIHSS and GCS scores, was observed to be a significant risk factor for END. There was a persistent rise in the median values of NIHSS scores in patients with END. The percentage of patients with a pre-specified NIHSS score of 12 or more at admission was also higher in the END group (n=19, 76%) as compared to patients without END (n=22, 22.4%) (p<0.001). Similarly, (n=20, 80%) of the patients with END had a GCS score of ≤ 12 at admission as compared to (n=22, 24.7%) of the patients without END (p<0.001).
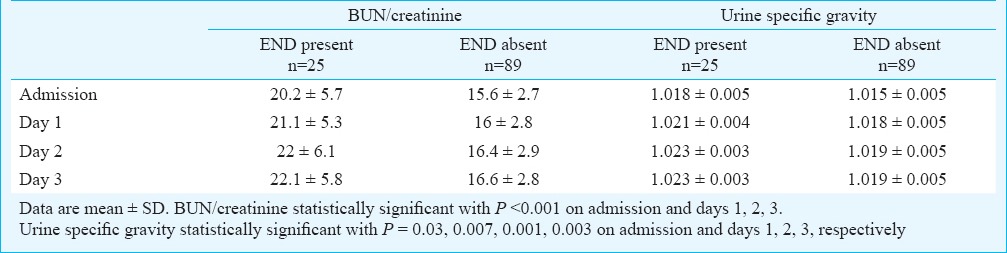
There was a significant difference (p<0.001) in the mean values of BUN/creatinine ratio at admission in patients with and without END. A significantly higher proportion of patients with END (n=18, 72%) had BUN/creatinine >15 as compared to (n=27, 30.3%) of the patients without END (p<0.001). A significant association was seen between USG >1.010 and END (p=0.009), as (n=20, 80%) of the patients with END had a USG >1.010 as compared to 44 (49.4%) patients without END. A weak positive correlation was found between BUN/creatinine and NIHSS score at admission (r = 0.305, P = 0.001). A persistent rise in the mean values of BUN/creatinine ratio and urine specific gravity was observed in patients with early neurological deterioration compared to those without END (Table II).
Table II.
Comparison of mean values of BUN/creatinine and urine specific gravity in patients with and without early neurological deterioration
A significantly higher proportion of patients with END (n=21, 84%) had positive CRP as compared to 56 (62.9%) patients without END (p<0.05). Similarly, 20 per cent (n=5) of the patients with END had positive d-dimer as compared to all the patients without END who had a negative d-dimer test result (p<0.001). A significant difference was seen between the mean values of blood glucose at admission in patients with and without END (p<0.001). Similar results were observed for HbA1c as a predictor of END. The mean value was higher in the group with END (p=0.007). A significant difference was also found in plasma osmolality between the two END groups (p=0.047).
All patients with END (100%) were observed to have involvement of MCA as compared to 66.3 per cent (n=59) patients without END. Approximately 80 per cent (n=20) of the patients with END had size of hypodensity >1/3rd of MCA territory as compared to 6.7 per cent (n=6) of the patients without END (p<0.001). Cerebral oedema was observed in 64 per cent (n=16) of the patients with END as compared to 10.1 per cent (n=9) of the patients without END (p<0.001). The presence of mid-line shift was seen in 28 per cent (n=7) of patients with END and was absent in the majority of patients without END (n=86, 96.6%) (p<0.001). None of the patients without END had presence of HMCA sign on NCCT head as compared to 20 per cent (n=5) patients with END who demonstrated the HMCA sign.
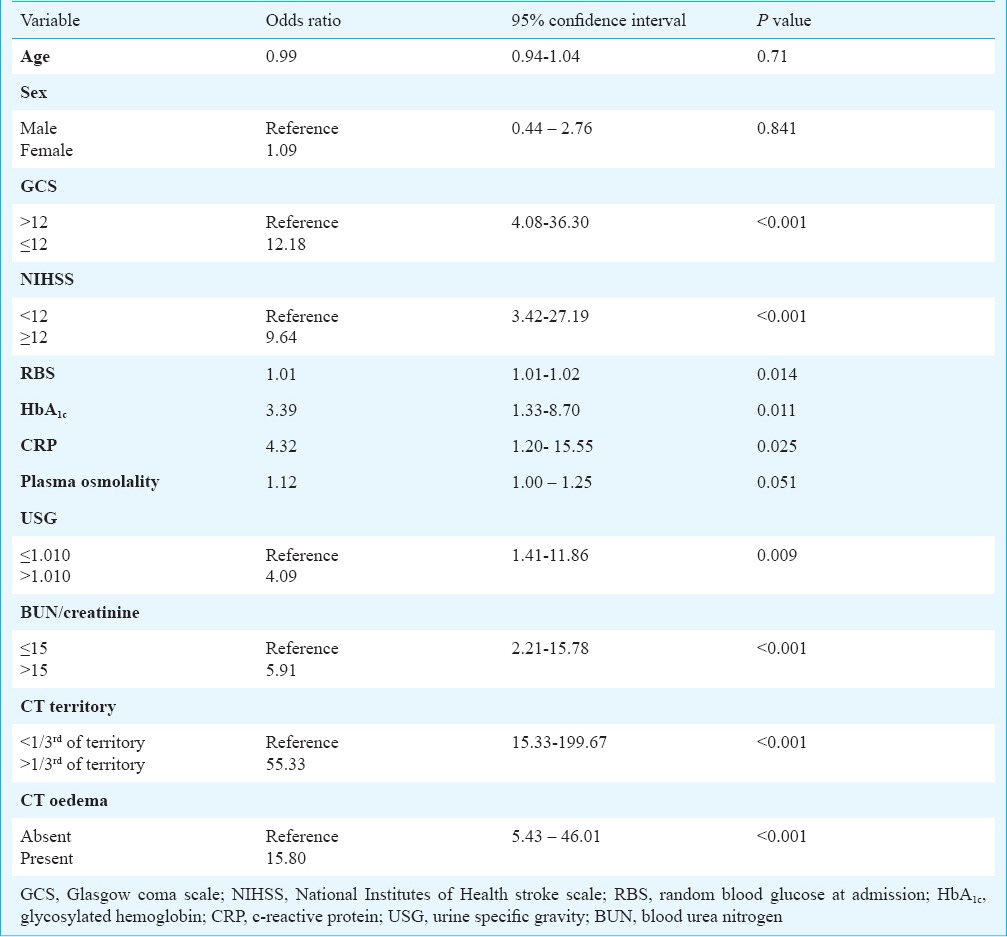
Bivariate logistic regression analysis was used to test for the factors potentially associated with END (Table III). Patients with a NIHSS score ≥12 at admission were 9.64 times more likely to develop END as compared to patients with NIHSS score <12. Similarly, patients with a GCS score ≤ 12 at admission who were 12.18 times more likely to develop END compared to those with GCS score >12. In addition, patients with BUN/creatinine >15 were 5.91 times more likely to develop END (p<0.001), whereas, patients with USG >1.010 were found to be 4.09 times more likely to develop END (p=0.009). High levels of blood glucose and glycosylated haemoglobin at admission were also found to be risk factors for END. The presence of cerebral oedema, mid line shift and size of hypodensity >1/3rd of the MCA territory on NCCT imaging of head were found to be significant risk factors for END on bivariate regression analysis.
Table III.
Bivariate logistic regression analysis of factors associated with early neurological deterioration
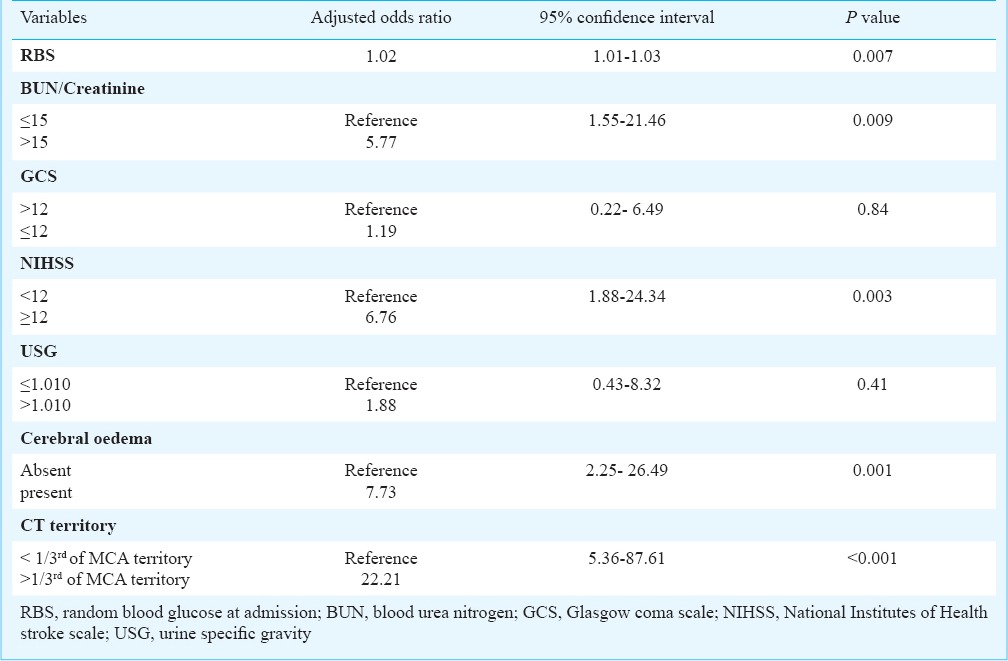
On multiple logistic regression analysis random blood glucose at admission, BUN/creatinine >15, NIHSS score ≥12 at admission, cerebral oedema on NCCT head and hypodensity >1/3rd of the MCA territory involved, were found to be independent risk factors for predicting END (Table IV).
Table IV.
Multivariable logistic regression analysis for possible factors associated with early neurological deterioration
Discussion
In our study, various clinical, biochemical and radiological parameters were analyzed as risk factors for END. The END was observed in 21.9 per cent patient and no significant association was found between age, sex and END in our study. These findings are similar to that of previously published studies5,8,13. High blood glucose levels at admission was found to be an independent predictor of END in our study, as reported earlier4,5,13. The detrimental effects of hyperglycaemia can be attributed to tissue acidosis secondary to anaerobic glycolysis, lactic acidosis, free radical production, disruption of the blood–brain barrier and the development of brain oedema23,24,25.
An increased stroke severity at presentation, as assessed by NIHSS and GCS scores, was observed in patients with END. However, only NIHSS ≥12 at presentation was found to be an independent risk factor predictive of END. Various studies have found a similar association between high initial stroke severity and END, and thus support our findings7,13,17,26 Lin et al17 also studied NIHSS ≥ 12 and GCS ≤ 12 as predictors of END. However, NIHSS ≥ 12 and GCS ≤ 12 were not found to be independent predictors of END on multivariable logistic regression analysis in their study. DeGraba et al6 took an initial threshold NIHSS score >7 as a risk factor for neurological worsening. It was observed that patients with an initial NIHSS of ≤ 7 experienced a 14.8 per cent worsening rate versus those with a score of > 7 with a 65.9 per cent worsening rate. Such a difference in findings can be possibly explained by a higher mean NIHSS score at admission (12.2 ± 2) in patients with END in our study.
Although BUN/creatinine >15 and urine specific gravity >1.010 have not been studied widely as predictors of END, BUN/creatinine >15 was found as an independent factor predictive of END is our study. As BUN/creatinine >15 is considered to be indicative of relative dehydration19, our findings support the role of volume depletion or relative dehydration as a risk factor contributing to END. Previous studies in stroke patients have elicited that dehydration after stroke is associated with increased BUN/creatinine ratio, decreased blood pressure, increase in blood viscosity, reduced circulation in collaterals and predisposition for venous thromboembolism; which worsens the ischaemic process and increases the risk of END22,27,28. Although urine specific gravity is also an indicator of hydration status of a patient, we did not observe USG >1.010 as an independent risk factor for END. This finding can partially be explained by the use of urine dipsticks for measuring urine specific gravity, which may not be a very reliable method. In a pilot study by Rowat et al21 involving 20 acute stroke patients, urine dipstick underestimated urine specific gravity as compared to refractometry. Also, change in urine specific gravity does not always precede a change in BUN/creatinine ratio, thereby indicating that urine specific gravity may not be a warning sign for dehydration always.
Though a significantly higher value of plasma osmolality was recorded in patients with END in our study, but plasma osmolality was not found to be an independent risk factor for END on logistic regression analysis. This was in contrast to the findings of the THIRST study16 and a study by Bhalla et al20 in which a correlation between plasma osmolality levels and worse outcome in stroke patients was reported.
Amongst radiological parameters, cerebral oedema and size of hypodensity >1/3rd of the MCA territory on NCCT head were found to be independent risk factors for END. In the European Cooperative Acute Stroke Study (ECASS) I8, size of infarct involving > 33 per cent of the MCA territory and brain swelling were found to be the only factors independently associated with early neurological worsening in the final logistic model.
One of the major limitations of our study was a small sample size, as only 114 patients with acute ischaemic stroke, were analyzed. In addition, urine specific gravity was estimated using urine reagent strips, which often underestimates urine specific gravity as compared to urine refractometry.
In conclusion, our findings suggested that a BUN/Cr ratio > 15 at admission was an independent risk factor for END in patients with acute ischaemic stroke. Dehydration being a treatable condition, the use of BUN/creatinine >15 as a marker of relative dehydration, can be helpful in detecting patients with dehydration early and thus may play a role in preventing neurological worsening. Hence, monitoring of the hydration status needs to be emphasized in the management of ischaemic stroke patients. Also, further studies are required to investigate whether acute correction of dehydration in such patients would play a role in preventing END and hence, improved outcomes.
References
- 1.Foulkes MA, Wolf PA, Price TR, Mohr JP, Hier DB. The stroke data bank: design, methods, and baseline characteristics. Stroke. 1988;19:547–54. doi: 10.1161/01.str.19.5.547. [DOI] [PubMed] [Google Scholar]
- 2.Shah B, Mathur P. Stroke surveillance in India. New Delhi, India. New Delhi: ICMR; 2006. Nov 13-15, Indian Council of Medical Research (ICMR). Workshop report prepared by. [Google Scholar]
- 3.Da ìvalos A, Castillo J. Current review of cerebrovascular disease. In: Fisher M, Bogousslavsky J, editors. Philadelphia: Current Medicine Inc; 1999. pp. 149–60. [Google Scholar]
- 4.Da ìvalos A, Cendra E, Teruel J, Martinez M, Genis D. Deteriorating ischemic stroke: risk factors and prognosis. Neurology. 1990;40:1865–9. doi: 10.1212/wnl.40.12.1865. [DOI] [PubMed] [Google Scholar]
- 5.Roquer J, Rodriguez-Campello A, Gomis M, Jiménez-Conde J, Cuadrado-Godia E, Vivanco R, et al. Acute stroke unit care and early neurological deterioration in ischemic stroke. J Neurol. 2008;255:1012–7. doi: 10.1007/s00415-008-0820-z. [DOI] [PubMed] [Google Scholar]
- 6.DeGraba TJ, Hallenbeck JM, Pettigrew KD, Dutka AJ, Kelly BJ. Progression in acute stroke: value of the initial NIH stroke scale score on patient stratification in future trials. Stroke. 1999;30:1208–12. doi: 10.1161/01.str.30.6.1208. [DOI] [PubMed] [Google Scholar]
- 7.Vila N, Castillo J, Dávalos A, Chamorro A. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke. 2000;31:2325–9. doi: 10.1161/01.str.31.10.2325. [DOI] [PubMed] [Google Scholar]
- 8.Dávalos A, Toni D, Iweins F, Lesaffre E, Bastianello S, Castillo J. Neurological deterioration in acute ischemic stroke: potential predictors and associated factors in the European cooperative acute stroke study (ECASS) I. Stroke. 1999;30:2631–6. doi: 10.1161/01.str.30.12.2631. [DOI] [PubMed] [Google Scholar]
- 9.Barber M, Langhorne P, Rumley A, Lowe GD, Stott DJ. D-dimer predicts early clinical progression in ischemic stroke: confirmation using routine clinical assays. Stroke. 2006;37:1113–5. doi: 10.1161/01.STR.0000209240.63821.1a. [DOI] [PubMed] [Google Scholar]
- 10.Barber M, Langhorne P, Rumley A, Lowe GD, Stott DJ. Hemostatic function and progressing ischemic stroke: D-dimer predicts early clinical progression. Stroke. 2004;35:1421–5. doi: 10.1161/01.STR.0000126890.63512.41. [DOI] [PubMed] [Google Scholar]
- 11.Stead LG, Gilmore RM, Decker WW, Weaver AL, Brown RD., Jr Initial emergency department blood pressure as predictor of survival after acute ischemic stroke. Neurology. 2005;65:1179–83. doi: 10.1212/01.wnl.0000180939.24845.22. [DOI] [PubMed] [Google Scholar]
- 12.Vila N, Castillo J, Dávalos A, Esteve A, Planas AM, Chamorro A. Levels of anti-inflammatory cytokines and neurological worsening in acute ischemic stroke. Stroke. 2003;34:671–5. doi: 10.1161/01.STR.0000057976.53301.69. [DOI] [PubMed] [Google Scholar]
- 13.Toni D, Fiorelli M, Gentile M, Bastianello S, Sacchetti ML, Argentino C, et al. Progressing neurological deficit secondary to acute ischemic stroke: a study on predictability, pathogenesis, and prognosis. Arch Neurol. 1995;52:670–5. doi: 10.1001/archneur.1995.00540310040014. [DOI] [PubMed] [Google Scholar]
- 14.Fisher M, Garcia JH. Evolving stroke and the ischemic penumbra. Neurology. 1996;47:884–8. doi: 10.1212/wnl.47.4.884. [DOI] [PubMed] [Google Scholar]
- 15.Mayer SA, Lignelli A, Fink ME, Kessler DB, Thomas CE, Swarup R, et al. Perilesional blood flow and edema formation in acute intracerebral hemorrhage: a SPECT study. Stroke. 1998;29:1791–8. doi: 10.1161/01.str.29.9.1791. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez GJ, Cordina SM, Vazquez G, Suri MF, Kirmani JF, Ezzeddine MA, et al. The Hydration Influence on the Risk of Stroke (THIRST) study. Neurocrit Care. 2009;10:187–94. doi: 10.1007/s12028-008-9169-5. [DOI] [PubMed] [Google Scholar]
- 17.Lin LC, Yang JT, Weng HH, Hsiao CT, Lai SL, Fann WC. Predictors of early clinical deterioration after acute ischemic stroke. Am J Emerg Med. 2011;29:577–81. doi: 10.1016/j.ajem.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 18.Lin LC, Fann WC, Chou MH, Chen HW, Su YC, Chen JC. Urine specific gravity as a predictor of early neurological deterioration in acute ischemic stroke. Med Hypotheses. 2011;77:11–4. doi: 10.1016/j.mehy.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Schrock JW, Glasenapp M, Drogell K. Elevated blood urea nitrogen/creatinine ratio is associated with poor outcome in patients with ischemic stroke. Clin Neurol Neurosurg. 2012;114:881–4. doi: 10.1016/j.clineuro.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 20.Bhalla A, Sankaralingam S, Dundas R, Swaminathan R, Wolfe CD, Rudd AG. Influence of raised plasma osmolality on clinical outcome after acute stroke. Stroke. 2000;31:2043–8. doi: 10.1161/01.str.31.9.2043. [DOI] [PubMed] [Google Scholar]
- 21.Rowat A, Smith L, Graham C, Lyle D, Horsburgh D, Dennis M. A pilot study to assess if urine specific gravity and urine colour charts are useful indicators of dehydration in acute stroke patients. J Adv Nurs. 2011;67:1976–83. doi: 10.1111/j.1365-2648.2011.05645.x. [DOI] [PubMed] [Google Scholar]
- 22.National Institutes of Health Stroke Scale. [accessed on April 10, 2014]. Available vailable from: http://www.ninds.nih.gov/doctors/nih_stroke_scale.pdf .
- 23.Baird TA, Parsons MW, Phanh T, Butcher KS, Desmond PM, Tress BM, et al. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke. 2003;34:2208–14. doi: 10.1161/01.STR.0000085087.41330.FF. [DOI] [PubMed] [Google Scholar]
- 24.Alawneh JA, Moustafa RR, Baron JC. Hemodynamic factors and perfusion abnormalities in early neurological deterioration. Stroke. 2009;40:e443–50. doi: 10.1161/STROKEAHA.108.532465. [DOI] [PubMed] [Google Scholar]
- 25.Parsons MW, Barber PA, Desmond PM, Baird TA, Darby DG, Byrnes G, et al. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol. 2002;52:20–8. doi: 10.1002/ana.10241. [DOI] [PubMed] [Google Scholar]
- 26.Kwan J, Hand P. Early neurological deterioration in acute stroke: clinical characteristics and impact on outcome. QJM. 2006;99:625–33. doi: 10.1093/qjmed/hcl082. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi T, Minematsu K, Hasegawa Y. General care in acute stroke. Cerebrovasc Dis. 1997;7(Suppl 3):12–7. [Google Scholar]
- 28.Kelly J, Hunt BJ, Lewis RR, Swaminathan R, Moody A, Seed PT, et al. Dehydration and venous thromboembolism after acute stroke. QJM. 2004;97:293–6. doi: 10.1093/qjmed/hch050. [DOI] [PubMed] [Google Scholar]


