Abstract
Background & objectives:
Ciprofloxacin is commonly used in clinical practice for the treatment of recurrent urinary tract infections caused by Escherichia coli. However, very often these recurrent infections are due to a failure in a complete eradication of the microorganisms colonizing the urinary tract, especially in catheterized patients. To enhance the bactericidal activity of ciprofloxacin against biofilm-forming uropathogenic E. coli (UPECs), we examined its effect in combination with two pentacyclic triterpenes – asiatic and ursolic acids.
Methods:
The anti-biofilm activity of ciprofloxacin and pentacyclic triterpenes - asiatic acid (AA) and ursolic acid (UA), as well as their synergistic effect were tested on two types of surfaces - polystyrene microtiter plates and silicone catheters. It was investigated using the time-killing and biofilm assays.
Results:
Anti-biofilm activity of ciprofloxacin was not observed on microtiter plates or on the catheters. Ciprofloxacin combined with ursolic acid inhibited the biofilm formation on microtitre plates. This mixture, however, did not express such a strong activity against the synthesis of biofilm on the surface of catheters. Ciprofloxacin combined with asiatic acid had very weak inhibiting effect on the synthesis of biofilm mass on microtitre plates as well as on the catheters. Despite this, both mixtures – ciprofloxacin and asiatic acid, as well as ciprofloxacin and ursolic acid, exhibited strong and significant impact on the eradication of mature biofilm (P < 0.05).
Interpretation & conclusions:
Although ciprofloxacin is recommended in the treatment of urinary tract infections caused by UPECs, but its efficacy is arguable. Subinhibitory concentrations of ciprofloxacin did not inhibit the formation of biofilm. Pentacyclic triterpenes used in combination with ciprofloxacin enhanced its anti-biofilm effectiveness. However, this anti-biofilm activity was found to depend on the type of surface on which biofilm was formed.
Keywords: Asiatic acid, biofilm, ciprofloxacin, UPEC, ursolic acid
Uropathogenic Escherichia coli strains (UPECs) are frequent cause of nosocomial infections associated with the use of urinary catheters. Due to the ability to form biofilm on biomaterials, UPECs are responsible for the recurrent and chronic infections of the urinary tract1. It is known that bacteria growing in biofilm are less susceptible to multifarious antimicrobial agents than their planktonic forms. Ciprofloxacin (CIP) is recommended empirical antibiotic used in urinary tract infections1. However, many reports indicate that the widespread use of CIP is contributing to the increasing number of resistant E. coli strains2,3. For this reason, many studies have been focused on identifying new compounds that have antimicrobial activity against microorganisms. Natural plant products and/or their combinations with antibiotics seem to be a promising solution. Pentacyclic triterpenes (PTs) present in plants occur in the free acid form or as aglycones. Among the free triterpenic acids, asiatic acid (2α,3β,23-trihydroxyurs-12-en-28-oic, AA) and ursolic acid (3β-hydroxyurs-12-en-28-oic, UA) are secondary metabolites of plants with a wide spectrum of pharmacological activities. AA is the main active constituent of the tropical medicinal plant Centella asiatica4. UA has been found in plants belonging to the Ericaceae, Rosaceae and Lamiaceae families5. Antimicrobial activities of AA and UA, mainly against Gram-positive bacteria, has been reported6,7,8,9. Little is known about their anti-biofilm activity. Previous studies have demonstrated that UA affects genes expression involved in sulphur metabolism, stress responses and biofilm formation10,11. AA has not previously been studied for its potential anti-biofilm properties.
The aim of the present investigation was to assess the anti-biofilm activity of CIP alone and in combination with AA and UA on biofilm formation by UPECs.
Material & Methods
This study was carried out in the Department of Biology and Medical Parasitology, Medical University, Wrocław, Poland from September 2011 to November 2012.
Bacterial strains: The uropathogenic reference E. coli CFT073 strain (ATCC 700928) and 10 clinical UPECs isolated from the urine specimens of patients with pyelonephritis hospitalized in the Academic Clinical Centre of the Wroclaw Medical University were used. E. coli identification was done by biochemical methods using the API-20E test kit (BioMιrieux, Poland). The strains were maintained on Mueller-Hinton (M-H) agar slopes (Oxoid, UK) at 4°C.
Phylogenetic classification and detection of biofilm-related genes: Total DNA was isolated from overnight bacterial culture using GeneMATRIX Bacterial & Yeast Genomic DNA Purification Kit (EURx, Poland). All PCR reactions were performed using DreamTaq™ DNA polymerase (Fermentas, Germany). Phylogenetic group was determined using primers specific for two genes (chuA and yjaA) and DNA fragment (TspE4.C2) according to Clermont et al12. The isolates were also screened for the presence of genes important for biofilm formation luxS, rpoS, sdiA, mqsR, ant43, yceP, yliH, cspG, bolA (www.ncbi.nlm.nih.gov/genbank). Sequence coding for 16SrRNA was used as a positive control13. Targeted genes as well as primer sequences for the amplification procedures are given in the Table. After separation in 1.5 per cent agarose gel, PCR amplification products were visualized and analyzed using the Quantity One Software (Bio-Rad, USA) (Fig. 1).
Table.
Primer sequences used in PCR for determination of E. coli phylogenetic group and detection of biofilm-related genes
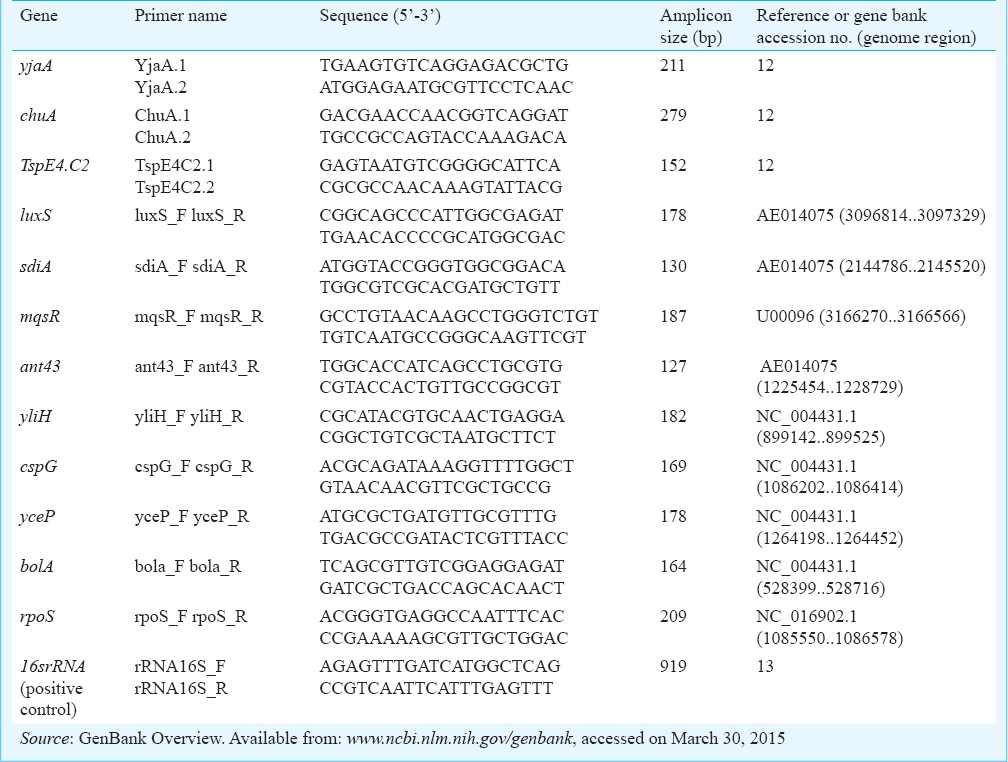
Fig. 1.
Agarose gel electrophoresis of PCR products. (A) phylogenetic analysis. Lanes: 1 – yjaA, 2 – chuA (upper band), TspE4.C2 (lower band), 3 – 16SrRNA (control), (B) biofilm-related genes. Lanes:1 – luxS, 2 – sdiA, 3 – mqsR, 4 – ant43, 5 – yliH, 6 – cspG, 7 – yceP, 8 – bolA, 9 – rpoS. M – molecular size markers (100 bp, Fermentas). Lane 1, 178 bp; Lane 2, 130 bp; Lane 3, 187 bp; Lane 4, 127 bp; Lane 5, 182 bp; Lane 6, 169 bp; Lane 7, 178 bp; Lane 8, 164 bp; Lane 9, 209 bp.
Antimicrobial agents: Ciprofloxacin lactate (Proxacin®, Poland) was used in the experiment. AA (purity ≥ 97%) and UA (purity ≥ 90%) were purchased from Sigma-Aldrich (Poland). The acids were dissolved in 96 per cent ethanol (heated to 70°C) as 10 mg/ml stock solutions and stored at -20°C. For all experiments CIP and PTs were prepared by diluting with Mueller-Hinton Broth (MHB).
MIC determination: MICs were determined by the broth microdilution method outlined by CLSI (Clinical and Laboratory Standards Institute)14. In our study the MICs of CIP were 0.007–0.031 μg/ml. MICs of PTs ranged from 512 to 1024 μg/ml.
Biofilm formation assay and quantification: The biofilm formation assay was performed according to O’Toole and Kolter15 with slight modifications. In brief, 20 μl of each diluted culture (1-2×108 cfu/ml) was inoculated into six wells of a 96-well polystyrene plate containing 200 μl of m0 ueller-Hinton broth (MHB). After incubation for 6, 12, 18, 24, 48, 72 and 96 h at 37°C the wells were rinsed thoroughly with phosphate buffered saline (PBS) to remove nonadherent bacteria. Bacterial cells bound to the walls of the wells were stained with one per cent (w/v) crystal violet (Sigma-Aldrich, Poland) for 15 min, and then rinsed thoroughly with PBS. The dye bound to the adherent bacterial cells was resolubilized with 95 per cent (v/v) ethanol. The optical density (OD) of each well was measured at 590 nm using a plate reader (Infinite® 200 PRO, TECAN, Switzerland). In each plate four wells were used as blanks containing MHB medium only. The cut-off OD (ODc) value was defined as three standard deviations (SD) above the mean OD of the negative control. In our study the ODc value was 0.04. On the basis of ODs of bacterial biofilms E. coli isolates were classified into four categories: OD ≤ ODc no biofilm producer; ODc < OD ≤ 2×ODc weak biofilm producer; 2×ODc < OD ≤ 4×ODc moderate biofilm producer; 4×ODc < OD strong biofilm producer16.
Biofilm formation on microtitre plates and count of live bacteria in biofilm in the presence of PTs and CIP: This experiment was performed according to the method described by di Bonaventura et al17. Briefly, AA and UA were tested at a concentration of 50 μg/ml, and CIP was tested at subinhibitory concentration (0.5×MIC) to study their effect on biofilm formation. The solutions of individual PT, CIP and their mixtures: CIP+AA, CIP+UA prepared in 200 μl of MHB were added to microtitre wells containing 20 μl of diluted culture of bacteria. After 6, 12, 18, 24, 48, 72 and 96 h of incubation, quantities of biofilms were measured. Drug-free medium was used in control wells. Bacterial survival in biofilm was established after each time of incubation. Bacteria were washed three times with sterile PBS to remove nonadherent bacteria. Biofilms were manually scraped using a sterile spatula and transferred into microtubes containing 10 μl of PBS. The tubes were centrifuged to separate cells from the biofilm matrix. The cfu (colony forming unit) was assessed by plating serial dilutions on nutrient agar. Each experiment was conducted in triplicate.
Visualization of biofilms by 4’,6-diamidino-2-phenylindole (DAPI) staining: For visualization by fluorescence microscopy, the biofilms were allowed to grow on polystyrene pieces (0.5×0.5 cm) placed in MHB supplemented with and without CIP, CIP+AA and CIP+UA. After 6, 12, 18, 24, 48, 72 and 96 h at 37°C the polystyrene pieces were washed with PBS and stained with DAPI solution (Merck, Germany). After 10 min of staining in the dark, the DAPI solution was removed by rinsing with PBS. The polystyrene pieces with biofilm were dried in the dark at room temperature and analysed by fluorescence microscopy (Nikon Eclipse 400, Poland). Biofilms were analysed with 1000-fold magnification18.
Biofilm formation on urological catheters and count of live bacteria in biofilm in the presence of PTs and CIP: To define the degree of biofilm production a silicone-coated latex Foley catheters and a one per cent TTC solution (2,3,5-triphenyltetrazolium chloride) were used19. Prepared sterile fragments of the catheters were placed into tubes containing a suspension of the tested isolate with the addition of CIP, PTs and their mixtures: CIP+AA and CIP+UA. After the specified period of incubation (6, 12, 18, 24, 48, 72 and 96 h) catheters were rinsed in sterile PBS, inserted into tubes containing MHB and TTC solution and incubated at 37°C for 24 h. Catheters were rinsed again with PBS and assessed for degree of reduction of TTC to the red formazan by viable bacteria growing in the biofilm according to the following scale: +1 - slight pink dots on the catheter surface, +2 – pink colouring of the entire surface, +3 – pink colouring of the entire surface of the biomaterial with the dark-coloured spots. A catheter fragment incubated in MHB without bacterial suspension was used as a negative control. In addition to the visual analysis of biofilm formation the number of viable bacteria in the nutrient agar plates was also determined. The biofilm was taken off from the surface of the catheters by sonication. The obtained suspensions were diluted and inoculated on nutrient agar plates. The results were given as the mean number of cfu/ml. Experiment was repeated thrice.
Effect of PTs and CIP on biofilm eradication: The 24 h catheter-biofilms were exposed to individual CIP, PTs and mixtures: CIP+AA and CIP+UA. After the next 24 h incubation all samples were sonicated. The obtained bacterial suspensions were diluted and plated on nutrient agar plates to calculate the average number of viable bacterial cells (cfu/ml). Each experiment was repeated thrice.
Statistical analysis: All values were expressed as a mean ± SD. The differences in biofilm formation and the number of viable bacteria exposed to antimicrobial agents and unexposed were analyzed by a parametric t-test for independent samples. Statistical calculations were made using Statistica 9.0. (Stat Soft, Poland)
Results
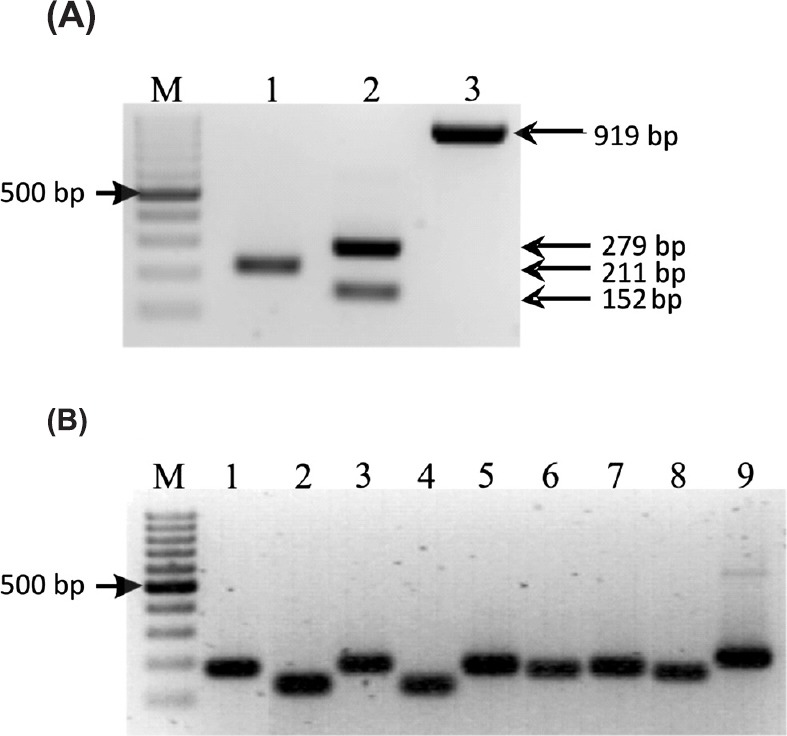
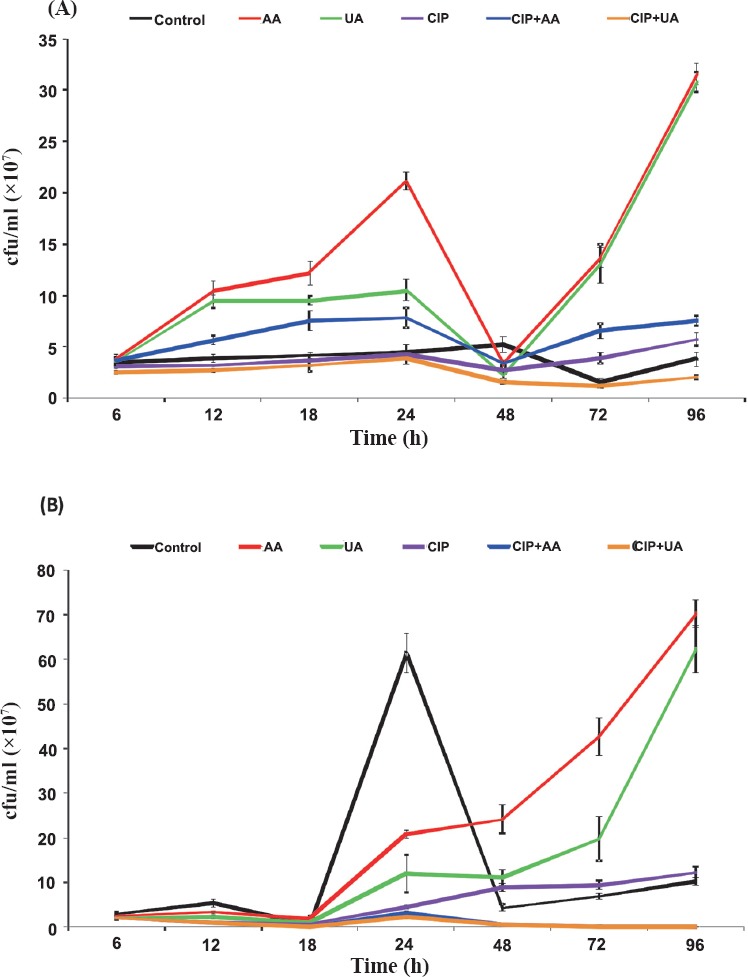
Biofilm formation on microtitre plates and count of live bacteria in biofilm in the presence of PTs and CIP: Results showing the influence of CIP, AA, UA and their mixtures CIP+AA and CIP+UA on biofilm formation in microtitre plates by clinical UPECs are given in Fig. 2A. After 6 h of incubation bacteria did not produce biofilm (OD < 0.04). In 12 h samples, the inhibitory effect of mixtures CIP+AA and CIP+UA on biofilm formation was noticed. After 18 h of incubation bacteria in all samples produced weak biofilm (0.04 < OD < 0.08). In 24 h samples bacteria produced moderate (control, CIP, AA, and UA) or weak (CIP+AA, CIP+UA) biofilms. The amount of biofilm decreased after 48 h of incubation. The mean OD values in five of six samples were 0.056–0.074. The bacteria treated with CIP+UA did not produce biofilm after 48 h incubation. The 72 h control sample and cultures containing CIP, AA, UA, and CIP+AA produced weak biofilm. Mean OD values ranged from 0.048 to 0.075. Bacteria treated with CIP+UA did not form biofilm mass in comparison with control sample (P<0.05). After 96 h incubation bacteria produced weak biofilm in the control and the samples containing CIP, AA, UA and CIP+AA (ODs 0.045–0.062). The isolates treated with CIP+UA did not form biofilm. OD value was 0.028.
Fig. 2.
Biofilm production (A) and bacterial survival (B) on microtitre plates. Values represent the mean ±SD for ten clinical UPECs. CIP, ciprofloxacin; AA, asiatic acid; UA, ursolic acid. *P≤ 0.05 compared with control.
The bacterial survival in biofilm mass was determined after each time of incubation (Fig. 2B). In the period between 6 and 18 h of incubation the number of bacteria increased and bacterial survival in all tested samples, except UA, was lower than 100 per cent in comparison with control. In the 24 h control sample the cfu/ml was 9.4×109. In all tested samples the percentage of bacterial survival was above 100 per cent. After 48 h of incubation the number of cells per ml decreased to 5.4×109 in the control suspension. In the examined samples the bacterial survival also decreased, but still was higher than the value of cfu/ml noticed in control sample. In 72 h control biofilm mass the cfu/ml was 4.7×109. The significant decrease (P < 0.05) in bacterial survival was observed only in the samples containing CIP+AA (29 per cent of control sample) and CIP+UA (7 per cent of control sample). After 96 h of incubation the number of bacterial cells per ml in the control was 3.8×109. The number of live bacteria decreased even more in comparison with 72 h biofilm samples. After 96 h of incubation the most effective bactericidal activities were detected in bacterial cultures treated with CIP+UA (0.11×109 cfu/ml) and CIP+AA (0.68×109 cfu/ml).
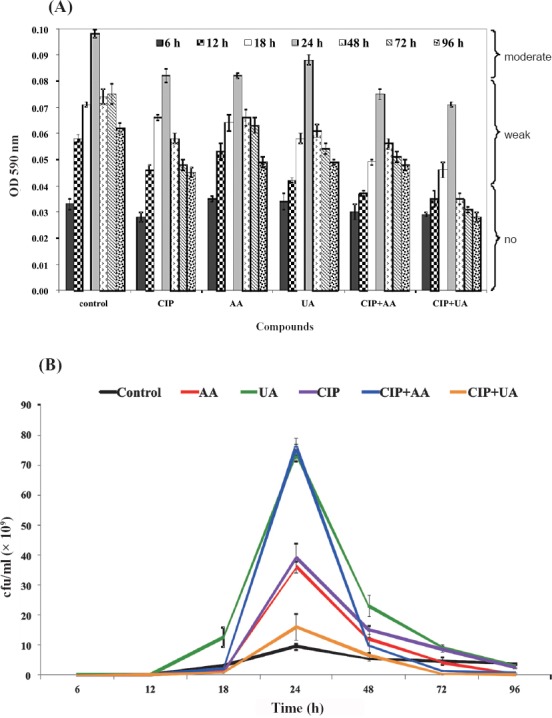
The synthesis of biofilm mass by reference CFT073 strain is shown in Fig. 3A. The OD values indicated that CFT073 was weaker biofilm producer than clinical UPECs. During the whole period of incubation (6-96 h) CFT073 produced weak biofilm in control samples (0.04 < OD < 0.08). The most effective activity against biofilm formation showed mixture containing CIP+UA. Bacteria incubated in the presence of these compounds did not produce biofilm after 6, 12, 24, 72 and 96 h. The only significant result was noticed for CIP+UA after 24 h of incubation.
Fig. 3.
Biofilm production (A) and bacterial survival (B) on microtitre plates. Values represent the mean ±SD of three separate experiments for CFT073. CIP, ciprofloxacin; AA, asiatic acid; UA, ursolic acid. *P≤ 0.05 compared with control.
The bacterial survival of CFT073 in biofilm decreased in control and all tested samples between 6-18 h of incubation (Fig. 3B). In 24 h cultures, only mixtures CIP+AA and CIP+UA decreased the number of viable bacteria in comparison with control sample. After 48 h of incubation in all examined samples the percentage of bacterial survival was lower than in the control. Similar results were noticed in 72 and 96 h cultures except bacteria treated with CIP. The most effective bactericidal activity were detected in bacterial biofilms treated with CIP+UA (0.0057×107 cfu/ml) and UA (0.013×107 cfu/ml).
Visualization of biofilms by DAPI staining: To gain information about the differences in the structural features of the biofilms formed by UPECs, 6, 12, 18, 24, 48, 72, and 96 h old biofilms were examined by fluorescence microscopy. Fig. 4 illustrates representative patterns for UPECs biofilm cells after 24 and 96 h of accumulation on polystyrene pieces. After 24 h of growth, UPECs formed large aggregates characteristic for mature biofilms. The formation of these aggregates was suppressed when these cells were grown in presence of CIP+AA and CIP+UA. Interestingly, in biofilms treated with CIP, morphologically altered bacterial cells were observed. The size of bacterial aggregates significantly decreased in 96 h control sample. Microscopic examination also confirmed that this effect was more evident in the biofilm cultures treated with CIP+AA and CIP+UA.
Fig. 4.
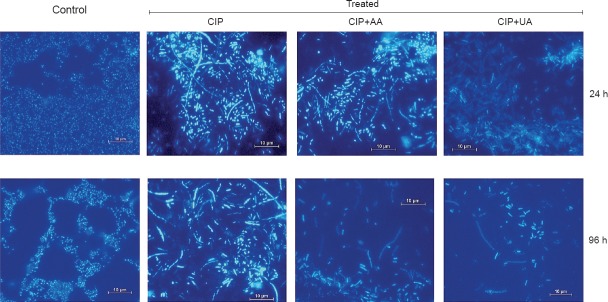
Fluorescence microscopy images (×1000) revealing the antibiofilm ability of CIP, CIP+AA, CIP+UA against clinical UPECs. CIP, ciprofloxacin; AA, asiatic acid; UA, ursolic acid.
Biofilm formation on urological catheters and count of live bacteria in biofilm in presence of PTs and CIP: A visual assessment of biofilm formation and its reduction after the application of PTs and CIP was done. The intensity of the catheter colour was interpreted as the degree of biofilm formation. The darker colour of the catheter surface corresponded to the higher number of live bacteria. It was observed that the bacterial survival on catheters marked as +1 was lower than 3.5×107 cfu/ml. The bacterial survival in biofilm on catheters marked as +2 ranged from 3.7×107 to 1.2×108 cfu/ml. The survival of bacteria noticed on catheters signed as +3 ranged from 1.3×108 to 3.2×108 cfu/ml. Simultaneously the number of viable bacteria in the biofilm mass formed on catheters was assessed (Fig. 5).
Fig. 5.
Bacterial survival in biofilm produced on urinary catheters by clinical UPECs (A) and CFT073 (B). Values shown in Fig. 5A are the mean ±SD for 10 UPECs. Values shown in Fig. 5B are the mean ±SD of three separate experiments for CFT073. CIP, ciprofloxacin; AA, asiatic acid; UA, ursolic acid.
The number of bacterial cells in control catheter biofilms depended on the phase of biofilm development. The cfu/ml were 3.5×107, 3.9×107, 4.2×107, 4.4×107, 5.3×107, 1.6×107, and 3.8×107 in 6, 12, 18, 24, 48, 72, and 96 h controls, respectively (Fig. 5A). After 6, 12, 18, and 24 h of incubation the bacterial survival decreased slightly only in examined samples containing CIP and CIP+UA. After the next day (48 h) of exposure of bacteria to antimicrobials a significant decrease of the bacterial survival was noticed in all tested biofilms (P < 0.05). The percentage of viable cells ranged from 29 to 65 per cent in comparison to the control sample. The bacterial survival in 72 h biofilms increased in all tested samples except the sample containing CIP+UA. In 96 h biofilm masses the viability of rods decreased, but the anti-biofilm activity was detected only in the case of CIP+UA (P < 0.05). The results of CFT073 strain survival are shown in Fig. 5B. The cfu/ml in controls were 3.0×107, 5.4×107, 4.9×106, 6.1×108, 4.4×107, 6.9×107, and 1.0×108 in 6, 12, 18, 24, 48, 72, and 96 h samples, respectively. After 6, 12 and 24 h of incubation the percentage of bacterial survival in all tested cultures was lower than in control. The mixtures of CIP+AA and CIP+UA showed significant (P<0.05) inhibitory effect on bacterial growth in catheter-biofilms after 18, 48, 72 and 96 h of incubation.
Effect of PTs and CIP on biofilm eradication: The synergistic effect of CIP and PTs in the process of eradication of biofilm from the surface of silicon urological catheters was examined. The mean cfu/ml in 24 h biofilms (controls) were 9.4×107 and 2.5×108 for clinical isolates and CFT073, respectively. CIP had no influence on biofilm eradication. The percentage of surviving bacteria was 192 in case of clinical UPECs and 188 for CFT073 (Fig. 6). Used individually, AA and UA slightly reduced biofilm mass. The most effective action was shown by a combination of CIP+AA, reducing the number of viable bacteria in biofilm mass to 12 per cent for clinical UPECs and 5 per cent in case of CFT073 (P ≤ 0.05). The mixture of CIP+UA reduced cells viability to 29 and 18 per cent for clinical UPECs and CFT073, respectively (P ≤ 0.05).
Fig. 6.
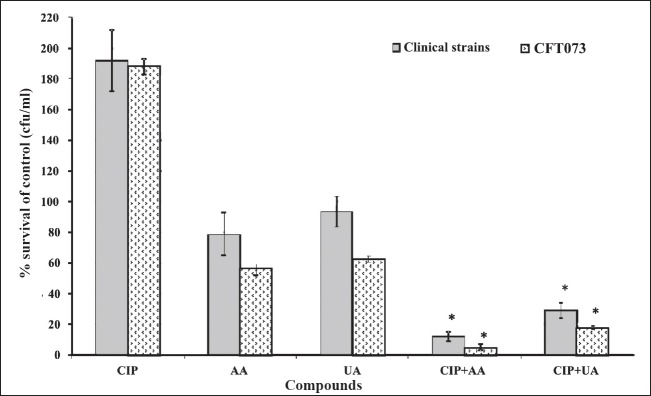
Effect of CIP and PTs on eradication of the biofilm mass from surface of catheters. Values represent the mean value for 10 clinical UPECs and the mean value of three separate experiments for CFT073. CIP, ciprofloxacin; AA, asiatic acid; UA, ursolic acid. *P<0.05 compared with control.
Discussion
E. coli are the most commonly isolated bacteria from catheterised patients20. CIP is one of the most effective antibiotics against UPECs infections1. However, eradication of the infection remains sometimes barely achievable with this antimicrobial. The reason of this phenomenon may be formation of antibiotic-resistant biofilm mass by bacteria causing infection. The concentrations of antimicrobials required to inactivate the biofilm cells are much higher than the concentrations of drugs used for the inactivation of planktonic forms. On the other hand, such high doses cannot be used in the treatment of infections due to their toxicity for the host. Therefore, the effectiveness of CIP and its combination with PT on the formation and eradication of biofilm was tested. AA and UA were selected for this study because of their antibacterial activities noticed in previous studies9,21. We established that UPECs showed the loss of virulence factors important in biofilm formation at the concentration of 50 μg/ml of AA and UA. It is also known that UA has anti-biofilm activity10,11,22 and AA increases the sensitivity of biofilms to antibiotics23. Ren et al10 found that UA inhibited biofilm formation in E. coli, Pseudomonas aeruginosa and Vibrio harveyi strains when added to inoculum or to 24 h biofilms. Zhou et al22 noticed that 1/4 MIC of UA affected biofilm formation by Streptococcus mutans and S. gordonii, while lower concentrations of this PT (1/8 and 1/16 MICs) hardly prevented biofilm development. Kurek et al24 demonstrated the synergistic effect of UA combined with ampicillin or oxacillin on biofilm formed by Staphylococcus epidermidis. The substantial decreases in biofilms formed by S. aureus and Listeria monocytogenes were noticed only in combination of UA and ampicillin. Neither UA+ampicillin nor UA+oxacillin affected the biofilm mass formation by P. aeruginosa.
Garo et al23 evaluated the activity of AA used alone and in combination with tobramycin and CIP on P. aeruginosa biofilms. When applied alone, AA did not reduce the cell viability of P. aeruginosa biofilms. However, this PT increased the susceptibility of biofilm bacteria to tobramycin and CIP.
The inhibitory effect of CIP and PTs on bacterial growth was found to be time incubation dependent. The lowest percentage of bacterial survival in biofilms growing on microtitre plates was noticed after 96 h of incubation of CFT073 and clinical UPECs with both, CIP+AA and CIP+UA. Such effect was observed on catheters’ surfaces for clinical isolates treated with CIP+UA, and CFT073 treated with CIP+AA and CIP+UA. The results showed that clinical isolates were less susceptible when treated with CIP and PTs. The visual analysis of biofilm formation on catheters’ surfaces showed that bacteria treated with CIP+AA and CIP+UA produced less biofilm mass than bacteria growing in the presence of CIP. The differences in the inhibition of the biofilm formation on the microtitre plates and the catheters could be due to the structure of the surface on which the biofilm was formed. Inhibition of biofilm formation was more effective on the polystyrene surface of microtitre plates than the silicone surface of catheters. These differences are probably due to the chemical composition and the topography of these surfaces. It is known that the level of biofilm production is material dependent25 and roughness of surfaces promotes the formation and maturation of biofilm26. In our study, UA combined with CIP more effectively inhibited biofilm formation than AA combined with CIP. Hou et al27 reported that methyl group present at C-23 of corosolic, oleanolic and maslinic acids was important in suppressing α-glucosidase activity. It is worth noting that UA used in our study contains CH3 group at C-23 what makes it more active against biofilm growth than AA.
The eradication of mature biofilms is a major problem in the treatment of chronic bacterial infections. The resistance of bacteria growing in biofilm to antibiotics can be caused by numerous factors28,29,30. Exopolysaccharide matrix is one of the main factors being a barrier for drug penetration31. CIP used in our study showed no inhibitory effect on the bacterial growth in mature biofilm. In contrast, Balaji et al32 reported that CIP at subinhibitory concentrations efficiently inhibited the biofilm formation by S. pyogenes. Del Pozo and Patel33 have established that the treatment of bacterial biofilms with antibiotics may lead to eradication of most of the susceptible and metabolically active bacteria. However, the small number of persister cells located in the deeper biofilm layers, exposed to sublethal doses of antimicrobials, can survive and be able to reconstitute the biofilm mass. It has been shown that CIP penetrates the biofilms formed by E. coli CFT073, P. aeruginosa, Klebsiella pneumoniae strains better than other antibiotics when it is used in concentrations above MICs34,35,36.
In our study, individually used PTs showed a negligible effect on biofilm eradication. The combination of CIP with PTs showed the ability to disrupt mature biofilm mass on catheters. The bacterial survival of clinical UPECs treated with the combination of CIP+AA decreased to 12 per cent, and to 29 per cent in case of CIP+UA. CFT073 used in our study displayed greater sensitivity to CIP+AA and CIP+UA. It was observed that the mixture of AA and CIP better eradicated mature biofilms than UA combined with CIP. It might be associated with the chemical nature of PTs. AA possesses three hydroxyl groups (at position C-2, C-3, C-23) that make it hydrophilic. UA has only one hydroxyl group (at position C-3) and, therefore, is hydrophobic. Probably due to its hydrophilic nature, AA better penetrates into biofilm structure and can improve antimicrobial activity of CIP. The bactericidal effect of CIP may also be enhanced by the acidic character of PTs. The changes in pH of the growth medium caused by PTs can disturb the functioning of membrane-bound proton pumps in bacterial cells and promote a biocidal effect of antimicrobials37. Such a mechanism of action might explain the synergistic effect of CIP and PT used in the current study.
Acknowledgment
The authors acknowledge Dr Andrzej Hendrich for intellectual discussions, and Dr Ewa Lewczyk, Head, Bacteriological Laboratory of the Academic Clinical Centre of the Wroclaw Medical University for isolation and species biochemical identification of bacterial strains. This research work was financially supported by a project from the Wroclaw Medical University, Wroclaw, Poland (protocol number: 5-S/PD-SN/2011).
References
- 1.Wagenlehner FME, Wullt B, Perletti G. Antimicrobials in urogenital infections. Int J Antimicrob Agents. 2011;38:3–10. doi: 10.1016/j.ijantimicag.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Mandal J, Acharya NS, Buddhapriya D, Parija SC. Antibiotic resistance pattern among common bacterial uropathogens with a special reference to ciprofloxacin resistant Escherichia coli. Indian J Med Res. 2012;136:842–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Pallett A, Hand K. Complicated urinary tract infections: practical solutions for the treatment of multi-resistant Gram-negative bacteria. J Antimicrob Chemother. 2010;65(Suppl 3):iii25–33. doi: 10.1093/jac/dkq298. [DOI] [PubMed] [Google Scholar]
- 4.Puttarak P, Panichayupakaranant P. Factors affecting the content of pentacyclic triterpenes in Centella asiatica raw materials. Pharm Biol. 2012;50:1508–12. doi: 10.3109/13880209.2012.685946. [DOI] [PubMed] [Google Scholar]
- 5.Babalola IT, Shode FO. Ubiquitous ursolic acid: a potential pentacyclic triterpene natural product. J Pharm Phytochem. 2013;2:214–22. [Google Scholar]
- 6.Fontanay S, Grare M, Mayer J, Finance C, Duval RE. Ursolic, oleanolic and betulinic acids: antibacterial spectra and selectivity indexes. J Ethnopharmacol. 2008;120:272–6. doi: 10.1016/j.jep.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Kurek A, Grudniak AM, Szwed M, Klicka A, Samluk £, Wolska KI. Oleanolic acid and ursolic acid affect peptidoglycan metabolism in Listeria monocytogenes. Anton Leeuw Int J G. 2010;97:61–8. doi: 10.1007/s10482-009-9388-6. [DOI] [PubMed] [Google Scholar]
- 8.Filocamo A, Bisignano C, D’Arrigo M, Ginestra G, Mandalari G, Galati EM. Norfloxacin and ursolic acid: in vitro association and postantibiotic effect against Staphylococcus aureus. Lett Appl Microbiol. 2011;53:193–7. doi: 10.1111/j.1472-765X.2011.03090.x. [DOI] [PubMed] [Google Scholar]
- 9.Wojnicz D, Tichaczek-Goska D, Kicia M. Effect of asiatic and ursolic acids on growth and virulence factors of uropathogenic Escherichia coli strains. Turk J Biol. 2013;37:556–64. [Google Scholar]
- 10.Ren D, Zuo R, Gonzalez Barrios AF, Bedzyk LA, Eldridge GR, Pasmore ME, et al. Differential gene expression for investigation of Escherichia coli biofilm inhibition by plant extract ursolic acid. Appl Environ Microbiol. 2005;71:4022–34. doi: 10.1128/AEM.71.7.4022-4034.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grudniak AM, Kurek A, Szarlak J, Wolska KI. Oleanolic and ursolic acids infuence the expression of the cysteine regulon and the stress response in Escherichia coli. Curr Microbiol. 2011;62:1331–6. doi: 10.1007/s00284-010-9866-0. [DOI] [PubMed] [Google Scholar]
- 12.Clermont O, Bonacorsi S, Bingen E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol. 2000;66:4555–8. doi: 10.1128/aem.66.10.4555-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li D, Liu B, Chen M, Guo D, Guo X, Liu F, et al. A multiplex PCR method to detect 14 Escherichia coli serogroups associated with urinary tract infections. J Microbiol Methods. 2010;82:71–7. doi: 10.1016/j.mimet.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Wayne, PA: CLSI; 2008. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. M100-S18. 17th Informational Supplement. [Google Scholar]
- 15.O’Toole GA, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 16.Stepanovic S, Vukovic D, Hola V, Di Bonaventura G, Djukic S, Cirkovic I, et al. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. Acta Path Micro Im B. 2007;115:891–9. doi: 10.1111/j.1600-0463.2007.apm_630.x. [DOI] [PubMed] [Google Scholar]
- 17.di Bonaventura G, Spedicato I, D’Antonio D, Robuffo I, Piccolomini R. Biofilm formation by Stenotrophomonas maltophita modulation by quinolones, trimetoprim-sulfamethoxazole, and ceftazidime. Antimicrob Agents Chemother. 2004;48:151–60. doi: 10.1128/AAC.48.1.151-160.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hannig C, Hannig M, Rehmer O, Brauna G, Hellwig E, Al-Ahmada A. Fluorescence microscopic visualization and quantification of initial bacterial colonization on enamel in situ . Arch Oral Biol. 2007;52:1048–56. doi: 10.1016/j.archoralbio.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Budzynska A, Wieckowska-Szakiel M, Sadowska B, Kalemba D, Rozalska B. Antibiofilm activity of selected plant essential oils and their major components. Pol J Microbiol. 2011;60:35–41. [PubMed] [Google Scholar]
- 20.Bonkat G, Widmer AF, Rieken M, van der Merwe A, Braissant O, Müller G, et al. Microbial biofilm formation and catheter-associated bacteriuria in patients with suprapubic catheterisation. World J Urol. 2013;31:565–71. doi: 10.1007/s00345-012-0930-1. [DOI] [PubMed] [Google Scholar]
- 21.Wojnicz D, Kicia M, Tichaczek-Goska D. Effect of asiatic and ursolic acids on morphology, hydrophobicity and adhesion of UPECs to uroepithelial cells. Folia Microbiol. 2013;58:245–52. doi: 10.1007/s12223-012-0205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou L, Ding Y, Chen W, Zhang P, Chen Y, Lv X. The in vitro study of ursolic acid and oleanolic acid inhibiting cariogenic microorganisms as well as biofilm. Oral Dis. 2013;19:494–500. doi: 10.1111/odi.12031. [DOI] [PubMed] [Google Scholar]
- 23.Garo E, Eldridge GR, Goering MG, de Lancey Pulcini E, Hamilton MA, Costerton JW, et al. Asiatic acid and corosolic acid enhance the susceptibility of Pseudomonas aeruginosa biofilms to tobramycin. Antimicrob Agents Chemother. 2007;51:1813–7. doi: 10.1128/AAC.01037-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurek A, Nadkowska P, Pliszka S, Wolska KI. Modulation of antibiotic resistance in bacterial pathogens by oleanolic acid and ursolic acid. Phytomedicine. 2012;19:515–9. doi: 10.1016/j.phymed.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Rogers J, Dowsett AB, Dennis PJ, Lee JV, Keevil CW. Influence of plumbing materials on biofilm formation and growth of Legionella pneumophila in potable water systems. Appl Environ Microbiol. 1994;60:1842–51. doi: 10.1128/aem.60.6.1842-1851.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor RL, Verran J, Lees GC, Ward AJP. The influence of substratum topography on bacterial adhesion to polymethyl methacrylate. J Mater Sci Mater Med. 1998;9:17–22. doi: 10.1023/a:1008874326324. [DOI] [PubMed] [Google Scholar]
- 27.Hou W, Li Y, Zhang Q, Wei X, Peng A, Chen L, et al. Triterpene acids isolated from Lagerstroemia speciosa leaves as α-glucosidase inhibitors. Phytother Res. 2009;23:614–8. doi: 10.1002/ptr.2661. [DOI] [PubMed] [Google Scholar]
- 28.Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother. 2001;45:999–1007. doi: 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–8. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 30.Stewart PS. Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbiol. 2002;292:107–13. doi: 10.1078/1438-4221-00196. [DOI] [PubMed] [Google Scholar]
- 31.Leid JG, Willson CJ, Shirtliff ME, Hassett DJ, Parsek MR, Jeffers AK. The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-gamma-mediated macrophage killing. J Immunol. 2005;175:7512–8. doi: 10.4049/jimmunol.175.11.7512. [DOI] [PubMed] [Google Scholar]
- 32.Balaji K, Thenmozhi R, Pandian SK. Effect of subinhibitory concentrations of fluoroquinolones on biofilm production by clinical isolates of Streptococcus pyogenes. Indian J Med Res. 2013;137:963–71. [PMC free article] [PubMed] [Google Scholar]
- 33.Del Pozo JL, Patel R. The challenge of treating biofilm-associated bacterial infections. Clin Pharmacol Ther. 2007;82:204–9. doi: 10.1038/sj.clpt.6100247. [DOI] [PubMed] [Google Scholar]
- 34.Rivardo F, Martinotti MG, Turner RJ, Ceri H. Synergistic effect of lipopeptide biosurfactant with antibiotics against Escherichia coli CFT073 biofilm. Int J Antimicrob Agents. 2011;37:324–31. doi: 10.1016/j.ijantimicag.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Walters MC, Roe F, Bugnicourt A, Franklin MJ, Stewart PS. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob Agents Chemother. 2003;47:317–23. doi: 10.1128/AAC.47.1.317-323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderl JN, Franklin MJ, Stewart PS. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob Agents Chemother. 2000;44:1818–24. doi: 10.1128/aac.44.7.1818-1824.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garrett TR, Bhakoo M, Zhang Z. Bacterial adhesion and biofilms on surfaces. Prog Nat Sci. 2008;18:1049–56. [Google Scholar]


