ABSTRACT
Porcine reproductive and respiratory syndrome virus (PRRSV) is one of the most economically important viruses affecting the swine industry worldwide. Our previous research showed that PRRSV downregulates the expression of heme oxygenase-1 (HO-1), a pivotal cytoprotective enzyme, postinfection and that overexpression of HO-1 inhibits PRRSV replication. MicroRNAs regulate gene expression at the posttranscriptional level and have recently been demonstrated to play vital roles in pathogen-host interactions. The present study sought to determine whether microRNAs modulate HO-1 expression and, by doing so, regulate PRRSV replication. Using bioinformatic prediction and experimental verification, we demonstrate that HO-1 expression is regulated by miR-24-3p. A direct interaction between miR-24-3p and HO-1 mRNA was confirmed using a number of approaches. Overexpression of miR-24-3p significantly decreased HO-1 mRNA and protein levels. PRRSV infection induced miR-24-3p expression to facilitate viral replication. The suppressive effect of HO-1 induction by protoporphyrin IX cobalt chloride (CoPP; a classical inducer of HO-1 expression) on PRRSV replication in MARC-145 cells and primary porcine alveolar macrophages could also be reversed by overexpression of miR-24-3p. Collectively, these results suggested that miR-24-3p promotes PRRSV replication through suppression of HO-1 expression, which not only provides new insights into virus-host interactions during PRRSV infection but also suggests potential new antiviral strategies against PRRSV infection.
IMPORTANCE MicroRNAs (miRNAs) play vital roles in viral infections by regulating the expression of viral or host genes at the posttranscriptional level. Heme oxygenase-1 (HO-1), a pivotal cytoprotective enzyme, has antiviral activity for a number of viruses, such as Ebola virus, hepatitis C virus, human immunodeficiency virus, and our focus, PRRSV, which causes great economic losses each year in the swine industry worldwide. Here, we show that PRRSV infection induces host miRNA miR-24-3p expression and that miR-24-3p regulates HO-1 expression through both mRNA degradation and translation repression. Suppression of HO-1 expression by miR-24-3p facilitates PRRSV replication. This work lends credibility to the hypothesis that an arterivirus can manipulate cellular miRNAs to enhance virus replication by regulating antiviral responses following viral infection. Therefore, our findings provide new insights into the pathogenesis of PRRSV.
INTRODUCTION
Porcine reproductive and respiratory syndrome virus (PRRSV) is one of the most economically important viruses affecting the swine industry worldwide, resulting in significant economic losses each year (1–3). PRRSV is a small, enveloped, linear, single positive-stranded RNA virus and a member of the order Nidovirales, family Arteriviridae (4). Current vaccination strategies cannot effectively control PRRSV infection because of the high antigenic heterogeneity (5, 6), the replication in and destruction of lung alveolar macrophages (7–9), and the observed antibody-dependent enhancement of PRRSV (10, 11). Therefore, it is imperative to study PRRSV pathogenesis mechanisms so that more effective control measures can be developed.
Heme oxygenase-1 (HO-1) is the rate-limiting enzyme of heme degradation, and it functions to catabolize free heme into biliverdin, carbon monoxide, and iron. HO-1 and its end products have antioxidant, anti-inflammatory, and antiviral properties, and it is known to be a pivotal cytoprotective enzyme (12). Upregulation of HO-1 expression suppresses replication of a number of viruses, including hepatitis C virus (HCV), HIV-1, hepatitis B virus (HBV), and influenza virus (13–17). Our previous work showed that PRRSV significantly downregulates HO-1 expression in vivo and in vitro (1, 18). Furthermore, overexpression or induction of HO-1 expression inhibits PRRSV replication (19), indicating that increased expression of HO-1 may provide a potential new antiviral strategy against PRRSV infection.
MicroRNAs (miRNAs) are evolutionarily conserved, small, endogenous, noncoding RNAs that regulate gene expression (20). Growing evidence indicates that miRNAs can modulate virus replication directly as well as the host cell response to viral infection in a proviral or antiviral manner (21, 22). It has been demonstrated that HCV replication depends on a liver-specific miRNA, miR-122 (23, 24). Kaposi's sarcoma-associated herpesvirus upregulates miR-132 expression that, in turn, inhibits the expression of p300, a transcriptional coactivator, to promote viral replication (25). miR-125b reduces PRRSV replication by negatively regulating the NF-κB pathway (26), and miR-181 inhibits PRRSV replication by targeting both viral genomic RNA and receptor CD163 (27, 28).
Recent reports show that HO-1 expression can be regulated by miRNAs in certain cell types. For example, miR-377 and miR-217 can decrease HO-1 expression in endothelial cells by a direct interaction with the 3′ untranslated region (UTR) of HO-1 mRNA (29), whereas miR-378 decreases HO-1 expression in lung cancer cells (30). We hypothesized that there may also be miRNAs targeting HO-1 in PRRSV-permissive cells, which may subsequently affect the regulation of PRRSV replication. In the present study, we demonstrate that miR-24-3p promoted PRRSV replication through the targeting of HO-1 in MARC-145 cells and porcine alveolar macrophages (PAMs). These data not only provide new insights into virus-host interactions during PRRSV infection but also suggest potential new antiviral strategies against PRRSV infection in the future.
MATERIALS AND METHODS
Cells, viruses, and reagents.
MARC-145 cells derived from African green monkey kidney cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Life Technologies Corp., Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic-antimycotic solution (Life Technologies Corp., Grand Island, NY, USA) at 37°C and 5% CO2. Porcine alveolar macrophages (PAMs) were isolated from healthy 6-week-old crossbred weaned (Landrace × Yorkshire) PRRSV-negative pigs using a lung lavage technique as previously described (31) with minor modifications (19). All animal work was in strict accordance with the guidelines of the Institutional Animal Care and Use Committee and approved by the Animal Care and Use Committee of Northwest A&F University. Viruses used in this study were a highly pathogenic PRRSV strain, SD16 (GenBank accession number JX087437), and a recombinant PRRSV. The recombinant PRRSV was based on the genetic background of SD16 that expressed enhanced green fluorescent protein (EGFP) as an additional open reading frame (ORF) (rHP-PRRSV/SD16/EGFP) (32).
Synthetic double-stranded RNAs that mimic endogenous miRNAs when transfected into cells (miRNA mimics), as well as synthetic miRNAs containing specific site mutations (miRNA mutant mimics) and a small interfering RNA (siRNA) targeting HO-1 (siHO-1) were synthesized by GenePharma (Shanghai, China). Protoporphyrin IX cobalt chloride (CoPP), a classical inducer of HO-1 gene expression, was purchased from Sigma-Aldrich (St. Louis, MO, USA).
In silico identification of miRNA binding sites.
The miRNA binding sites in the HO-1 3′ UTR from both Macaca mulatta and Sus scrofa were predicted using RNAhybrid (33), TargetScan (34), and a microRNA database (35). This prediction is based on an algorithm that determined the optimal complementarity between miRNAs and a given mRNA. The predicted target sites were chosen when the hybrid free energy was less than −30 kcal/mol and when they were identified in at least two databases.
Construction of psiCheck2 target luciferase reporters.
The full-length HO-1 3′ UTRs of monkey (XM_001113241; 611 bp, position +868 to +1478) and pig (NM_001004027; 611 bp, position +942 to +1552) were amplified from cDNA in MARC-145 cells and PAMs, subcloned into psiCheck2 vector (Promega, Madison, WI, USA) using NotI and XhoI enzymes, and designated psiCheck2-mHO-1-WT (where WT is wild type) and psiCheck2-pHO-1-WT, respectively.
To generate the miRNA target site mutants, mutations were introduced into the 3′ UTR segments containing the miR-24-3p potential binding site of murine HO-1 (mHO-1) and porcine HO-1 (pHO-1). Then these segments were synthesized, annealed into double strands, and subcloned into the psiCheck2 vector (Promega, Madison, WI, USA) using NotI and XhoI enzymes; the constructs were designated psiCheck2-mHO-1-Mut (where Mut is mutant) and psiCheck2-pHO-1-Mut, respectively.
Luciferase reporter assay.
A luciferase reporter assay was processed as described previously (36) with the following modifications. HEK293FT cells were cotransfected with 50 ng of psiCheck2-HO-1-WT or psiCheck2-HO-1-Mut plasmid and 100 mM concentrations of miR-24-3p mimics or miR-24-3p-Mut mimics or negative-control (NC) mimics using X-tremeGENE HP DNA transfection reagent (Roche, Mannheim, Germany). At 36 h posttransfection, cells were lysed, and luciferase expression was measured on a Synergy HT Multi-Mode Microplate Reader (BioTek, Winooski, VT) using a dual-luciferase reporter assay system (Promega, Madison, WI, USA) according to the manufacturer's instructions.
Coimmunoprecipitation with Ago2.
An RNA-induced silencing complex (RISC) immunoprecipitation assay was performed as previously described (27, 37) with the following modifications. MARC-145 cells were seeded in a six-well plate at a density of 2 × 105 cells/well. After 24 h, cells were transfected with 150 nM concentrations (final) of the miR-24-3p mimics, miR-24-3p-Mut mimics, or NC mimics for 36 h using X-tremeGENE siRNA transfection reagent (Roche, Mannheim, Germany) according to the manufacturer's instructions. Mouse anti-Ago2 monoclonal antibody (clone 2E12-1C9; Abnova) was used for measurement of Ago2 protein in input cell lysates. IgG was used as an isotype control.
Flow cytometry assay.
MARC-145 cells were seeded in a 24-well chamber slide at a density of 0.5 × 105 cells/well. After 24 h, cells were transfected with 150 nM concentrations (final) of the miR-24-3p mimics, NC mimics, or siHO-1 (as positive control) for 12 h using X-tremeGENE siRNA transfection reagent (Roche, Mannheim, Germany) and then infected with rHP-PRRSV/SD16/EGFP at a multiplicity of infection (MOI) of 0.01, followed by treatment for 48 h at 37°C with 5% CO2 in the presence of CoPP (20 μM) from 1 h postinfection (hpi) onward. EGFP-PRRSV-positive cells were detected by flow cytometry.
Quantitative reverse transcriptase PCR (qRT-PCR).
Total RNA from MARC-145 cells, PAMs, or supernatant PRRSV RNA was isolated using TRIzol reagent and reverse transcribed using a Primescript RT reagent kit (TaKaRa, Dalian, China) according to the manufacturer's instructions. Quantitative PCR (qPCR) was performed with a Step One Plus real-time PCR system (Applied Biosystems, Foster City, CA, USA) and FastStart Universal SYBR green master (Roche, Basle, Switzerland), with the forward and reverse primers for HO-1 and ORF7 of PRRSV quantitative PCR (qPCR), as described previously (19). β-Actin mRNA was used as an internal reference.
For detection of miR-24-3p, total RNA was reverse transcribed with the specific primer 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCTGTTCCT-3′. The qPCRs were performed using FastStart Universal SYBR green master mix (Roche, Basle, Switzerland) with the miR-24-3p-specific forward primer 5′-ACACTCCAGCTGGGTGGCTCAGTTCAGCAG-3′ and the universal reverse primer 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAG-3′. The abundance of the U6 RNA served as an internal reference.
For detection of supernatant PRRSV RNA, a plasmid bearing a 372-bp fragment of the PRRSV ORF7 sequence was used to generate a standard curve. The standard curve was plotted from the results of parallel PCRs performed on serial dilutions of standard DNA. RNA absolute quantities were calculated by normalization to the standard curve.
Western blot analysis.
Western blotting was performed as described previously (38) with minor modifications. MARC-145 cells or PAMs were lysed, and the cellular proteins were separated by SDS-PAGE and transferred onto a polyvinylidene difluoride (PVDF) membrane. The PVDF membrane was probed with one of the following primary antibodies: mouse anti-HO-1 polyclonal antibody at a 1:1,000 dilution (19), a monoclonal antibody (6D10) (32) to the PRRSV N protein at a 1:2,000 dilution, or an anti-α-tubulin antibody at a 1:5,000 dilution (Abcam, Cambridge, MA, USA). Horseradish peroxidase (HRP)-conjugated anti-mouse IgG at a 1:2,000 dilution (Jackson, West Grove, PA, USA) was used to label primary antibody binding. Immunolabeled proteins were visualized using enhanced chemiluminescence (ECL) reagent (Pierce, Rockford, IL, USA).
Virus titration.
Virus progeny production was determined by titration as described previously (38) with minor modifications. MARC-145 cells were trypsinized and seeded in a 96-well plate 24 h before virus infection. Virus supernatants were prepared through serial dilutions, and 100 μl of solution was added to each well in replicates of eight. Six days after infection, the 50% tissue culture infective dose (TCID50) was calculated by the Reed-Muench method.
Statistical analysis.
All experiments were performed with at least three independent experiments. Statistical significance was determined by Student's t test when only two groups were compared or by one-way analysis of variance (ANOVA) when more than two groups were compared. A P value of <0.05 was considered statistically significant.
RESULTS
Prediction and selection of HO-1 miRNA regulators.
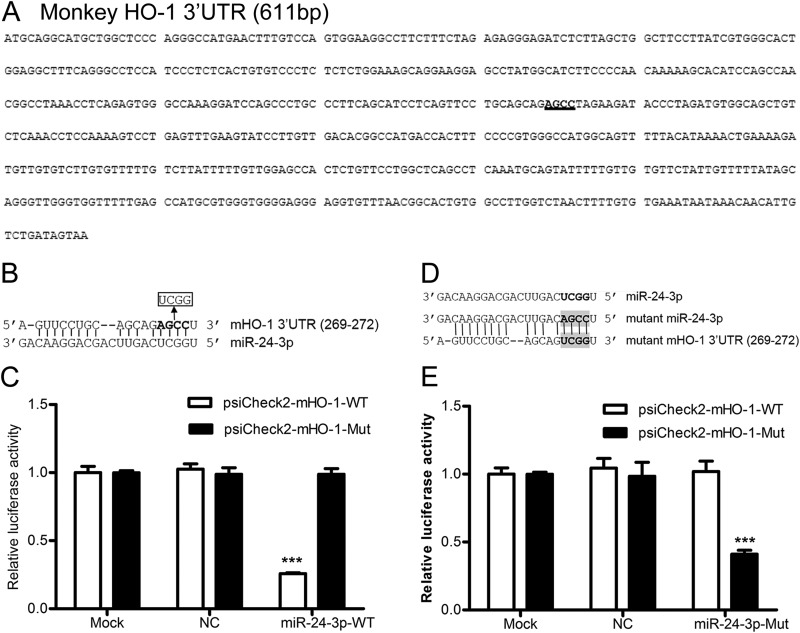
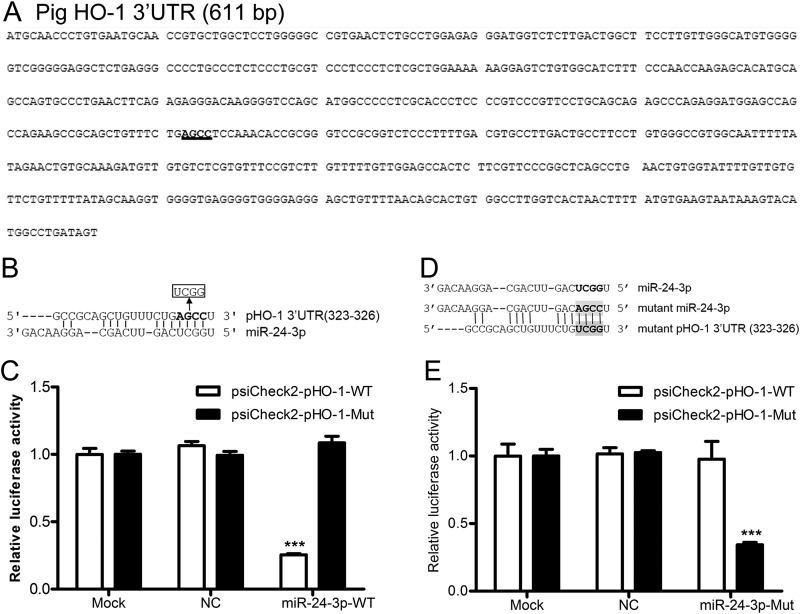
To identify miRNAs with complementarity to the monkey and pig HO-1 3′ UTRs, we performed in silico analysis using a number of bioinformatics tools. We found five monkey miRNAs and two pig miRNAs with potential binding sites in the 3′ UTRs of monkey and pig HO-1, respectively. The miRNAs identified as potentially targeting monkey HO-1 were miR-24-3p, miR-217b, miR-377, miR-760, and miR-762, whereas those potentially targeting pig HO-1 were miR-24-3p and miR-92b-5p. The mature sequence of miR-24-3p is predicted to interact with the HO-1 3′ UTR from positions 269 to 272 (monkey) and 323 to 326 (pig) via a seed match interaction (see Fig. 2A and 3A). There are at least two putative miR-217 sites and one putative miR-377 seed match site in the monkey HO-1 3′ UTR. One miR-760 and five predicted miR-762 binding sites were found in the HO-1 3′ UTR of monkey. Four predicted miR-92-5p binding sites were found in the HO-1 3′ UTR of pig (data not shown).
FIG 2.
miR-24-3p targets the 3′ UTR of monkey HO-1. (A) The 3′ UTR of monkey HO-1 contains one binding site (highlighted) for miR-24-3p. (B) Four nucleotides in the seed binding site of the monkey HO-1 3′ UTR were replaced. (C) Luciferase activity in lysates of HEK293FT cells cotransfected with psiCheck2-mHO-1-WT or psiCheck2-mHO-1-Mut and 100 nM concentrations of NC mimics or miR-24-3p mimics. (D) Four-nucleotide mutations were introduced to the seed target site of miR-24-3p, and restoration of seed binding between the mutant miR-24-3p and mutant 3′ UTR of monkey HO-1 mRNA is shown. (E) Luciferase activity in lysates of HEK293FT cells cotransfected with psiCheck2-mHO-1-WT or psiCheck2-mHO-1-Mut and 100 nM concentrations of NC mimics or miR-24-3p-Mut mimics. Data in panels C and E are expressed as means ± standard deviations of four independent experiments; P values were calculated using Student's t test. ***, P < 0.001 (relative to the mock control).
FIG 3.
miR-24-3p targets the 3′ UTR of pig HO-1. (A) The 3′ UTR of pig HO-1 contains one binding site (highlighted) for miR-24-3p. (B) Four nucleotides in the seed binding site of pig HO-1 3′ UTR were replaced. (C) Luciferase activity in lysates of HEK293FT cells cotransfected with psiCheck2-pHO-1-WT or psiCheck2-pHO-1-Mut and 100 nM concentrations of NC mimics or miR-24-3p mimics. (D) Four-nucleotide mutations were introduced to the seed target site of miR-24-3p, and restoration of seed binding between mutant miR-24-3p and the mutant 3′ UTR of pig HO-1 mRNA is shown. (E) Luciferase activity in lysates of HEK293FT cells cotransfected with psiCheck2-pHO-1-WT or psiCheck2-pHO-1-Mut and 100 nM concentrations of NC mimics or miR-24-3p-Mut mimics. Data in panels C and E are expressed as means ± standard deviations of four independent experiments; P values were calculated using Student's t test. ***, P < 0.001 (relative to the mock control).
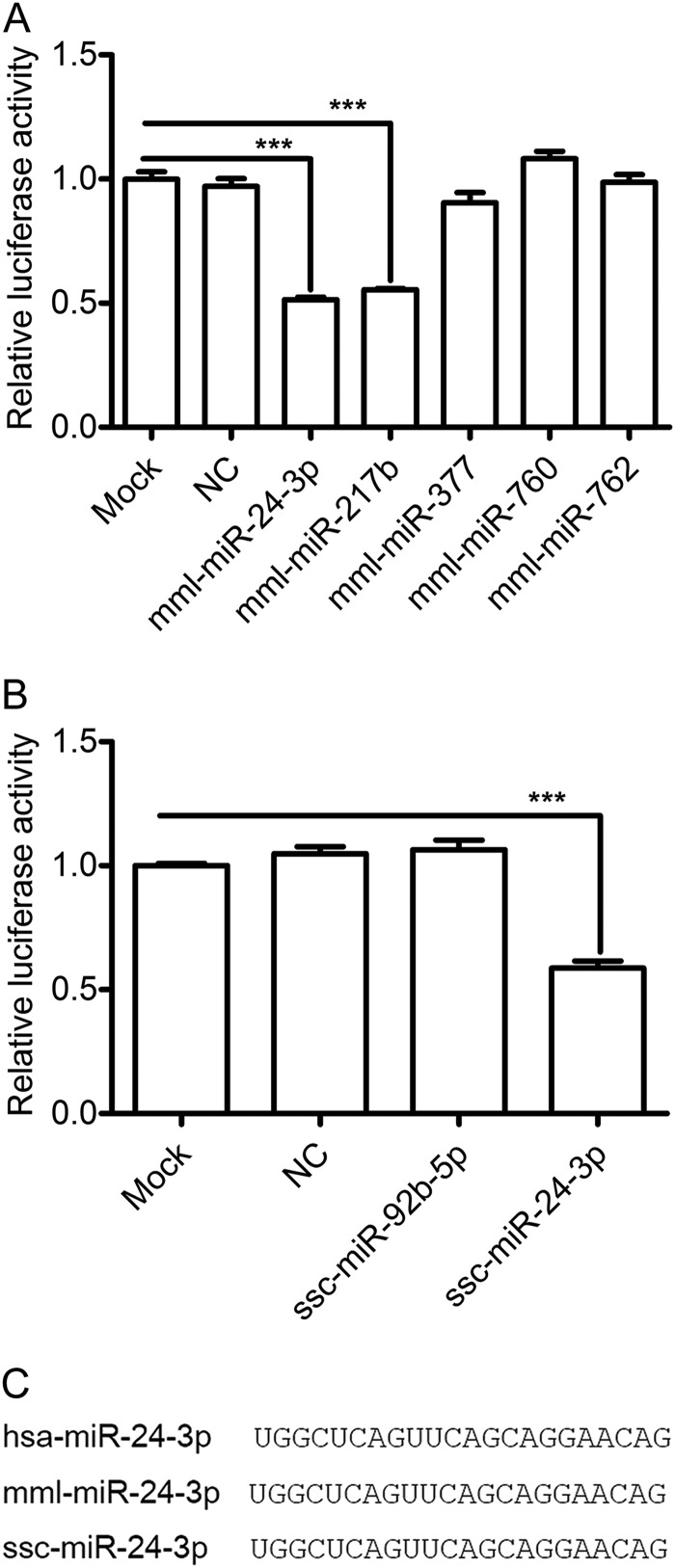
To investigate whether the candidate miRNAs could potentially decrease HO-1 expression by binding to the 3′ UTR, a plasmid-based luciferase reporter assay was used to examine the effects overexpression of the miRNAs on reporter gene expression in cells. The “mml-” prefix was used to denote the miRNAs from Macaca mulatta (monkey), and the “ssc-” prefix was used to denote those from Sus scrofa (pig). The effect of the various miRNAs on reporter gene expression was monitored by cotransfection of cells with miRNA mimics and a luciferase reporter containing either the monkey HO-1 3′ UTR (psiCheck2-mHO-1-WT) or pig HO-1 3′ UTR (psiCheck2-pHO-1-WT). As shown in Fig. 1A, overexpression of mml-miR-24-3p and mml-miR-217b resulted in 49% and 45% decreases in luciferase expression from psiCheck2-mHO-1-WT, respectively. In contrast, cotransfection with the other three miRNAs or a negative control (NC) had no effect. Only ssc-miR-24-3p significantly decreased luciferase expression from psiCheck2-pHO-1-WT by 42% (Fig. 1B). Since miR-24-3p is well conserved among different host species (Fig. 1C) and significantly inhibited luciferase expression from both psiCheck2-mHO-1-WT (Fig. 1A) and psiCheck2-pHO-1-WT (Fig. 1B), it was chosen for further validation studies.
FIG 1.
Luciferase reporter activity assay identifies miR-24-3p as a regulator of HO-1. (A) HEK293FT cells were cotransfected with indicated mimics and psiCheck2-mHO-1-WT (a luciferase reporter containing the monkey HO-1 3′ UTR). (B) HEK293FT cells were cotransfected with indicated mimics and psiCheck2-pHO-1-WT (a luciferase reporter containing the pig HO-1 3′ UTR). After 36 h, cells were lysed, and luciferase activity was measured. Data in panels A and B are expressed as means ± standard deviations of four independent experiments; P values were calculated using Student's t test. ***, P < 0.001 (relative to the mock control). (C) Sequence homology of miR-24-3p in human, monkey, and pig. NC was used to denote the negative control. The “hsa-” prefix was used to denote the miRNAs from Homo sapiens (human), the “mml-” prefix was used to denote those from Macaca mulatta (monkey), and the “ssc-” prefix was used to denote those from Sus scrofa (pig).
miR-24-3p directly interacts with the 3′ UTR of HO-1 mRNA.
To investigate whether miR-24-3p directly targets the 3′ UTR of HO-1 mRNA, which contained the predicted seed match site for miR-24-3p (Fig. 2A and 3A), the effect of mutating the seed sequence in the HO-1 3′ UTR of both monkey (Fig. 2B) and pig (Fig. 3B) was examined. As expected, miR-24-3p mimics significantly inhibited the luciferase activity of psiCheck2-mHO-1-WT by ∼62 to 75% (Fig. 2C). A similar effect was observed using the construct containing the pig HO-1 3′ UTR, psiCheck2-pHO-1-WT. miR-24-3p mimics significantly decreased the luciferase expression from psiCheck2-pHO-1-WT by ∼62 to 75% (Fig. 3C). Disruption of the seed sequence in plasmids containing the monkey HO-1 3′ UTR (psiCheck2-mHO-1-Mut) or the pig HO-1 3′ UTR (psiCheck2-pHO-1-Mut) ablated the ability of miR-24-3p mimics to inhibit the expression of luciferase (Fig. 2C and 3C, respectively).
To confirm the above results, the mutant miR-24-3p (miR-24-3p-Mut) restoring base complementarity with mutated HO-1 3′ UTR sequences (Fig. 2D and 3D) was generated, and its effect on WT and mutant HO-1 3′ UTR reporter activity was examined. As shown in Fig. 2E, miR-24-3p-Mut mimics did not affect the luciferase expression from psiCheck2-mHO-1-WT. Similar results were observed when miR-24-3p-Mut and psiCheck2-pHO-1-WT were cotransfected into HEK293FT cells (Fig. 3E). In contrast, miR-24-3p-Mut mimics significantly decreased the luciferase activity of psiCheck2-mHO-1-Mut (Fig. 2E) and psiCheck2-pHO-1-Mut (Fig. 3E). These results demonstrated that miR-24-3p could directly target the 3′ UTR of HO-1 mRNA.
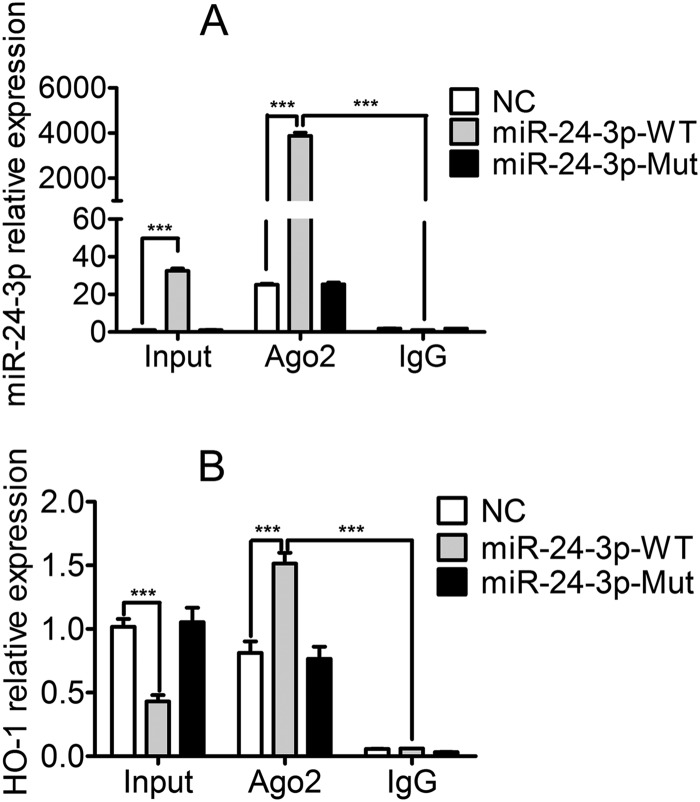
To further demonstrate the direct interaction between miR-24-3p and the 3′ UTR of HO-1 mRNA, an Ago2 coimmunoprecipitation analysis was performed to detect whether RNA-induced silencing complex (RISC) could retain HO-1 mRNA when miR-24-3p was overexpressed. MARC-145 cells were transfected with either miR-24-3p mimics, miR-24-3p-Mut mimics, or NC mimics and used to immunoprecipitate Ago2. As shown in Fig. 4A, the miR-24-3p level associated with Ago2 protein was increased by 4,000-fold compared to that of the IgG isotype control. In input cell lysates, the HO-1 mRNA level in cells transfected with miR-24-3p mimics was about 60% lower than that in cells transfected with NC mimics or miR-24-3p-Mut mimics (Fig. 4B). However, the abundance of HO-1 mRNA increased by approximately 2-fold in mRNAs from Ago2 coimmunoprecipitation in the presence of miR-24-3p mimics compared to levels with NC mimics or miR-24-3p-Mut mimics (Fig. 4B). These results demonstrated that miR-24-3p directly interacted with monkey HO-1 mRNA in the RISC.
FIG 4.
miR-24-3p physically binds to HO-1 mRNA in the RISC. qPCR analysis of miR-24-3p (A) and HO-1 mRNA (B) among RNAs extracted from input cells, Ago2 coimmunoprecipitation, or IgG (isotype control) immunoprecipitation of lysates from MARC-145 cells. The level of miR-24-3p was normalized to the level of U6. The level of HO-1 mRNA was normalized to the level of β-actin. Data are expressed as means ± standard deviations of three independent experiments. P values were calculated using Student's t test. ***, P < 0.001.
miR-24-3p downregulates expression of HO-1 in MARC-145 cells.
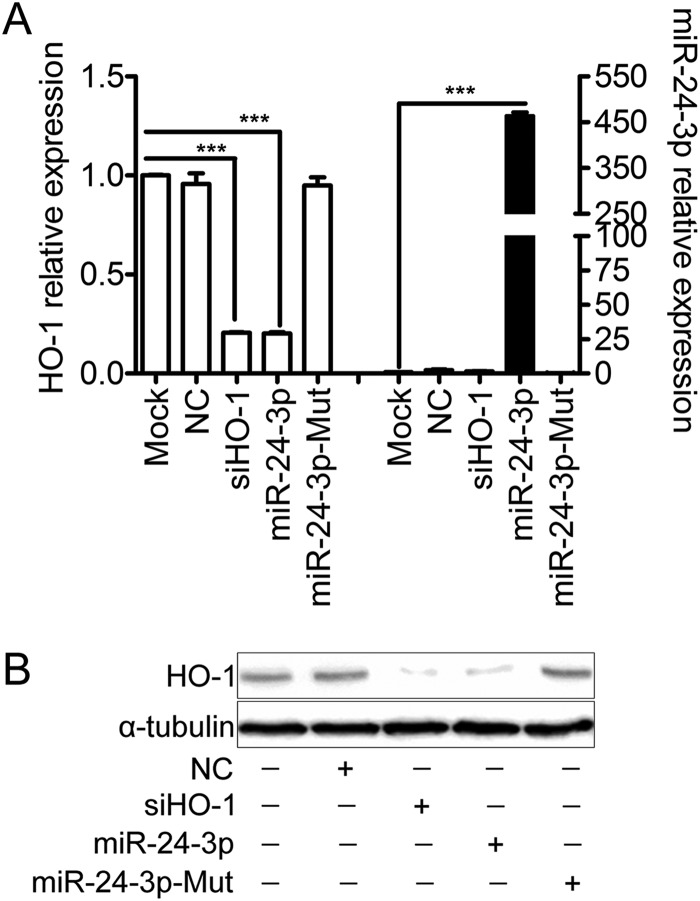
To determine if overexpression of miR-24-3p decreases HO-1 expression, MARC-145 cells were transfected with miR-24-3p mimics, miR-24-3p-Mut mimics, NC mimics, or siHO-1 (as positive control), and the expression of HO-1 was examined. As shown in Fig. 5, miR-24-3p mimics and siHO-1 transfection led to significant reduction of HO-1 mRNA expression (Fig. 5A). When MARC-145 cells were transfected with miR-24-3p mimics, the expression level of HO-1 mRNA was decreased by 80% (Fig. 5A). NC mimics or miR-24-3p-Mut mimics had no effect on HO-1 expression. Western blot analysis was used to confirm the observed effects of HO-1 siRNAs and miR-24-3p on HO-1 protein levels (Fig. 5B).
FIG 5.
Overexpression of miR-24-3p decreases HO-1 mRNA and protein level in MARC-145 cells. Relative expression levels of HO-1 and miR-24-3p in MARC-145 cells transfected with 200 nM concentrations of miR-24-3p mimics, miR-24-3p-Mut mimics, NC mimics, or siHO-1 (as positive control) at 12 h were determined by qRT-PCR (A) or Western blotting (B). α-Tubulin was used as the loading control. Data in panel A are expressed as means ± standard deviations of three independent experiments; P values were calculated using Student's t test. ***, P < 0.001 (relative to the mock control).
PRRSV infection induces miR-24-3p expression.
To determine whether PRRSV infection affects the expression level of miR-24-3p, miR-24-3p levels in the MARC-145 cells were quantified by qPCR during PRRSV infection. As shown in Fig. 6, miR-24-3p was upregulated in MARC-145 cells infected with PRRSV as early as 2 h postinfection (hpi); it reached its peak expression at 12 hpi (600%), and then its expression decreased gradually as the infection progressed but still remained significantly higher than the level in the mock control. In addition, the proportion of positive cells infected with recombinant EGFP PRRSV was measured by flow cytometry. The results showed that the percentage of EGFP-positive MARC-145 cells infected with PRRSV at 36 hpi was 34% (data not shown). Importantly, however, PRRSV inactivated by UV irradiation did not alter miR-24-3p expression (Fig. 6).
FIG 6.

PRRSV infection induces miR-24-3p expression. MARC-145 cells were infected with PRRSV strain SD16 (MOI of 1.0), and then miR-24-3p levels at the indicated time points were determined by qPCR. Data are expressed as means ± standard deviations of three independent experiments. P values were calculated using Student's t test. **, P < 0.01; ***, P < 0.001 (relative to the mock control).
miR-24-3p reverses the suppressive effect of HO-1 induction on PRRSV infection.
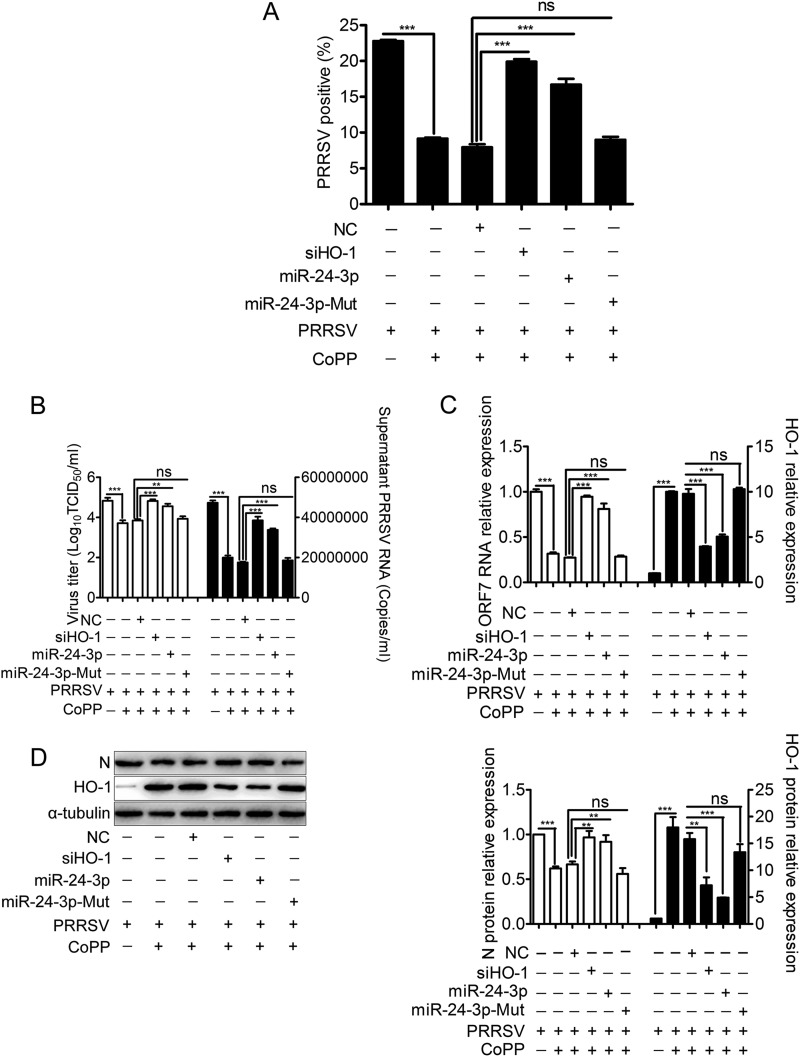
Protoporphyrin IX cobalt chloride (CoPP) is a classical inducer of HO-1 gene expression. Our previous study showed that induction of HO-1 by CoPP effectively inhibited PRRSV infection, which was reversed by prior siRNA knockdown (19). To examine whether overexpression of miR-24-3p reverses the inhibitory effect of HO-1 on PRRSV infection, MARC-145 cells were pretreated with miR-24-3p mimics, followed by treatment with CoPP, and then infected with PRRSV expressing EGFP. As shown in Fig. 7A, pretreatment with miR-24-3p mimics or siHO-1 in CoPP-treated MARC-145 cells resulted in a 110% or 150% increase of EGFP-PRRSV-positive cells, respectively, while miR-24-3p-Mut mimics had no significant effect.
FIG 7.
miR-24-3p reverses the suppressive effect of HO-1 induction on PRRSV infection. MARC-145 cells were transfected with 200 nM concentrations of miR-24-3p mimics, miR-24-3p-Mut mimics, NC mimics, or siHO-1 (as a positive control) for 12 h and then infected with either rHP-PRRSV/SD16/EGFP (MOI of 0.01) for 48 h or PRRSV strain SD16 (MOI of 0.01) for 24 h, followed by treatment with CoPP (20 μM) from 1 hpi onward. The percentage of EGFP-positive cells infected with PRRSV 48 hpi was measured by flow cytometry (A). PRRSV replication was evaluated by qRT-PCR for extracellular PRRSV ORF7 RNA and virus titers in the supernatants (B) and for intracellular PRRSV ORF7 RNA (C). (D) Western blotting for intracellular PRRSV N protein was performed. α-Tubulin was used as the loading control. Results in panels A to C are expressed as means ± standard deviations of three independent experiments. P values were calculated using Student's t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant.
To confirm whether miR-24-3p reverses the effect of HO-1 induction on wild-type (WT) PRRSV replication, virus replication was evaluated by HO-1 induction in MARC-145 cells after transfection with miR-24-3p mimics, NC mimics, or HO-1 siRNAs as a positive control. Pretreatment with miR-24-3p mimics or siHO-1 in CoPP-treated MARC-145 cells significantly increased the virus titers (Fig. 7B, left) and levels of PRRSV RNA in the supernatants (Fig. 7B, right), intracellular PRRSV RNA (Fig. 7C), and intracellular PRRSV protein (Fig. 7D) compared with levels in cells treated with NC mimics while treatment with miR-24-3p-Mut mimics had no effect. Virus titers in the supernatants increased by 0.71 log10, and expression levels of extracellular PRRSV ORF7 RNA in CoPP-treated MARC-145 cells pretreated with miR-24-3p mimics were upregulated by 93% (Fig. 7B). Expression levels of intracellular PRRSV ORF7 RNA in CoPP-treated MARC-145 cells pretreated with miR-24-3p mimics were increased by 200% (Fig. 7C). Expression levels of intracellular PRRSV N protein in CoPP-treated MARC-145 cells pretreated with miR-24-3p mimics were increased by 38% (Fig. 7D).
A similar experiment was performed with WT PRRSV in PAMs, and similar results were observed (Fig. 8). Expression levels of extracellular PRRSV ORF7 RNA in CoPP-treated PAMs pretreated with miR-24-3p mimics were increased by 55% (Fig. 8A). The levels of intracellular PRRSV ORF7 RNA in CoPP-treated PAMs pretreated with miR-24-3p mimics were increased by approximately 55% (Fig. 8B). Expression levels of intracellular PRRSV N protein in CoPP-treated PAMs pretreated with miR-24-3p mimics were also increased by 44% (Fig. 8C).
FIG 8.
miR-24-3p reverses the suppressive effect of HO-1 induction on PRRSV replication in PAMs. PAMs were transfected with 200 nM concentrations of miR-24-3p mimics, miR-24-3p-Mut mimics, NC mimics, or siHO-1 (as positive control) for 12 h and then infected with PRRSV strain SD16 (MOI of 0.01) for 24 h, followed by treatment with CoPP (5 μM) from 1 hpi onward. PRRSV replication was evaluated by qRT-PCR for extracellular PRRSV ORF7 RNA in the supernatants (A) and for intracellular PRRSV ORF7 RNA (B). Western blotting for intracellular PRRSV N protein was performed, with α-tubulin used as the control (C). Results are expressed as means ± standard deviations of three independent experiments. P values were calculated using Student's t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant.
DISCUSSION
The regulation of HO-1 expression occurs mainly at the transcriptional level (39); however, recent reports reveal that expression of HO-1 can also be regulated posttranscriptionally by miRNAs in some cell types (40). Gene expression is decreased by miRNAs through recognition of complementary sequence target elements. The present study shows that miRNAs targeting HO-1 may regulate PRRSV replication. Using both in silico and in vitro methods, we demonstrated that miR-24-3p is a key regulator of HO-1 expression. Both mml-miR-24-3p and mml-miR217b regulated luciferase expression by targeting the monkey HO-1 3′ UTR, and ssc-miR-24-3p regulated expression by targeting the pig HO-1 3′ UTR (Fig. 1). Previous studies indicated that miR-377, alone or in combination with miR-217, targeted the human HO-1 3′ UTR (29). Our results showed that overexpression of mml-miR-217b, but not mml-miR-377, markedly decreased luciferase expression from a luciferase reporter containing the monkey HO-1 3′ UTR (Fig. 1), despite the presence a predicted binding site in the 3′ UTR of monkey HO-1. Importantly, both monkey and pig HO-1 genes have predicted binding sites for miR-24-3p in their 3′ UTRs, and their ability to regulate HO-1 expression was validated through a luciferase activity assay (Fig. 1 to 3) and Ago2 coimmunoprecipitation analysis (Fig. 4). In addition, miR-24-3p is well conserved among different host species (Fig. 1C).
miRNAs downregulate gene expression either by inhibiting mRNA translation or by inducing mRNA degradation (41, 42). miR-377 and miR-217 were previously shown to downregulate HO-1 expression by translation repression and not mRNA degradation in both HEK293 cells and primary human umbilical vein endothelial cells (HUVEC) (29). miR-196 and let-7 miRNA directly target the 3′ UTR of Bach-1 mRNA, a basic leucine zipper mammalian transcriptional repressor of HO-1, and translationally repress expression of this protein, thereby upregulating HO-1 gene expression (43, 44). Our results showed that overexpression of miR-24-3p in MARC-145 cells significantly downregulated both HO-1 mRNA and protein expression (Fig. 5). These results suggested that miR-24-3p might decrease HO-1 expression through both mRNA degradation and translation repression. In addition, CoPP treatment markedly downregulated miR-24-3p expression in MARC-145 cells and PAMs (data not shown). Collectively, these results demonstrated that there is an inverse correlation between the expression of miR-24-3p and HO-1 levels.
miR-24-3p has been investigated for its role in cell proliferation and differentiation (45–47) as well as for its role in stress responses under conditions of hypoxia and microbe infection (48, 49). Through microRNA sequencing analysis, miR-24-3p was identified as being linked to a hypoxia gene signature in breast cancer (48). In this study, infection of MARC-145 cells with PRRSV markedly induced miR-24-3p (Fig. 6). HO-1 plays an important role in protecting the host against viral infection (13, 15–17). Upregulation of HO-1 by CoPP treatment, pre- and postinfection, remarkably inhibits Ebola virus (EBOV) transcription/replication (50). Induction or overexpression of HO-1 also suppresses hepatitis C virus (HCV) replication and protects hepatocytes from oxidative damage (14). In addition, the HO-1 product biliverdin inhibits HCV replication by increasing the antiviral interferon response (51, 52). Our previous work showed that HO-1 induction using different concentrations of CoPP greatly decreases both recombinant PRRSV and wild-type PRRSV replication in a dose-dependent manner. HO-1 knockdown using a specific siRNA reverses the suppressive effect of HO-1 on PRRSV replication (19). These results suggested that there is a direct correlation between HO-1 expression and the inhibition of PRRSV replication. In the present study, we investigated whether miR-24-3p could reverse the inhibitory effect of HO-1 on PRRSV replication. As expected, pretreatment with either miR-24-3p or siHO-1 significantly reversed the suppression of PRRSV replication following HO-1 induction by CoPP in MARC-145 cells (Fig. 7) and PAMs (Fig. 8). These results indicated that miR-24-3p promotes PRRSV replication through suppression of HO-1 expression.
In summary, we demonstrated for the first time that HO-1 expression is regulated by miR-24-3p. Infection of PRRSV-permissive cells with PRRSV induces miR-24-3p expression. miR-24-3p then binds to the functional sites in the 3′ UTR of HO-1 and downregulates HO-1 gene expression to facilitate PRRSV replication. These findings not only provide new insights into virus-host interactions during PRRSV infection but also suggest potential new control measures for future PRRSV infection.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (31472173, 31430084, 31101690, 31302060, 31302103), the National High-Tech R&D Program of China (2011AA10A208), the Natural Science Basic Research Plan in Shaanxi Province of China (2014JQ3088), the Northwest A&F University talent special fund (Z111021201), and the Fundamental Research Fund (QN2013028). I.G.G. is a Wellcome Senior Fellow and supported by funding from the Wellcome Trust (WT097997MA).
REFERENCES
- 1.Xiao S, Jia J, Mo D, Wang Q, Qin L, He Z, Zhao X, Huang Y, Li A, Yu J, Niu Y, Liu X, Chen Y. 2010. Understanding PRRSV infection in porcine lung based on genome-wide transcriptome response identified by deep sequencing. PLoS One 5:e11377. doi: 10.1371/journal.pone.0011377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Normile D. 2007. Virology China, Vietnam grapple with “rapidly evolving” pig virus. Science 317:1017. doi: 10.1126/science.317.5841.1017. [DOI] [PubMed] [Google Scholar]
- 3.Tian K, Yu X, Zhao T, Feng Y, Cao Z, Wang C, Hu Y, Chen X, Hu D, Tian X, Liu D, Zhang S, Deng X, Ding Y, Yang L, Zhang Y, Xiao H, Qiao M, Wang B, Hou L, Wang X, Yang X, Kang L, Sun M, Jin P, Wang S, Kitamura Y, Yan J, Gao GF. 2007. Emergence of fatal PRRSV variants: unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS One 2:e526. doi: 10.1371/journal.pone.0000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snijder EJ, Kikkert M, Fang Y. 2013. Arterivirus molecular biology and pathogenesis. J Gen Virol 94:2141–2163. doi: 10.1099/vir.0.056341-0. [DOI] [PubMed] [Google Scholar]
- 5.Brar MS, Shi M, Hui RK, Leung FC. 2014. Genomic evolution of porcine reproductive and respiratory syndrome virus (PRRSV) isolates revealed by deep sequencing. PLoS One 9:e88807. doi: 10.1371/journal.pone.0088807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leng CL, Tian ZJ, Zhang WC, Zhang HL, Zhai HY, An TQ, Peng JM, Ye C, Sun L, Wang Q, Sun Y, Li L, Zhao HY, Chang D, Cai XH, Zhang GH, Tong GZ. 2014. Characterization of two newly emerged isolates of porcine reproductive and respiratory syndrome virus from Northeast China in 2013. Vet Microbiol 171:41–52. doi: 10.1016/j.vetmic.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Gomez-Laguna J, Salguero FJ, Barranco I, Pallares FJ, Rodriguez-Gomez IM, Bernabe A, Carrasco L. 2010. Cytokine expression by macrophages in the lung of pigs infected with the porcine reproductive and respiratory syndrome virus. J Comp Pathol 142:51–60. doi: 10.1016/j.jcpa.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu Q, Bai J, Zhang L, Liu J, Jiang Z, Michal JJ, He Q, Jiang P. 2012. Two-dimensional liquid chromatography-tandem mass spectrometry coupled with isobaric tags for relative and absolute quantification (iTRAQ) labeling approach revealed first proteome profiles of pulmonary alveolar macrophages infected with porcine reproductive and respiratory syndrome virus. J Proteome Res 11:2890–2903. doi: 10.1021/pr201266z. [DOI] [PubMed] [Google Scholar]
- 9.Genini S, Delputte PL, Malinverni R, Cecere M, Stella A, Nauwynck HJ, Giuffra E. 2008. Genome-wide transcriptional response of primary alveolar macrophages following infection with porcine reproductive and respiratory syndrome virus. J Gen Virol 89:2550–2564. doi: 10.1099/vir.0.2008/003244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoon KJ, Wu LL, Zimmerman JJ, Hill HT, Platt KB. 1996. Antibody-dependent enhancement (ADE) of porcine reproductive and respiratory syndrome virus (PRRSV) infection in pigs. Viral Immunol 9:51–63. doi: 10.1089/vim.1996.9.51. [DOI] [PubMed] [Google Scholar]
- 11.Bao D, Wang R, Qiao S, Wan B, Wang Y, Liu M, Shi X, Guo J, Zhang G. 2013. Antibody-dependent enhancement of PRRSV infection down-modulates TNF-alpha and IFN-beta transcription in macrophages. Vet Immunol Immunopathol 156:128–134. doi: 10.1016/j.vetimm.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Chung SW, Hall SR, Perrella MA. 2009. Role of haem oxygenase-1 in microbial host defence. Cell Microbiol 11:199–207. doi: 10.1111/j.1462-5822.2008.01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt WN, Mathahs MM, Zhu Z. 2012. Heme and HO-1 inhibition of HCV, HBV, and HIV. Front Pharmacol 3:129. doi: 10.3389/fphar.2012.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Z, Wilson AT, Mathahs MM, Wen F, Brown KE, Luxon BA, Schmidt WN. 2008. Heme oxygenase-1 suppresses hepatitis C virus replication and increases resistance of hepatocytes to oxidant injury. Hepatology 48:1430–1439. doi: 10.1002/hep.22491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devadas K, Dhawan S. 2006. Hemin activation ameliorates HIV-1 infection via heme oxygenase-1 induction. J Immunol 176:4252–4257. doi: 10.4049/jimmunol.176.7.4252. [DOI] [PubMed] [Google Scholar]
- 16.Hashiba T, Suzuki M, Nagashima Y, Suzuki S, Inoue S, Tsuburai T, Matsuse T, Ishigatubo Y. 2001. Adenovirus-mediated transfer of heme oxygenase-1 cDNA attenuates severe lung injury induced by the influenza virus in mice. Gene Ther 8:1499–1507. doi: 10.1038/sj.gt.3301540. [DOI] [PubMed] [Google Scholar]
- 17.Protzer U, Seyfried S, Quasdorff M, Sass G, Svorcova M, Webb D, Bohne F, Hosel M, Schirmacher P, Tiegs G. 2007. Antiviral activity and hepatoprotection by heme oxygenase-1 in hepatitis B virus infection. Gastroenterology 133:1156–1165. doi: 10.1053/j.gastro.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Xiao S, Gao J, Liu M, Zhang X, Li M, Zhao G, Mo D, Liu X, Chen Y. 2014. Inhibition of replication of porcine reproductive and respiratory syndrome virus by hemin is highly dependent on heme oxygenase-1, but independent of iron in MARC-145 cells. Antiviral Res 105:39–46. doi: 10.1016/j.antiviral.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Xiao S, Zhang A, Zhang C, Ni H, Gao J, Wang C, Zhao Q, Wang X, Wang X, Ma C, Liu H, Li N, Mu Y, Sun Y, Zhang G, Hiscox JA, Hsu WH, Zhou EM. 2014. Heme oxygenase-1 acts as an anti-viral factor for porcine reproductive and respiratory syndrome virus infection and over-expression inhibits virus replication in vitro. Antiviral Res 110:60–69. doi: 10.1016/j.antiviral.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Bartel DP. 2009. MicroRNAs: target recognition and regulatory functions. Cell 136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottwein E, Cullen BR. 2008. Viral and cellular microRNAs as determinants of viral pathogenesis and immunity. Cell Host Microbe 3:375–387. doi: 10.1016/j.chom.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skalsky RL, Cullen BR. 2010. Viruses, microRNAs, and host interactions. Annu Rev Microbiol 64:123–141. doi: 10.1146/annurev.micro.112408.134243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. 2005. Modulation of hepatitis C virus RNA abundance by a liver-specific microRNA. Science 309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 24.Jangra RK, Yi M, Lemon SM. 2010. Regulation of hepatitis C virus translation and infectious virus production by the microRNA miR-122. J Virol 84:6615–6625. doi: 10.1128/JVI.00417-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lagos D, Pollara G, Henderson S, Gratrix F, Fabani M, Milne RS, Gotch F, Boshoff C. 2010. miR-132 regulates antiviral innate immunity through suppression of the p300 transcriptional co-activator. Nat Cell Biol 12:513–519. doi: 10.1038/ncb2054. [DOI] [PubMed] [Google Scholar]
- 26.Wang D, Cao L, Xu Z, Fang L, Zhong Y, Chen Q, Luo R, Chen H, Li K, Xiao S. 2013. miR-125b reduces porcine reproductive and respiratory syndrome virus replication by negatively regulating the NF-κB pathway. PLoS One 8:e55838. doi: 10.1371/journal.pone.0055838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao L, Guo XK, Wang L, Zhang Q, Li N, Chen XX, Wang Y, Feng WH. 2013. MicroRNA 181 suppresses porcine reproductive and respiratory syndrome virus (PRRSV) infection by targeting PRRSV receptor CD163. J Virol 87:8808–8812. doi: 10.1128/JVI.00718-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo XK, Zhang Q, Gao L, Li N, Chen XX, Feng WH. 2013. Increasing expression of microRNA 181 inhibits porcine reproductive and respiratory syndrome virus replication and has implications for controlling virus infection. J Virol 87:1159–1171. doi: 10.1128/JVI.02386-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beckman JD, Chen C, Nguyen J, Thayanithy V, Subramanian S, Steer CJ, Vercellotti GM. 2011. Regulation of heme oxygenase-1 protein expression by miR-377 in combination with miR-217. J Biol Chem 286:3194–3202. doi: 10.1074/jbc.M110.148726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skrzypek K, Tertil M, Golda S, Ciesla M, Weglarczyk K, Collet G, Guichard A, Kozakowska M, Boczkowski J, Was H, Gil T, Kuzdzal J, Muchova L, Vitek L, Loboda A, Jozkowicz A, Kieda C, Dulak J. 2013. Interplay between heme oxygenase-1 and miR-378 affects non-small cell lung carcinoma growth, vascularization, and metastasis. Antioxid Redox Signal 19:644–660. doi: 10.1089/ars.2013.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wensvoort G, Terpstra C, Pol JM, ter Laak EA, Bloemraad M, de Kluyver EP, Kragten C, van Buiten L, den Besten A, Wagenaar F, et al. 1991. Mystery swine disease in The Netherlands: the isolation of Lelystad virus. Vet Q 13:121–130. doi: 10.1080/01652176.1991.9694296. [DOI] [PubMed] [Google Scholar]
- 32.Wang C, Huang B, Kong N, Li Q, Ma Y, Li Z, Gao J, Zhang C, Wang X, Liang C, Dang L, Xiao S, Mu Y, Zhao Q, Sun Y, Almazan F, Enjuanes L, Zhou EM. 2013. A novel porcine reproductive and respiratory syndrome virus vector system that stably expresses enhanced green fluorescent protein as a separate transcription unit. Vet Res 44:104. doi: 10.1186/1297-9716-44-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rehmsmeier M, Steffen P, Hochsmann M, Giegerich R. 2004. Fast and effective prediction of microRNA/target duplexes. RNA 10:1507–1517. doi: 10.1261/rna.5248604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. 2003. Prediction of mammalian microRNA targets. Cell 115:787–798. doi: 10.1016/S0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 35.Betel D, Wilson M, Gabow A, Marks DS, Sander C. 2008. The microRNA.org resource: targets and expression. Nucleic Acids Res 36:D149–D153. doi: 10.1093/nar/gkm995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qin L, Chen Y, Niu Y, Chen W, Wang Q, Xiao S, Li A, Xie Y, Li J, Zhao X, He Z, Mo D. 2010. A deep investigation into the adipogenesis mechanism: profile of microRNAs regulating adipogenesis by modulating the canonical Wnt/beta-catenin signaling pathway. BMC Genomics 11:320. doi: 10.1186/1471-2164-11-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang WX, Wilfred BR, Hu Y, Stromberg AJ, Nelson PT. 2010. Anti-Argonaute RIP-Chip shows that miRNA transfections alter global patterns of mRNA recruitment to microribonucleoprotein complexes. RNA 16:394–404. doi: 10.1261/rna.1905910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao S, Wang Q, Gao J, Wang L, He Z, Mo D, Liu X, Chen Y. 2011. Inhibition of highly pathogenic PRRSV replication in MARC-145 cells by artificial microRNAs. Virol J 8:491. doi: 10.1186/1743-422X-8-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gozzelino R, Jeney V, Soares MP. 2010. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol 50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 40.Kozakowska M, Szade K, Dulak J, Jozkowicz A. 2014. Role of heme oxygenase-1 in postnatal differentiation of stem cells: a possible cross-talk with microRNAs. Antioxid Redox Signal 20:1827–1850. doi: 10.1089/ars.2013.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bartel DP. 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 42.Fabian MR, Sonenberg N, Filipowicz W. 2010. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem 79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 43.Hou W, Tian Q, Zheng J, Bonkovsky HL. 2010. MicroRNA-196 represses Bach1 protein and hepatitis C virus gene expression in human hepatoma cells expressing hepatitis C viral proteins. Hepatology 51:1494–1504. doi: 10.1002/hep.23401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hou W, Tian Q, Steuerwald NM, Schrum LW, Bonkovsky HL. 2012. The let-7 microRNA enhances heme oxygenase-1 by suppressing Bach1 and attenuates oxidant injury in human hepatocytes. Biochim Biophys Acta 1819:1113–1122. doi: 10.1016/j.bbagrm.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu W, Liu M, Peng X, Zhou P, Zhou J, Xu K, Xu H, Jiang S. 2013. miR-24-3p and miR-27a-3p promote cell proliferation in glioma cells via cooperative regulation of MXI1. Int J Oncol 42:757–766. [DOI] [PubMed] [Google Scholar]
- 46.Jensen K, Brusletto BS, Aass HC, Olstad OK, Kierulf P, Gautvik KM. 2013. Transcriptional profiling of mRNAs and microRNAs in human bone marrow precursor B cells identifies subset- and age-specific variations. PLoS One 8:e70721. doi: 10.1371/journal.pone.0070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye SB, Li ZL, Luo DH, Huang BJ, Chen YS, Zhang XS, Cui J, Zeng YX, Li J. 2014. Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget 5:5439–5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Camps C, Saini HK, Mole DR, Choudhry H, Reczko M, Guerra-Assuncao JA, Tian YM, Buffa FM, Harris AL, Hatzigeorgiou AG, Enright AJ, Ragoussis J. 2014. Integrated analysis of microRNA and mRNA expression and association with HIF binding reveals the complexity of microRNA expression regulation under hypoxia. Mol Cancer 13:28. doi: 10.1186/1476-4598-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin W, Ibeagha-Awemu EM, Liang G, Beaudoin F, Zhao X, Guan le L. 2014. Transcriptome microRNA profiling of bovine mammary epithelial cells challenged with Escherichia coli or Staphylococcus aureus bacteria reveals pathogen directed microRNA expression profiles. BMC Genomics 15:181. doi: 10.1186/1471-2164-15-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hill-Batorski L, Halfmann P, Neumann G, Kawaoka Y. 2013. The cytoprotective enzyme heme oxygenase-1 suppresses Ebola virus replication. J Virol 87:13795–13802. doi: 10.1128/JVI.02422-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lehmann E, El-Tantawy WH, Ocker M, Bartenschlager R, Lohmann V, Hashemolhosseini S, Tiegs G, Sass G. 2010. The heme oxygenase 1 product biliverdin interferes with hepatitis C virus replication by increasing antiviral interferon response. Hepatology 51:398–404. doi: 10.1002/hep.23339. [DOI] [PubMed] [Google Scholar]
- 52.Zhu Z, Wilson AT, Luxon BA, Brown KE, Mathahs MM, Bandyopadhyay S, McCaffrey AP, Schmidt WN. 2010. Biliverdin inhibits hepatitis C virus nonstructural 3/4A protease activity: mechanism for the antiviral effects of heme oxygenase? Hepatology 52:1897–1905. doi: 10.1002/hep.23921. [DOI] [PMC free article] [PubMed] [Google Scholar]