ABSTRACT
Middle East respiratory syndrome (MERS) is a highly lethal pulmonary infection. Serum from convalescent MERS patients may provide some benefit but is not readily available. In contrast, nearly all camels in the Middle East have been infected with MERS-CoV. Here, we show that sera obtained from MERS-immune camels augment the kinetics of MERS-CoV clearance and reduce the severity of pathological changes in infected lungs, with efficacy proportional to the titer of MERS-CoV-neutralizing serum antibody.
IMPORTANCE Middle East respiratory syndrome, caused by a coronavirus, is highly lethal, with a case fatality rate of 35 to 40%. No specific therapy is available, and care is generally supportive. One promising approach is passive administration of sera from convalescent human MERS patients or other animals to exposed or infected patients. The vast majority of, if not all, camels in the Middle East have been infected with MERS-CoV, and some contain high titers of antibody to the virus. Here, we show that this antibody is protective if delivered either prophylactically or therapeutically to mice infected with MERS-CoV, indicating that this may be a useful intervention in infected patients.
TEXT
A decade after the emergence of the severe acute respiratory syndrome (SARS), a novel beta coronavirus was isolated from a patient with a fatal viral pneumonia in Saudi Arabia in 2012 (1). The disease is now designated Middle East respiratory syndrome (MERS), and the causative virus is MERS coronavirus (MERS-CoV). So far (as of 7 February 2015), 971 confirmed cases, 356 of them fatal, have been reported to the World Health Organization (http://www.who.int/csr/disease/coronavirus_infections/mers-5-february-2015.pdf?ua=1). Primary human cases have been reported from a number of countries in the Arabian peninsula and the Middle East region, but travel-associated cases and limited human-to-human transmission from such cases have been reported from other countries in Europe, Africa, and Asia. While clusters of human cases with limited human-to-human transmission within health care facilities or families have been reported (2), index cases in the transmission chains remain of presumed zoonotic origin.
MERS-CoV-like viruses are widespread in dromedary camels, with seroepidemiological studies indicating seroprevalence of >90% in adult animals (3). Viruses isolated from dromedaries are genetically and phenotypically closely related to viruses isolated from humans and retain the capacity to infect ex vivo cultures of the human airways (4). Other domestic livestock in affected areas, including cattle, goats, sheep, and equids, have no evidence of MERS-CoV infection. There is no convincing evidence of MERS-CoV in bats, although a genetically related virus, albeit with a divergent spike protein, has been detected in Neoromicia capensis bats from Africa (5).
Infection in dromedaries has been reported to precede human infection in a few instances (6). Given the ubiquitous nature of infection in dromedaries, human exposure to MERS-CoV must be common; however, human disease remains rare (7). Furthermore, MERS-CoV remains endemic in dromedaries in East and North Africa (3), although locally acquired human cases have not been reported in countries in these regions. It is unclear whether this represents a lack of recognition or a true absence of disease. Thus, while dromedaries are recognized as a natural host of MERS-CoV, the modes of transmission to humans remain unclear.
The apparent case fatality of MERS appears to be high (approximately 37%), with age and underlying disease conditions, including diabetes, respiratory or cardiovascular diseases, and immunocompromised status, being risk factors (8). When human case clusters have been intensively investigated, it has become apparent that milder cases are not uncommon and that such cases are probably undiagnosed in the general population (2). Thus, the overall severity of MERS may be milder than reflected from hitherto-diagnosed cases. The repeated emergence of clusters of human-to-human MERS transmission is reminiscent of the emergence of SARS in late 2002, when clusters of human cases from the animal reservoir emerged and then went extinct, until the virus finally adapted to acquire the capacity for sustained human-to-human transmission. Virus then spread globally to infect more than 8,000 persons in >28 countries or territories (reviewed in reference 9). Within the past 200 years, other animal coronaviruses have adapted to humans and have spread globally, viz., human coronaviruses 229E and OC43 (10). Thus, zoonotic MERS-CoV remains a concern for global public health.
So far, no clinically effective therapeutics have been identified. Some drugs, including some licensed for human use in other clinical indications, have activity in vitro, but it is unclear whether their pharmacology and toxicity would allow therapeutic efficacy in humans (11, 12). Passive immunotherapy using convalescent-phase human plasma is being considered for a number of emerging infectious diseases (e.g., MERS, influenza, and Ebola) (11, 13). It was used for treatment of SARS with potentially promising results, although in the absence of controlled clinical trials, the results remain inconclusive (13, 14). The limited number of patients surviving MERS who are fit to donate plasma and have low convalescent-phase-antibody titers has constrained its use in MERS. On the other hand, dromedaries in the Middle East and in parts of Africa have high seroprevalence, and many of them have very high neutralizing antibody titers, presumably maintained through repeated reinfections. They have unusual single-chain immunoglobulins that may have theoretical advantages for passive immunotherapy (15). In this study, we used a mouse model sensitized to MERS-CoV though transduction of the human DPP4 receptor (16) to investigate the prophylactic and therapeutic efficacy of dromedary serum containing high-titer neutralizing antibodies to MERS-CoV in reducing viral load, weight loss, and lung pathology.
Assay for neutralizing antibody in dromedary camels.
Serum samples were collected from dromedaries in Egypt (collected in 2013) and Australia (collected in 2014) as previously described (17, 18). Samples from Egypt contained antibody to MERS-CoV, while sera from Australian camels served as negative controls, since all dromedary camels from Australia tested thus far are negative for MERS-CoV-specific antibody. Multiple aliquots were prepared to avoid repeated freezing and thawing of the sera. Titers of antibody to MERS-CoV were determined using a well-characterized and validated lentivirus-based pseudoparticle neutralization test as previously described (18). In this assay, pseudoparticles expressing the MERS-CoV spike protein of the EMC/2012 virus strain are exposed to antibodies under investigation, and neutralizing titers are calculated. A panel of sera was selected to represent a range of MERS-CoV antibody titers found in dromedaries sampled in the field (Table 1). Sera from Egypt (no. 1 to 6) had reciprocal antibody titers of 1:160 to 1:1,280, while sera from Australia (no. 21 and 23) were, as expected, anti-MERS-CoV antibody negative.
TABLE 1.
Titers of MERS-CoV-neutralizing antibody in dromedary camel sera assayed in a MERS-CoV spike pseudoparticle microneutralization test
| Serum ID | Geographic origin | Reciprocal neutralizing antibody titer |
|---|---|---|
| 1 | Egypt | 1:640 |
| 2 | Egypt | 1:1,280 |
| 3 | Egypt | 1:640 |
| 4 | Egypt | 1:640 |
| 5 | Egypt | 1:160 |
| 6 | Egypt | 1:320 |
| 21 | Australia | <1:10 |
| 23 | Australia | <1:10 |
Protective efficacy in MERS-CoV-infected mice.
Mice are resistant to infection with MERS-CoV but can be rendered susceptible if the human dipeptidyl peptidase receptor (hDPP4) is supplied exogenously. We showed previously that transduction with an adenovirus vector expressing hDPP4 sensitizes mice to subsequent challenge with the MERS-CoV strain EMC/2012 (16). Virus is cleared within 7 to 10 days, with inflammatory cell infiltration being apparent on histological examination of the lungs. Six- to 10-week-old immunocompetent mice lose minimal amounts of weight and show no signs of clinical disease. However, mice lacking expression of the type 1 interferon receptor (IFNAR−/− mice) are more susceptible to the infection, showing weight loss and more extensive inflammatory cell infiltration and edema on pathological examination of lung tissue. Initially, we assessed whether antibody from MERS immune camels was protective if 200 μl was delivered to immunocompetent BALB/c mice 1 day prior to infection with 1 × 105 PFU MERS-CoV (all protocols were approved by the University of Iowa Animal Care and Utilization Committee). Virus titers were measured at day 3 postinfection (p.i.), since we showed previously that this is a useful time for assessing efficacy of anti-MERS-CoV prophylactic treatment (16). As shown in Fig. 1A, virus was partially or completely cleared by day 3 p.i. from infected mice, with the protective ability of each serum being proportional to the neutralizing titer measured in vitro. Further testing showed that serum from camel 2 could be diluted 4-fold without loss of ability to effect virus clearance by day 3 p.i. (Fig. 1B). Additionally, serum dilution did not show any evidence of antibody-mediated enhancement of virus replication.
FIG 1.
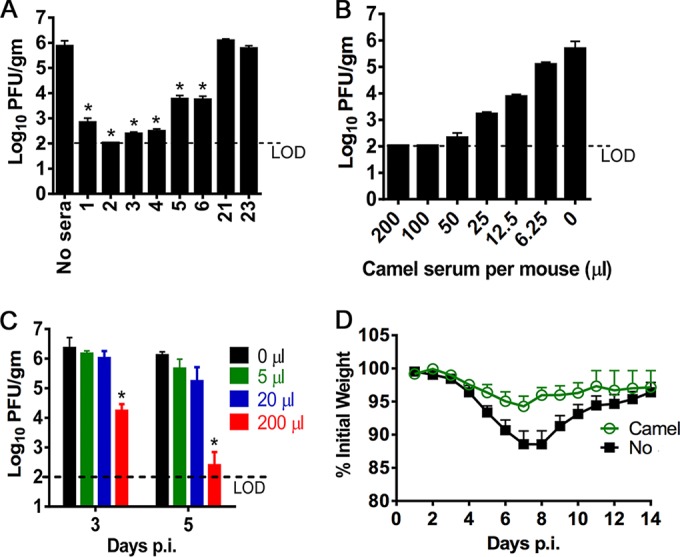
Enhanced kinetics of MERS-CoV clearance after treatment with sera from convalescent camels. Mice were sensitized to infection with MERS-CoV by transduction with Ad5-hDPP4. Five days later, mice were then challenged intranasally with 1 × 105 PFU of MERS-CoV EMC/2012, kindly provided by Bart Haagmans, Erasmus Medical Center, The Netherlands. All work with infectious MERS-CoV was performed in a biosafety level 3 (BSL3) laboratory. (A) A 200-μl portion of camel serum was transferred intraperitoneally into Ad5-hDPP4-transduced 6- to 8-week-old female BALB/c mice 24 h before MERS-CoV infection. Virus titers in the lungs were measured at day 3 postinfection. Titers are expressed as PFU per gram of tissue. There were 3 mice/group/time point. *, P < 0.05 compared to the no-treatment group. (B) A 200-μl portion of camel serum 2 diluted in phosphate-buffered saline (PBS) was transferred into Ad5-hDPP4-transduced 6- to 8-week-old BALB/c mice, as described for panel A. Virus titers were measured at day 3 p.i. There were 3 mice/group/time point. (C). A 200-μl portion of camel serum 2 diluted in PBS was transferred intraperitoneally into 6- to 10-week-old Ad5-hDPP4-transduced IFNAR−/− mice 1 day after intranasal infection with 1 × 105 PFU MERS-CoV. Titers were measured at days 3 and 5 p.i. There were 3 mice/group/time point. (D) A 200-μl portion of camel serum 2 was transferred intraperitoneally into Ad5-hDPP4 transduced IFNAR−/− mice 1 day after MERS-CoV infection. Mice were monitored daily for mortality (there was none) and weight loss. There were 5 mice per group.
If camel serum is to be useful in patients, it will need to be delivered therapeutically. Since Ad5-hDPP4-transduced mice lacking expression of the IFN-α/β receptor (IFNAR−/−) are more susceptible to MERS-CoV than immunocompetent mice, we treated IFNAR−/− mice with serum from camel 2 at 24 h after infection. Under these conditions, treatment with undiluted serum accelerated the kinetics of virus clearance, with MERS-CoV being nearly undetectable by 5 days after challenge (Fig. 1C). Unlike BALB/c mice, IFNAR−/− mice exhibit weight loss after MERS-CoV infection. Treated MERS-CoV-infected mice exhibited less weight loss than untreated mice (Fig. 1D). Consistent with these results, decreased amounts of perivascular and peribronchial inflammatory cell infiltration, hemorrhage, and edema were apparent on histological examination of infected lungs when treated and untreated infected IFNAR−/− mice were compared (Fig. 2).
FIG 2.
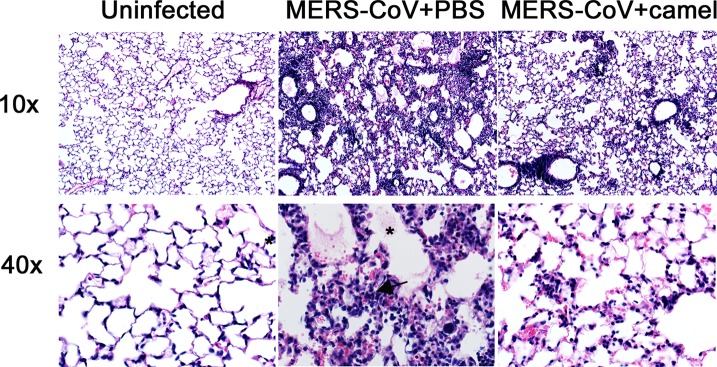
Decreased severity of histological changes in MERS-CoV infected mice after therapeutic treatment with sera from convalescent camels. IFNAR−/− mice were transduced with Ad5-hDPP4 and were left untreated (PBS) or treated with 200 μl camel serum (MERS-CoV+camel) 2 1 day before infection with MERS-CoV EMC/2012 (1 × 105 PFU). At 7 days postinfection, mice were anesthetized and perfused via the right ventricle with PBS followed by zinc formalin. Lungs were removed, fixed in zinc formalin, and paraffin embedded. Sections were stained with hematoxylin and eosin for histological analysis. (Left) Lungs from uninfected mice. (Middle and right) Lungs from MERS-CoV-infected mice at 7 days postchallenge that were either untreated (middle) or treated with 200 μl of camel serum 2 intraperitoneally (right). Multifocal peribronchial and perivascular infiltration, hemorrhage, and edema (*) are observed in untreated samples (middle), but only minimal cellular infiltration is detected in mice that received MERS-CoV-specific camel antibody (right). Magnification, 10× (top) or 40× (bottom).
Implications for MERS-CoV-infected patients.
In this study, we show that prophylactic or therapeutic treatment with high-titer MERS immune camel serum is able to diminish weight loss and lung histological changes and effect virus clearance in mice infected with MERS-CoV EMC/2012. At this point, it is not possible to determine the effects of any treatment on severe clinical disease. Nonhuman primates and rabbits can be infected with MERS-CoV, but none, except perhaps marmosets, develop severe clinical disease (19, 20). Marmosets variably develop severe disease and are not readily available. Transgenic mice expressing hDPP4 may provide an alternative approach, but these mice either remain asymptomatic (our unpublished results) or develop clinical disease, the significance of which is confounded by the concomitant development of severe encephalitis (reference 21 and our unpublished data). Our experiments were performed with a single strain of MERS-CoV, but it is likely that other strains will be neutralized as well. While evidence for evolution of human MERS-CoV has been reported, virtually no mutations are detected in the receptor binding domain, the most important site of neutralization. The most consistent change, at amino acid 1020 of the spike protein (22), is in the putative fusion domain, but even this change may reflect tissue culture adaptation. Comparison of titers of cross-neutralizing antibody against MERS-CoV from Saudi Arabia belonging phylogenetically to clade B, and genetically diverse viruses from Egypt demonstrated no reduction in neutralizing potency of dromedary camel serum against the latter (17).
While changes in clinical disease and blood laboratory parameters have been well documented in MERS in patients, little is known about changes in virus load and tissue damage as disease progresses, because human specimens are not available. However, based on information acquired from the 2002-2003 epidemic of severe acute respiratory syndrome, it is likely that a favorable outcome will occur when virus clearance occurs rapidly, providing time for the development of a protective antibody and T cell responses, which then definitively clear the virus. Camel serum clearly reduces virus titers when delivered either prophylactically or therapeutically. Use of camel serum has several advantages, including ready availability in the Arabian peninsula, the site of all initial infection thus far, and the presence of high titers of MERS-CoV S-specific antibodies. High titers of anti-virus antibody are believed to reflect repeated infection of camels, perhaps during the birthing season (23). Based on a study of experimentally infected camels (24), MERS-CoV causes a moderate rhinitis with high virus loads detected in the nasal secretions, which would facilitate repeated infections. Camel antibodies have certain other advantages, including a long CDR3, which enhances the ability to recognize structures not detected by conventional antibodies, increased stability compared to conventional antibodies, and relative ease of high-level production (15). Heterologous (e.g., equine) antibodies have been successfully used in the past for passive immunotherapy or immunoprophylaxis of diseases such as rabies, tetanus, and snake bites, but such therapy carries a potential, though low, risk from hypersensitivity to parenteral injection of protein from a different species. In the longer term, recombinant camelid antibodies can be expressed and multimerized, which results in enhanced avidity, and humanized to reduce the risk of hypersensitivity (25).
In summary, our results provide proof of concept that sera from MERS-CoV immune dromedary camels are potentially useful in treatment of patients with MERS. Efficacy is most likely if the sera are delivered early in the course of illness (11). Furthermore, camels immunized with MERS-CoV can serve as the initial source for developing recombinant, humanized single-stranded antibodies and as an additional tool for prophylactic or therapeutic treatment of exposed or infected patients, respectively.
ACKNOWLEDGMENTS
This work was funded in part by the National Institute of Allergy and Infectious Diseases (grants PO1 AI060699 and RO1 AI0901322 [S.P.] and contract HHSN272201400006C [M.P. and G.K.]).
REFERENCES
- 1.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. 2012. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 2.Drosten C, Meyer B, Muller MA, Corman VM, Al-Masri M, Hossain R, Madani H, Sieberg A, Bosch BJ, Lattwein E, Alhakeem RF, Assiri AM, Hajomar W, Albarrak AM, Al-Tawfiq JA, Zumla AI, Memish ZA. 2014. Transmission of MERS-coronavirus in household contacts. N Engl J Med 371:828–835. doi: 10.1056/NEJMoa1405858. [DOI] [PubMed] [Google Scholar]
- 3.Reusken CB, Messadi L, Feyisa A, Ularamu H, Godeke GJ, Danmarwa A, Dawo F, Jemli M, Melaku S, Shamaki D, Woma Y, Wungak Y, Gebremedhin EZ, Zutt I, Bosch BJ, Haagmans BL, Koopmans MP. 2014. Geographic distribution of MERS coronavirus among dromedary camels, Africa. Emerg Infect Dis 20:1370–1374. doi: 10.3201/eid2008.140590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan RW, Hemida MG, Kayali G, Chu DK, Poon LL, Alnaeem A, Ali MA, Tao KP, Ng HY, Chan MC, Guan Y, Nicholls JM, Peiris JS. 2014. Tropism and replication of Middle East respiratory syndrome coronavirus from dromedary camels in the human respiratory tract: an in-vitro and ex-vivo study. Lancet Respir Med 2:813–822. doi: 10.1016/S2213-2600(14)70158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corman VM, Ithete NL, Richards LR, Schoeman MC, Preiser W, Drosten C, Drexler JF. 2014. Rooting the phylogenetic tree of middle East respiratory syndrome coronavirus by characterization of a conspecific virus from an African bat. J Virol 88:11297–11303. doi: 10.1128/JVI.01498-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azhar EI, El-Kafrawy SA, Farraj SA, Hassan AM, Al-Saeed MS, Hashem AM, Madani TA. 2014. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med 370:2499–2505. doi: 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- 7.Hemida MG, Al-Naeem A, Perera RA, Chin A, Poon LM, Peiris M. 12 February 2015. Lack of Middle East respiratory syndrome coronavirus transmission from infected camels. Emerg Infect Dis doi: 10.3201/eid2104.141949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hui DS, Memish ZA, Zumla A. 2014. Severe acute respiratory syndrome vs. the Middle East respiratory syndrome. Curr Opin Pulm Med 20:233–241. doi: 10.1097/MCP.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 9.Peiris JS, Guan Y, Yuen KY. 2004. Severe acute respiratory syndrome. Nat Med 10:S88–97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drexler JF, Corman VM, Drosten C. 2014. Ecology, evolution and classification of bat coronaviruses in the aftermath of SARS. Antiviral Res 101:45–56. doi: 10.1016/j.antiviral.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayden FG, Farrar J, Peiris JS. 2014. Towards improving clinical management of Middle East respiratory syndrome coronavirus infection. Lancet Infect Dis 14:544–546. doi: 10.1016/S1473-3099(14)70793-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omrani AS, Saad MM, Baig K, Bahloul A, Abdul-Matin M, Alaidaroos AY, Almakhlafi GA, Albarrak MM, Memish ZA, Albarrak AM. 2014. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis 14:1090–1095. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw FM, Lim WS, Makki S, Rooney KD, Beck CR. 2015. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis 211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stockman LJ, Bellamy R, Garner P. 2006. SARS: systematic review of treatment effects. PLoS Med 3:e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tillib SV, Ivanova TI, Vasilev LA, Rutovskaya MV, Saakyan SA, Gribova IY, Tutykhina IL, Sedova ES, Lysenko AA, Shmarov MM, Logunov DY, Naroditsky BS, Gintsburg AL. 2013. Formatted single-domain antibodies can protect mice against infection with influenza virus (H5N2). Antiviral Res 97:245–254. doi: 10.1016/j.antiviral.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Zhao J, Li K, Wohlford-Lenane C, Agnihothram SS, Fett C, Zhao J, Gale MJ Jr, Baric RS, Enjuanes L, Gallagher T, McCray PB Jr, Perlman S. 2014. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci U S A 111:4970–4975. doi: 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemida MG, Perera RA, Al Jassim RA, Kayali G, Siu LY, Wang P, Chu KW, Perlman S, Ali MA, Alnaeem A, Guan Y, Poon LL, Saif L, Peiris M. 2014. Seroepidemiology of Middle East respiratory syndrome (MERS) coronavirus in Saudi Arabia (1993) and Australia (2014) and characterisation of assay specificity. Euro Surveill 19(23):pii=20828 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perera RA, Wang P, Gomaa MR, El-Shesheny R, Kandeil A, Bagato O, Siu LY, Shehata MM, Kayed AS, Moatasim Y, Li M, Poon LL, Guan Y, Webby RJ, Ali MA, Peiris JS, Kayali G. 2013. Seroepidemiology for MERS coronavirus using microneutralisation and pseudoparticle virus neutralisation assays reveal a high prevalence of antibody in dromedary camels in Egypt, June 2013. Euro Surveill 18(36):pii=20574 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20574. [DOI] [PubMed] [Google Scholar]
- 19.de Wit E, Rasmussen AL, Falzarano D, Bushmaker T, Feldmann F, Brining DL, Fischer ER, Martellaro C, Okumura A, Chang J, Scott D, Benecke AG, Katze MG, Feldmann H, Munster VJ. 2013. Middle East respiratory syndrome coronavirus (MERS-CoV) causes transient lower respiratory tract infection in rhesus macaques. Proc Natl Acad Sci U S A 110:16598–16603. doi: 10.1073/pnas.1310744110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falzarano D, de Wit E, Feldmann F, Rasmussen AL, Okumura A, Peng X, Thomas MJ, van Doremalen N, Haddock E, Nagy L, LaCasse R, Liu T, Zhu J, McLellan JS, Scott DP, Katze MG, Feldmann H, Munster VJ. 2014. Infection with MERS-CoV causes lethal pneumonia in the common marmoset. PLoS Pathog 10:e1004250. doi: 10.1371/journal.ppat.1004250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agrawal AS, Garron T, Tao X, Peng BH, Wakamiya M, Chan TS, Couch RB, Tseng CT. 14 January 2015. Generation of transgenic mouse model of Middle East respiratory syndrome coronavirus infection and disease. J Virol doi: 10.1128/JVI.03427-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cotten M, Watson SJ, Zumla AI, Makhdoom HQ, Palser AL, Ong SH, Al Rabeeah AA, Alhakeem RF, Assiri A, Al-Tawfiq JA, Albarrak A, Barry M, Shibl A, Alrabiah FA, Hajjar S, Balkhy HH, Flemban H, Rambaut A, Kellam P, Memish ZA. 2014. Spread, circulation, and evolution of the Middle East respiratory syndrome coronavirus. mBio 5:e01062–13. doi: 10.1128/mBio.01062-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemida MG, Chu DK, Poon LL, Perera RA, Alhammadi MA, Ng HY, Siu LY, Guan Y, Alnaeem A, Peiris M. 2014. MERS coronavirus in dromedary camel herd, Saudi Arabia. Emerg Infect Dis 20:1231–1234. doi: 10.3201/eid2007.140571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adney DR, van Doremalen N, Brown VR, Bushmaker T, Scott D, de Wit E, Bowen RA, Munster VJ. 2014. Replication and shedding of MERS-CoV in upper respiratory tract of inoculated dromedary camels. Emerg Infect Dis 20:1999–2005. doi: 10.3201/eid2012.141280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanlandschoot P, Stortelers C, Beirnaert E, Ibanez LI, Schepens B, Depla E, Saelens X. 2011. Nanobodies(R): new ammunition to battle viruses. Antiviral Res 92:389–407. doi: 10.1016/j.antiviral.2011.09.002. [DOI] [PubMed] [Google Scholar]


