Abstract
Here, we show that a CD40L-adjuvanted DNA/modified vaccinia virus Ankara (MVA) simian immunodeficiency virus (SIV) vaccine enhances protection against a pathogenic neutralization-resistant mucosal SIV infection, improves long-term viral control, and prevents AIDS. Analyses of serum IgG antibodies to linear peptides of SIV Env revealed a strong response to V2, with targeting of fewer epitopes in the immunodominant region of gp41 (gp41-ID) and the V1 region as a correlate for enhanced protection. Greater expansion of antiviral CD8 T cells in the gut correlated with long-term viral control.
TEXT
We recently demonstrated that CD40L, a costimulatory molecule for dendritic cells (DCs) and B cells, can serve as an adjuvant for enhancing protection against mucosal challenge with a moderately neutralization-sensitive heterologous simian immunodeficiency virus (SIV), SIVsmE660, in rhesus macaques (1, 2). Here, we investigated the potential of CD40L as an adjuvant to enhance protection mediated by a DNA/modified vaccinia virus Ankara (MVA) SIV vaccine against neutralization-resistant intrarectal SIVmac251 infection. In the present study, we adjuvanted both DNA and MVA vaccines, whereas in a previous study (1) we adjuvanted only the DNA vaccine.
Three groups (n = 10 per group) of Indian rhesus macaques (RMs) were studied. The DM group was inoculated intramuscularly at weeks 0 and 8 with 3 mg of a DNA SIV vaccine (DNA/SIV) and boosted with 108 PFU of an MVA SIV vaccine (MVA/SIV) at weeks 16 and 24. At the same times, the D40LM40L group was inoculated with 3 mg of DNA/SIV plus the CD40L adjuvant (DNA/SIV-40L) (1) and 108 PFU of MVA/SIV premixed with 106 PFU of CD40L-expressing MVA vaccine (MVA/CD40L). We chose to use a very low dose of MVA/CD40L to prevent overactivation and potential apoptosis of DCs. The DNA vaccine expressed SIVmac239 Gag, protease (PR), reverse transcriptase (RT), envelope (Env), Tat, and Rev (3). The MVA vaccine expressed SIVmac239 Gag, PR, RT, and Env (4). The DNA/SIV-40L (1) and MVA/CD40L vaccines (data not shown) additionally expressed a membrane-bound form of macaque CD40L. A group of SIV-naive RMs served as controls. Four RMs in each of the vaccine and control groups were positive for Mamu A*01 (Mamu A*01+). One RM in each vaccine group was Mamu B*08+ or B*17+. Eight weekly moderate-dose intrarectal challenges with SIVmac251 were initiated at 22 to 24 weeks after the last immunization using 647 50% tissue culture infective doses (TCID50) (1.25 × 107 copies of viral RNA; 2006-Day 9 stock), which infected nearly 30% of naive RMs after the first exposure (Fig. 1A). All animals were housed at the Yerkes National Primate Research Center according to the standards of the U.S. National Research Council's Guide for the Care and Use of Laboratory Animals (5) and protocols approved by the Emory University (Atlanta, GA) Institutional Animal Care and Use Committee under protocol number 092-2010Y. Statistical analyses were conducted using Prism (GraphPad Software). The Wilcoxon-Mann-Whitney U test was used to compare immune responses and viral RNA levels between groups. Spearman's rank correlation method was used for nonparametric data correlations (indicated as r values on graphs in several of the figures). A two-sided P value of <0.05 was considered significant.
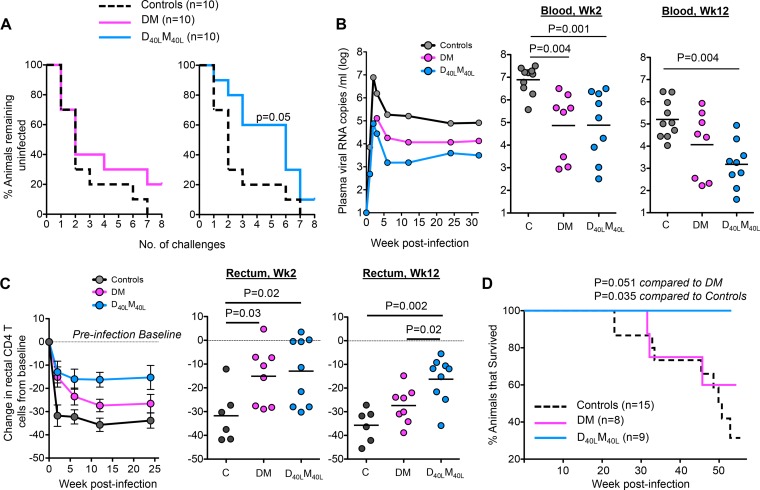
FIG 1.
CD40L-adjuvanted DNA/MVA SIVmac239 vaccine enhances protection against SIVmac251 infection. (A) Kaplan-Meier plots showing the number of SIV challenges required for acquisition of SIVmac251 infection. The P value reflects a significantly lower hazard ratio than that for controls when the log rank (Mantel-Cox) method was used. (B) Geometric mean values for viral RNA in plasma. Scatter plots show number of copies of viral RNA for individual animals postinfection. (C) Change in percentage of rectal CD4 T cells from preinfection baseline levels in SIV-infected animals. The CD4 T cell level was measured as a percentage of total CD3 cells (3). Data reflect means ± the standard error of the mean (SEM). Scatter plots show changes in rectal CD4 T cell levels for individual animals postinfection. Preinfection data were not available for 4 control animals and thus were not included in the analysis. However, we observed a profound depletion of CD4 T cells in the rectums of these 4 animals as early as 2 weeks postinfection. P values show differences between the indicated groups. (D) Survival of animals post-SIVmac251 infection. The P values indicate a higher survival rate in adjuvanted animals than in nonadjuvanted and unvaccinated animals as determined by the log rank (Mantel-Cox) method. Five additional unvaccinated controls that were challenged simultaneously for a parallel study were included. The P value is 0.052 without these additional controls.
Acquisition of SIVmac251 infection was significantly slower in the D40LM40L group than in controls, and the D40LM40L group had an estimated per-challenge vaccine efficacy of 50% (Fig. 1A). This delay in acquisition of infection was not evident in the DM group, although a similar percentage (10 to 20%) of animals in both vaccine groups remained protected after 8 challenges. Two challenges were sufficient to achieve infection of 50% of the animals in the control and DM groups, whereas 6 challenges were needed for infection of 50% of the animals in the CD40L-adjuvanted group (Fig. 1A). In addition, CD40L-adjuvanted animals showed profound control of virus replication (Fig. 1B). Both vaccine groups exhibited a 2-log-lower peak viremia than controls. However, only the adjuvanted animals maintained this viral control long term. At 12 weeks postinfection (p.i.), the majority of animals (7 out of 9) in the adjuvant group had controlled viremia to below 104 RNA copies/ml, whereas most DM animals (5/8) and all controls (10/10) had virus loads above this level. Consistent with enhanced viral control, better preservation of CD4 T cells in the rectum during peak and set point phases of infection (Fig. 1C) and enhanced survival (Fig. 1D) were shown by the adjuvanted animals. All animals in the study were euthanized 45 to 55 weeks p.i. At this time, none of the adjuvanted animals exhibited symptoms of AIDS, although 38% of the DM group and 90% of controls had to be euthanized due to AIDS. These results demonstrate that the CD40L adjuvant not only enhanced protection against acquisition of neutralization-resistant mucosal SIV infection but also provided better long-term control of viral replication and prevention of disease.
The D40LM40L vaccine did not significantly enhance serum IgG binding antibody titers (Fig. 2A), IgG avidity against SIVmac239 Env (data not shown), or neutralizing antibody titers against an easy-to-neutralize TCLA-SIVmac251 strain (data not shown). Neither vaccine elicited neutralizing antibodies against a difficult-to-neutralize SIVmac251 WY:30 isolate. It is interesting to note that in our previous study, where we adjuvanted only DNA vaccine with CD40L (1), we observed an improvement in avidity, and this was not the case when we adjuvanted both DNA and MVA vaccines. The reasons for this difference are not clear and need further investigation. The vaccines also did not produce significantly different anti-gp140 Env serum or rectal IgA responses (data not shown). These were transient (rectal) or present in extremely low concentrations (serum), and no associations with rate of infection acquisition were found.
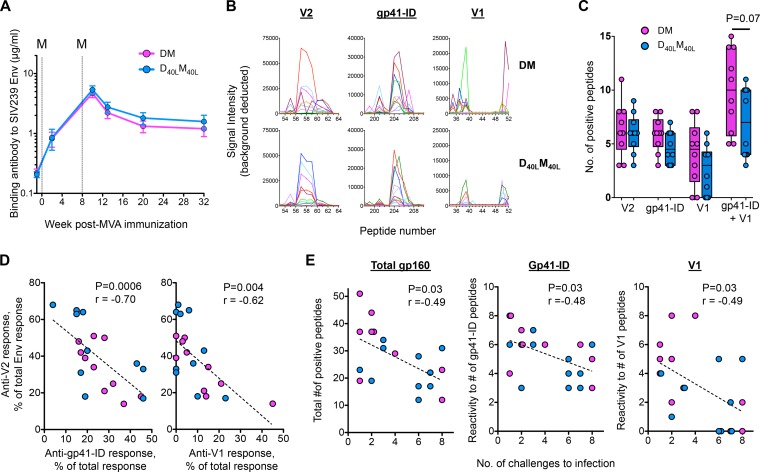
FIG 2.
D40LM40L vaccine alters binding-antibody specificity and promotes a V2-focused IgG response. (A) Geometric mean anti-Env IgG binding antibodies in serum after MVA boosting. Concentrations of Env-specific IgG were measured by enzyme-linked immunosorbent assay (ELISA) (1) using rgp140mac239 (Immune Technologies). Error bars represent SEM. M, MVA vaccine; dashed lines, day of MVA vaccination. (B) Serum binding IgG responses in the nonadjuvanted (DM) and adjuvanted (D40LM40L) animals against 15-mer peptides (overlapping by 12 amino acids) specific to the V1 and V2 variable regions of gp120 and the immunodominant region of gp41 (gp41-ID) of SIVmac239. Each line represents an individual animal. Linear epitope mapping analyses were performed as previously described (6) with modifications, including background correction and inclusion of a positivity cutoff of ≥500 signal intensity units. (C) Breadth of IgG response specific to individual regions of gp160. Tukey box plots and individual animals in each vaccination group are represented. (D) Correlations between the binding intensity of antibody to V2 peptides and the binding intensity of antibody to gp41-ID or V1 peptides at 2 weeks after the second MVA boost. (E) Correlation between the breadth of reactivity to total Env, gp41-ID, or V1 peptides and the number of challenges required for productive infection. For correlation tests, uninfected animals were assigned the number 8.
We then performed linear epitope-mapping studies using overlapping peptides (15 mers overlapping by 12 amino acids) to define regions of SIV Env targeted by IgG at 2 weeks following the second MVA boost in each animal (Fig. 2B). In nonadjuvanted animals, the majority of responses (by magnitude and breadth) were targeted to V2, followed by the immunodominant region of gp41 (gp41-ID) and V1 (Fig. 2B and C). In adjuvanted animals, there was a relatively dominant response to V2, whereas responses to gp41-ID and V1 targeted fewer epitopes than in nonadjuvanted animals (Fig. 2C). In addition, animals that showed strong responses to V2 showed weak responses to gp41-ID or V1 (Fig. 2D). Interestingly, animals with broader Env peptide binding activity showed a faster acquisition of infection than animals with fewer targeted peptides (Fig. 2E). More specifically, all animals had comparable anti-V2 binding activities (Fig. 2C), but animals with a relatively greater breadth of responses targeted to non-V2 regions such as gp41-ID and V1 showed more rapid acquisition of infection (Fig. 2E). These findings can likely be extended to IgG levels in rectal secretions because gp140-specific prechallenge rectal and serum IgG levels were highly correlated (P < 0.0001; r = 0.9) (data not shown), suggesting that the rectal IgG was derived from serum. The results point to an important association between specificity of vaccine-induced antibody binding to regions of SIV Env and protection against a neutralization-resistant SIV infection.
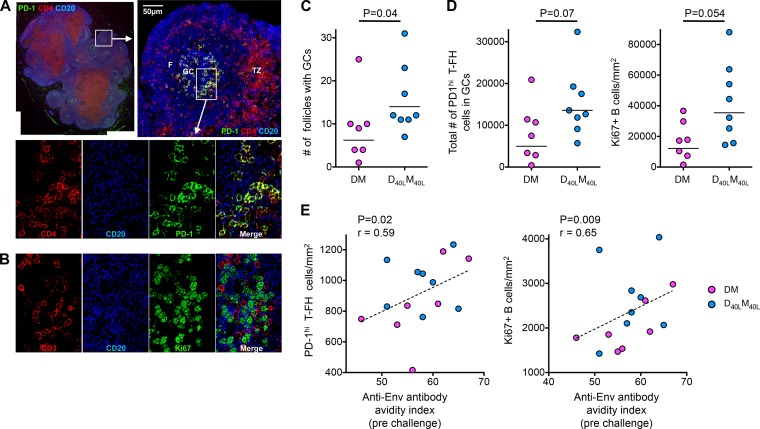
We characterized T follicular helper (T-FH) responses and germinal center (GC) B cell responses in nondraining lymph nodes at 2 weeks after the second MVA boost using immunohistochemistry (Fig. 3A and B). Impressively, the adjuvant enhanced the number of B cell follicles with GCs, with a trend toward a higher number of T-FH and proliferating GC B cells (Fig. 3C and D). The density of T-FH and GC B cells correlated directly with the avidity of prechallenge anti-Env antibody (Fig. 3E), suggesting a role for these cells in promoting the avidity of the antibody response.
FIG 3.
D40LM40L vaccine enhances T-FH and germinal center B cell responses. (A) Immunofluorescence imaging of a nondraining lymph node from a CD40L-adjuvanted animal at 2 weeks after the final MVA boost shows germinal center follicles with T-FH cells. Sections were stained with CD4 (red), CD20 (blue), and PD-1 (green) as described previously (7, 8). The merged image shows PD-1high CD4 T-FH cells abundantly present within germinal centers of lymphoid follicles. F, follicle; GC, germinal center; TZ, T cell zone. Scale bars = 50 microns. (B) Sections of lymph node germinal center follicles stained with CD3 (red), CD20 (blue), and Ki67 (green) show proliferating (Ki67+) B cells in germinal center follicles. (C) Numbers of follicles with germinal centers in nonadjuvanted (DM) and adjuvanted (D40LM40L) animals. The P value reflects significance as determined by the Mann-Whitney t test. (D) Total number of T-FH cells and density of Ki67+ B cells/mm2 of section. The total number of T-FH cells was calculated as a product of the density of T-FH cells/mm2 and the number of follicles in the section. (E) Correlation of the density of T-FH cells and Ki67+ B cells with the avidity index of anti-SIVmac239 Env IgG at the time of challenge. P values reflect significance as determined by Spearman's rank correlation test. These analyses were performed on seven DM and eight D40LM40L animals.
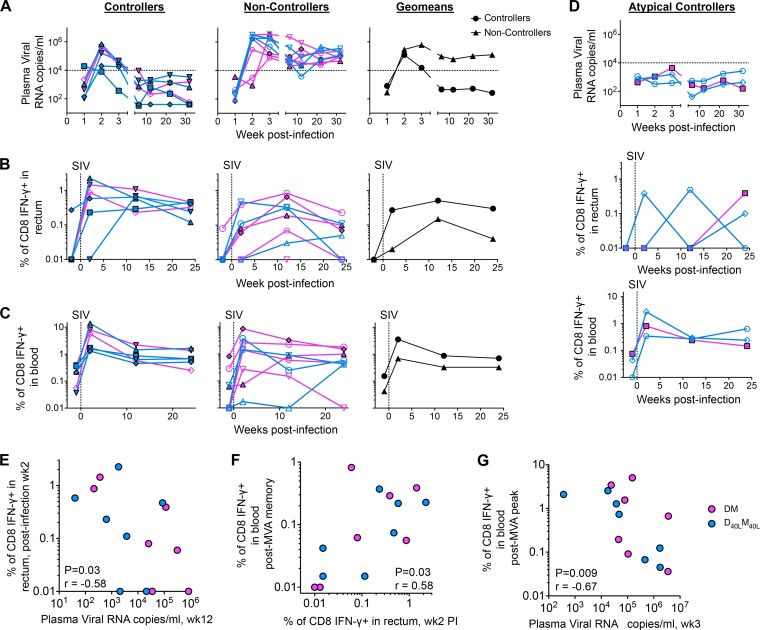
The D40LM40L vaccine did not enhance the magnitude of postvaccine SIV Gag-specific gamma interferon (IFN-γ)-producing CD8 and CD4 T cell responses in blood (data not shown). However, postinfection expansion of SIV Gag-specific CD8 T cell responses in rectal tissue was strongly associated with enhanced long-term viral control (Fig. 4). For these analyses, we excluded three animals (one from the DM group; two from the D40LM40L group) that showed atypical viral kinetics with no real peak and with marked viral control throughout (Fig. 4D). We divided vaccinated animals into controllers and noncontrollers based on a set point plasma viral load of 104 copies/ml. Controllers (viral load of <104 copies/ml) exhibited a 7-fold-greater expansion of SIV Gag-specific CD8 T cell responses in the rectum at 2 weeks p.i. than animals that showed diminished viral control (>104 copies/ml at set point) (Fig. 4A and B). This distinction was not obvious for CD8 T cells in blood (Fig. 4C). The frequency of SIV Gag-specific CD8 T cells in the rectum at 2 weeks p.i. correlated inversely with set point viremia (Fig. 4E). Animals with greater postvaccination SIV Gag-specific CD8 T cell responses in blood showed greater expansion of responses in the rectum at 2 weeks p.i. (Fig. 4F). In addition, vaccine-elicited CD8 T cell responses correlated strongly with viral control at 3 weeks p.i. (Fig. 4G). Collectively, these results demonstrate a critical role for the early expansion of vaccine-elicited rectal CD8 T cells in the long-term control of pathogenic SIVmac251 infection.
FIG 4.
Enhanced control of SIVmac251 is associated with rapid expansion of SIV Gag-specific CD8 T cells in the rectum. (A) Temporal viral RNA levels in controllers (<104 copies/ml at set point) and noncontrollers (>104 copies/ml at set point). Results for nonadjuvanted animals are shown in pink and for CD40L-adjuvanted animals in blue. Results for A*01 animals are indicated by filled symbols with a black border and for non-A*01 animals by open symbols. Viral RNA levels were determined using qPCR (9). Dashed lines indicate the viral load threshold used to define controllers and noncontrollers. (B) Temporal frequencies of postinfection Gag-specific CD8 IFN-γ+ T cells in the rectum determined using an intracellular-cytokine-staining (ICS) assay (1). Dashed lines indicate the day of productive SIVmac251 infection. (C) Temporal frequencies of postinfection Gag-specific CD8 IFN-γ+ T cells in the blood determined using an ICS assay. Dashed lines indicate the day of productive SIVmac251 infection. (D) Temporal viral RNA levels in blood (top panel) and expansion of Gag-specific CD8 T cells in the rectum (middle panel) and blood (bottom panel) of atypical controllers postinfection. The vertical dashed lines indicate the day of productive SIVmac251 infection. (E) Correlation between Gag-specific CD8 IFN-γ T cells in rectum at 2 weeks postinfection and viral set point at week 12 postinfection. (F) Correlation between post-MVA vaccination memory IFN-γ+ CD8 T cells in blood and postinfection IFN-γ+ CD8 T cells in rectum. (G) Correlation between post-MVA vaccination peak IFN-γ+ CD8 T cells in blood and viral RNA copies at 3 weeks postinfection. P values for panels E through G were obtained using the two-tailed Spearman rank correlation test.
In conclusion, our results show that adjuvanting a DNA/MVA SIV vaccine with CD40L significantly delays acquisition of neutralization-resistant intrarectal SIVmac251 infection, improves long-term viral control, and prevents AIDS. Interestingly, immune correlate analyses of antibody binding to linear peptides of SIV Env revealed a strong V2 response with narrower binding to the gp41-ID and V1 regions as a correlate of enhanced protection against acquisition of mucosal SIVmac251 infection. Given the proximity of V1 to V2, one hypothesis is that anti-V1 IgG may interfere with protective functions of anti-V2 IgG (e.g., blocking interaction between the viral envelope and CD4 receptor and clearance of infected cells by antibody-dependent cell-mediated cytotoxicity). Another hypothesis is that a strong V2 response with narrower binding to non-V2 regions is a surrogate for another immune response, not measured here, that corresponds to a vaccine-induced protective state. Together, these data raise hypotheses that need to be tested in further studies.
ACKNOWLEDGMENTS
We thank Sunil Kannanganat for help with the monkey study, Ronald C. Desrosiers (New England National Primate Research Center) and Nancy Miller (NIAID, NIH) for providing the SIVmac251 stock, Jeffrey Americo (NIH) and Laurie Donald (GeoVax, Inc.) for help with production of MVA, Robert L. Wilson (LSUHSC) for technical support, Helen Drake-Perrow for administrative support, the Yerkes Division of Research Resources for animal care, the Emory CFAR Virology Core for viral load assays, the NIH AIDS Research and Reference Reagent Program for the provision of peptides, and the Nonhuman Primate Resource Reagent Program for antibodies.
This work was supported by National Institutes of Health grant P01 AI088575 to R.R.A., Yerkes National Primate Research Center base grant P51 RR00165, and Emory CFAR grant P30 AI050409. Funding for the neutralization assays was provided by NIAID, NIH, contract no. HHSN27201100016C to D.C.M. Partial support for MVA construction was provided by the Division of Intramural Research, NIAID, NIH.
R.R.A. is a coinventor of DNA/MVA vaccine technology that has been licensed to GeoVax, Inc., by Emory University.
REFERENCES
- 1.Kwa S, Lai L, Gangadhara S, Siddiqui M, Pillai VB, Labranche C, Yu T, Moss B, Montefiori DC, Robinson HL, Kozlowski PA, Amara RR. 2014. CD40L-adjuvanted DNA/modified vaccinia virus Ankara simian immunodeficiency virus SIV239 vaccine enhances SIV-specific humoral and cellular immunity and improves protection against a heterologous SIVE660 mucosal challenge. J Virol 88:9579–9589. doi: 10.1128/JVI.00975-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopker M, Easlick J, Sterrett S, Decker JM, Barbian H, Learn G, Keele BF, Robinson JE, Li H, Hahn BH, Shaw GM, Bar KJ. 2013. Heterogeneity in neutralization sensitivities of viruses comprising the simian immunodeficiency virus SIVsmE660 isolate and vaccine challenge stock. J Virol 87:5477–5492. doi: 10.1128/JVI.03419-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kannanganat S, Nigam P, Velu V, Earl PL, Lai L, Chennareddi L, Lawson B, Wilson RL, Montefiori DC, Kozlowski PA, Moss B, Robinson HL, Amara RR. 2010. Preexisting vaccinia virus immunity decreases SIV-specific cellular immunity but does not diminish humoral immunity and efficacy of a DNA/MVA vaccine. J Immunol 185:7262–7273. doi: 10.4049/jimmunol.1000751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Rompay KK, Greenier JL, Cole KS, Earl P, Moss B, Steckbeck JD, Pahar B, Rourke T, Montelaro RC, Canfield DR, Tarara RP, Miller C, McChesney MB, Marthas ML. 2003. Immunization of newborn rhesus macaques with simian immunodeficiency virus (SIV) vaccines prolongs survival after oral challenge with virulent SIVmac251. J Virol 77:179–190. doi: 10.1128/JVI.77.1.179-190.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 6.Tomaras GD, Binley JM, Gray ES, Crooks ET, Osawa K, Moore PL, Tumba N, Tong T, Shen X, Yates NL, Decker J, Wibmer CK, Gao F, Alam SM, Easterbrook P, Abdool Karim S, Kamanga G, Crump JA, Cohen M, Shaw GM, Mascola JR, Haynes BF, Montefiori DC, Morris L. 2011. Polyclonal B cell responses to conserved neutralization epitopes in a subset of HIV-1-infected individuals. J Virol 85:11502–11519. doi: 10.1128/JVI.05363-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong JJ, Amancha PK, Rogers K, Ansari AA, Villinger F. 2012. Spatial alterations between CD4+ T follicular helper, B, and CD8+ T cells during simian immunodeficiency virus infection: T/B cell homeostasis, activation, and potential mechanism for viral escape. J Immunol 188:3247–3256. doi: 10.4049/jimmunol.1103138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mylvaganam GH, Velu V, Hong J-J, Sadagopal S, Kwa S, Basu R, Lawson B, Villinger F, Amara RR. 2014. Diminished viral control during simian immunodeficiency virus infection is associated with aberrant PD-1hi CD4 T cell enrichment in the lymphoid follicles of the rectal mucosa. J Immunol 193:4527–4536. doi: 10.4049/jimmunol.1401222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amara RR, Villinger F, Altman JD, Lydy SL, O'Neil SP, Staprans SI, Montefiori DC, Xu Y, Herndon JG, Wyatt LS, Candido MA, Kozyr NL, Earl PL, Smith JM, Ma HL, Grimm BD, Hulsey ML, Miller J, McClure HM, McNicholl JM, Moss B, Robinson HL. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69–74. doi: 10.1126/science.1058915. [DOI] [PubMed] [Google Scholar]