Abstract
Background & objectives:
Early identification of bacterial infection in patients with fever is important for prompt treatment. However, the available parameters such as C-reactive protein (CRP) and leukocyte counts are not very specific. This study was aimed to assess the diagnostic value of procalcitonin (PCT), CRP, interleukin-6 (IL-6) and serum amyloid A (SAA) for bacterial infection in febrile patients.
Methods:
Serum samples were collected from febrile patients between January and December 2012 and processed for blood cultures. PCT, IL-6, CRP and SAA levels were measured. The patients were divided into three groups according to the final diagnosis: bacteraemia group (group1), bacterial infection with negative blood culture (group 2) and non-bacterial infection group (group 3).
Results:
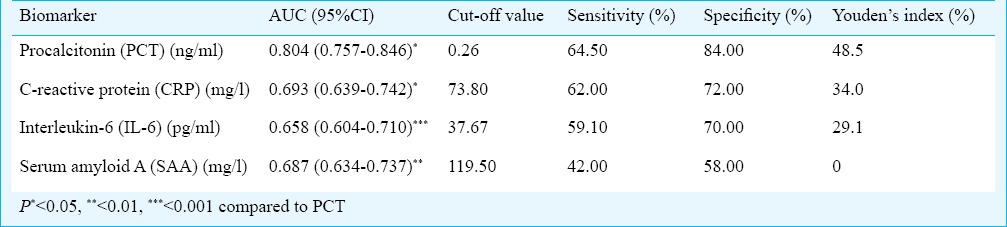
There were significant (P<0.05) difference in the levels of PCT, CRP, IL-6 and SAA among the three groups. The PCT levels of patients with Gram-positive bacterial infections were lower than Gram-negative bacterial infections (0.53 vs 2.13, P < 0.01). The best cut-off value to detect bacterial infections was 0.26 ng/ml for PCT. PCT, CRP, IL-6 and SAA had areas under the curve of 0.804, 0.693, 0.658 and 0.687, respectively.
Interpretation & conclusions:
Our results showed PCT as a valuable marker of bacterial infections in febrile patients. PCT was superior to CRP, IL-6 or SAA in the early identification of bacterial infection. More prospective and large scale studies are warranted to confirm these findings.
Keywords: C-reactive protein, fever, infection, interleukin-6, procalcitonin, serum amyloid A
Promp identification of early bacterial infection in fever is very important, since appropriate aetiological treatment and avoidance of unnecessary antimicrobial therapy could not only reduce the morbidity, mortality and costs to patients, but also can reduce the emergence of antibiotic-resistant bacteria. The traditional diagnostic tools, such as C-reactive protein (CRP) and leukocyte count, are not specific enough for differentiating bacterial infections from viral infections and systemic inflammation1,2. Microbiologic culture requires at least 24-48 h, and negative cultures do not exclude the presence of infection3. Moreover, only 5-10 per cent of blood cultures performed in hospitals show microorganisms4. Therefore, there is an obvious need for more specific biomarkers of bacterial infections in febrile patients.
Procalcitonin (PCT), the precursor of the hormone calcitonin, is produced by C-cells of the thyroid gland or neuroendocrine cells in the lung or intestine5, and its level in blood of healthy people is less than 0.01 ng/ml. Levels of PCT rise dramatically during bacterial infections, whereas low levels were detected during viral infections or non-infectious febrile conditions6,7. Many studies showed the diagnostic property of PCT superior to CRP6,8,9,10. Despite many studies, it remains unclear whether PCT adds significantly to the discriminative properties of the already used set of diagnostic biomarkers, and further studies need to be done to determine the specific diagnostic cut-off value. Though the negative cut-off value for PCT is generally believed to be < 0.5 ng/ml, it is not consistant since “negative” PCT value has been observed in some patients with bacterial infection11. Interleukin-6 (IL-6) is an important proinflammatory cytokine in the early phase of inflammation, which increases in local and blood circulation after stimulus such as infection, surgery and trauma12. Serum amyloid A (SAA) is an acute phase response protein and produced by activated macrophages and fibroblasts after stimulus such as IL-1, IL-6 and tumour necrosis factor (TNF), which, similar to CRP, is a sensitive index for early inflammatory disease13. Therefore, the detection of PCT, CRP, IL-6 and SAA may be a better combination to identify early bacterial infection in febrile patients. We, therefore, undertook this study to compare the diagnostic properties and assess the optimum cut-off values of PCT, CRP, IL-6 and SAA to detect bacterial infections early in febrile patients presenting to the Infectious Diseases department.
Material & Methods
Study design and settings: The study was conducted between January and December, 2012, in the Center of Infectious Diseases, West China Hospital, Sichuan University, PR China. The Ethics Committee for Research and Clinical Practice in West China Hospital approved the protocol for this research. Written informed consent was obtained from every enrolled patient.
Patients: All adult patients (aged 18 to 85 yr) with fever (temperature >38.0°C) in the Infectious Diseases department of West China Hospital, Sichuan University within 24 h after admitting to the ward were enrolled. Exclusion criteria were temperature < 38.0°C, absence of informed consent, pregnancy, thyroid tumour, discharge diagnosis for fever of unknown origin (FUO) and fungal infection. Some cases of fever of unknown origin (FUO) who remained unsolved at the time of discharge, were also excluded.
Measurement: Clinical data were collected, routine laboratory measurements were performed. Blood samples (20 ml) were aseptically collected for aerobic and anaerobic blood cultures. Bacterial culture was done in clinical microbiology laboratory of our hospital. All samples were collected within 24 h after registration to the department of Infectious Diseases. MicroScan WalkAway-96 System (Siemens, USA) was used to identify the bacterial species. PCT levels were detected by a chemiluminescence sandwich immunoassay and were measured by the Roche Elecsys E170 electrochemiluminescence analytical instruments (Roche Diagnostics Gmbtt Mannheim, Germany). The lower limit of detection of the assay was 0.05 ng/ml. The concentration of CRP in serum was determined by IMMAGE 800 specific protein analysis system (Beckman Coulter company, USA). IL-6 levels were measured by the Roche Elecsys E170 electrochemiluminescence analytical instruments. The concentration of SAA in serum was determined by BN II System (Dade Behring Marburg GmbH, Germany).
Group classification: The patients were divided into three groups according to the final diagnosis. The patients with bacterial infection were divided into two groups (groups 1 and 2) according to the result of blood culture. Group 1 represented patients with bacteraemia defined by a positive blood culture. Group 2 represented patients with bacterial infections but with negative blood culture. The patients in group 3 had no bacterial infection. The groups were comparable in age and sex. Groups 1 and 2 were pooled against group 3 prior to drawing ROC (receiver operating characteristic) curves.
Statistical analysis: Data were analyzed by SPSS version 17.0 software package (SPSS Inc., Chicago, USA). The Shapiro-Wilk normality test was performed for all quantitative variables14. In the presence of non-normally distributed data, non-parametric statistical methods were used. Significance testing was carried out using the Kruskal-Wallis H test and Wilcoxon two-sample test. The association between PCT and CRP or SAA was tested based on Spearman's rank correlation. ROC curves were analysed with the Medcalc software version 11.5.1.0 (Medcalc software bvba, Belgium). ROC curves were plotted for PCT, CRP, IL-6 and SAA. Diagnostic accuracy was assessed by calculating the areas under the ROC curves (AUC). ROC curve analysis was carried out using the method of DeLong et al15 An AUC of > 0.9 was considered excellent; 0.8-0.9, very good; 0.7-0.8, good; 0.6-0.7, average; < 0.6, poor16. The best cut-off point is the point where sum of specificity and sensitivity is maximum, when equal weight is given to both17. A two-tailed P < 0.05 was considered significant.
Results
A total of 326 febrile patients (mean age: 45.26 ± 19.03 yr; 179 male) were included in this study. Demographic and laboratory characteristics of patients with fever in this study are listed in Table I. Bacteraemia was confirmed in 58 patients (17.79%), bacterial infection was excluded in 50 patients (15.34%). The final diagnoses are shown in Table II, most common diagnosis was bacterial pneumonia (128/326, 39.26%). Of the 276 patients with bacterial infection, there were 161 cases (58.33%) of hospital acquired infection and 115 cases (41.67%) of community acquired infection. There were significant differences in the levels of PCT, CRP, IL-6 and SAA among the three groups (P < 0.001) (Table I). The correlation between PCT and CRP was moderate (r = 0.532), and those between PCT and SAA (r = 0.331) or IL-6 (r = 0.419) were weak.
Table I.
Demographic and laboratory characteristics of enrolled patients (n=326) with fever
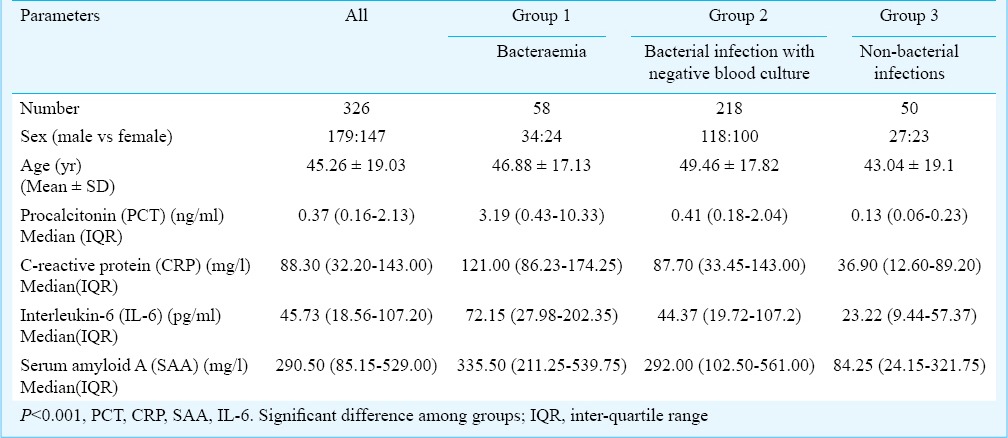
Table II.
Final diagnosis of enrolled patients (n=326) with fever
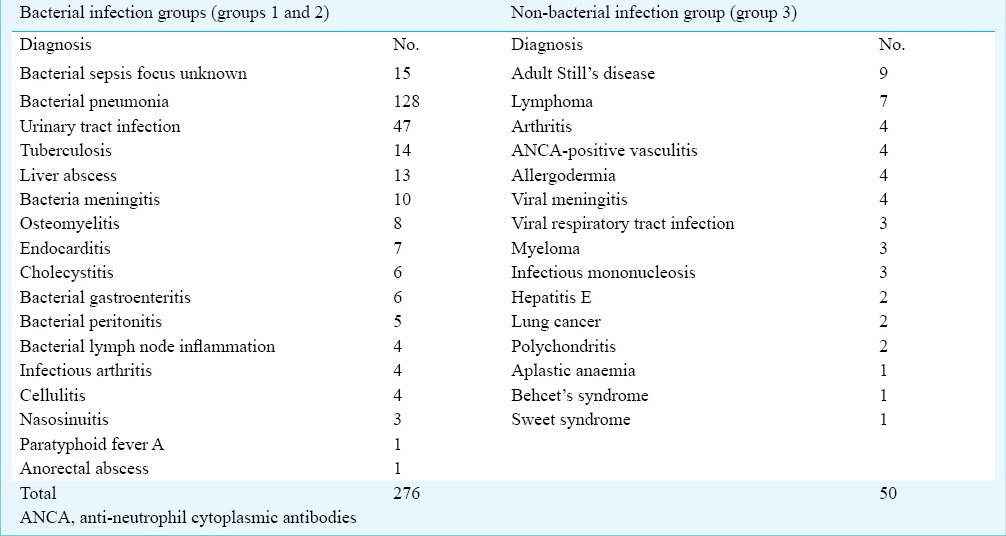
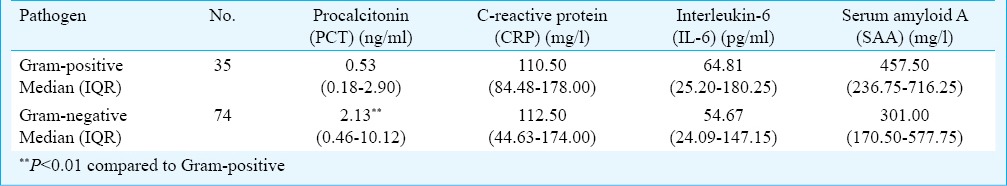
The pathogens isolated from cultures are shown in Fig. 1. The most frequently isolated pathogen was Escherichia coli (25/276, 9.06%), followed by Acinetobacter baumannii/calcoaceticus (21/276, 7.61%). PCT, CRP, IL-6 and SAA concentrations according to pathogens in patients with bacterial infection are shown in Table III. The PCT levels in Gram-positive and Gram-negative bacterial infections were 0.53 ng/ml and 2.13 ng/ml, respectively, with the levels significantly higher in Gram-negative bacterial infections (P < 0.01).
Fig. 1.
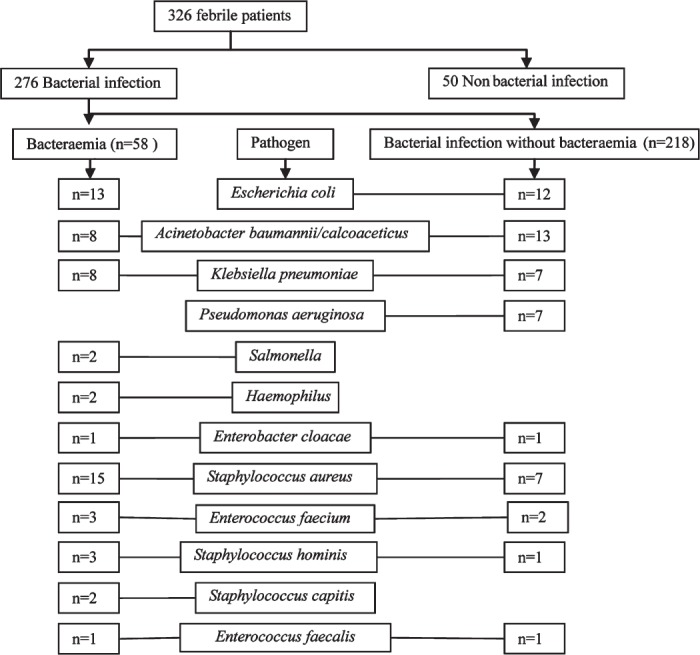
Bacterial pathogens isolated from the enrolled febrile patients.
Table III.
Differences of PCT, CRP, IL-6 and SAA concentrations based on gram-positive and gram -negative bacterial infections
The ROC curves of PCT, CRP, IL-6 and SAA concentrations for discrimination between patients in the bacterial infection group and non-bacterial infection group are shown in Fig. 2. The best cut-off value to detect bacterial infection was 0.26 ng/ml for PCT. The AUC values for the studied biomarkers are listed in Table IV. PCT was better than CRP (P < 0.05), IL-6 (P < 0.001) and SAA (P < 0.01) for detecting bacterial infection.
Fig. 2.
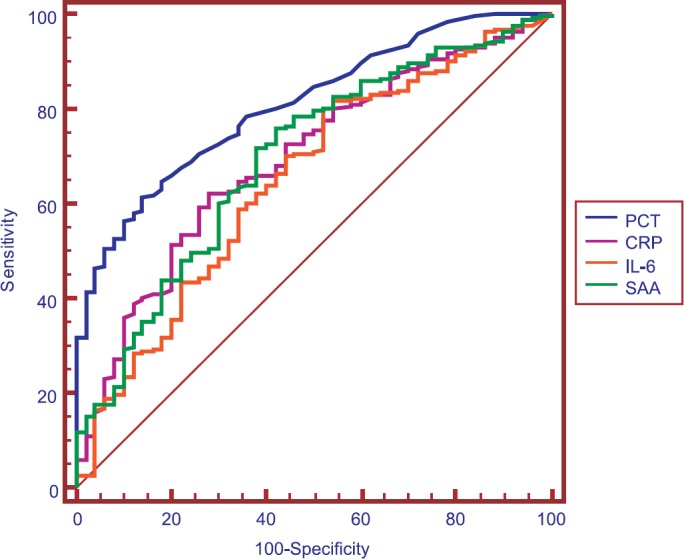
Receiver operating characteristic (ROC) curve demonstrating sensitivity as a function of one-specificity for distinction between patients in the bacterial infection groups (group 1 and 2) and patients in the non-bacterial infection group (group 3) based on procalcitonin (PCT), C-reactive protein (CRP), interleukin-6 (IL-6) and serum amyloid A (SAA) levels. PCT, CRP, IL-6 and SAA had areas under the ROC curve of 0.804, 0.693, 0.658 and 0.687, respectively.
Table IV.
Area under the curves (AUC) of the receiver operating characteristic (ROC) for procalcitonin (PCT), C-reactive protein (CRP), interleukin-6 (IL-6), serum amyloid A (SAA) and the best cut-off values to detect bacterial infection from febrile patients in the infectious diseases department
Discussion
Early identification of bacterial infection in patients with fever is important for the therapy, however, laboratory parameters, such as CRP level and leukocyte counts have less value for early diagnosis. Many previous studies have shown that PCT level can be used to identify bacterial infections in patients with sepsis18,19,20 and in patients admitted to intensive care units (ICUs)20 or the emergency department (ED)8,21.
Our study suggested PCT as a valuable biomarker in detecting bacterial infection in febrile patients. This observation is consistent with the findings of previous researches6,8,9,10 and may be explained by the fact that interferon-gamma (IFN-γ) inhibits IL-1 beta-induced calcitonin mRNA expression and PCT secretion, so serum PCT levels increase less in viral infections as compared with bacterial infections22. Besides, CRP and IL-6 were also found to be of value in detecting bacterial infection in febrile patients. Previous studies have also used CRP level to identify bacterial infections in febrile patients23,24 and PCT was superior to CRP in identifying bacterial infection6,8,9,10. Though CRP is the most commonly measured acute parameter in infection and sepsis, it is not a reliable marker in identifying bacterial infection because of its low sensitivity and specificity. There was no study using SAA as a marker for distinction between infectious and non-infectious fever. SAA has the same initial kinetics as CRP in the acute phase response. This study showed that SAA might not be a valuable biomarker of identifying bacterial infection in patients with fever. A previous study showed IL-6 as a better prognostic marker of bacterial infection than CRP in patients with febrile neutropenia25. This inconsistency may be due to different testing time. In addition, there was no significant association between PCT and CRP or SAA or IL-6, which also might be related to the different peak time and plasma half-life of different biomarkers after a stimulus. In bacterial infections, IL-6 levels rise 2h after endotoxin administration, and then gradually decline26. PCT is probably synthesized in the liver or monocytes, in response to cytokines such as IL-6 and TNF-α27,28. PCT increases in blood six hours after a stimulus, reaches a plateau between 12 and 48 h, and then decreases if the stimulus stops. CRP increased four hours later than PCT7. In general, biomarker measurement has some disadvantages. Probably the combination of biomarkers would lead to a better sensitivity and specificity to predict bacterial infections.
In our study Acinetobacter baumannii/calcoaceticus was isolated from 21 patients, as reported in previous studies also11,29. Our hospital is a large general hospital, many elderly patients hospitalized in other departments or other hospitals, if suffer from fever during their hospitalization and with a suspicion of infection are then admitted to the Infectious Diseases department. Therefore, there were some patients with hospital acquired infection and some isolated pathogens were not common community acquired pathogens.
Our study showed that PCT levels in Gram-negative bacterial infections were significantly higher than that in Gram-positive bacterial infections, consistent with results of previous studies30,31. The TNF-α plays a pivotal role in the cytokine response to Gram-negative bacteria, and also in the release of PCT from various cells in bacterial infection30. Previous experiment showed that PCT peak value was significantly higher in rats stimulated with lipopolysaccharide (LPS), a component of the outer membrane of the Gram-negative bacteria, than in those stimulated with muramyl dipeptide, a component of the outer membrane of the Gram-positive bacteria32. Therefore, the PCT level was possibly related to the characteristics of the pathogen.
There were some limitations concerning this study. Firstly, the study was based on a large hospital (4300 ward beds) statistics and not on a specified population in a region, which might have caused selection bias. Second, the cut-off values from ROC curves are based on sensitivity and specificity which are sensitive to prevalence of a disease and the same is not known.
In conclusion, PCT may be a valuable biomarker of bacterial infections in febrile patients, with greater predictive value than CRP and other biomarkers of infection. SAA may not be a valuable marker of identifying bacterial infection. More prospective and large scale studies are needed to better define the usefulness of PCT in identifying the causes of fever and guiding the rational use of antibiotics.
Acknowledgment
The authors thank the nurses at the Center of Infectious Disease, West China Hospital, Sichuan University for clinical assistance.
References
- 1.Marnell L, Mold C, Du Clos TW. C-1. reactive protein: ligands, receptors and role in inflammation. Clin Immunol. 2005;7:104–11. doi: 10.1016/j.clim.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Meisner M. Biomarkers of sepsis: clinically useful? Curr Opin Crit Care. 2005;11:473–80. doi: 10.1097/01.ccx.0000176694.92883.ce. [DOI] [PubMed] [Google Scholar]
- 3.Mitaka C. Clinical laboratory differentiation of infectious versus non-infectious systemic inflammatory response syndrome. Clin Chim Acta. 2005;351:17–29. doi: 10.1016/j.cccn.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Reimer LG, Wilson ML, Weinstein MP. Update on detection of bacteremia and fungemia. Clin Microbiol Rev. 1997;10:444–65. doi: 10.1128/cmr.10.3.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reinhart K, Karzai W, Meisner M. Procalcitonin as a marker of the systemic inflammatory response to infection. Intensive Care Med. 2000;26:1193–200. doi: 10.1007/s001340000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briel M, Schuetz P, Mueller B, Young J, Schild U, Nusbaumer C, et al. Procalcitonin-guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch Intern Med. 2008;168:2000. doi: 10.1001/archinte.168.18.2000. [DOI] [PubMed] [Google Scholar]
- 7.Limper M, de Kruif MD, Duits AJ, Brandjes DP, van Gorp EC. The diagnostic role of procalcitonin and other biomarkers in discriminating infectious from non-infectious fever. J Infect. 2010;60:409–16. doi: 10.1016/j.jinf.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 8.de Kruif MD, Limper M, Gerritsen H, Spek CA, Brandjes DP, ten Cate H, et al. Additional value of procalcitonin for diagnosis of infection in patients with fever at the emergency department. Crit Care Med. 2010;38:457–63. doi: 10.1097/CCM.0b013e3181b9ec33. [DOI] [PubMed] [Google Scholar]
- 9.Lee CC, Chen SY, Tsai CL, Wu SC, Chiang WC, Wang JL, et al. Prognostic value of mortality in emergency department sepsis score, procalcitonin, and C-reactive protein in patients with sepsis at the emergency department. Shock. 2008;29:322–7. doi: 10.1097/shk.0b013e31815077ca. [DOI] [PubMed] [Google Scholar]
- 10.Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. 2004;39:206–17. doi: 10.1086/421997. [DOI] [PubMed] [Google Scholar]
- 11.Kim MH, Lim G, Kang SY, Lee WI, Suh JT, Lee HJ. Utility of procalcitonin as an early diagnostic marker of bacteremia in patients with acute fever. Yonsei Med J. 2011;52:276–81. doi: 10.3349/ymj.2011.52.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kushner I. Regulation of the acute phase response by cytokines. Perspect Biol Med. 1993;36:611–22. doi: 10.1353/pbm.1993.0004. [DOI] [PubMed] [Google Scholar]
- 13.Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem. 1999;265:501–23. doi: 10.1046/j.1432-1327.1999.00657.x. [DOI] [PubMed] [Google Scholar]
- 14.Ghasemi A, Zahediasl S. Normality tests for statistical analysis: a guide for non-statisticians. Int J Endocrinol Metab. 2012;10:486–9. doi: 10.5812/ijem.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 16.Ying GS, Maguire M, Quinn G, Kulp MT, Cyert L Vision in Preschoolers (VIP) Study Group. ROC analysis of the accuracy of Noncycloplegic retinoscopy, Retinomax Autorefractor, and SureSight Vision Screener for preschool vision screening. Invest Ophthalmol Vis Sci. 2011;52:9658–64. doi: 10.1167/iovs.11-8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar R, Indrayan A. Receiver operating characteristic (ROC) curve for medical researchers. Indian Pediatr. 2011;48:277–87. doi: 10.1007/s13312-011-0055-4. [DOI] [PubMed] [Google Scholar]
- 18.Brunkhorst FM, Wegscheider K, Forycki ZF, Brunkhorst R. Procalcitonin for early diagnosis and differentiation of SIRS, sepsis, severe sepsis, and septic shock. Intensive Care Med. 2000;26(Suppl 2):S148–52. doi: 10.1007/BF02900728. [DOI] [PubMed] [Google Scholar]
- 19.Endo S, Aikawa N, Fujishima S, Sekine I, Kogawa K, Yamamoto Y, et al. Usefulness of procalcitonin serum level for the discrimination of severe sepsis from sepsis: a multicenter prospective study. J Infect Chemother. 2008;14:244–9. doi: 10.1007/s10156-008-0608-1. [DOI] [PubMed] [Google Scholar]
- 20.Muller B, Becker KL, Schachinger H, Rickenbacher PR, Huber PR, Zimmerli W, et al. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit Care Med. 2000;28:977–83. doi: 10.1097/00003246-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Limper M, de Kruif MD, Ajubi NE, van Zanten AP, Brandjes DP, Duits AJ, et al. Procalcitonin as a potent marker of bacterial infection in febrile Afro-Caribbean patients at the emergency department. Eur J Clin Microbiol Infect Dis. 2011;30:831–6. doi: 10.1007/s10096-010-1150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linscheid P, Seboek D, Nylen ES, Langer I, Schlatter M, Becker KL, et al. In vitro and in vivo calcitonin I gene expression in parenchymal cells: a novel product of human adipose tissue. Endocrinology. 2003;144:5578–84. doi: 10.1210/en.2003-0854. [DOI] [PubMed] [Google Scholar]
- 23.Lee CC, Hong MY, Lee NY, Chen PL, Chang CM, Ko WC. Pitfalls in using serum C-reactive protein to predict bacteremia in febrile adults in the ED. Am J Emerg Med. 2012;30:562–9. doi: 10.1016/j.ajem.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Nahum E, Livni G, Schiller O, Bitan S, Ashkenazi S, Dagan O. Role of C-reactive protein velocity in the diagnosis of early bacterial infections in children after cardiac surgery. J Intensive Care Med. 2012;27:191–6. doi: 10.1177/0885066610396642. [DOI] [PubMed] [Google Scholar]
- 25.von Lilienfeld-Toal M, Dietrich MP, Glasmacher A, Lehmann L, Breig P, Hahn C, et al. Markers of bacteremia in febrile neutropenic patients with hematological malignancies: procalcitonin and IL-6 are more reliable than C-reactive protein. Eur J Clin Microbiol Infect Dis. 2004;23:539–44. doi: 10.1007/s10096-004-1156-y. [DOI] [PubMed] [Google Scholar]
- 26.Dandona P, Nix D, Wilson MF, Aljada A, Love J, Assicot M, et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79:1605–8. doi: 10.1210/jcem.79.6.7989463. [DOI] [PubMed] [Google Scholar]
- 27.Oberhoffer M, Stonans I, Russwurm S, Stonane E, Vogelsang H, Junker U, et al. Procalcitonin expression in human peripheral blood mononuclear cells and its modulation by lipopolysaccharides and sepsis-related cytokines in vitro. J Lab Clin Med. 1999;134:49–55. doi: 10.1016/s0022-2143(99)90053-7. [DOI] [PubMed] [Google Scholar]
- 28.Whicher J, Bienvenu J, Monneret G. Procalcitonin as an acute phase marker. Ann Clin Biochem. 2001;38:483–93. doi: 10.1177/000456320103800505. [DOI] [PubMed] [Google Scholar]
- 29.Su CP, Chen TH, Chen SY, Ghiang WC, Wu GH, Sun HY, et al. Predictive model for bacteremia in adult patients with blood cultures performed at the emergency department: a preliminary report. J Microbiol Immunol Infect. 2011;44:449–55. doi: 10.1016/j.jmii.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Charles PE, Ladoire S, Aho S, Quenot JP, Doise JM, Prin S, et al. Serum procalcitonin elevation in critically ill patients at the onset of bacteremia caused by either Gram negative or Gram positive bacteria. BMC Infect Dis. 2008;8:38. doi: 10.1186/1471-2334-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prat C, Sancho JM, Dominguez J, Xicoy B, Gimenez M, Ferra C, et al. Evaluation of procalcitonin, neopterin, C-reactive protein, IL-6 and IL-8 as a diagnostic marker of infection in patients with febrile neutropenia. Leuk Lymphoma. 2008;49:1752–61. doi: 10.1080/10428190802258956. [DOI] [PubMed] [Google Scholar]
- 32.Tavares E, Maldonado R, Ojeda ML, Minano FJ. Circulating inflammatory mediators during start of fever in differential diagnosis of Gram-negative and Gram-positive infections in leukopenic rats. Clin Diagn Lab Immunol. 2005;12:1085–93. doi: 10.1128/CDLI.12.9.1085-1093.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


