Abstract
Background & objectives:
Pleural effusion is a common occurrence in patients with late-stage chronic kidney disease (CKD). In developing countries, many effusions remain undiagnosed after pleural fluid analysis (PFA) and patients are empirically treated with antitubercular therapy. The aim of this study was to evaluate the role of adenosine deaminase (ADA), nucleic acid amplification tests (NAAT) and medical thoracoscopy in distinguishing tubercular and non-tubercular aetiologies in exudative pleural effusions complicating CKD.
Methods:
Consecutive stage 4 and 5 CKD patients with pleural effusions underwent PFA including ADA and PCR [65 kDa gene; multiplex (IS6110, protein antigen b, MPB64)]. Patients with exudative pleural effusion undiagnosed after PFA underwent medical thoracoscopy.
Results:
All 107 patients underwent thoracocentesis with 45 and 62 patients diagnosed as transudative and exudative pleural effusions, respectively. Twenty six of the 62 patients underwent medical thoracoscopy. Tuberculous pleurisy was diagnosed in six while uraemic pleuritis was diagnosed in 20 subjects. The sensitivity and specificity of pleural fluid ADA, 65 kDa gene PCR, and multiplex PCR were 66.7 and 90 per cent, 100 and 50 per cent, and 100 and 100 per cent, respectively. Thoracoscopy was associated with five complications in three patients.
Interpretation & conclusions:
Uraemia remains the most common cause of pleural effusion in CKD even in high TB prevalence country. Multiplex PCR and thoracoscopy are useful investigations in the diagnostic work-up of pleural effusions complicating CKD while the sensitivity and/or specificity of ADA and 65 kDa gene PCR is poor.
Keywords: ADA, chronic renal failure, CKD, PCR, pleurisy, thoracoscopy, tuberculosis
Pleural effusion is a frequent occurrence in chronic kidney disease (CKD) patients1 with common causes being fluid overload, cardiac failure, hypoproteinaemia, bacterial infection, tuberculosis and inadequate dialysis2. Although there are no published data, most frequently patients with exudative pleural effusions in India are treated empirically with anti-tuberculosis therapy because tuberculosis is the most common cause of pleural effusion in the general population. A definitive diagnosis of tuberculous pleurisy requires demonstration of either Mycobacterium tuberculosis in pleural specimens or granulomatous inflammation in the pleura3.
The sensitivity of acid-fast staining and culture for M.tuberculosis in pleural fluid is low4. Elevated levels of adenosine deaminase (ADA) in the pleural fluid exhibit sensitivity and specificity values exceeding 90 per cent for diagnosis of pleural tuberculosis5,6. The activity and thus the diagnostic efficacy of ADA, is decreased in patients with uraemia, even after three sessions of haemodialysis7. In chronically dialsed patients these effects may be more pronounced. Nucleic acid amplification tests (NAATs) for M.tuberculosis in pleural fluid have poor sensitivity (43-77%) but good specificity (96-98%) for the diagnosis of tuberculous pleuritis8.
Medical thoracoscopy is a valuable tool in the evaluation of undiagnosed exudative pleural effusions as one can obtain large pleural biopsy specimens under vision9. In some studies, a diagnosis of pleural tuberculosis could be made in 100 per cent patients with thoracoscopy, which was higher than the yield of closed pleural biopsy (51-79%)10,11. The percentage of so-called “idiopathic” pleural effusions can be reduced to 4 per cent with the use of thoracoscopy12.
In our tertiary care centre in north India, closed pleural biopsy is not performed routinely and all patients with undiagnosed pleural effusions are subjected to medical thoracoscopy13. The aim of this study was to investigate the utility of ADA, NAAT and medical thoracoscopy in distinguishing tuberculous and non-tuberculous aetiologies among exudative pleural effusions complicating CKD.
Material & Methods
This prospective observational study was conducted in the Department of Pulmonary Medicine, Postgraduate Institute of Medical Education and Research, Chandigarh, India, between July 2010 and December 2011. The Institute Ethics Committee approved the protocol and written informed consent was obtained from all patients. Consecutive adult stage 4 and 5 CKD patients (glomerular filtration rate, GFR <30 ml/min or dialysis-dependent) with pleural effusion were enrolled in the study from the Renal Clinic of this Institute. All patients underwent thoracentesis and pleural fluid was analyzed for leucocyte count and differential, protein, glucose, lactate dehydrogenase (LDH), Gram stain and bacterial culture, smear for acid-fast bacilli (AFB) by Ziehl-Neelsen (ZN) staining, culture on Lowenstein-Jensen (L-J) medium, ADA levels, cytology and NAAT for M. tuberculosis. Light's criteria14 were used in classifying transudative and exudative pleural effusion. If the criteria for an exudative effusion were borderline, the protein gradient value (between serum and pleural fluid) greater than 3.1 g/dl was used for classifying transudate. Pleural effusions were arbitrarily classified as minimal (blunting of the costophrenic angle and <10 mm height on lateral decubitus film), small (<1/3rd of hemithorax), moderate(1/3rd-2/3rd of hemithorax) and large (>2/3rd of hemithorax) based on the chest radiograph.
Patients with inconclusive Gram-stain, bacterial culture, AFB and cytology underwent medical thoracoscopy and biopsy of the parietal pleura. Thoracoscopy was not performed in those with coagulation abnormalities (platelet count <75,000/μl; prothrombin time or activated partial thromboplastin time, four or 10 sec, greater than control, respectively), acute coronary syndromes within the last six weeks, refractory cough, extensive adhesions on imaging (chest radiograph, ultrasonography or computed tomography of the chest) and failure to provide informed consent.
Thoracoscopy was performed in the morning after overnight fast under local anaesthesia and conscious sedation as previously described13. Biopsy specimens were sent for histopathological examination, AFB smear, culture on L-J medium and NAAT for M. tuberculosis.
A diagnosis of parapneumonic effusion was made on the presence of a positive Gram stain or culture and/or low pleural fluid glucose (<40 mg/dl) associated with bacterial pneumonia or lung abscess. A diagnosis of pleural tuberculosis (gold standard) was made on demonstration of granulomatous inflammation on histological examination of pleural biopsy specimen or demonstration of AFB or growth of M.tuberculosis on pleural biopsy or pleural fluid specimen. A diagnosis of uraemic pleural effusion was made on the finding of non-specific pleuritis on pleural biopsy, and no growth of any microorganism on culture. All patients were followed up for at least three months following the procedure.
ADA estimation: ADA activity was measured by the method of Guisti et al15. Two ml of pleural fluid was collected in sterile container and was either immediately analysed or refrigerated at 4°C and analyzed within two days. An ADA value >40 U/l was taken as the cut-off for calculating sensitivity and specificity.
Nucleic acid amplification tests: Samples were processed in triplicates and were considered positive if amplification was seen in two of the three samples. PCR for β-actin gene16 (forward primer- 5’-TGACGGGGTCACCCACACTGTGCCCATCTA-3’; reverse primer- 5’-CTAGAAGCATTGGCGGTGG-ACGATGGAGGG-3’) was performed to ensure quality of DNA extraction, and samples were considered inappropriate if it was negative. To ensure PCR quality and to rule out extraneous contamination, M. tuberculosis H37Rv genomic DNA (positive control) and a null sample (negative control) were included with every batch of PCR.
Polymerase chain reaction for M.tuberculosis 65 kDa gene was performed on pleural fluid specimens as previously described17. The primers used were: forward primer- 5’-GAGATCGAGCTGGAGGATCC-3’, and reverse primer- 5’-AGCTGCAGCCCAAAGGTG TTA-3’. The PCR conditions were an initial denaturation step at 94°C for 7 min and 35 cycles of 94°C for one minute, 60°C for two minutes and 72°C for two minutes. Final extension was done at 72°C for 10 minutes. PCR product was visualized under ultra violet (UV) light after electrophoresis on 1.2 per cent agarose gel with ethidium bromide.
Multiplex PCR designed to amplify IS6110, protein antigen b, and MPB64 was carried out as described previously18. The sequence of primers used for proteinb PCR were: 5’-ACCACCGAGCGGTTCGCCTGA-3’ and 5’-GATCTGCGGGTCGTCCCAGGT-3’ respectively. Primers used for IS6110 were: 5’-CCTGCGAGCGTAGGCGT-3’, 5’-CTCGTCCAGCGCCGCTTCGG-3’. Primers used for MPB64 were: 5’-TCCGCTGCCAGTCGTCTTCC-3’ and MPB2: 5’-GTCCTCGCGAGTCTAGGCCA-3’. DNA amplification was performed for 40 cycles following an initial denaturation step at 94°C for 5 minutes in a thermocycler using the following programme: denaturation at 94°C for one minute, annealing at 65°C for 1.5 min, extension at 72°C for 1.5 min, and final extension at 72°C for 10 min. The amplified product was detected on 1.5 per cent agarose gel stained with ethidium bromide. The stained gel was examined under UV light to look for bands 123 bp for IS6110, 419 bp for protein b, and 240 bp for MPB64 using a molecular weight marker of 100-bp ladder. Presence of at least one of these bands under ultraviolet transillumination was considered positive.
Patients with a diagnosis of tuberculous pleural effusion were treated with isoniazid, rifampicin, pyrazinamide and ethambutol for two months followed by isoniazid and rifampicin for four months. Those with uraemic pleuritis were treated with intensification of dialysis.
Statistical analysis was performed using the statistical package StatsDirect (version 2.7.9, UK). The differences between continuous and categorical variables was analyzed using Mann-Whitney U and chi-square tests, respectively. The diagnostic performance of ADA and NAAT are presented as sensitivity and specificity.
Results
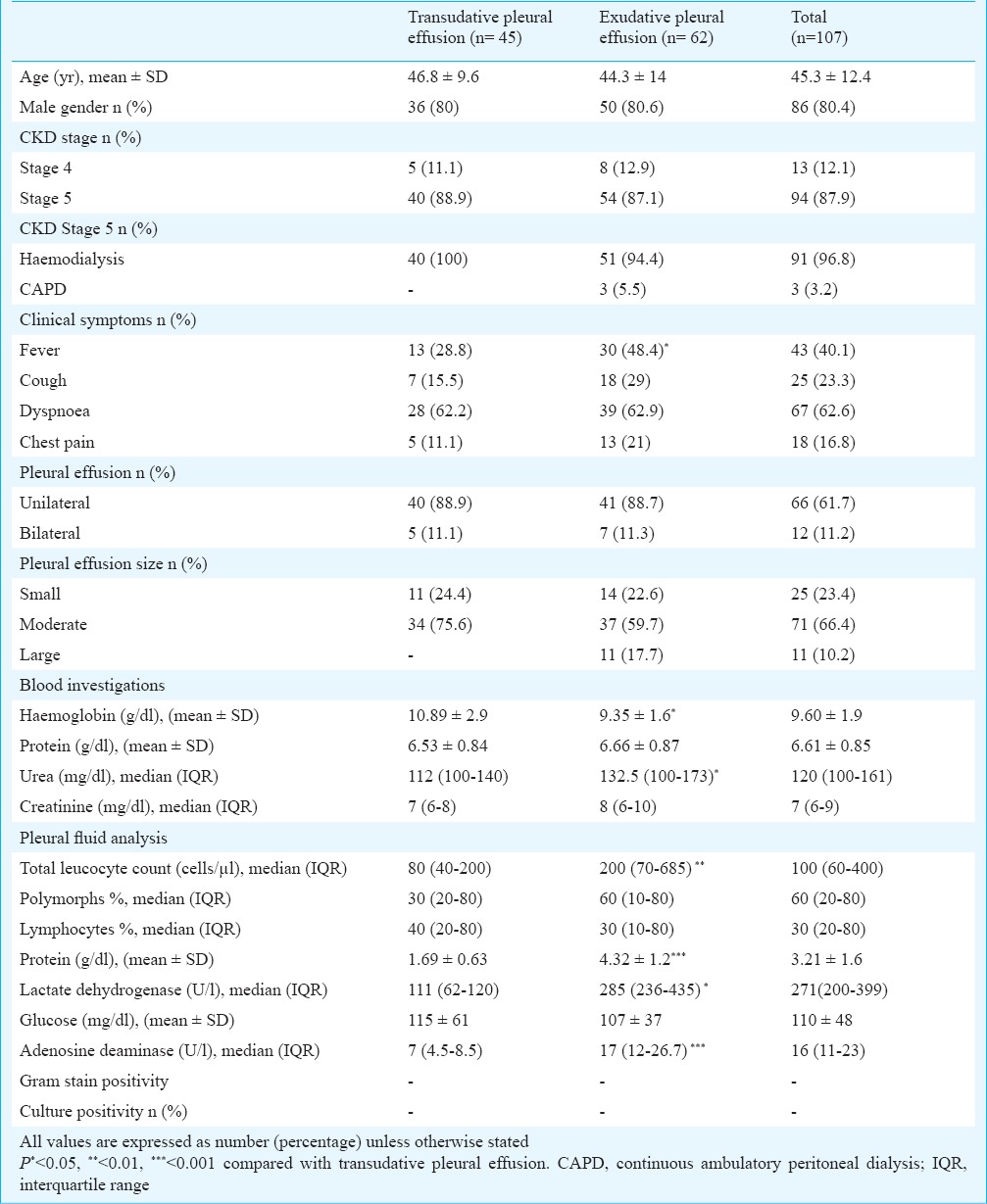
During the study period, a total of 107 CKD patients [stage 4: 13 (12%); stage 5: 94 (88%)] with mean age of 45.2 years were detected to have pleural effusion. The causes of CKD included diabetes (n=36), chronic glomerulonephritis (n=22), hypertension (n=20), chronic interstitial nephritis (n=11), obstructive nephropathy (n=8) and unknown (n=10). The demographic and clinical characteristics of the patients are shown in the Table. Of the 94 stage 5 CKD patients, 96.8 per cent were on maintenance haemodialysis, while three patients were on peritoneal dialysis. HIV serology was negative in all. Pleural effusions were unilateral in 88, 62 per cent were right-sided, and 66 per cent were moderate in size. The commonest presenting feature was dyspnoea followed by fever, cough and chest pain. The effusion was transudative in 45 (42.1%) and exudative in 62 (57.9%) patients. There was no difference in demographic and clinical characteristics except lower haemoglobin and higher serum LDH in patients with exudative pleural effusion. Pleural fluid AFB and culture on L-J medium was negative in all patients with exudative pleural effusion.
Table.
Demographic and clinical characteristics of the study population
Thoracoscopy was performed in 26 (42%) patients with exudative pleural effusion (Figure). Of these, 25 were on dialysis (24 haemodialysis, 1 peritoneal dialysis). Adhesions were found in 11 (42.3%) and diffusely scattered small nodules were encountered in five patients. A diagnosis of pleural tuberculosis was made in six (23.1%) patients while a diagnosis of non-specific pleuritis [fibrinous pleuritis (n=8), mesothelial cell proliferation with inflammation (n=6) and organising inflammation (n=6)] was made in the remaining. The diagnosis of pleural tuberculosis was made on the finding of necrotic granulomas on histopathology in all patients. Smear for AFB was positive in three patients while culture of the pleural tissue yielded growth of M.tuberculosis in three patients. Presence of pleural nodules (5/6 patients with pleural tuberculosis) had 83 per cent sensitivity and 100 per cent specificity fordiagnosis of tuberculous pleuritis. Five complications [(haemorrhage (n=2), empyema (n=3)] were encountered in three patients, all in those with adhesions, which were treated with chest tube insertion and antibiotic therapy.
Fig.
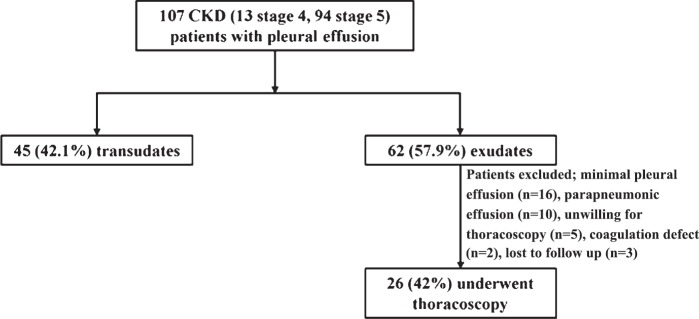
Algorithm depicting the flow of patients in the study.
Sensitivity and specificity of ADA in pleural fluid: Four of six patients with tuberculosis had ADA ≥40 U/l while two patients had ADA values of 38 and 14 U/l, respectively. Two patients with non-specific pleuritis had ADA >40 U/l (48 and 50 U/l, respectively). The sensitivity and specificity of ADA for the diagnosis of tuberculosis was 66.7 and 90 per cent respectively.
Sensitivity and specificity of TB-PCR in pleural fluid/biopsy: PCR for 65 kDa gene was positive in 16 patients: all six with pleural TB and 10 of the 20 patients with non-specific pleuritis giving a sensitivity and specificity of 100 and 50 per cent, respectively. On the other hand, the multiplex PCR was positive only in patients with tuberculous pleural effusion, providing a sensitivity and specificity of 100 per cent.
Follow up of patients: The mean (IQR) duration of followup was five (4-9) months. All six patients with pleural tuberculosis showed clinical response in terms of symptoms and resolution of effusion after institution of antitubercular therapy. Of the 20 patients with non-specific pleuritis, 13 patients responded to intensification of haemodialysis session, three of whom underwent kidney transplantation whereas the remaining seven patients continued to have recurrent pleural effusions. No patient in the non-specific pleuritis group showed evidence of tuberculosis during followup.
Discussion
In the current study, the commonest cause of exudative pleural effusions was uraemic rather than infections, similar to previously reported experience19,20. We found poor sensitivity and specificity (67 and 90%, respectively) of pleural fluid ADA at a cut-off of 40 units per liter. In the largest study on ADA in pleural effusions, Porcel et al21 reported the sensitivity and specificity of pleural fluid ADA >35U/l of 93 and 90 per cent, respectively. In two meta-analyses, the sensitivity and specificity of ADA in diagnosis of tuberculous pleurisy have been reported at 47-100 and 41-100 per cent, respectively5,6. There are no studies evaluating ADA in pleural fluid of CKD patients. In a study on renal transplant recipients with tuberculous pleural effusion, a sensitivity of 91.3 per cent has been reported for ADA22.
In our study, although the sensitivity of 65 kDa PCR was 100 per cent, the specificity was only 50 per cent. In contrast, multiplex PCR showed 100 per cent sensitivity and specificity. In a meta-analysis8 on the role of NAAT in diagnosis of tuberculous pleuritis the sensitivity and specificity of commercial NAAT were 62 and 98 per cent, respectively, whereas the in-house NAAT had sensitivity and specificity of 71 and 93 per cent, respectively. Studies using 65kDa gene PCR reported sensitivity and specificity ranging from 20-100 and 78-100 per cent, respectively8. The multiplex PCR employing IS6110, protein antigen b, and MPB64 has been shown to have excellent sensitivity and specificity17,23,24.
Pleural fluid smear for AFB was negative and culture for M.tuberculosis was sterile in all our patients finally diagnosed to have pleural tuberculosis. Routine smears of pleural fluid for mycobacteria are almost always negative unless the patient has tuberculous empyema25,26. In most studies on tuberculous pleuritis, the cultures were positive in less than 40 per cent27. In one study on 248 patients with tuberculous pleuritis who underwent needle biopsy of the pleura, the biopsy showed granulomas in 80 per cent, the AFB stain and culture of the biopsy tissue was positive in 25.8 and 56 per cent respectively26.
Thoracoscopic pleural biopsy with histopathological examination remains the most sensitive investigation for diagnosis of tuberculous pleural effusion with 100 per cent sensitivity, and is currently the reference standard3,11,28. Tuberculosis was encountered in only a minority of CKD patients with exudative pleural effusion, contrary to the high prevalence of tuberculosis in exudative pleural effusion in general population in India29. Tuberculosis was diagnosed in six of 107 (5.6%) patients; this figure was high compared with the general population29. On the other hand, the most common cause of pleural effusion was CKD-related. There may be several reasons for this, the most common being inadequate dialysis and co-existing cardiovascular disease. All our patients were receiving twice a week dialysis, as being practiced in India30.
Our study showed medical thoracoscopy to be a safe and effective technique in the diagnostic work-up of uraemic pleural effusion. This procedure prevented unnecessary exposure of CKD patients to potentially toxic and prolonged course of antitubercular therapy. A variety of complications with thoracoscopy have been described31. However, there was no mortality associated with diagnostic thoracoscopy while the major complication rate was about two per cent31. In our study, five complications were reported in three patients, all in those with adhesions. Hence, thoracoscopy should be performed as early as possible, before adhesions have had a time to develop13. Uraemia is associated with platelet dysfunction and increased risk of haemorrhagic events32 as also seen in our study where bleeding was seen despite normal coagulation parameters in patients receiving haemodialysis the day before the procedure.
The results of this study can only be considered as hypothesis generating because of the small sample size and the conduct of the study at a single centre.
In conclusion, the results of this study suggest that uraemic pleural effusion is the most common cause of pleural effusion in CKD, even in high TB prevalence area. Multiplex PCR and thoracoscopy are useful investigations in the diagnostic work-up of pleural effusions complicating uraemia to identify tuberculous aetiology. Where multiplex PCR is not available, thoracoscopy should be routinely performed in the diagnostic work-up of pleural effusions complicating uraemia. The multiplex PCR and the thoracoscopy approach requires validation in larger preferably multicentric trials with uniform methodology.
References
- 1.Maher JF. Uremic pleuritis. Am J Kidney Dis. 1987;10:19–22. doi: 10.1016/s0272-6386(87)80005-7. [DOI] [PubMed] [Google Scholar]
- 2.Rashid-Farokhi F, Pourdowlat G, Nikoonia MR, Behzadnia N, Kahkouee S, Nassiri AA, et al. Uremic pleuritis in chronic hemodialysis patients. Hemodial Int. 2013;17:94–100. doi: 10.1111/j.1542-4758.2012.00722.x. [DOI] [PubMed] [Google Scholar]
- 3.McGrath EE, Anderson PB. Diagnostic tests for tuberculous pleural effusion. Eur J Clin Microbiol Infect Dis. 2010;29:1187–93. doi: 10.1007/s10096-010-0986-z. [DOI] [PubMed] [Google Scholar]
- 4.Sahn SA, Huggins JT, San Jose ME, Alvarez-Dobano JM, Valdes L. Can tuberculous pleural effusions be diagnosed by pleural fluid analysis alone? Int J Tuberc Lung Dis. 2013;17:787–93. doi: 10.5588/ijtld.12.0892. [DOI] [PubMed] [Google Scholar]
- 5.Greco S, Girardi E, Masciangelo R, Capoccetta GB, Saltini C. Adenosine deaminase and interferon gamma measurements for the diagnosis of tuberculous pleurisy: a meta-analysis. Int J Tuberc Lung Dis. 2003;7:777–86. [PubMed] [Google Scholar]
- 6.Liang QL, Shi HZ, Wang K, Qin SM, Qin XJ. Diagnostic accuracy of adenosine deaminase in tuberculous pleurisy: a meta-analysis. Respir Med. 2008;102:744–54. doi: 10.1016/j.rmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Dussol B, Fenouillet E, Brunet P, Purgus R, Sauze N, Carrega L, et al. Kinetic study of adenosine concentrations and the expression of adenosine deaminase in mononuclear cells during hemodialysis. Kidney Int. 2004;66:1640–6. doi: 10.1111/j.1523-1755.2004.00930.x. [DOI] [PubMed] [Google Scholar]
- 8.Pai M, Flores LL, Hubbard A, Riley LW, Colford JM., Jr Nucleic acid amplification tests in the diagnosis of tuberculous pleuritis: a systematic review and meta-analysis. BMC Infect Dis. 2004;4:6. doi: 10.1186/1471-2334-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loddenkemper R. Thoracoscopy--state of the art. Eur Respir J. 1998;11:213–21. doi: 10.1183/09031936.98.11010213. [DOI] [PubMed] [Google Scholar]
- 10.Loddenkemper R, Grosser H, Gabler A, Mai J, Presseuler H, Brandt HJ. Prospective evaluation of biopsy methods in diagnosis of malignant pleural effusions. intra-patient comparison between pleural fluid cytology, blind needle biopsy and thoracoscopy. Am Rev Respir Dis. 1983;127(Suppl 4):114–1983. [Google Scholar]
- 11.Diacon AH, Van de Wal BW, Wyser C, Smedema JP, Bezuidenhout J, Bolliger CT, et al. Diagnostic tools in tuberculous pleurisy: a direct comparative study. Eur Respir J. 2003;22:589–91. doi: 10.1183/09031936.03.00017103a. [DOI] [PubMed] [Google Scholar]
- 12.Boutin C, Astoul P, Seitz B. The role of thoracoscopy in the evaluation and management of pleural effusions. Lung. 1990;168(Suppl):1113–21. doi: 10.1007/BF02718251. [DOI] [PubMed] [Google Scholar]
- 13.Mootha VK, Agarwal R, Singh N, Aggarwal AN, Gupta D, Jindal SK. Medical thoracoscopy for undiagnosed pleural effusions: experience from a tertiary care hospital in north India. Indian J Chest Dis Allied Sci. 2011;53:21–4. [PubMed] [Google Scholar]
- 14.Light RW, Macgreggor MI, Luchsinger PC, Ball WC., Jr Plural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med. 1972;77:508–13. doi: 10.7326/0003-4819-77-4-507. [DOI] [PubMed] [Google Scholar]
- 15.Giusti G. Adenosine deaminase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. 2nd ed. New York: Academic Press, Inc.; 1974. pp. 1092–9. [Google Scholar]
- 16.Mootha VK, Agarwal R, Aggarwal AN, Gupta D, Ahmed J, Verma I, et al. The Sarcoid-Tuberculosis link: evidence from a high TB prevalence country. J Infect. 2010;60:501–3. doi: 10.1016/j.jinf.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Jung HC, Eckmann L, Yang SK, Panja A, Fierer J, Morzycka-Wroblewska E, et al. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kusum S, Aman S, Pallab R, Kumar SS, Manish M, Sudesh P, et al. Multiplex PCR for rapid diagnosis of tuberculous meningitis. J Neurol. 2011;258:1781–7. doi: 10.1007/s00415-011-6010-4. [DOI] [PubMed] [Google Scholar]
- 19.Berger HW, Rammohan G, Neff MS, Buhain WJ. Uremic pleural effusion. A study in 14 patients on chronic dialysis. Ann Intern Med. 1975;82:362–4. doi: 10.7326/0003-4819-82-3-362. [DOI] [PubMed] [Google Scholar]
- 20.Bakirci T, Sasak G, Ozturk S, Akcay S, Sezer S, Haberal M. Pleural effusion in long-term hemodialysis patients. Transplant Proc. 2007;39:889–91. doi: 10.1016/j.transproceed.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 21.Porcel JM, Esquerda A, Bielsa S. Diagnostic performance of adenosine deaminase activity in pleural fluid: a single-center experience with over 2100 consecutive patients. Eur J Intern Med. 2010;21:419–23. doi: 10.1016/j.ejim.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Chung JH, Kim YS, Kim SI, Park K, Park MS, Kim SK, et al. The diagnostic value of the adenosine deaminase activity in the pleural fluid of renal transplant patients with tuberculous pleural effusion. Yonsei Med J. 2004;45:661–4. doi: 10.3349/ymj.2004.45.4.661. [DOI] [PubMed] [Google Scholar]
- 23.Sharma K, Sharma A, Sharma SK, Sen RK, Dhillon MS, Sharma M. Does multiplex polymerase chain reaction increase the diagnostic percentage in osteoarticular tuberculosis. A prospective evaluation of 80 cases? Int Orthop. 2012;36:255–9. doi: 10.1007/s00264-011-1241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma K, Sharma A, Singh M, Ray P, Dandora R, Sharma SK, et al. Evaluation of polymerase chain reaction using protein b primers for rapid diagnosis of tuberculous meningitis. Neurol India. 2010;58:727–31. doi: 10.4103/0028-3886.72189. [DOI] [PubMed] [Google Scholar]
- 25.Escudero Bueno C, Garcia Clemente M, Cuesta Castro B, Molinos Martin L, Rodriguez Ramos S, Gonzalez Panizo A, et al. Cytologic and bacteriologic analysis of fluid and pleural biopsy specimens with Cope's needle. Study of 414 patients. Arch Intern Med. 1990;150:1190–4. doi: 10.1001/archinte.150.6.1190. [DOI] [PubMed] [Google Scholar]
- 26.Valdes L, Alvarez D, San Jose E, Penela P, Valle JM, Garcia-Pazos JM, et al. Tuberculous pleurisy: a study of 254 patients. Arch Intern Med. 1998;158:2017–21. doi: 10.1001/archinte.158.18.2017. [DOI] [PubMed] [Google Scholar]
- 27.Light RW. Update on tuberculous pleural effusion. Respirology. 2010;15:451–8. doi: 10.1111/j.1440-1843.2010.01723.x. [DOI] [PubMed] [Google Scholar]
- 28.Keys C, McLeod E, Pesti C, Armstrong D. Thoracoscopic pleural biopsy as an aid to diagnosis in pediatric tuberculosis with pleural involvement. Eur J Pediatr Surg. 2012;22:315–7. doi: 10.1055/s-0032-1315802. [DOI] [PubMed] [Google Scholar]
- 29.Aggarwal AN, Gupta D, Jindal SK. Diagnosis of tuberculous pleural effusion. Indian J Chest Dis Allied Sci. 1999;41:89–100. [PubMed] [Google Scholar]
- 30.Jha V. End-stage renal care in developing countries: the India experience. Ren Fail. 2004;26:201–8. doi: 10.1081/jdi-120039516. [DOI] [PubMed] [Google Scholar]
- 31.Rahman NM, Ali NJ, Brown G, Chapman SJ, Davies RJ, Downer NJ, et al. Local anaesthetic thoracoscopy: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(Suppl 2):ii54–60. doi: 10.1136/thx.2010.137018. [DOI] [PubMed] [Google Scholar]
- 32.Jalal DI, Chonchol M, Targher G. Disorders of hemostasis associated with chronic kidney disease. Semin Thromb Hemost. 2010;36:34–40. doi: 10.1055/s-0030-1248722. [DOI] [PubMed] [Google Scholar]


