ABSTRACT
The nonenveloped simian virus 40 (SV40) hijacks the three endoplasmic reticulum (ER) membrane-bound J proteins B12, B14, and C18 to escape from the ER into the cytosol en route to successful infection. How C18 controls SV40 ER-to-cytosol membrane penetration is the least understood of these processes. We previously found that SV40 triggers B12 and B14 to reorganize into discrete puncta in the ER membrane called foci, structures postulated to represent the cytosol entry site (C. P. Walczak, M. S. Ravindran, T. Inoue, and B. Tsai, PLoS Pathog 10:e1004007, 2014). We now find that SV40 also recruits C18 to the virus-induced B12/B14 foci. Importantly, the C18 foci harbor membrane penetration-competent SV40, further implicating this structure as the membrane penetration site. Consistent with this, a mutant SV40 that cannot penetrate the ER membrane and promote infection fails to induce C18 foci. C18 also regulates the recruitment of B12/B14 into the foci. In contrast to B14, C18's cytosolic Hsc70-binding J domain, but not the lumenal domain, is essential for its targeting to the foci; this J domain likewise is necessary to support SV40 infection. Knockdown-rescue experiments reveal that C18 executes a role that is not redundant with those of B12/B14 during SV40 infection. Collectively, our data illuminate C18's contribution to SV40 ER membrane penetration, strengthening the idea that SV40-triggered foci are critical for cytosol entry.
IMPORTANCE Polyomaviruses (PyVs) cause devastating human diseases, particularly in immunocompromised patients. As this virus family continues to be a significant human pathogen, clarifying the molecular basis of their cellular entry pathway remains a high priority. To infect cells, PyV traffics from the cell surface to the ER, where it penetrates the ER membrane to reach the cytosol. In the cytosol, the virus moves to the nucleus to cause infection. ER-to-cytosol membrane penetration is a critical yet mysterious infection step. In this study, we clarify the role of an ER membrane protein called C18 in mobilizing the simian PyV SV40, a PyV archetype, from the ER into the cytosol. Our findings also support the hypothesis that SV40 induces the formation of punctate structures in the ER membrane, called foci, that serve as the portal for cytosol entry of the virus.
INTRODUCTION
While polyomaviruses (PyVs) are known to establish asymptomatic persistent infections in the kidney, blood, skin, and brain of healthy individuals, they carry the potential to cause debilitating diseases, especially during immunosuppression. For example, infections caused by the human BK, JC, and Merkel cell PyVs can lead to PyV-associated nephropathy, progressive multifocal leukoencephalopathy, and Merkel cell carcinoma, respectively (1, 2). Simian virus 40 (SV40) traditionally has been used as a model for studying this virus family and has structural and genetic similarities to human PyVs. SV40 and all other PyVs are nonenveloped icosahedral particles, approximately 45 nm in diameter, and contain a double-stranded DNA genome. When fully assembled, the outer capsid contains 360 copies of the major capsid protein VP1 arranged as 72 pentamers; in turn, these pentamers are stabilized by hydrophobic interactions, disulfide bonds, and calcium ions. Residing beneath each pentamer is a minor coat protein, either VP2 or VP3, which is not exposed on the surface of a native virus (3, 4, 5). To cause infection, SV40 binds to the glycolipid ganglioside GM1 receptor on the host cell surface and becomes internalized (6–8). The virus then traffics to the lumen of the endoplasmic reticulum (ER) (9–11), where it coopts cellular machineries to cross the ER membrane and reach the cytosol as a mostly intact particle (12). From the cytosol, further disassembly of the virus generates a subviral particle (containing its viral genome) that is transferred through the nuclear pore complex into the nucleus (13). In this compartment, transcription and replication of viral genes ensue, leading to lytic infection or cellular transformation.
Viral trafficking through the ER for entry into the cytosol, a strategy unique to SV40 and other PyVs, represents a decisive infection step. Insights into how ER membrane penetration occurs have emerged recently. Several studies pinpointed select ER protein quality control components responsible for inducing conformational changes to the virus. Specifically, members of the protein disulfide isomerase (PDI) family use either their oxidoreductase or chaperone activities to disrupt the forces that stabilize the VP1 pentamers (14–18). These reactions expose the minor coat proteins VP2/3, generating a hydrophobic viral particle that binds to and integrates into the ER membrane (16, 19–23); viral integration with the ER membrane thereby initiates the membrane penetration process. Membrane penetration proceeds when the embedded Glu residue of VP2 serves as a trigger to recruit an ER transmembrane protein, called BAP31, and a subset of additional factors involved in the ER-associated degradation (ERAD) process (23). ERAD is a quality control process that functions to eliminate misfolded proteins from the ER by retrotranslocating them into cytosol for proteasomal degradation (24). SV40 and other PyVs utilize selective ERAD components, as well as the ER membrane-bound J proteins DnaJB12 (B12), DnaJB14 (B14), and DnaJC18 (C18), to reach the cytosol and cause infection (23–30). The individual contribution of each membrane-bound J protein, their potential redundancy, and how they may cooperate to promote successful PyV membrane penetration and infection are largely unknown.
Through transient interactions with its J domain, a J protein stimulates the ATPase and consequently the substrate binding activity of the Hsc70 chaperone family. The J domains of B12 and B14 are localized on the cytosolic face of the ER membrane to engage cytosolic Hsc70 (29, 30). B12 and B14 also physically interact as a multiprotein complex (31). In the context of SV40 entry, we recently reported that B12 and B14 reorganize into discrete puncta, called foci, in the ER membrane, a phenomenon originally reported with BAP31 (23, 31). Importantly, focus formation is largely selective for those cellular components required in facilitating SV40's ER-to-cytosol transport. This structure contains a higher concentration of ER membrane components essential for catalyzing the viral membrane penetration step. By concentrating to a specific region in the ER membrane, these cellular membrane components may efficiently recruit cytosolic factors to the membrane penetration site to effectively mobilize the viral particle into the cytosol. Consistent with this hypothesis, we identified the cytosolic cochaperone SGTA as a binding partner of the B12-B14 complex, which acts as an important player for PyV ER membrane penetration (31). In order to promote SV40 infection, the B12-B14 complex must be able to form foci on the ER membrane and bind to cytosolic chaperones, including Hsc70 and the SGTA cochaperone.
Despite sharing a high degree of sequence homology with B12 and B14, C18's contribution to SV40 infection is unclear. Here, we focused on the role of C18 in promoting SV40 ER membrane penetration. Our data indicate that C18 becomes recruited to SV40-induced foci containing BAP31, B12, and B14. Importantly, these foci contain the membrane penetration-competent SV40, suggesting they represent sites of membrane penetration. C18 also regulates the recruitment of B12/B14 into the foci. Mutational analysis indicated that C18 has different domain requirements than B12/B14 during recruitment to the foci, consistent with functional rescue studies demonstrating that C18 executes a role that is not redundant with those of B12/B14 in promoting SV40 infection.
MATERIALS AND METHODS
Antibodies.
Monoclonal SV40 large T antigen antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal VP1 antibody was kindly provided by Walter Scott (University of Miami). Rabbit anti-VP2/3 antibody and anti-S-tag antibody were purchased from Abcam (Cambridge, MA). Polyclonal DnaJB14, DnaJB12, and SGTA antibodies were purchased from Proteintech Group (Chicago, IL). Monoclonal BAP31 and polyclonal Hsc70 antibodies were purchased from Pierce (Rockford, IL), and anti-FLAG tag antibody was obtained from Sigma (St. Louis, MO).
Reagents.
Dulbecco's modified Eagle's medium (DMEM), Opti-MEM, and 0.25% trypsin-EDTA were purchased from Invitrogen (Carlsbad, CA). Fetal clone III (FC) was from HyClone (Logan, UT). Complete-mini EDTA-free protease inhibitor cocktail tablets were purchased from Roche. Dithiothreitol was purchased from Sigma (St. Louis, MO). Deoxy Big CHAP and S-agarose beads were obtained from Calbiochem (Billerica, MA) and Novagen (San Diego, CA), respectively.
Preparation of WT and ΔVP3 SV40.
Wild-type (WT) SV40 was prepared using an OptiPrep gradient system as described previously (12). To prepare ΔVP3 SV40, the SV40 mutant genome lacking VP3 (i.e., ΔVP3) was transfected into CV-1 cells. Five days after transfection, cells were lysed in a buffer containing 50 mM HEPES (pH 7.5), 150 mM NaCl, and 0.5% Brij58 to isolate the mutant virus from the cells. Purification of the mutant virus was described previously (12).
siRNA transfection and DNA plasmids.
AllStars Negative, purchased from Qiagen (Valencia, CA), was used as the control short interfering RNA (siRNA) (labeled as scrambled). The following custom siRNA sequences were generated and purchased from Dharmacon (Pittsburgh, PA) or Invitrogen: C18 siRNA, 5′ GCUAUGAUGAAUACGGAGAUU 3′ and 5′ UCUCCGUAUUCAUCAUAGCUU 3′; B12 siRNA, 5′ GGCAGAGUGGGAACUUGAAACUGUU 3′ and 5′ AACAGUUUCAAGUUCCCACUCUGCC 3′; and B14 siRNA, 5′ GGUUCCUGAAAUCUUGGACUGUUUA 3′ and 5′ UAAACAGUCCAAGAUUUCAGGAACC 3′.
Using Lipofectamine RNAiMAX (Invitrogen), 50 nM control or custom siRNAs were reverse transfected into CV-1 cells. Infection or biochemical assays were carried out at 48 or 72 h posttransfection. C18 and B12 were amplified from the HEK293T cDNA pool and cloned into pcDNA3.1(−) (Invitrogen) vector with a C-terminal S-tag or N-terminal FLAG tag. B14 WT and mutant constructs were previously reported (31). Site-directed mutagenesis was performed on His 110 of C18 and His 138 of B12 to yield H110Q C18 and H138Q B12 mutants, respectively. The Δ lumenal FLAG-C18 contains residues 1 to 261 and Δ lumenal FLAG-B12 contains residues 1 to 276, and the constructs were generated using standard cloning methods.
Interaction studies by S-agarose bead affinity purification.
To study physical interactions between BAP31-J proteins C18-B12, C18-B14, C18-Hsc70, and C18-SGTA, HEK293T cells were transfected with the respective S-tagged plasmids. Cells were lysed in a buffer containing 50 mM Tris, pH 7.4, 150 mM NaCl, 1 mM EDTA, protease inhibitors, and 1% Deoxy Big CHAP (Calbiochem, Billerica, MA). The cleared lysates were incubated with S beads for 2 h at 4°C and then washed with the lysis buffer containing 0.1% Deoxy Big CHAP. SDS sample buffer was used for elution at 95°C. Interacting proteins of interest were analyzed by immunoblotting using the respective endogenous antibodies.
Immunofluorescence microscopy for focus formation.
CV-1 cells were grown in 12- or 24-well plates for 12 h, treated with SV40 for the indicated time, and washed with PBS, followed by fixation with 1% formaldehyde at room temperature. Cells then were permeabilized with 0.2% Triton X-100 and blocked with 5% milk with 0.2% Tween. Primary antibodies were incubated for 1 h at room temperature, followed by fluorescence-conjugated secondary antibodies for 30 min at room temperature. Coverslips were mounted with ProLong Gold (Invitrogen). Images were taken using an inverted epifluorescence microscope (Nikon Eclipse TE2000-E) equipped with 60× and 100× 1.40-numeric-aperture (NA) objectives and a Photometrics CoolSnap HQ camera. For C18 and other overexpression studies, cells were transfected with the desired plasmid with FuGene (Promega) at least 24 h prior to infection. For knockdown studies, cells were reverse transfected with the desired siRNA using Lipofectamine RNAiMAX (Invitrogen) at the time of cell seeding. ImageJ software (NIH) was used for image processing, analysis, and assembly.
Knockdown-rescue experiments.
CV-1 cells were reverse transfected with the indicated siRNA using Lipofectamine RNAiMAX (Invitrogen). Twenty-four h after siRNA transfection, cells were transfected with FLAG- or S-tagged green fluorescent protein (GFP) or J protein constructs (C18, B14, and B12). Twenty-four h after DNA transfection, cells were infected with SV40, and at 20 h postinfection (p.i.), cells were observed with immunofluorescence microscopy using SV40 T antigen and FLAG or S-tag antibody. For quantification, T antigen-positive cells were counted among FLAG or S-tagged protein-expressing cells.
Statistics.
Quantitative data are presented as the means from at least three independent experiments with standard deviations (SD). Paired two-tailed Student's t tests were used to acquire P values.
RESULTS
SV40 recruits C18 to the virus-induced B12/B14 foci that harbor membrane penetration-competent virus.
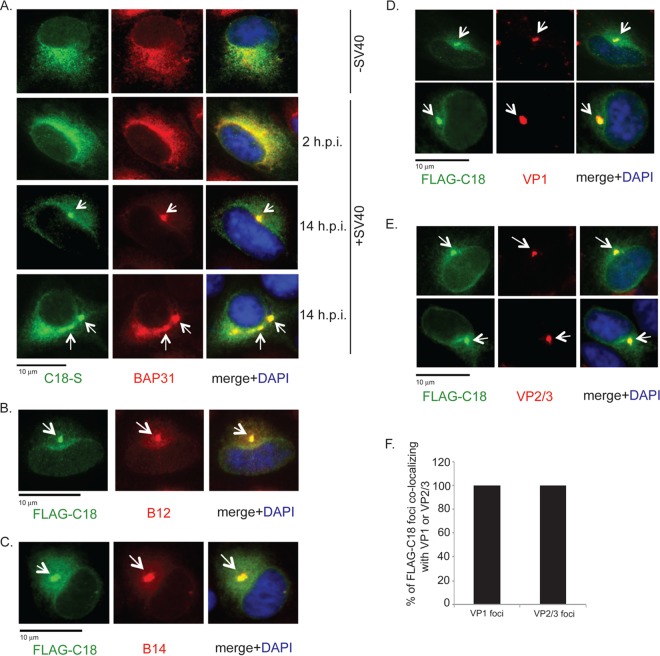
We recently demonstrated that SV40 triggers B12 and B14 to form foci that colocalize with the previously reported BAP31 foci (23, 31). As C18 also was implicated in facilitating SV40 ER membrane penetration and infection (26), we first examined the localization of C18 using immunofluorescence microscopy. S-tagged C18 was transfected in CV-1 cells that were left uninfected or were infected with SV40 for 2 or 14 h. In uninfected cells, C18 colocalizes with endogenous BAP31 in a diffuse pattern (Fig. 1A, top row), indicating that transfected C18 is localized to the ER. In cells infected with SV40 for 2 h, an early infection time point when the virus has yet to reach the ER, C18 displayed a similar diffuse colocalization with BAP31 (Fig. 1A, second row). In contrast, in cells infected for 14 h when SV40 has reached the ER, discrete regions of concentrated C18 and BAP31 were observed (Fig. 1A, third and fourth rows). These SV40-induced foci start to appear at approximately 6 to 8 h p.i. (data not shown), as previously reported for the B14 foci (31). FLAG-tagged C18 foci colocalized with the endogenous B12 (Fig. 1B) and B14 (Fig. 1C) foci. Colocalization of BAP31, B12, B14, and C18 in punctate structures within the ER membrane upon SV40 infection suggests that these factors all cooperate to guide SV40 across the ER membrane.
FIG 1.
SV40 recruits C18 to the virus-induced B12/B14 foci which also harbor membrane penetration-competent virus. (A) CV-1 cells were transfected with S-tagged C18, and at 12 h after transfection, cells mock infected or infected with SV40 (MOI, ∼50) for 2 h and 14 h were fixed, stained with anti-S antibody and anti-BAP31 antibodies, and analyzed by immunofluorescence microscopy. (B and C) CV-1 cells were transfected with FLAG-tagged C18. At 12 h after transfection, cells infected with SV40 (MOI, ∼50) for 14 h were fixed, stained with anti-FLAG and anti-B12 (B) or anti-FLAG and anti-B14 antibodies (C), and analyzed by immunofluorescence microscopy. DAPI, 4′,6-diamidino-2-phenylindole (DAPI). (D and E) CV-1 cells were transfected with FLAG-tagged C18 and infected with SV40 (MOI, ∼50) for 14 h, fixed, stained with anti-FLAG and anti-VP1 (D) or anti-FLAG and anti-VP2/3 antibodies (E), and analyzed by immunofluorescence microscopy. (F) Quantification of the percentage of FLAG-C18 foci colocalizing with VP1 or VP2/3 foci. Values represent means ± SD from three independent experiments.
Using antibodies directed against SV40 capsid proteins, we observed that foci containing C18 harbor not only VP1 (Fig. 1D, first and second rows; two examples are shown) but also the minor coat proteins VP2/3 (Fig. 1E, first and second rows; two examples are shown). Importantly, VP2/3 is exposed and recognized by antibodies in immunofluorescence experiments only when PyVs are activated by cellular factors in the ER lumen that induce conformational changes to the virus (19). Due to its hydrophobicity, VP2 exposure enables PyV to engage the ER membrane (16, 19–22). Therefore, within these foci are viruses that are likely to be membrane embedded and in the process of undergoing ER membrane penetration. Quantification of the data revealed that nearly every detectable C18 focus contained both VP1 and VP2/3 (Fig. 1F). These results correlate with the observation that SV40 VLPs (lacking both VP2/3) are noninfectious and do not induce foci (23). Collectively, our findings demonstrate that C18 is a component of the SV40-induced focus structure in the ER membrane that contains BAP31, B12, and B14. The C18 foci also harbor VP2/3-exposed, membrane penetration-competent virus, consistent with the notion that the foci represent the site from which SV40 penetrates into the cytosol.
VP3 is required to induce C18 foci.
To further interrogate the correlation between focus formation and ER-to-cytosol transport, we asked whether a mutant SV40 that cannot penetrate the ER membrane triggers C18 focus formation. We previously demonstrated that an SV40 mutant lacking VP3 but harboring VP2 (i.e., ΔVP3 SV40) reaches the ER from the cell surface but fails to gain entry into the cytosol and consequently cannot cause infection (12). Using this mutant, we assessed whether VP3 contributes to focus formation. WT and ΔVP3 SV40 were compared in their ability to cause focus formation. Immunoblot analysis confirmed the absence of VP3 in ΔVP3 SV40 compared to WT SV40 as expected, with equal amounts of VP2 and VP1 between the two (Fig. 2A). When cells were infected with the ΔVP3 SV40, exposure of its VP2 was observed (Fig. 2B), indicating that this mutant virus is able to reach the ER from the cell surface, consistent with a previous observation (12). However, we found a lack of FLAG-C18 foci in cells infected with ΔVP3 SV40 compared to cells infected with an equal amount of WT SV40 (Fig. 2C; quantified in D). Similarly, endogenous BAP31 did not form foci when infected with ΔVP3 SV40 (Fig. 2E). We conclude that VP3 is required to recruit selective cellular components to the foci in the ER membrane. As VP3 is essential for focus formation and virus ER-to-cytosol membrane transport, these findings further support the hypothesis that focus formation is functionally linked to the ER membrane penetration process.
FIG 2.
SV40 lacking VP3 does not induce C18 foci. (A) Purified WT SV40 and ΔVP3 SV40 were immunoblotted using the indicated antibodies. (B) CV-1 cells were mock infected or infected with ΔVP3 SV40 for 8 h, fixed, stained with anti-VP1 or VP2/3 antibodies, and analyzed by immunofluorescence microscopy. (C) FLAG-C18-transfected CV-1 cells were infected with the same amount (20 μg) of either purified WT or ΔVP3 SV40 for 8 h, fixed, stained with anti-FLAG and anti-VP1 antibodies, and analyzed by immunofluorescence microscopy. (D) Quantification data showed the percentage of FLAG-C18-expressing cells with C18 foci after WT SV40 or ΔVP3 SV40 infection. Cells were scored positive if at least one focus was present in the cell. Values represent means ± SD from three independent experiments. (E) CV-1 cells were infected with the same amount (20 μg) of either purified WT or ΔVP3 SV40 for 8 h, fixed, stained with anti-BAP31 and anti-VP1 antibodies, and analyzed by immunofluorescence microscopy.
C18 regulates B12, B14, and BAP31 focus formation.
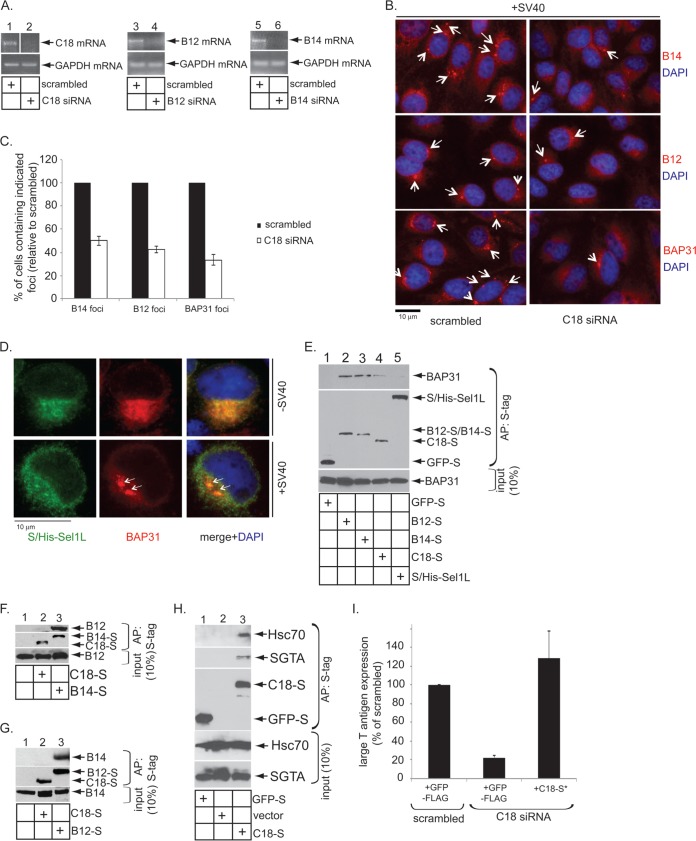
Given that C18, as well as BAP31, B14, and B12, all are targeted to the foci, we asked whether C18 assists in recruiting these other ER membrane proteins into the foci. To this end, CV-1 cells were transfected with a control siRNA (scrambled) or a C18-specific siRNA. As expected, the C18 mRNA level was markedly reduced after transfection with C18 siRNA but not control siRNA (Fig. 3A, top, compare lane 2 to 1), demonstrating the efficiency of the siRNA construct; the C18 siRNA specifically targets C18 (see below). Cells then were infected with SV40 for 14 h. Focus formation of B12, B14, and BAP31 again was monitored by immunofluorescence microscopy using antibodies against the endogenous proteins. In cells transfected with an siRNA against C18, an obvious reduction in the number of cells containing foci was observed compared to cells transfected with the scrambled siRNA (Fig. 3B). When quantified, cells transfected with C18 siRNA displayed a 50 to 60% reduction in BAP31-, B14-, or B12-positive foci compared to control siRNA-transfected cells (Fig. 3C). These findings demonstrate that C18 plays a key role in recruiting B14, B12, and BAP31 to the SV40-induced focus structure.
FIG 3.
C18 regulates B12, B14, and BAP31 focus formation. (A) RT-PCR results showing the knockdown efficiency of C18 (lanes 1 and 2)-, B12 (lanes 3 and 4)-, and B14 (lanes 5 and 6)-specific siRNAs. The expression of GAPDH mRNA was used as a loading control. (B) CV-1 cells were reverse transfected with scrambled or C18 siRNA. After 48 h of transfection, cells were infected with SV40 (MOI, ∼15) for 14 h, fixed, stained with the indicated antibodies, and analyzed by immunofluorescence microscopy. (C) Quantification data from panel B, where cells were scored positive if at least one focus was present in the cell. Values represent means ± SD from three independent experiments. (D) S/His-tagged Sel1L-transfected CV-1 cells were infected with SV40 (MOI, ∼50), fixed at 14 hpi, stained with anti-S and anti-BAP31 antibodies, and analyzed by immunofluorescence microscopy. (E) BAP31 interaction with C18, B14, and B12 were analyzed by using lysates derived from HEK 293T cells transfected with the indicated construct. The S-tagged proteins were affinity purified (AP) using S-agarose beads, and the precipitated samples were immunoblotted with the indicated antibodies. (F, G, and H) Interaction between C18-S/B14-S with endogenous B12 (F), C18-S/B12-S with endogenous B14 (G), and C18-S with endogenous Hsc70 and SGTA (H) were assessed by affinity purification using HEK 293T lysates harboring the indicated construct using S-agarose beads, followed by immunoblotting the precipitated material with the indicated antibodies. (I) S-tagged C18-expressing vector can rescue the C18 knockdown effect. CV-1 cells were reverse transfected with either scrambled or C18 siRNA (50 nM) for 24 h. Cells then were transfected with the indicated construct for 24 h, infected with SV40 (MOI, ∼0.5) for 20 h, fixed, and stained using anti-large T antigen, anti-FLAG, or anti-S antibodies. The percentages of large T antigen-positive cells were determined in cells expressing either GFP-FLAG or C18-S* by immunofluorescence microscopy. C18-S* is a construct designed to be resistant to the C18 siRNA. Values represent means ± SD from three independent experiments.
C18 might be important for focus formation, because it physically interacts with BAP31, B12, and/or B14 to stabilize the overall focus architecture. To test this possibility, transfected S-tagged constructs were subjected to S-affinity purification. We found that precipitation of B12-S and B14-S pulled down endogenous BAP31, while S-tagged GFP and the ER membrane-bound protein Sel1L, which is not recruited into foci by SV40 (Fig. 3D), did not (Fig. 3E, top, compare lanes 2 and 3 to 1 and 5). C18-S also pulled down endogenous BAP31, albeit to a lesser extent (Fig. 3E, compare lane 4 to lanes 2 and 3). Additional binding studies revealed that precipitation of B14-S but not C18-S pulled down endogenous B12 (Fig. 3F, top, compare lane 3 to 2), and precipitation of B12-S but not C18-S pulled down endogenous B14 (Fig. 3G, top, compare lane 3 to 2). C18-S's weak (or lack of) binding to other cellular factors in the foci likely is not due to C18-S's improper insertion into the ER membrane. C18-S was observed to interact with the known B12/B14 cytosolic binding partners Hsc70 and SGTA (Fig. 3H, lane 3), demonstrating that C18's J domain is properly displayed on the cytosolic surface of the ER membrane. Moreover, expression of a C18-S construct designed to be resistant to the C18-specific siRNA (C18-S*) functionally restores SV40 infection in cells transfected with the C18 siRNA (Fig. 3I); infection was monitored by assessing the expression of the virally encoded large T antigen in the host nucleus. These data show the general integrity of S-tagged C18. We conclude C18 interacts weakly with BAP31 and appears not to be part of the B12-B14 complex. C18's weak interaction with BAP31 may explain why knockdown of C18 partially decreased the recruitment of BAP31 into the foci. As BAP31 interacts with B12 and B14, a decrease in BAP31 recruitment to the foci (by knocking down C18) also might affect B12/B14 recruitment into the foci.
C18, B12, and B14 display various domain requirements to recruit into foci.
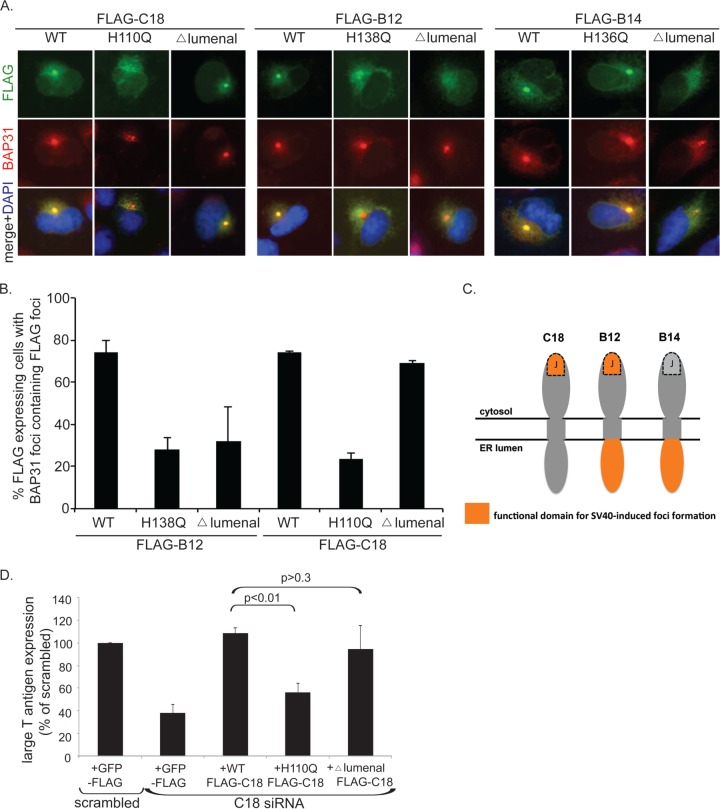
We next investigated the molecular determinant(s) within C18 that allows it to be recruited to the foci during infection. To this end, we expressed WT and mutant C18 constructs and examined their ability to colocalize with the endogenous BAP31 foci. As expected, when FLAG-tagged WT C18 was transfected into CV-1 cells followed by the addition of SV40, discrete FLAG signal was present within the BAP31 foci (Fig. 4A, first column). Disrupting the HPD motif within the highly conserved J domain of C18 with an H110Q point mutation eliminated its ability to colocalize with BAP31 foci (second column); the HPD motif in the J domain is important for binding to its cytosolic interacting partner, Hsc70 (32). In contrast, truncating what is predicted to be the lumenal domain of C18 (i.e., Δ lumenal) did not abolish C18 focus formation (third column). These findings suggest that an intact J domain, but not lumenal domain, is required for C18 to be recruited to the foci. Interestingly, these results are in complete contrast to those for B14, which requires its lumenal domain and not the HPD motif for recruitment into the SV40-induced foci (31; repeated here in the 7th to 9th columns). This striking difference prompted us to investigate B12 in a similar fashion. Akin to C18, the HPD motif was required for transfected B12 to form foci (compare the fourth and fifth columns), and analogous to B14, the lumenal region also was required (compare the fourth to the sixth column). These data were quantified by scoring FLAG-positive cells (which contain BAP31 foci) for the presence or absence of colocalizing FLAG foci (Fig. 4B); the quantification for FLAG-B14 was previously reported (31). Taken together, our results indicate that C18 displays different domain requirements than B12 and B14 to recruit into the foci. A summary of the functional domains within each J protein required for SV40-induced focus formation is depicted in Fig. 4C.
FIG 4.
C18, B12, and B14 display various domain requirements for recruitment into foci. (A) FLAG-tagged WT or the indicated mutant forms of C18, B12, or B14 transfected in CV-1 cells were infected with SV40 (MOI, ∼50) for 14 h. Cells then were fixed, stained with the indicated antibodies, and analyzed by immunofluorescence microscopy. (B) Quantification of data from panel A showing the focus-forming ability of WT and mutant C18 and B12. Values represent means ± SD from three independent experiments. (C) Schematic diagram depicting functional domains (orange) of C18, B12, and B14 essential for SV40-induced focus formation. (D) CV-1 cells were reverse transfected with scrambled or C18 siRNA for 24 h prior to transfection with the indicated constructs for 24 h. Cells then were infected with SV40 (MOI, ∼0.5) for 20 h, fixed, and stained with anti-FLAG and anti-large T antigen antibodies. The percentages of large T antigen-positive cells were determined in GFP-expressing or WT or mutant C18-expressing cells by using immunofluorescence microscopy. Values represent means ± SD from three independent experiments.
We envisioned that if C18 focus formation was required for SV40 infection, expression of a C18 mutant defective in focus formation would be unable to replace endogenous C18 in promoting infection. To test this, we performed knockdown-rescue experiments where endogenous C18 was downregulated with a C18 siRNA. Cells expressing FLAG-tagged GFP or C18 constructs resistant to the C18 siRNA were infected with SV40. Infection levels were assessed by immunofluorescence microscopy and quantification of large T antigen expression in the nucleus of cells expressing the FLAG protein. FLAG-GFP-expressing cells transfected with C18 siRNA had a greater than 60% reduction in infection compared to the control condition using scrambled siRNA (Fig. 4D). Similar to experiments with S-tagged C18 (Fig. 3G), expression of siRNA-resistant WT FLAG-C18 completely restored infection (Fig. 4D). In contrast, H110Q C18 expression failed to restore infection (Fig. 4D). When the Δ lumenal C18 variant was tested, we observed an almost complete rescue of infection (Fig. 4D). These results indicate that the integrity of the J but not the lumenal domain is essential for C18 to support SV40 infection. Moreover, the observation that C18's ability to form foci completely correlates with its ability to promote SV40 infection further reinforces the notion that virus-triggered foci represent a productive viral membrane penetration site in the ER.
C18, B12, and B14 have nonredundant functions in promoting SV40 infection.
Individual knockdown of C18, B12, or B14 markedly attenuated SV40 infection (26), demonstrating that each J protein is required for infection. However, whether they play unique roles during viral entry is not entirely clear. Moreover, the observation that all three J proteins display various domain requirements for recruitment into the virus-induced foci (Fig. 4) raises the possibility that these J proteins act in a nonredundant manner during SV40 infection. To test this hypothesis definitively, we performed additional knockdown-rescue experiments monitoring SV40 infection. In cells transfected with C18 siRNA, expression of siRNA-resistant C18 but not B12 or B14 rescued infection in C18-compromised cells (Fig. 5A). Similarly, in cells transfected with a B12-specfic siRNA (directed against the 3′ untranslated region B12 sequence) to downregulate B12 mRNA expression (Fig. 3A, compare lane 4 to 3), the expression of B12, but not of B14 or C18, restored infection in B12 knockdown cells (Fig. 5B). Finally, in cells transfected with a B14-specific siRNA (directed against the 3′ untranslated region B14 sequence) to downregulate B14 mRNA expression (Fig. 3A, compare lane 6 to 5), expressing only B14 but not B12 or C18, restored infection in B14 knockdown cells (Fig. 5C). These results conclusively demonstrate that C18, B12, and B14 function in a nonredundant manner to facilitate SV40 infection.
FIG 5.

C18, B12, and B14 play nonredundant roles during SV40 infection. (A, B, and C) CV-1 cells were reverse transfected with scrambled or the indicated siRNAs for 24 h prior to transfection with the indicated FLAG-tagged constructs for 24 h. Cells then were infected with SV40 (MOI, ∼0.5) for 20 h, fixed, and stained with anti-FLAG and anti-large T antigen antibodies. The percentages of T antigen-positive cells were determined in GFP-, C18-, B12-, or B14-expressing cells by using immunofluorescence microscopy. Values represent means ± SD from three independent experiments. (D) CV-1 cells were transfected with scrambled or the indicated siRNAs for 48 h prior to infection with SV40 (MOI, ∼0.5) for 20 h. Cells were fixed and stained with anti-large T antigen antibodies, and the percentages of large T antigen-positive cells were scored by using immunofluorescence microscopy. Values represent means ± SD from three independent experiments.
Finally, we conducted concurrent knockdown studies to assess whether C18 functions independently of B12 and B14 during SV40 infection. We reasoned that if C18 affects SV40 infection independent of B12 and B14, additional silencing of C18 in cells where B12 and B14 are knocked down should lead to a more severe block in infection compared to knocking down only B12 and B14. However, we find that knockdown of C18, B12, and B14 resulted in a similar degree of block in infection compared to knocking down B12 and B14 (Fig. 5D). This finding suggests that C18 is unlikely to play a role independent of those of B12 and B14.
DISCUSSION
Membrane penetration represents a decisive yet enigmatic step during entry of nonenveloped viruses. This is elegantly illustrated in the case of the nonenveloped SV40, where it must penetrate the host ER membrane to access the cytosol in order to cause infection. To cross the ER membrane, the coordinated actions of a series of ER lumenal factors impart conformational changes to the virus to generate a hydrophobic viral particle (14–22). This enables the virus to bind to and integrate into the ER membrane, thereby initiating membrane transport. However, events within the ER membrane and in the cytosol that propel the virus into the cytosol are not well characterized. In this context, the three ER membrane-bound J proteins B12, B14, and C18 were reported to play a crucial role in ejecting SV40 and other PyVs into the cytosol (26). The role of C18 is the least understood among these three. This study provides insight into how C18 participates in this process.
Our findings reveal that C18 is recruited to discrete puncta in the ER membrane, referred to as foci, during SV40 infection. Similar targeting of B12 and B14 (as well as BAP31) into this structure was described previously (23, 31). Current evidence suggests a functional role of foci during ER membrane penetration of SV40. Specifically, there is a strong correlation between host components essential for SV40 ER membrane penetration and their recruitment into foci (23, 31). Conversely, membrane proteins, including Hrd1, calnexin, and Sec61α, which are not required to promote viral ER-to-cytosol transport (23), are not recruited to these structures (31). In addition, the kinetics of focus formation temporally coincides with the arrival of SV40 in the cytosol (31). Our study here further supports this view. First, we found that the foci contain VP2/3-exposed SV40, which represents the membrane penetration-competent form of SV40 (20–22). Second, an SV40 mutant that fails to undergo ER-to-cytosol transport cannot induce focus formation. Third, the C18 domains that are essential for targeting to the foci and promoting productive infection are the same. Collectively, these data strongly implicate the focus structures as cytosol entry sites for SV40.
Our analyses also revealed that C18 impacts the recruitment of B12 and B14, as well as BAP31, to the foci. The binding studies demonstrate that C18 interacts modestly with BAP31 (Fig. 3E and 6A). C18, however, does not appear to engage the B12-B14 complex (Fig. 3F and G), yet it controls the recruitment of B12 and B14 to the foci during SV40 infection. One possible explanation is that C18 guides BAP31 to the foci (Fig. 6B). Therefore, a lack of C18 would decrease BAP31 in the foci. Because BAP31 also interacts with B12 and B14 (Fig. 3E and 6A), the lack of BAP31 in the foci (due to C18 knockdown) may affect B12/B14 recruitment to the foci as a consequence. Alternatively, C18 could stabilize a reversible intermediate form of SV40 in the foci that permits recognition by the B12-B14 complex; this scenario would operate independently of BAP31's function. Clearly, the precise molecular mechanism enabling C18 to control the recruitment of B12 and B14 to the foci, as well as whether B12/B14/BAP31 reciprocally regulate C18's recruitment to the foci, requires further investigation.
FIG 6.
Working model describing C18's role during SV40 infection. (A) At steady state, C18 binds to BAP31, which also interacts with the B12-B14 complex. (B) During ER membrane penetration of SV40, the VP2/3-exposed viral particle promotes C18, along with the B12-B14 complex and BAP31, to rearrange into foci in the bilayer of the ER membrane. Formation of foci requires both VP2 and VP3. While the precise mechanism by which C18 controls the recruitment of the B12-B14 complex and BAP31 to the foci remains unclear, it may be due to interaction of C18 with BAP31, which also binds to the B12-B14 complex. Regardless, focus formation likely serves to concentrate cytosolic Hsc70 machinery used to eject the virus into the cytosol, as suggested by our earlier study (31). Functional domains of C18, B12, and B14 essential for SV40-induced focus formation are colored in orange.
We found that in contrast to B14, both B12 and C18 require an intact HPD motif within their J domains to be recruited into the foci. This motif allows interaction with cytosolic Hsc70 in order to stimulate its ATPase activity (32). These findings suggest that recruitment of chaperones/cochaperones of the Hsp70 family plays a role in reinforcing the focus structure. Interestingly, a recent report found that a portion of overexpressed B14 and B12 relocalize to form globular-shaped nuclear membrane structures, referred to as DJANGOs, in an HPD-dependent manner (33). While the relationship between these observations is not entirely clear, this finding nonetheless suggests that the interactions of J proteins with Hsc70 chaperones play an important role in regulating their dynamics within cellular membranes. We note that in analyzing the ability of the transfected J proteins to localize to the foci, we examined only cells that appeared healthy and where the J proteins displayed a perinuclear-ER localization. The lumenal domain of B12 and B14, but not that of C18, plays a role in recruitment to the foci. Our findings clearly indicate that C18 displays a different set of domain requirements than B12 and B14 to recruit into foci.
Consistent with this, we found, using knockdown-rescue experiments, that C18 exerts a role that is not redundant with those of B12 and B14 during SV40 infection, despite the three proteins sharing high sequence similarity. These data suggest that each J protein executes a distinct set of actions in ejecting SV40 from the ER into the cytosol. For example, in the ER lumen, the lumenal domain of B12 and B14, but not C18, may act to recognize the incoming SV40. Once the virus is integrated into the membrane bilayer, the transmembrane domain of each J protein could act to further guide the virus across the ER membrane, perhaps by recognizing different structural motifs within the membrane-embedded virus. Finally, on the cytosolic side, recruitment of different cytosolic components, including the Hsc70 chaperone family, by C18, B12, and B14 would coordinately solubilize the virus into the cytosol. Pinpointing the specific cytosolic interacting partners of the J proteins would provide additional insights into this final phase of the viral membrane transport process.
Several host components required by PyVs for infection are implicated in ERAD, including BAP31, B12, and B14 (23–30, 34). However, the precise functions of these proteins in ERAD are not clear, and whether C18 participates in ERAD is completely unknown. Additional studies are needed to determine whether the normal cellular roles of these factors are directly coopted by PyVs or if these ERAD components exert different activities when encountering the viral particle. If PyVs indeed are masquerading as a genuine substrate for ERAD in order to gain access to the cytosol, then the viral particle would reflect a more specialized substrate (e.g., multiprotein aggregate) and not a canonical misfolded protein. While little is known about the host machinery required to eliminate these protein aggregates from the ER, B12 recently was reported to physically connect with a component of the autophagy pathway in order to clear a misfolded substrate that is soluble but resistant to ERAD (35). This finding raises the possibility that PyVs hijack the ERAD-coupled autophagy pathway (36) en route to successful infection, as suggested by the potential use of this pathway by the human BK PyV (37).
ACKNOWLEDGMENTS
B.T. is funded by the NIH (AI064296). This study also was partially supported by the Protein Folding Disease Initiative of the University of Michigan Medical School.
REFERENCES
- 1.DeCaprio JA, Garcea RL. 2013. A cornucopia of human polyomaviruses. Nat Rev Microbiol 11:264–276. doi: 10.1038/nrmicro2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang M, Abend JR, Johnson SF, Imperiale MJ. 2009. The role of polyomaviruses in human disease. Virology 384:266–273. doi: 10.1016/j.virol.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen XS, Stehle T, Harrison SC. 1998. Interaction of polyomavirus internal protein VP2 with the major capsid protein VP1 and implications for participation of VP2 in viral entry. EMBO J 17:3233–3240. doi: 10.1093/emboj/17.12.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stehle T, Gamblin SJ, Yan Y, Harrison SC. 1996. The structure of simian virus 40 refined at 3.1 A resolution. Structure 4:165–182. doi: 10.1016/S0969-2126(96)00020-2. [DOI] [PubMed] [Google Scholar]
- 5.Liddington RC, Yan Y, Moulai J, Sahli R, Benjamin TL, Harrison SC. 1991. Structure of simian virus 40 at 3.8-A resolution. Nature 354:278–284. doi: 10.1038/354278a0. [DOI] [PubMed] [Google Scholar]
- 6.Smith AE, Lilie H, Helenius A. 2003. Ganglioside-dependent cell attachment and endocytosis of murine polyomavirus-like particles. FEBS Lett 555:199–203. doi: 10.1016/S0014-5793(03)01220-1. [DOI] [PubMed] [Google Scholar]
- 7.Tsai B, Gilbert JM, Stehle T, Lencer W, Benjamin TL, Rapoport TA. 2003. Gangliosides are receptors for murine polyoma virus and SV40. EMBO J 22:4346–4355. doi: 10.1093/emboj/cdg439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Low JA, Magnuson B, Tsai B, Imperiale MJ. 2006. Identification of gangliosides GD1b and GT1b as receptors for BK virus. J Virol 80:1361–1366. doi: 10.1128/JVI.80.3.1361-1366.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert J, Benjamin T. 2004. Uptake pathway of polyomavirus via ganglioside GD1a. J Virol 78:12259–12267. doi: 10.1128/JVI.78.22.12259-12267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian M, Cai D, Verhey KJ, Tsai B. 2009. A lipid receptor sorts polyomavirus from the endolysosome to the endoplasmic reticulum to cause infection. PLoS Pathog 5:e1000465. doi: 10.1371/journal.ppat.1000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kartenbeck J, Stukenbrok H, Helenius A. 1989. Endocytosis of simian virus 40 into the endoplasmic reticulum. J Cell Biol 109:2721–2729. doi: 10.1083/jcb.109.6.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inoue T, Tsai B. 2011. A large and intact viral particle penetrates the endoplasmic reticulum membrane to reach the cytosol. PLoS Pathog 5:e1002037. doi: 10.1371/journal.ppat.1002037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakanishi A, Clever J, Yamada M, Li PP, Kasamatsu H. 1996. Association with capsid proteins promotes nuclear targeting of simian virus 40 DNA. Proc Natl Acad Sci U S A 93:96–100. doi: 10.1073/pnas.93.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson CD, Derdowski A, Maginnis MS, O'Hara BA, Atwood WJ. 2012. The VP1 subunit of JC polyomavirus recapitulates early events in viral trafficking and is a novel tool to study polyomavirus entry. Virology 428:30–40. doi: 10.1016/j.virol.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schelhaas M, Malmstrom J, Pelkmans L, Haugstetter J, Ellgaard L, Grünewald K, Helenius A. 2007. Simian virus 40 depends on ER protein folding and quality control factors for entry into host cells. Cell 131:516–529. doi: 10.1016/j.cell.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 16.Magnuson B, Rainey EK, Benjamin T, Baryshev M, Mkrtchian S, Tsai B. 2005. ERp29 triggers a conformational change in polyomavirus to stimulate membrane binding. Mol Cell 20:289–300. doi: 10.1016/j.molcel.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 17.Walczak CP, Tsai B. 2011. A PDI family network acts distinctly and coordinately with ERp29 to facilitate polyomavirus infection. J Virol 85:2386–2396. doi: 10.1128/JVI.01855-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilbert J, Ou W, Silver J, Benjamin T. 2006. Downregulation of protein disulfide isomerase inhibits infection by the mouse polyomavirus. J Virol 80:10868–10870. doi: 10.1128/JVI.01117-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norkin LC, Anderson HA, Wolfrom SA, Oppenheim A. 2002. Caveolar endocytosis of simian virus 40 is followed by brefeldin A-sensitive transport to the endoplasmic reticulum, where the virus disassembles. J Virol 76:5156–5166. doi: 10.1128/JVI.76.10.5156-5166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rainey-Barger EK, Magnuson B, Tsai B. 2007. A chaperone-activated nonenveloped virus perforates the physiologically relevant endoplasmic reticulum membrane. J Virol 81:12996–13004. doi: 10.1128/JVI.01037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuksin D, Norkin LC. 2012. Disassembly of simian virus 40 during passage through the endoplasmic reticulum and in the cytoplasm. J Virol 86:1555–1562. doi: 10.1128/JVI.05753-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daniels R, Rusan NM, Wadsworth P, Hebert DN. 2006. SV40 VP2 and VP3 insertion into ER membranes is controlled by the capsid protein VP1: implications for DNA translocation out of the ER. Mol Cell 24:955–966. doi: 10.1016/j.molcel.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Geiger R, Andritschke D, Friebe S, Herzog F, Luisoni S, Heger T, Helenius A. 2011. BAP31 and BiP are essential for dislocation of SV40 from the endoplasmic reticulum to the cytosol. Nat Cell Biol 13:1305–1314. doi: 10.1038/ncb2339. [DOI] [PubMed] [Google Scholar]
- 24.Olzmann JA, Kopito RR, Christianson JC. 2012. The mammalian endoplasmic reticulum-associated degradation system. Cold Spring Harb Perspect Biol 5:a013185. doi: 10.1101/cshperspect.a013185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirsch C, Gauss R, Horn SC, Neuber O, Sommer T. 2009. The ubiquitylation machinery of the endoplasmic reticulum. Nature 458:453–460. doi: 10.1038/nature07962. [DOI] [PubMed] [Google Scholar]
- 26.Goodwin EC, Lipovsky A, Inoue T, Magaldi TG, Edwards Van Goor APKE, Paton AW, Paton JC, Atwood WJ, Tsai B, DiMaio D. 2011. BiP and multiple DNAJ molecular chaperones in the endoplasmic reticulum are required for efficient simian virus 40 infection. mBio 2:e00101-11. doi: 10.1128/mBio.00101-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett SM, Jiang M, Imperiale MJ. 2013. Role of cell-type-specific endoplasmic reticulum-associated degradation in polyomavirus trafficking. J Virol 87:8843–8852. doi: 10.1128/JVI.00664-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lilley BN, Gilbert JM, Ploegh HL, Benjamin TL. 2006. Murine polyomavirus requires the endoplasmic reticulum protein Derlin-2 to initiate infection. J Virol 80:8739–8744. doi: 10.1128/JVI.00791-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sopha P, Kadokura H, Yamamoto YH, Takeuchi M, Saito M, Tsuru A, Kohno K. 2012. A novel mammalian ER-located J-protein, DNAJB14, can accelerate ERAD of misfolded membrane proteins. Cell Struct Funct 37:177–187. doi: 10.1247/csf.12017. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto YH, Kimura T, Momohara S, Takeuchi M, Tani T, Kimata Y, Kadokura H, Kohno K. 2010. A novel ER J-protein DNAJB12 accelerates ER-associated degradation of membrane proteins including CFTR. Cell Struct Funct 35:107–116. doi: 10.1247/csf.10023. [DOI] [PubMed] [Google Scholar]
- 31.Walczak CP, Ravindran MS, Inoue T, Tsai B. 2014. A cytosolic chaperone complexes with dynamic membrane J-proteins and mobilizes a nonenveloped virus out of the endoplasmic reticulum. PLoS Pathog 10:e1004007. doi: 10.1371/journal.ppat.1004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai J, Douglas MG. 1996. A conserved HPD sequence of the J-domain is necessary for YDJ1 stimulation of Hsp70 ATPase activity at a site distinct from substrate binding. J Biol Chem 271:9347–9354. doi: 10.1074/jbc.271.16.9347. [DOI] [PubMed] [Google Scholar]
- 33.Goodwin EC, Motamedi N, Lipovsky A, Fernández-Busnadiego R, DiMaio D. 2014. Expression of DNAJB12 or DNAJB14 causes coordinate invasion of the nucleus by membranes associated with a novel nuclear pore structure. PLoS One 9:e94322. doi: 10.1371/journal.pone.0094322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grove DE, Fan CY, Ren HY, Cyr DM. 2011. The endoplasmic reticulum-associated Hsp40 DNAJB12 and Hsc70 cooperate to facilitate RMA1 E3-dependent degradation of nascent CFTRDeltaF508. Mol Biol Cell 22:301–314. doi: 10.1091/mbc.E10-09-0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Houck SA, Ren HY, Madden VJ, Bonner JN, Conlin MP, Janovick JA, Conn PM, Cyr DM. 2014. Quality control autophagy degrades soluble ERAD-resistant conformers of the misfolded membrane protein GnRHR. Mol Cell 54:166–179. doi: 10.1016/j.molcel.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kruse KB, Brodsky JL, McCracken AA. 2006. Autophagy: an ER protein quality control process. Autophagy 2:135–137. doi: 10.4161/auto.2.2.2388. [DOI] [PubMed] [Google Scholar]
- 37.Bouley SJ, Maginnis MS, Derdowski A, Gee GV, O'Hara BA, Nelson CD, Bara AM, Atwood WJ, Dugan AS. 2014. Host cell autophagy promotes BK virus infection. Virology 456-457:87–95. doi: 10.1016/j.virol.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]