ABSTRACT
When HIV-1 vaccine candidates that include soluble envelope glycoproteins (Env) are tested in humans and other species, the resulting antibody responses to Env are sifted for correlates of protection or risk. One frequently used assay measures the reduction in antibody binding to Env antigens by an added chaotrope (such as thiocyanate). Based on that assay, an avidity index was devised for assessing the affinity maturation of antibodies of unknown concentration in polyclonal sera. Since a high avidity index was linked to protection in animal models of HIV-1 infection, it has become a criterion for evaluating antibody responses to vaccine candidates. But what does the assay measure and what does an avidity index mean? Here, we have used a panel of monoclonal antibodies to well-defined epitopes on Env (gp120, gp41, and SOSIP.664 trimers) to explore how the chaotrope acts. We conclude that the chaotrope sensitivity of antibody binding to Env depends on several properties of the epitopes (continuity versus tertiary- and quaternary-structural dependence) and that the avidity index has no simple relationship to antibody affinity for functional Env spikes on virions. We show that the binding of broadly neutralizing antibodies against quaternary-structural epitopes is particularly sensitive to chaotrope treatment, whereas antibody binding to epitopes in variable loops and to nonneutralization epitopes in gp41 is generally resistant. As a result of such biases, the avidity index may at best be a mere surrogate for undefined antibody or other immune responses that correlate weakly with protection.
IMPORTANCE An effective HIV-1 vaccine is an important goal. Such a vaccine will probably need to induce antibodies that neutralize typically transmitted variants of HIV-1, preventing them from infecting target cells. Vaccine candidates have so far failed to induce such antibody responses, although some do protect weakly against infection in animals and, possibly, humans. In the search for responses associated with protection, an avidity assay based on chemical disruption is often used to measure the strength of antibody binding. We have analyzed this assay mechanistically and found that the epitope specificity of an antibody has a greater influence on the outcome than does its affinity. As a result, the avidity assay is biased toward the detection of some antibody specificities while disfavoring others. We conclude that the assay may yield merely indirect correlations with weak protection, specifically when Env vaccination has failed to induce broad neutralizing responses.
INTRODUCTION
Most vaccines that protect humans from viral infection induce effective neutralizing antibody (NAb) responses (1), but human immunodeficiency virus type 1 (HIV-1) vaccine candidates based on the viral envelope glycoproteins (Env) have so far failed to induce broadly neutralizing antibodies (bNAbs) (2–4). Passive immunization with bNAbs, either systemically or topically, protects robustly against virus acquisition in animal models of HIV-1 infection, whereas nonneutralizing antibodies (non-NAbs) do not (5–10). Accordingly, it is reasonable to conclude that vaccine-induced bNAb responses would be important for protection. Also of note is that bNAbs arise in a minority of HIV-1-infected people (4, 11–15). These bNAbs evolve by iterated B-cell cycling through germinal centers of lymph nodes, and their affinity maturation involves a high degree of somatic hypermutation, including deletions and insertions in complementarity-determining regions (CDRs) and mutations in the normally conserved framework regions (4, 16, 17).
Epitopes that can bind NAbs must be located on the exterior of virions and be accessible on the surface of functional Env proteins at some stage before viral entry is completed (18). Conversely, epitopes that become exposed only when a functional Env protein is denatured will not bind NAbs (19). Although epitopes on folded protein molecules are unlikely to be formed exclusively from residues that are adjacent in the polypeptide chain, they can be subdivided into two general subcategories. Continuous epitopes are contained within a local stretch of polypeptide and can be mimicked by short peptides (6 to 20 residues). Discontinuous, or composite, epitopes consist of discrete clusters of amino acid residues that are widely separated in the polypeptide sequence but are brought into close proximity when the protein folds (19–23). Discontinuous epitopes are most sensitive to conformational changes; they can also be formed by sequences in different subunits of an oligomeric protein, e.g., the HIV-1 Env trimer; such quaternary-structural epitopes are particularly sensitive to changes in protein conformation.
Antibodies (Abs) to discontinuous epitopes are prevalent in HIV-1-positive human sera and include bNAbs directed to gp120 (4, 12–15, 24). Abs generated to HIV-1 during early infection include those to predominantly continuous V3 epitopes, but as the response matures, Abs emerge to discontinuous epitopes, such as the CD4-binding site (CD4bs), CD4-induced (CD4i), glycan-dependent, and quaternary-structural epitopes (2, 4, 17). Some quaternary-structural epitopes on Env are formed where the three gp120 subunits meet at the trimer apex (25–30). Overall, complex and conformationally sensitive epitopes are far more likely than simpler ones to be relevant to the design of Env vaccines capable of inducing bNAbs.
Perhaps because of the failure of Env vaccine candidates to guide the immune response toward the generation of bNAbs, parameters other than neutralization have gained prominence as measures of vaccine performance. They include Ab-binding titers and the resistance of Ab binding to chaotrope treatment in what are known as avidity assays (31–37). The average affinity of serum or plasma Abs directed to any particular antigen (Ag) is difficult to ascertain. Thus, a half-maximal binding titer for a polyclonal serum in an enzyme-linked immunosorbent assay (ELISA) is the product both of the functional affinities of the constituent Abs and of their concentrations. Although the concentration and affinity of an Ab to a specific epitope cluster can both increase early in infections, these two variables are differently regulated and can be directly measured only when pure monospecific Abs are available (18, 38–40). Can, then, the strength of binding somehow be indirectly determined without knowledge of the specific Ab concentrations? It has long been known that Ab binding to Ag can be reversed by chaotropic ions, such as thiocyanate (SCN−) (41). Building on this effect, Pullen et al. used the sensitivity of bound Ab to chaotrope-induced dissociation in an ELISA as a measurement of the strength of the binding; they designated the assay endpoint the avidity index (42).
The avidity index has been used to monitor the affinity maturation of the Ab responses induced by rubella, mumps, and influenza virus vaccines (42–44). Perhaps because of their value in these other vaccine contexts, avidity assays have become widely used for evaluating HIV-1 vaccine candidates. When the assay is adapted to new uses, however, it should be noted how qualitative demands on NAb responses and the complexity and lability of Ags differ drastically among viruses. The Env glycoproteins may be particularly sensitive to conformational perturbations and denaturation. Indeed, long ago, the use of urea, a chaotrope, during the purification of HIV-1 gp160 for human clinical trials was cautioned against because of the resulting denaturation (45). Nonetheless, the avidity index has been correlated with protection from the acquisition of infection (32, 35, 36, 46) or with viremic suppression postinfection (31, 33, 34) in the absence of effective NAb responses in various simian models of HIV-1 infection. It is of note, however, that the lack of such correlations has also been reported (47).
A previous study indicated that Ab binding to continuous HIV-1 Env epitopes is more chaotrope resistant than binding to discontinuous ones (48). Here, we have corroborated this observation, expanded it to other epitopes, including quaternary-structural epitopes, and investigated its underlying mechanism by varying the conditions of chaotrope pulsing in binding assays incorporating a panel of lectins and Abs. We show that chaotropes have a far more drastic effect on the binding to conformationally sensitive epitopes than to more continuous ones, a finding that is partly attributable to a perturbation of Env protein structure and not just to the direct dissociation of Ab-Env complexes (41). We have also investigated the relationship between chaotrope resistance and the valency of binding, i.e., avidity in a stricter sense than for the term avidity index. Here, we found that higher valency or avidity does confer some chaotrope resistance, although chaotrope resistance and functional affinity did not correlate well.
We conclude that an avidity index based on the chaotrope resistance of Ab binding to HIV-1 Env proteins is a highly complex measurement. Many confounding factors make the chaotrope-based avidity assay a problematic tool for evaluating Ab responses to Env and for improving the design of HIV-1 vaccines.
MATERIALS AND METHODS
Antibodies, soluble CD4, lectins, Env proteins, and peptides.
The following monoclonal antibodies (MAbs) were obtained through the NIH AIDS Reagent Program: 268-D and 257-D (isolated with a 23-mer peptide representing the N-terminal part of V3 [MN] [49, 50]); 2191 (isolated with a disulfide-bonded and glycosylated V3 sequence grafted onto a truncated form of murine leukemia virus gp70 [25]); 246-D (gp41 cluster I [51]) from S. Zolla-Pazner; F105 (directed to the CD4-binding site [CD4bs]) and F240 (gp41 cluster I) (52, 53) from M. Posner; F425 B4e8 (directed to the tip of the V3 loop and capable of neutralizing a subset of primary isolates from subtypes B, C, and D [54, 55]); F425A1g8 (directed to a CD4-induced epitope [CD4i]) and b12 (CD4bs [54–56]) from D. Burton; 17b, E51, and A32 (CD4i [24, 57–59]) from J. Robinson; and NIH45-46 GW54 (CD4bs [60]) from J. Mascola. The MAbs 2G12 (glycan epitope), b6 (CD4bs), 15e (CD4bs), PGT128 (V3 base, glycan), and PGT135 (high-mannose glycan) (29, 61–64) were provided by the IAVI Neutralizing Antibody Consortium Repository. HIVIG, a polyclonal IgG preparation from HIV-positive sera, was obtained from the NIH AIDS Reagent Program (catalog number 3957). D7324, an affinity-purified sheep Ab to the C-terminal region of gp120, was purchased from Aalto Bioreagents (Dublin, Ireland). CD4-IgG2 and soluble CD4 (sCD4) were obtained from Progenics Pharmaceuticals. Biotin-conjugated concanavalin A (ConA) (catalog number C2272) and snowdrop (Galanthus nivalis) lectin (catalog number LA71100-4) were purchased from Sigma-Aldrich and United Biosystems, respectively.
gp120 (JR-FL) expressed in CHO cells was provided by Progenics. Extensive structural and epitope-mapping information is available for this protein in truncated forms that include its V3 region (65). A soluble gp41 trimer derived from the HIV-1 SF162 sequence (75 kDa, streptavidin tagged) was produced and purified as described elsewhere (66). A synthetic peptide with the sequence N-DQQLLGIWGCSGKLICTTAVPWNC-C, representing part of epitope cluster I in the ectodomain of gp41, and a negative control with the same residues in scrambled order, N-WNCGTQGPTSKCDIWCLGLILVAQ-C, were made by Primm Biotech. The two D7324 epitope-tagged Env proteins BG505 SOSIP.664-D7324 (SOSIP.664 is also referred to as SOSIP.R6) (27, 28) and WT.SEKS-D7324 (italics indicate an uncleaved construct) were expressed and purified as previously described (27, 28). The structure of a deglycosylated form of the BG505 SOSIP.664 trimer in complex with the V3 base- and glycan-specific Fab PGT122 has been determined by crystallography to a resolution of 4.7 Å. The structure of fully glycosylated trimer in complex with the CD4bs-specific Fab PGV04 was resolved to 5.8 Å by cryo-electron microscopy; the epitopes of several other MAbs have also been delineated on this trimer (67, 68). The sequence modifications in BG505 SOSIP.664, including a disulfide bridge between gp120 and gp41 (SOS), the trimer-stabilizing IP change, and the truncation at residue 664, have been described previously (28). WT.SEKS has the natural REKR cleavage site replaced by the nonscissile motif SEKS but lacks the SOS and I559P (change of I to P at position 559) changes (27). SOSIP.664 adopts nativelike trimeric structures, while WT.SEKS exhibits aberrant, nonnative, variable shapes (27, 67, 68).
ELISA with chaotrope pulsing.
Capture ELISA was performed as described elsewhere (69, 70). Briefly, Costar 96-well white plates were coated overnight with 10 μg/ml D7324 (for capture of gp120 or Env trimers via C-terminal epitopes) in 100 mM NaHCO3, pH 9.5. Plates were blocked with 200 μl B3T buffer (150 mM NaCl, 50 mM Tris-HCl, 1 mM EDTA, 3.3% fetal bovine serum [FBS], 2% bovine serum albumin [BSA], and 0.07% Tween 20) for 1 h at room temperature, washed twice with Tris-buffered saline (TBS), and then incubated for 2 h with 600 ng/ml JR-FL gp120, BG505 SOSIP.664, or WT.SEKS Env proteins in TBS containing 10% FBS.
Direct-coating ELISA was performed as described above for the Ab capture assay except that the wells were coated overnight with Ag. The gp41 cluster I peptide or its scrambled counterpart (see above for sequences) was applied at 2 μg/ml in 100 mM NaHCO3, pH 9.5. In all peptide ELISAs with the gp41 cluster I peptide, the background signal obtained with the scrambled counterpart was subtracted. The gp120 monomer was applied at 600 ng/ml gp120, and the soluble gp41 trimer at 500 ng/ml, both in TBS. In all cases, the wells were subsequently blocked with B3T buffer.
The choice of the Ag concentrations specified above for both capture and direct-coating ELISAs was based on pretitrations. Thus, the selected Ag densities used fell in a zone of approximately constant 50% effective concentrations (EC50s) for the Abs. Each EC50 is, therefore, a good approximation of the dissociation constant, Kd, which describes the functional affinity.
The MAbs, lectins, and HIVIG were titrated in 3-fold serial dilutions (over different concentration ranges, as indicated in the figures) in Ab buffer (20% sheep serum, 4% milk, and 0.5% Tween 20 in TBS) and were then allowed to bind immobilized Ag for 2 h at room temperature. Soluble CD4 was titrated down in constant 4-fold molar excess over the MAbs to the CD4i epitopes. After Ab or lectin incubation, duplicate wells were washed twice with TBS and treated with either phosphate-buffered saline (PBS) or 1.5 M sodium thiocyanate (NaSCN) for 15 min and then washed twice again with TBS before the addition of the secondary Ab. That procedure is referred to herein as posttreatment. In the pretreatment, the wells were washed twice after gp120 was exposed to PBS or 1.5 M NaSCN and before the addition of the titrated MAb. We compared post- and pretreatment of both captured Ag and Ag used to directly coat wells, because direct coating might itself partly denature the Ag (71) and therefore diminish any additional effect of the chaotrope. Since the pretreatment chaotrope pulse was followed by washes to avoid residual direct effects of the reagent on Ab binding, some renaturation of the Ag might instead occur.
For detection of bound MAb and CD4-IgG2, we used anti-human IgG (Fc specific) and highly cross-adsorbed alkaline phosphatase (AP)-conjugated goat Ab (catalog number SAB3701277; Sigma-Aldrich) diluted 1:1,000. All detection Abs were diluted in TBS with 0.05% Tween 20 and incubated for 1 h at room temperature. Bound sCD4 was detected with anti-human CD4 biotin-conjugated MAb OKT4, followed by two TBS washes and then AP-conjugated streptavidin (catalog number 43-4322; Invitrogen). Fabs were detected by anti-human IgG F(ab′)2 highly cross-adsorbed AP-conjugated rabbit Ab (catalog number SAB3701250; Sigma-Aldrich). Plates were then washed four times with PBS containing 0.1% Tween 20 and then twice with Tropix (Invitrogen). The AP-mediated catalysis of the chemiluminescent substrate (Invitrogen) was allowed to proceed for 10 min before the plates were read on a luminometer. Luminescence values for test wells were corrected by subtracting first the control values obtained without Ag (but with capture Ab where applicable) or with the scrambled peptide and then the control values obtained without primary Ab. The resulting background-corrected values were plotted against Ab concentration and fitted with a three-parameter sigmoidal function that had the bottom plateau constrained to 0 (Prism; GraphPad). R2 values were >0.9.
Because chaotropic effects on interactions other than those between test MAbs and Env would make it hard to interpret the experiments, we investigated whether the chaotrope influenced the coating of the ELISA wells with the sheep capture Ab D7324. Wells coated as described above were pulsed with the chaotrope (1.5 M NaSCN) or PBS for 5, 10, 15, and 20 min. The remaining bound D7324 was then detected with AP-conjugated anti-sheep IgG. The outcome was that the chaotrope treatment did not reduce the amount of D7324 attached to the wells compared with the results for the PBS control (data not shown).
Correlations were investigated by calculating the nonparametric Spearman's rank coefficients, and their significance by performing two-tailed tests (Prism; GraphPad).
RESULTS
Differing effects of chaotrope treatment on MAb and lectin binding to different epitope clusters in gp120.
The degree of Ab occupancy is known to influence how sensitive an Ab-Ag complex is to dissociation by a chaotrope (72). To avoid that bias, we titrated MAbs and lectins over a wide concentration range when we measured their binding to D7324-captured gp120 (JR-FL) with and without a 15-min chaotrope (NaSCN) pulse (Fig. 1). The chaotrope had differing effects on two fitted binding measurements: the plateau value (Bmax) and the half-maximal binding concentration (EC50). At the Ag densities used, the EC50s approximate the dissociation constants, Kds, and thus describe the functional affinities of the MAbs, which result from the weighted averages over their mixed bivalent and monovalent modes of binding.
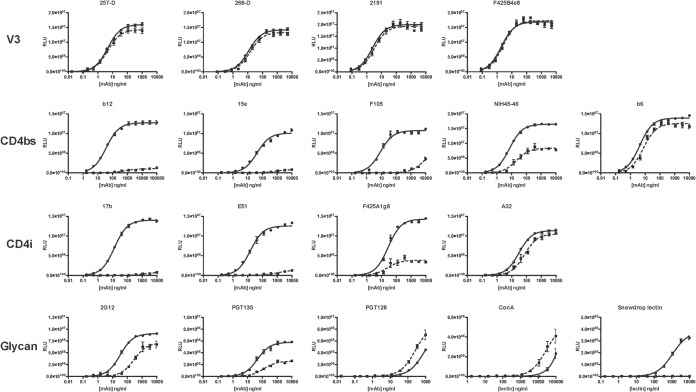
FIG 1.
Chaotrope effects on the binding of gp120-specific MAbs and lectins. The MAbs and lectins were titrated against D7324-captured gp120 (JR-FL) in ELISAs. Quantification of bound MAb, detected as luminescence (relative light units [RLU]), is expressed on the y axis as a function of MAb concentration (ng/ml) on the x axis. Ligand binding after chaotrope (NaSCN) or control (PBS) treatment is represented by dashed and solid lines, respectively. The data points are the mean results from three intra-assay replicates, and the error bars show the standard errors of the means (SEM). Each plot is representative of ≥2 replicate experiments.
When serum or plasma samples with unknown specific Ab concentrations are studied, it is common to calculate an avidity index, which quantifies the effect of the chaotrope on Ab-Ag binding. Specifically, the avidity index is the serum or plasma dilution factor for half-maximal binding after chaotrope exposure divided by the corresponding value in the absence of chaotrope, expressed as a percentage (33-36, 46, 73–76). Accordingly, we calculated here the corresponding value, i.e., 1/EC50 with chaotrope relative to the result without, expressed as a percentage. The resulting value would ideally be a measure of the relative functional affinity of the test Ab for the Ag in the presence of chaotrope. We refer to it as the functional affinity index [FAI]. In some other studies, the level of Ab binding in the presence of a constant or varied chaotrope concentration relative to the level of binding without chaotrope is reported (42, 44, 47, 48, 73, 77–84). Accordingly, we also calculated the reduction in Ab-binding plateaus, or Bmax values, and defined the Bmax index (BMI) as the Bmax with chaotrope divided by the Bmax without, expressed as a percentage.
We first assessed the effect of chaotrope exposure on the binding of MAbs to the V3 region of gp120 (Fig. 1). V3 is a dominant target for autologous, strain-specific, neutralizing responses that appear early in HIV-1 infection (85–88). Typically, V3 MAbs efficiently neutralize only sensitive (tier 1) isolates, because their epitopes are occluded on the trimers of more resistant (tier 2) viruses. These MAbs usually recognize V3 on gp120 monomers and, often, also V3 peptides of various lengths (28, 30, 89–92). In general, epitopes in loops or protruding regions of proteins tend to be continuous and, therefore, are mimicked well by short peptides (20, 21, 93). For all four V3 MAbs tested against JR-FL gp120, chaotrope exposure gave FAI and BMI values close to 100% (Fig. 1). Specifically, MAbs 257-D, 268-D (49, 50), 2191 (25), and F425-B4e8 (54, 55) gave BMI values of 95 to 102% and FAI values of ∼100%. Thus, the gp120 binding of all of the anti-V3 MAbs tested was highly chaotrope resistant. This result also provides an internal control by demonstrating that the chaotrope does not reverse the capture of gp120 by the Ab D7324 that is adsorbed to the ELISA wells.
In contrast to the binding of V3-specific MAbs, which was uniformly chaotrope resistant, MAb binding to the CD4bs epitopes differed widely in chaotrope sensitivity (Fig. 1). CD4bs MAbs bind to most gp120 monomers (e.g., JR-FL gp120), but the CD4bs Abs most prevalent in HIV-1-positive sera neutralize only tier 1 isolates because their epitopes are occluded on the trimers of tier 2 viruses (e.g., JR-FL). Some rare CD4bs bNAbs do, however, neutralize tier 2 viruses (24, 52, 56, 94). We used MAbs 15e, F105, and b6 as representatives of the first category and b12 and NIH45-46 to represent the second. The gp120 binding of these five CD4bs MAbs spanned a spectrum of chaotrope sensitivities. Thus, the binding of b12, 15e, and F105 was nearly eliminated by the chaotrope, NIH45-46 binding was partly sensitive (BMI of 51% and FAI of 34%), and b6 binding was chaotrope resistant (BMI of 90% and FAI of 57%). Chaotrope resistance did not, however, track with the capacity of the same MAbs to neutralize HIV-1 JR-FL. Thus, b12 and NIH45-46 potently neutralize this tier 2 virus but 15e, F105, and b6 do not (56). Still, the drastically divergent chaotrope sensitivities must reflect differences in how the MAb paratopes interact with the corresponding epitopes on the gp120 monomer. Although the epitopes for the five CD4bs MAbs overlap, they are certainly not identical. For example, b12, unlike b6 and NIH45-46, inserts its CDR3 into the same hydrophobic pocket that CD4 itself plugs with its Phe43 residue (63).
We also studied the chaotrope sensitivity of MAb binding to CD4i epitopes (Fig. 1). In these experiments, sCD4 was present in 4-fold molar excess over the MAb to induce the exposure of the CD4i epitopes on gp120 (see Materials and Methods). Given CD4 induction of these epitopes, all the CD4i-specific MAbs yielded binding plateaus such that both their BMI and FAI values could be determined for quantitative comparisons. The MAbs 17b, E51, and F425A1g8 recognize discontinuous epitopes that overlap the conserved coreceptor-binding site on the bridging sheet, which is formed between the inner and outer domains of gp120 upon CD4 binding (59). These three MAbs generally neutralize tier 1 but not tier 2 viruses (54, 57, 59, 95). Like the CD4bs MAbs, the CD4i MAbs bound to gp120 with various chaotrope sensitivities. Thus, for both 17b and E51, the BMI was <10% and the FAI was <0.1%, but for F425A1g8, the BMI was 27% and the FAI ∼100% (i.e., the EC50 for this MAb was unaffected by the chaotrope).
The A32 MAb also requires sCD4 for induction of its epitope but differs from the three CD4i MAbs discussed above by not neutralizing even tier 1 viruses and by recognizing a less complex epitope with segments in the C1 and C4 regions of gp120 (57, 96, 97). We found that A32 binding was resistant to the chaotrope (BMI of 92% and FAI of 42%). Hence, the binding of 17b, E51, and F425A1g8 to their conformationally sensitive, discontinuous CD4i epitopes in the bridging sheet was markedly more chaotrope sensitive than the binding of A32 to its CD4i epitope, which consists of two relatively continuous segments.
The glycan-dependent binding of MAbs and lectins also varied in chaotrope sensitivity (Fig. 1). The bNAb 2G12 interacts with the high-mannose glycans at N295, N332, and N392; its Fab arms are entwined through an unusual VH domain swap that confers higher affinity than other glycan-binding Abs display and renders the IgG functionally monovalent (61, 62). 2G12 binding to gp120 was partly chaotrope resistant (BMI of 78% and FAI of 12%). PGT135, also a bNAb, interacts with glycans at N332, N386, and N392 but contacts the polypeptide surface as well (67, 98). Like 2G12, PGT135 bound in a partly chaotrope-resistant manner (BMI of 45% and FAI of 37%). PGT128 interacts with the N301 and N332 glycans at the base of V3 and, also, with the polypeptide surface (67). This bNAb bound weakly to gp120, but its reactivity was increased by chaotrope treatment (BMI and FAI values could not be determined). Chaotrope exposure similarly enhanced the binding of the mannose-specific lectin ConA (99) but obliterated the reactivity of a second mannose-reactive ligand, snowdrop lectin (100). The markedly different, indeed sometimes opposing, effects on the binding of these bNAbs and lectins to gp120 suggest that chaotrope exposure modulates the orientations of the glycans in a complex manner.
In general, while the effect of chaotrope treatment varied both within and among the various gp120 epitope clusters, MAb binding to conformationally dependent, composite epitopes was more affected than the binding to predominantly continuous ones.
The binding of Abs to cluster I epitopes in gp41 is weakly enhanced by chaotrope treatment.
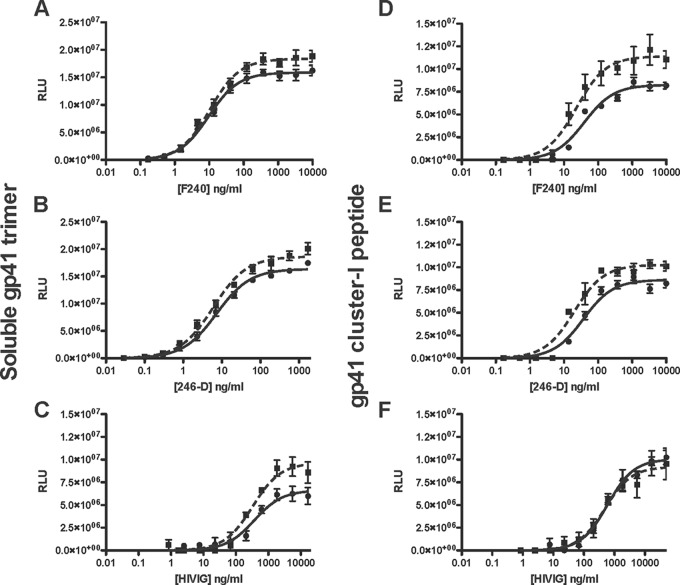
The immunodominant, nonneutralization epitopes in gp41 cluster I (residues 579 to 613) are predominantly continuous and can therefore be mimicked by short synthetic peptides (22, 23). The binding of MAbs F240 and 246-D and of a polyclonal HIV-1 IgG (HIVIG) to a soluble gp41 ectodomain trimer and to a peptide encompassing the cluster I epitopes was invariably and strongly chaotrope resistant. Indeed, chaotrope treatment enhanced F240, 246-D, and HIVIG binding to the gp41 trimer, yielding BMI values of 116%, 114%, and 146%, respectively; the relative functional affinities were not detectably affected, i.e., the FAI values were ∼100% (Fig. 2A to C). The binding of F240 and 246-D but not of HIVIG to the gp41 peptide was also enhanced by the chaotrope; the respective BMI values were 143%, 127%, and 91.7%, while the FAI values were again ∼100% (Fig. 2D to F). Since only the Bmax and not the functional affinity was affected by the chaotrope, the enhanced binding to the gp41 Ags may be caused by an increase in the number of epitopes that become available for Ab binding through the chaotrope treatment and remain so throughout the ELISA washes.
FIG 2.
Chaotrope effects on the binding of gp41-specific MAbs and HIVIG. The MAbs and HIVIG were titrated against wells directly coated with gp41 trimer (A to C) or gp41 peptide (D to F) in ELISAs. Bound Ab, detected as luminescence (RLU), is expressed on the y axis as a function of Ab concentration (ng/ml) on the x axis (MAb F240, MAb 246-D, or HIVIG as indicated). Ab binding after chaotrope (NaSCN) or control (PBS) treatment is represented by dashed and solid lines, respectively. The data points are the mean results from three intra-assay replicates, and the error bars show the SEM. Each plot is representative of ≥2 replicate experiments.
Affinity does not correlate with chaotrope resistance.
The original purpose of the chaotrope assay was to assess the functional affinity of Abs of unknown concentration in sera or plasma; i.e., to dissect out the respective contributions that affinity and concentration make to the increases in Ab binding titers that can be measured over time after infection or vaccination. The validity of that application for anti-Env antibodies can be tested by the use of various MAbs of known concentration. We therefore explored how well the two measurements of the chaotrope effect, i.e., the FAI and the BMI (derived from data in Fig. 1 and 2A and B), correlated with the EC50, which gives an estimate of Kd (Fig. 3A and B).
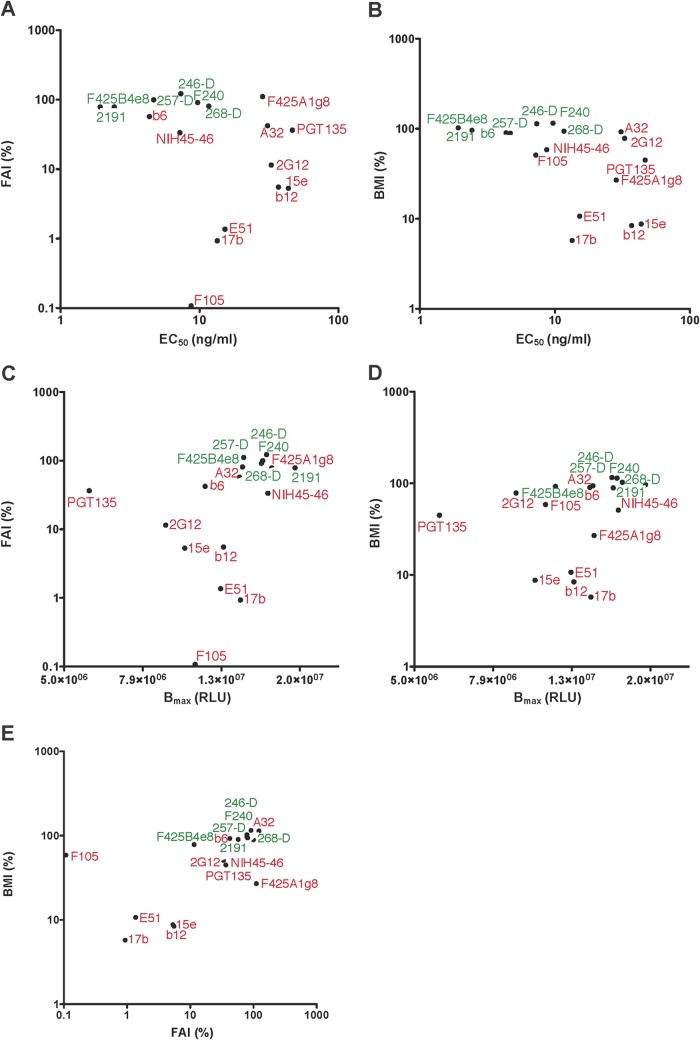
FIG 3.
Resistance of binding to the chaotrope does not correlate well with MAb affinity. EC50 and Bmax values were calculated by fitting a sigmoid function to the data shown in Fig. 1 and 2A and B. The functional affinity index (FAI) and the Bmax index (BMI) are two different measurements of the resistance to chaotrope treatment of MAb binding to the Env Ag. FAI (%) was plotted against EC50 (ng/ml) (A), BMI (%) against EC50 (ng/ml) (B), FAI (%) against Bmax (RLU) (C), BMI (%) against Bmax (RLU) (D), and FAI (%) against BMI (%) (E). Symbols representing Abs targeting the more continuous epitopes (V3 and gp41) and the more composite epitopes are labeled in green and red, respectively.
We found that the FAI did not correlate well with EC50, although the tendency was in the expected negative direction (r = −0.36, P = 0.16) (Fig. 3A). Hence, the FAI is a poor surrogate for the functional affinity of Abs for Env proteins. The BMI correlated more strongly, and also negatively, with EC50 (r = −0.58, P = 0.016) (Fig. 3B). Furthermore, FAI correlated fairly well with the absolute Bmax values (r = 0.59, P = 0.012) (Fig. 3C). This correlation agrees with the dependence of the chaotrope effect on the degree of Ab occupancy of the Ag or, more generally, on the amount of the Ab-Ag complex present (72). Hence, these confounding factors further diminish the value of the avidity index for evaluating affinity maturation.
There was a corresponding but weaker tendency for Bmax values to correlate with the BMI (r = 0.42, P = 0.090) (Fig. 3D), again agreeing with the finding that the more Ab that is bound to an Ag, the weaker is its proportional sensitivity to dissociation upon chaotrope exposure (72). The two measurements of chaotrope resistance, FAI and Bmax, did, however, correlate well with each other (r = 0.67, P = 0.0030) (Fig. 3E), suggesting that they identify largely interdependent aspects of Ab binding to Env proteins, in spite of multiple examples of discordant BMI and FAI values (see above). Furthermore, the scatterplot of BMI versus FAI showed that MAbs directed to different types of epitopes segregate (Fig. 3E). Thus, the CD4bs MAbs (except b6) and those to the CD4i (bridging sheet) epitopes fell to the lower left and the glycan-specific MAbs in the middle, whereas A32, the V3-specific MAbs, and the gp41-directed ones fell to the upper right of the plots. Of note is that the latter set is composed only of non-NAbs.
Partial denaturation of gp120 contributes to the chaotrope effect on Ab binding.
Since we found that Ab reactivity with composite epitopes is particularly sensitive to chaotrope treatment, we hypothesized that the chaotrope partly denatures gp120, thereby disrupting discontinuous epitopes while affecting continuous ones less drastically. Furthermore, if the Ag is partly denatured by the use of it to directly coat the plastic, additional denaturation by the chaotrope should be more limited than with captured Ag. To test these hypotheses, we compared the effects of applying the chaotrope pulse before and after MAb binding to gp120 monomers, both following capture by D7324 and in directly coated wells (see Materials and Methods).
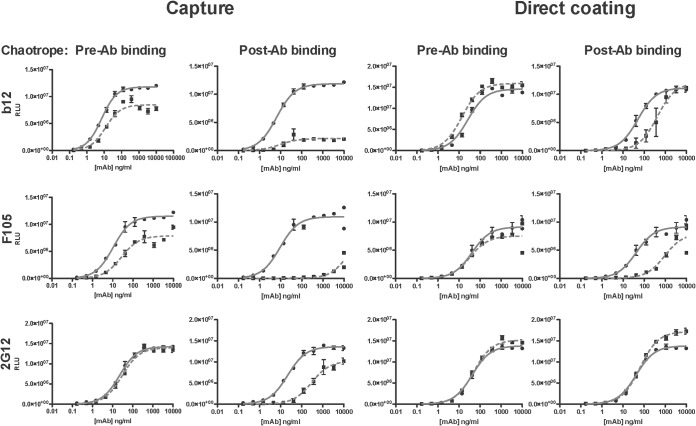
To allow clearly detectable effects, we chose three gp120-specific MAbs that bound with intermediate or stronger chaotrope sensitivity: b12 and F105, which recognize discontinuous epitopes in a chaotrope-sensitive manner, and 2G12, which binds to a glycan epitope in a partly chaotrope-resistant way. The distinct conditions of binding and chaotrope treatment yielded different results for the three MAbs (Fig. 4). In the D7324 capture assay, the pre-Ab chaotrope pulse reduced the binding of b12 and F105 but not of 2G12. Hence, for b12 and F105 the extensive effect of the post-Ab pulse can be partly attributed to denaturation of the gp120 Ag.
FIG 4.
Antigenic perturbation by the chaotrope contributes to reduced binding of MAbs to gp120. The plots on the left show MAb binding to gp120 in capture ELISA, and those on the right show the binding in the direct-coating ELISA, as indicated. For each type of assay, the results of the pre- and post-Ab-binding chaotrope (NaSCN) treatments are shown under the respective labels in the left and right columns (the ELISA conditions for the post-MAb-binding condition in the capture assay were like those in the experiments whose results are shown in Fig. 1.) In each assay, luminescence (RLU) is expressed on the y axis as a function of MAb concentration (ng/ml) on the x axis. The MAb binding after chaotrope or control (PBS) treatment is represented by dashed and solid lines, respectively. The data points are the mean results from three intra-assay replicates, and the error bars show the SEM. Each plot is representative of ≥2 replicate experiments.
In the direct-coating assay, the pre-Ab chaotrope pulses had negligible effects on all three Ab-gp120 interactions (the plateaus for b12 and 2G12 were marginally raised). For b12 and F105, however, the post-Ab pulse had a partial effect in the direct-coating assay, different from that in the D7324 capture assay, the FAI rather than the Bmax being reduced. In contrast, the post-Ab pulse raised the 2G12-binding plateau somewhat in the direct-coating assay. That effect resembles what we observed for PGT128 and ConA in the D7324 capture assay and for F240 and 246-D in the direct-coating gp41 assay, the plateau elevation of the latter having occurred under the same conditions as described here for 2G12 (Fig. 1, 2, and 4).
Overall, this analysis demonstrates that some effects of the chaotrope can be attributed to a perturbation of the Env antigen and not exclusively to the disruption of the interaction between intact Ab and Env proteins. Thus, the chaotrope acts at least partly, and sometimes substantially, by denaturing Env proteins. We note that while denaturation of the MAb might also contribute, those effects will not differentiate between MAbs stringently according to the complexity of their epitopes. And as a further illustration of how complex the chaotrope effects are, the changes in BMI and FAI values sometimes diverged (Fig. 1 to 3). Theoretically, those discrepancies could be related to the degrees of perturbation over the populations of Ag molecules. Thus, a complete denaturation of some epitopes ought to result in a reduced BMI, whereas a more partial disturbance—still compatible with some MAb binding—would merely reduce the FAI.
Multivalent binding increases chaotrope resistance.
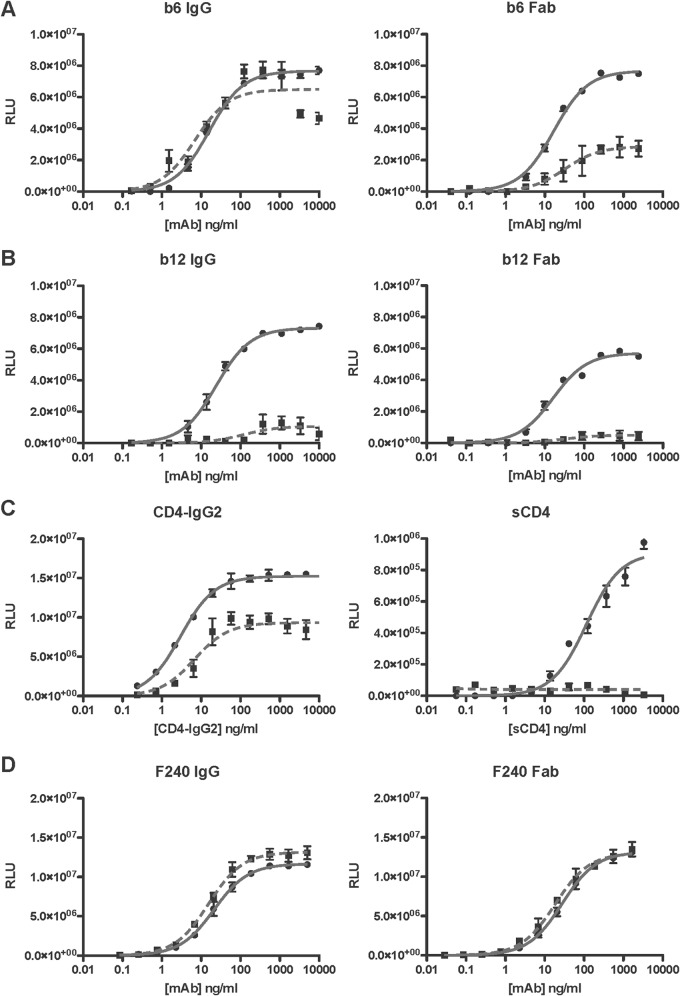
By comparing ligands capable of mono-, bi-, and tetravalent binding, we next investigated whether the avidity in a strict sense affected chaotrope resistance (Fig. 5). The binding of IgG (bivalent) of the CD4bs non-NAb b6 was resistant to the chaotrope (BMI of 84%), but the corresponding Fab (monovalent) bound in a chaotrope-sensitive manner (BMI of 37%) (Fig. 5A). Chaotrope treatment, which already had a profound effect with b12 IgG (BMI of 15%), further reduced the binding of the b12 Fab (BMI of 8.3%) (Fig. 5B). Likewise, for the tetravalent CD4-IgG2 protein, the BMI was relatively high, 61%, but for monovalent sCD4 it was only 4.5% (Fig. 5C). In contrast, the binding of either form of MAb F240 was chaotrope resistant; it was slightly enhanced for the IgG and less so for the Fab.
FIG 5.
Chaotrope resistance increases with valency. Mono-, bi-, and tetravalent ligands were titrated against D7324-captured gp120 (JR-FL) in ELISAs. Binding, detected as luminescence (RLU), is expressed on the y axis as a function of ligand concentration (ng/ml) on the x axis. The potentially bi- or tetravalent binding of IgG and CD4-IgG2 is shown on the left, and the monovalent binding of Fabs and sCD4 on the right. The ligands were b6 IgG/Fab (A), b12 IgG/Fab (B), CD4-IgG2/sCD4 (C), and F24 IgG/Fab (D). Ligand binding after chaotrope (NaSCN) or control (PBS) treatment is represented by dashed and solid lines, respectively. The data points are the mean results from three intra-assay replicates, and the error bars show the SEM. Each plot is representative of ≥2 replicate experiments.
The EC50 (nM) ratios for Fab over IgG were similar (∼2 to 3) for b6 and b12. This low ratio suggests partial but similar degrees of bivalency for these two MAbs. Thus, the greater chaotrope resistance of b6 than of b12 cannot be attributed to a greater degree of bivalent binding. The corresponding ratio for CD4-IgG2 versus sCD4 was much greater (∼13), a reflection of tetra- versus monovalent binding capability. The differential effect of chaotrope treatment was also much greater for CD4-IgG2 versus sCD4 than for b6 or b12 IgG versus Fab, a result illustrating how a high valency can confer chaotrope resistance. The chaotrope resistance of the F240 Fab (EC50 ratio for Fab over IgG of 2.3) shows that the intrinsic properties of the paratope-epitope interaction can still dominate, however.
Ab binding to captured Env trimers is chaotrope sensitive.
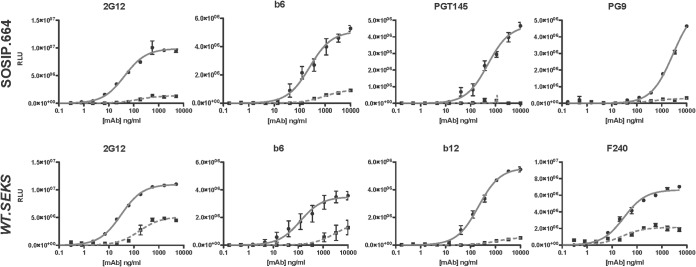
Soluble, recombinant BG505 SOSIP.664 trimers have a nativelike structure (26, 28, 67, 68). Most non-NAbs bind minimally to these trimers, whereas all NAbs that are active against the corresponding virus bind well (28, 30). One exception is the non-NAb b6, which, although it does not bind to SOSIP.664 trimers as detected by surface plasmon resonance (SPR), does bind to an extent in D7324 capture ELISA (28, 30). In contrast, uncleaved gp140 proteins lacking the SOSIP trimer-stabilizing modifications assume nonnativelike structures and fail to bind quaternary structure-dependent or -specific NAbs, such as PG9 and PGT145, but do bind several non-NAbs, such as b6 and F240 (27, 30). They also bind the broadly active NAb b12, which does not neutralize the BG505 virus (27). Against that background, we thought it worthwhile to compare the chaotrope sensitivity of MAb binding to nativelike, cleaved BG505 SOSIP.664-D7324 trimers and nonnativelike, uncleaved BG505 WT.SEKS-D7324 gp140 proteins (see Materials and Methods) (27). We selected 2G12 and b6, which recognize both forms of Env, PGT145 and PG9, which react only with BG505 SOSIP.664-D7324 trimers, and b12 and F240, which bind only to BG505 WT.SEKS-D7324 gp140 proteins.
We found that 2G12 binding to either form of D7324-captured Env was largely chaotrope sensitive (Fig. 6). Thus, for the SOSIP.664 trimers and WT.SEKS gp140, the FAI values were 28% and 18%, respectively, while the Bmax indices were 14% and 47%. The binding of b6 to SOSIP.664 trimers was chaotrope sensitive (FAI of 26% and BMI of 19%), whereas the chaotrope had a greater effect on b6 affinity for WT.SEKS gp140 than on its degree of binding (FAI of 4.1% and BMI of 48%) (Fig. 6). Hence, the binding of b6 to BG505 trimers was more chaotrope sensitive than its binding to JR-FL gp120 monomers (Fig. 1). The binding of b12 to WT.SEKS gp140 was highly chaotrope sensitive (FAI of <20% and Bmax of 10%), to roughly the same extent as b12 binding to gp120 (negligible in the presence of chaotrope). The binding of the gp41 MAb F240 to WT.SEKS gp140 was partially chaotrope sensitive, as reflected only in the BMI (FAI of 120% and BMI of 32%; note that F240 does not bind the SOSIP.664 trimers). Finally, and notably, the binding of the strictly trimer-specific bNAb PGT145 and of the trimer-preferring bNAb PG9 to SOSIP.664 trimers was obliterated by chaotrope treatment (Fig. 6). Thus, the chaotrope disproportionately disrupts the binding of some bNAbs or destroys their complex epitopes, which depend on a native structure, in this case at the trimer apex.
FIG 6.
The binding of MAbs to Env trimers is chaotrope sensitive. The MAbs were titrated against D7324-captured, nativelike BG505 SOSIP.664-D7324 trimers (top row) or uncleaved, nonnative BG505 WT.SEKS-D7324 gp140 proteins (bottom row). Bound MAb, detected as luminescence (RLU), is expressed on the y axis as a function of MAb concentration (ng/ml) on the x axis. The MAb binding after chaotrope (NaSCN) or control (PBS) treatment is represented by dashed and solid lines, respectively. Note the differences in y-axis scale between diagrams. The data points are the mean results from three intra-assay replicates, and the error bars show the SEM. Each plot is representative of ≥2 replicate experiments.
Overall, the examples described above show that chaotrope exposure can have a variety of effects on how well MAbs interact with Env proteins of various designs. Both the affinity and the extent of Ab binding can be affected. The complexity and oligomeric state of the Env protein are decisive variables in that binding to simple peptides, gp120 monomers, uncleaved gp140s, and nativelike trimers responds differentially to chaotrope exposure.
DISCUSSION
The chaotrope-based avidity assay has gained a prominent role in the evaluation of HIV-1 and simian immunodeficiency virus (SIV) vaccine candidates (31–37, 46, 47, 101, 102). In spite of its widespread use, however, it has been unclear what the avidity index actually measures, in particular with regard to complex and conformationally fragile antigens, such as HIV-1 Env. Clearly, the original rationale for this assay was to monitor the affinity maturation of polyclonal Abs specific for certain Ags without the need to know the constituent Ab concentrations and, thus, to dissect the two factors, concentration and affinity, that together determine binding titers.
Notably, the term “avidity” is widely used in two quite distinct senses; in one case very loosely, as a synonym for any Ab affinity, i.e., its meaning in the term “avidity index” (72), and in the other more strictly, to describe the strengthening of binding through two or multipoint interactions. But the capacity for bi- or polyvalent Ab binding, or avidity in the strict sense, does not tend to improve with affinity maturation. On the contrary, the more avidly binding isotypes dominate in the early response to infection and evanesce as the response matures (3). Thus, IgM can bind more avidly than bivalent IgG by virtue of its potential for decavalency. Within the IgG response, the main relevant difference among the subclasses is the greater hinge flexibility of IgG3, which can confer greater avidity under certain conditions of epitope density and orientation. We found that higher valency of binding is associated with chaotrope resistance (Fig. 5). In spite of its name, however, the avidity assay was not intended to detect effects of this kind. Indeed, a quite plausibly superior method by which to assess affinity maturation and, thereby, to distinguish recent from longstanding HIV-1 infections eliminates the influence of valency, or strict avidity, by reducing the Ag density (83). In an ELISA in which wells have a sufficiently sparse coating of Ag, the functional affinity, describing the binding of the potentially bivalent IgG, approaches the intrinsic affinity, i.e., that of the monovalent paratope-epitope interaction (71). In the avidity assays as conventionally performed, however, bivalent binding by IgG can occur to various extents, and as we demonstrated, this will influence the outcome.
We therefore tested the validity of the chaotrope-based index as a measurement of functional affinity. Thus, by using MAbs of known concentration rather than polyclonal sera, we found that the chaotrope resistance of antibody binding to HIV-1 Env proteins has no simple relationship to Ab-Ag affinity. The binding of MAbs to monomeric gp120 yielded a poor correlation between chaotrope resistance and functional affinity (Fig. 3A).
At the level of the Env trimer, a comparison with SPR analyses provides other instances of this disconnection. For example, the binding of 2G12 to the BG505 SOSIP.664 trimer was reduced but not abolished by chaotrope treatment, whereas the binding of PGT145 to its trimer-specific epitope was obliterated. In contrast to that pronounced difference, the two bNAbs bind to the trimer with high and similar affinities as assessed by SPR (30). The Kd value for the functionally monovalent 2G12 was 1.3 nM, whereas for PGT145, it was 2.0 to 2.9 nM for the monovalent binding of its IgG or Fab and 0.16 nM for its mixed bi- and monovalent binding as IgG. Hence, in these cases, chaotrope sensitivity is not directly determined by the bNAb-trimer affinity but appears more influenced by the quaternary-structural dependence of the epitope, which is strongest for PGT145 and weakest for 2G12.
Why would chaotrope resistance not generally reflect Ab affinity for HIV-1 Env proteins? Since different chaotropes disrupt different bonds, qualitative differences in bond compositions among paratope-epitope interactions and not just the binding energy (directly related to the affinity) are expected to influence chaotrope sensitivity (103). Thus, Abs with the same affinity might have widely different chaotrope sensitivity profiles depending on which Env epitope they interact with or how they bind within the same epitope cluster.
In general, continuous epitopes are less conformationally sensitive than discontinuous ones, making their ligation more chaotrope resistant. Many continuous epitopes are located in hydrophilic surface loops, as exemplified by the V3 epitopes in this study. It is pertinent to HIV-1 vaccine studies that the binding of Abs to the most variable epitopes will thus be among the most chaotrope resistant, as we show here. Another immunodominant cluster of continuous epitopes, however, is located on the highly conserved ectodomain of gp41. Although these cluster I epitopes are covered by gp120 in the Env complex, when presented as peptides or on gp41 fragments, they can bind Abs in a highly chaotrope-resistant and even -enhanced manner, as demonstrated here. Overall, surface loop- and cluster I-directed Abs would be overrepresented in responses yielding high avidity indices, whereas Abs to discontinuous gp120 epitopes would be underrepresented, because their binding is usually more chaotrope sensitive.
The primary explanation for this dichotomy is that the chaotrope acts partly by perturbing the conformation of the antigen or even by unfolding it (Fig. 4).This general difference in the chaotrope sensitivities of Ab binding to continuous and discontinuous epitopes thus also explains why, in studies of SIV vaccination with combined vector and gp120 boost regimens, the avidity index for postimmunization sera was lowest against a V1V2-deletion mutant of the gp120 monomer used as the Ag, intermediate against the full-length gp120 monomer, and highest against a V1V2 peptide (31, 34). When the V1V2 loops are deleted from gp120, the proportion of continuous epitopes decreases; the peptide antigens, in contrast, present these continuous variable-loop epitopes in isolation. The chaotrope resistance (i.e., avidity index) thus correlates with the relative abundance of and reactivity with simple, continuous epitopes on the Env antigen used in the binding assay.
Furthermore, we found that Ab binding to quaternary-structural epitopes was even more chaotrope sensitive than the binding to the other conformationally dependent sites (Fig. 6). In addition, the chaotrope sensitivity differed for the same epitope in different oligomeric contexts. Thus, 2G12 binding to gp120 monomers was considerably more chaotrope resistant than its binding to the SOSIP.664 trimer, suggesting that the epitope is more easily perturbed in the trimer than in the monomer, either because of differences in glycan composition or the conformational influence of the subunit interactions. In accordance with the latter explanation, the chaotrope affected the binding of b6 to gp120 negligibly, but it markedly reduced b6 binding to SOSIP.664 trimers and to the WT.SEKS gp140 protein. As another example, F240 binding to the gp41 trimer and cluster I peptide was somewhat enhanced by the chaotrope, but its binding to WT.SEKS gp140 was substantially reduced. Hence, the outcome of the avidity assay strongly depends on the complexity of the HIV-1 Env molecule it is based on.
Crucially, the correlations of the chaotrope-based index with protection or dampened viremia in HIV-1 Env vaccine experiments on nonhuman primates have so far been obtained in the absence of broad and potent neutralizing responses (31–36). And yet, it behooves us to consider what the index would detect if such responses were successfully induced. If a polyclonal serum from an HIV-1 vaccine recipient yields a high avidity index for Ab binding to Env, the most prevalent Abs probably recognize relatively continuous, simple epitopes. This result, in turn, translates into a preponderance of Abs to the variable loop regions and the N- and C-terminal segments of gp120, as well as to gp41 cluster I epitopes when these are included in the Ag. Such Abs generally do not neutralize or do so narrowly. It is notable that, of the MAbs to discontinuous epitopes, the CD4bs-reactive MAb b6 bound with the greatest chaotrope resistance, and A32, recognizing a CD4-induced epitope comprising two continuous segments at the termini of gp120, also bound a in a resistant manner. Neither of those MAbs generally neutralizes primary isolates of HIV-1. It is particularly noteworthy that the binding of bNAbs to quaternary-structural epitopes on native Env trimers would not be detected at all in a chaotrope-based avidity assay. Indeed, the inclusion of native trimers in chaotrope-based avidity assays may be ineffectual, whether the trimers are made as soluble recombinant proteins or incorporated into viruslike particles (33, 36). Overall, avidity indices would be skewed upwards by high ratios of non-bNAb over bNAb responses.
A variant of the index based on ammonium thiocyanate elution of Ab from wells directly coated with gp140 did, however, correlate with the development of cross-reactive NAbs in a group of treatment-naïve, HIV-1-infected people (88). That observation raises the question whether the binding of the bNAbs themselves in those sera was more chaotrope resistant than that of nonneutralizing or narrowly neutralizing responses. If not, the avidity index may be a mere surrogate marker for the bNAb responses, directly detecting increasingly strong binding by other Abs that develop in parallel. Our results favor the latter possibility, although we note the exception of the chaotrope-enhanced binding by the bNAb PGT128 to gp120. Such atypical Abs could enhance the net average chaotrope resistance of a polyclonal serum, but such exceptional results also raise the question whether other chaotropes would yield aberrant effects with other Abs.
The range of avidity indices found to correlate either with protection from infection or with substantial viremic suppression in plasma is remarkably narrow: 20 to 50% (31, 32, 34, 36, 46). To put this into perspective, it can be noted that in the corresponding index, FAI, the neutralizing and nonneutralizing Abs in our panel varied from 0.1 to 116%, i.e., >1,000-fold (Fig. 1 to 3). Against that background, it seems unlikely that an approximately 2-fold shift in the chaotrope resistance of an Ab interaction with a nonnative Env protein would identify the cause of the profound biological effects observed. It is quite plausible that other variables indirectly and inconstantly link the index to protection. After all, neither chaotrope resistance nor V1V2 peptide reactivity nor antibody-dependent cellular cytotoxicity, which in some assays is preferentially detected with A32-like and loop-directed Abs, is universally associated with protection (47, 104–107).
We conclude that strong chaotrope resistance of Ab binding to an Env protein may be attributed to multiple factors: Ab specificity for continuous epitopes, certain glycan interactions, and the degree of bivalent binding. The HIV-1 Env trimers are extraordinarily complex and fragile and, therefore, chaotrope sensitive. In particular, the quaternary-structural epitopes, such as those at the trimer apex, may be utterly destroyed by chaotropes. For studies based on HIV-1 Env, we argue that directly linking an avidity index to protective Ab responses is not feasible; it would require a better mechanistic understanding of specific chaotrope effects. For example, what categories of Env-specific Ab does the test Ag bind, and what does the chaotrope do to the structure of the corresponding epitopes? Because of these uncertainties, the avidity index is a problematic analytical tool for evaluating current HIV-1 Env vaccine candidates and for designing new ones.
ACKNOWLEDGMENTS
This work was supported by NIH grants R37 AI36082 and P01 AI82362.
We thank Al Cupo for advice on Env protein production and purification.
REFERENCES
- 1.Plotkin SA. 2008. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis 47:401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 2.Burton DR, Ahmed R, Barouch DH, Butera ST, Crotty S, Godzik A, Kaufmann DE, McElrath MJ, Nussenzweig MC, Pulendran B, Scanlan CN, Schief WR, Silvestri G, Streeck H, Walker BD, Walker LM, Ward AB, Wilson IA, Wyatt R. 2012. A blueprint for HIV vaccine discovery. Cell Host Microbe 12:396–407. doi: 10.1016/j.chom.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klasse PJ, Sanders RW, Cerutti A, Moore JP. 2012. How can HIV-type-1-Env immunogenicity be improved to facilitate antibody-based vaccine development? AIDS Res Hum Retroviruses 28:1–15. doi: 10.1089/aid.2011.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein F, Mouquet H, Dosenovic P, Scheid JF, Scharf L, Nussenzweig MC. 2013. Antibodies in HIV-1 vaccine development and therapy. Science 341:1199–1204. doi: 10.1126/science.1241144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho MW, Martin MA. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med 5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 6.Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q, Ketas T, Marx PA, Klasse PJ, Burton DR, Moore JP. 2003. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med 9:343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 7.Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, Koff WC, Watkins DI, Burton DR. 2009. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog 5:e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med 6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 9.Burton DR, Hessell AJ, Keele BF, Klasse PJ, Ketas TA, Moldt B, Dunlop DC, Poignard P, Doyle LA, Cavacini L, Veazey RS, Moore JP. 2011. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc Natl Acad Sci U S A 108:11181–11186. doi: 10.1073/pnas.1103012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pegu A, Yang ZY, Boyington JC, Wu L, Ko SY, Schmidt SD, McKee K, Kong WP, Shi W, Chen X, Todd JP, Letvin NL, Huang J, Nason MC, Hoxie JA, Kwong PD, Connors M, Rao SS, Mascola JR, Nabel GJ. 2014. Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Sci Transl Med 6:243ra288. doi: 10.1126/scitranslmed.3008992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burton DR, Desrosiers RC, Doms RW, Koff WC, Kwong PD, Moore JP, Nabel GJ, Sodroski J, Wilson IA, Wyatt RT. 2004. HIV vaccine design and the neutralizing antibody problem. Nat Immunol 5:233–236. doi: 10.1038/ni0304-233. [DOI] [PubMed] [Google Scholar]
- 12.Simek MD, Rida W, Priddy FH, Pung P, Carrow E, Laufer DS, Lehrman JK, Boaz M, Tarragona-Fiol T, Miiro G, Birungi J, Pozniak A, McPhee DA, Manigart O, Karita E, Inwoley A, Jaoko W, Dehovitz J, Bekker LG, Pitisuttithum P, Paris R, Walker LM, Poignard P, Wrin T, Fast PE, Burton DR, Koff WC. 2009. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol 83:7337–7348. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doria-Rose NA, Klein RM, Daniels MG, O'Dell S, Nason M, Lapedes A, Bhattacharya T, Migueles SA, Wyatt RT, Korber BT, Mascola JR, Connors M. 2010. Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables. J Virol 84:1631–1636. doi: 10.1128/JVI.01482-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mikell I, Sather DN, Kalams SA, Altfeld M, Alter G, Stamatatos L. 2011. Characteristics of the earliest cross-neutralizing antibody response to HIV-1. PLoS Pathog 7:e1001251. doi: 10.1371/journal.ppat.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piantadosi A, Panteleeff D, Blish CA, Baeten JM, Jaoko W, McClelland RS, Overbaugh J. 2009. Breadth of neutralizing antibody response to human immunodeficiency virus type 1 is affected by factors early in infection but does not influence disease progression. J Virol 83:10269–10274. doi: 10.1128/JVI.01149-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein F, Diskin R, Scheid JF, Gaebler C, Mouquet H, Georgiev IS, Pancera M, Zhou T, Incesu RB, Fu BZ, Gnanapragasam PN, Oliveira TY, Seaman MS, Kwong PD, Bjorkman PJ, Nussenzweig MC. 2013. Somatic mutations of the immunoglobulin framework are generally required for broad and potent HIV-1 neutralization. Cell 153:126–138. doi: 10.1016/j.cell.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medina-Ramirez M, Sanders RW, Klasse PJ. 2014. Targeting B-cell germlines and focusing affinity maturation: the next hurdles in HIV-1-vaccine development? Expert Rev Vaccines 13:449–452. doi: 10.1586/14760584.2014.894469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klasse PJ, Sattentau QJ. 2002. Occupancy and mechanism in antibody-mediated neutralization of animal viruses. J Gen Virol 83(Pt 9):2091–2108. [DOI] [PubMed] [Google Scholar]
- 19.Laver WG, Air GM, Webster RG, Smith-Gill SJ. 1990. Epitopes on protein antigens: misconceptions and realities. Cell 61:553–556. doi: 10.1016/0092-8674(90)90464-P. [DOI] [PubMed] [Google Scholar]
- 20.Atassi MZ. 1984. Antigenic structures of proteins. Their determination has revealed important aspects of immune recognition and generated strategies for synthetic mimicking of protein binding sites. Eur J Biochem 145:1–20. doi: 10.1111/j.1432-1033.1984.tb08516.x. [DOI] [PubMed] [Google Scholar]
- 21.Berzofsky JA. 1985. Intrinsic and extrinsic factors in protein antigenic structure. Science 229:932–940. doi: 10.1126/science.2410982. [DOI] [PubMed] [Google Scholar]
- 22.Goudsmit J, Meloen RH, Brasseur R. 1990. Map of sequential B cell epitopes of the HIV-1 transmembrane protein using human antibodies as probe. Intervirology 31:327–338. [DOI] [PubMed] [Google Scholar]
- 23.Klasse PJ, Pipkorn R, Blomberg J. 1991. A cluster of continuous antigenic structures in the transmembrane protein of HIV-1: individual patterns of reactivity in human sera. Mol Immunol 28:613–622. doi: 10.1016/0161-5890(91)90130-C. [DOI] [PubMed] [Google Scholar]
- 24.Moore JP, Ho DD. 1993. Antibodies to discontinuous or conformationally sensitive epitopes on the gp120 glycoprotein of human immunodeficiency virus type 1 are highly prevalent in sera of infected humans. J Virol 67:863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorny MK, Williams C, Volsky B, Revesz K, Cohen S, Polonis VR, Honnen WJ, Kayman SC, Krachmarov C, Pinter A, Zolla-Pazner S. 2002. Human monoclonal antibodies specific for conformation-sensitive epitopes of V3 neutralize human immunodeficiency virus type 1 primary isolates from various clades. J Virol 76:9035–9045. doi: 10.1128/JVI.76.18.9035-9045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Julien JP, Lee JH, Cupo A, Murin CD, Derking R, Hoffenberg S, Caulfield MJ, King CR, Marozsan AJ, Klasse PJ, Sanders RW, Moore JP, Wilson IA, Ward AB. 2013. Asymmetric recognition of the HIV-1 trimer by broadly neutralizing antibody PG9. Proc Natl Acad Sci U S A 110:4351–4356. doi: 10.1073/pnas.1217537110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ringe RP, Sanders RW, Yasmeen A, Kim HJ, Lee JH, Cupo A, Korzun J, Derking R, van Montfort T, Julien JP, Wilson IA, Klasse PJ, Ward AB, Moore JP. 2013. Cleavage strongly influences whether soluble HIV-1 envelope glycoprotein trimers adopt a native-like conformation. Proc Natl Acad Sci U S A 110:18256–18261. doi: 10.1073/pnas.1314351110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanders RW, Derking R, Cupo A, Julien JP, Yasmeen A, de Val N, Kim HJ, Blattner C, de la Pena AT, Korzun J, Golabek M, de Los Reyes K, Ketas TJ, van Gils MJ, King CR, Wilson IA, Ward AB, Klasse PJ, Moore JP. 2013. A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog 9:e1003618. doi: 10.1371/journal.ppat.1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong CH, Phogat S, Wrin T, Simek MD, Koff WC, Wilson IA, Burton DR, Poignard P. 2011. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yasmeen A, Ringe R, Derking R, Cupo A, Julien JP, Burton DR, Ward AB, Wilson IA, Sanders RW, Moore JP, Klasse PJ. 2014. Differential binding of neutralizing and non-neutralizing antibodies to native-like soluble HIV-1 Env trimers, uncleaved Env proteins, and monomeric subunits. Retrovirology 11:41. doi: 10.1186/1742-4690-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon SN, Doster MN, Kines RC, Keele BF, Brocca-Cofano E, Guan Y, Pegu P, Liyanage NP, Vaccari M, Cuburu N, Buck CB, Ferrari G, Montefiori D, Piatak M Jr, Lifson JD, Xenophontos AM, Venzon D, Robert-Guroff M, Graham BS, Lowy DR, Schiller JT, Franchini G. 2014. Antibody to the gp120 V1/V2 loops and CD4+ and CD8+ T cell responses in protection from SIVmac251 vaginal acquisition and persistent viremia. J Immunol 193:6172–6183. doi: 10.4049/jimmunol.1401504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai L, Kwa S, Kozlowski PA, Montefiori DC, Ferrari G, Johnson WE, Hirsch V, Villinger F, Chennareddi L, Earl PL, Moss B, Amara RR, Robinson HL. 2011. Prevention of infection by a granulocyte-macrophage colony-stimulating factor coexpressing DNA/modified vaccinia Ankara simian immunodeficiency virus vaccine. J Infect Dis 204:164–173. doi: 10.1093/infdis/jir199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai L, Vodros D, Kozlowski PA, Montefiori DC, Wilson RL, Akerstrom VL, Chennareddi L, Yu T, Kannanganat S, Ofielu L, Villinger F, Wyatt LS, Moss B, Amara RR, Robinson HL. 2007. GM-CSF DNA: an adjuvant for higher avidity IgG, rectal IgA, and increased protection against the acute phase of a SHIV-89.6P challenge by a DNA/MVA immunodeficiency virus vaccine. Virology 369:153–167. doi: 10.1016/j.virol.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pegu P, Vaccari M, Gordon S, Keele BF, Doster M, Guan Y, Ferrari G, Pal R, Ferrari MG, Whitney S, Hudacik L, Billings E, Rao M, Montefiori D, Tomaras G, Alam SM, Fenizia C, Lifson JD, Stablein D, Tartaglia J, Michael N, Kim J, Venzon D, Franchini G. 2013. Antibodies with high avidity to the gp120 envelope protein in protection from simian immunodeficiency virus SIV(mac251) acquisition in an immunization regimen that mimics the RV-144 Thai trial. J Virol 87:1708–1719. doi: 10.1128/JVI.02544-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao P, Zhao J, Patterson LJ, Brocca-Cofano E, Venzon D, Kozlowski PA, Hidajat R, Demberg T, Robert-Guroff M. 2010. Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J Virol 84:7161–7173. doi: 10.1128/JVI.00410-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao J, Lai L, Amara RR, Montefiori DC, Villinger F, Chennareddi L, Wyatt LS, Moss B, Robinson HL. 2009. Preclinical studies of human immunodeficiency virus/AIDS vaccines: inverse correlation between avidity of anti-Env antibodies and peak postchallenge viremia. J Virol 83:4102–4111. doi: 10.1128/JVI.02173-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ross TM, Xu Y, Green TD, Montefiori DC, Robinson HL. 2001. Enhanced avidity maturation of antibody to human immunodeficiency virus envelope: DNA vaccination with gp120-C3d fusion proteins. AIDS Res Hum Retroviruses 17:829–835. doi: 10.1089/088922201750252025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butler JE, Feldbush TL, McGivern PL, Stewart N. 1978. The enzyme-linked immunosorbent assay (ELISA): a measure of antibody concentration or affinity. Immunochemistry 15:131–136. doi: 10.1016/0161-5890(78)90053-6. [DOI] [PubMed] [Google Scholar]
- 39.Engvall E, Perlmann P. 1972. Enzyme-linked immunosorbent assay, ELISA. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol 109:129–135. [PubMed] [Google Scholar]
- 40.Katz FE, Steward MW. 1976. Studies on the genetic control of antibody affinity: the independent control of antibody levels and affinity in Biozzi mice. J Immunol 117:477–479. [PubMed] [Google Scholar]
- 41.Edgington TS. 1971. Dissociation of antibody from erythrocyte surfaces by chaotropic ions. J Immunol 106:673–680. [PubMed] [Google Scholar]
- 42.Pullen GR, Fitzgerald MG, Hosking CS. 1986. Antibody avidity determination by ELISA using thiocyanate elution. J Immunol Methods 86:83–87. doi: 10.1016/0022-1759(86)90268-1. [DOI] [PubMed] [Google Scholar]
- 43.Khurana S, Verma N, Yewdell JW, Hilbert AK, Castellino F, Lattanzi M, Del Giudice G, Rappuoli R, Golding H. 2011. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci Transl Med 3:85ra48. doi: 10.1126/scitranslmed.3002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Narita M, Matsuzono Y, Takekoshi Y, Yamada S, Itakura O, Kubota M, Kikuta H, Togashi T. 1998. Analysis of mumps vaccine failure by means of avidity testing for mumps virus-specific immunoglobulin G. Clin Diagn Lab Immunol 5:799–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore J, Lewis GK, Robinson J. 1993. Which gp160 vaccine? Nature 361:503. doi: 10.1038/361503a0. [DOI] [PubMed] [Google Scholar]
- 46.Xiao P, Patterson LJ, Kuate S, Brocca-Cofano E, Thomas MA, Venzon D, Zhao J, DiPasquale J, Fenizia C, Lee EM, Kalisz I, Kalyanaraman VS, Pal R, Montefiori D, Keele BF, Robert-Guroff M. 2012. Replicating adenovirus-simian immunodeficiency virus (SIV) recombinant priming and envelope protein boosting elicits localized, mucosal IgA immunity in rhesus macaques correlated with delayed acquisition following a repeated low-dose rectal SIV(mac251) challenge. J Virol 86:4644–4657. doi: 10.1128/JVI.06812-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Letvin NL, Rao SS, Montefiori DC, Seaman MS, Sun Y, Lim SY, Yeh WW, Asmal M, Gelman RS, Shen L, Whitney JB, Seoighe C, Lacerda M, Keating S, Norris PJ, Hudgens MG, Gilbert PB, Buzby AP, Mach LV, Zhang J, Balachandran H, Shaw GM, Schmidt SD, Todd JP, Dodson A, Mascola JR, Nabel GJ. 2011. Immune and genetic correlates of vaccine protection against mucosal infection by SIV in monkeys. Sci Transl Med 3:81ra36. doi: 10.1126/scitranslmed.3002351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Binley JM, Arshad H, Fouts TR, Moore JP. 1997. An investigation of the high-avidity antibody response to glycoprotein 120 of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses 13:1007–1015. doi: 10.1089/aid.1997.13.1007. [DOI] [PubMed] [Google Scholar]
- 49.Gorny MK, Xu JY, Gianakakos V, Karwowska S, Williams C, Sheppard HW, Hanson CV, Zolla-Pazner S. 1991. Production of site-selected neutralizing human monoclonal antibodies against the third variable domain of the human immunodeficiency virus type 1 envelope glycoprotein. Proc Natl Acad Sci U S A 88:3238–3242. doi: 10.1073/pnas.88.8.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gorny MK, Xu JY, Karwowska S, Buchbinder A, Zolla-Pazner S. 1993. Repertoire of neutralizing human monoclonal antibodies specific for the V3 domain of HIV-1 gp120. J Immunol 150:635–643. [PubMed] [Google Scholar]
- 51.Robinson WE Jr, Gorny MK, Xu JY, Mitchell WM, Zolla-Pazner S. 1991. Two immunodominant domains of gp41 bind antibodies which enhance human immunodeficiency virus type 1 infection in vitro. J Virol 65:4169–4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Posner MR, Cavacini LA, Emes CL, Power J, Byrn R. 1993. Neutralization of HIV-1 by F105, a human monoclonal antibody to the CD4 binding site of gp120. J Acquir Immune Defic Syndr 6:7–14. [PubMed] [Google Scholar]
- 53.Cavacini LA, Emes CL, Wisnewski AV, Power J, Lewis G, Montefiori D, Posner MR. 1998. Functional and molecular characterization of human monoclonal antibody reactive with the immunodominant region of HIV type 1 glycoprotein 41. AIDS Res Hum Retroviruses 14:1271–1280. doi: 10.1089/aid.1998.14.1271. [DOI] [PubMed] [Google Scholar]
- 54.Bell CH, Pantophlet R, Schiefner A, Cavacini LA, Stanfield RL, Burton DR, Wilson IA. 2008. Structure of antibody F425-B4e8 in complex with a V3 peptide reveals a new binding mode for HIV-1 neutralization. J Mol Biol 375:969–978. doi: 10.1016/j.jmb.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pantophlet R, Aguilar-Sino RO, Wrin T, Cavacini LA, Burton DR. 2007. Analysis of the neutralization breadth of the anti-V3 antibody F425-B4e8 and re-assessment of its epitope fine specificity by scanning mutagenesis. Virology 364:441–453. doi: 10.1016/j.virol.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burton DR, Pyati J, Koduri R, Sharp SJ, Thornton GB, Parren PW, Sawyer LS, Hendry RM, Dunlop N, Nara PL, Lamacchia M, Garratty E, Stiehm ER, Bryson YJ, Cao Y, Moore JP, Ho DD, Barbas CF III. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 57.Wyatt R, Moore J, Accola M, Desjardin E, Robinson J, Sodroski J. 1995. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J Virol 69:5723–5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thali M, Moore JP, Furman C, Charles M, Ho DD, Robinson J, Sodroski J. 1993. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J Virol 67:3978–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang CC, Venturi M, Majeed S, Moore MJ, Phogat S, Zhang MY, Dimitrov DS, Hendrickson WA, Robinson J, Sodroski J, Wyatt R, Choe H, Farzan M, Kwong PD. 2004. Structural basis of tyrosine sulfation and VH-gene usage in antibodies that recognize the HIV type 1 coreceptor-binding site on gp120. Proc Natl Acad Sci U S A 101:2706–2711. doi: 10.1073/pnas.0308527100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diskin R, Scheid JF, Marcovecchio PM, West AP Jr, Klein F, Gao H, Gnanapragasam PN, Abadir A, Seaman MS, Nussenzweig MC, Bjorkman PJ. 2011. Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science 334:1289–1293. doi: 10.1126/science.1213782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Calarese DA, Scanlan CN, Zwick MB, Deechongkit S, Mimura Y, Kunert R, Zhu P, Wormald MR, Stanfield RL, Roux KH, Kelly JW, Rudd PM, Dwek RA, Katinger H, Burton DR, Wilson IA. 2003. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science 300:2065–2071. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 62.Sanders RW, Venturi M, Schiffner L, Kalyanaraman R, Katinger H, Lloyd KO, Kwong PD, Moore JP. 2002. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J Virol 76:7293–7305. doi: 10.1128/JVI.76.14.7293-7305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pantophlet R, Ollmann Saphire E, Poignard P, Parren PW, Wilson IA, Burton DR. 2003. Fine mapping of the interaction of neutralizing and nonneutralizing monoclonal antibodies with the CD4 binding site of human immunodeficiency virus type 1 gp120. J Virol 77:642–658. doi: 10.1128/JVI.77.1.642-658.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ho DD, McKeating JA, Li XL, Moudgil T, Daar ES, Sun NC, Robinson JE. 1991. Conformational epitope on gp120 important in CD4 binding and human immunodeficiency virus type 1 neutralization identified by a human monoclonal antibody. J Virol 65:489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang CC, Tang M, Zhang MY, Majeed S, Montabana E, Stanfield RL, Dimitrov DS, Korber B, Sodroski J, Wilson IA, Wyatt R, Kwong PD. 2005. Structure of a V3-containing HIV-1 gp120 core. Science 310:1025–1028. doi: 10.1126/science.1118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Q, Zeng M, Duan L, Voss JE, Smith AJ, Pambuccian S, Shang L, Wietgrefe S, Southern PJ, Reilly CS, Skinner PJ, Zupancic ML, Carlis JV, Piatak M Jr, Waterman D, Reeves RK, Masek-Hammerman K, Derdeyn CA, Alpert MD, Evans DT, Kohler H, Muller S, Robinson J, Lifson JD, Burton DR, Johnson RP, Haase AT. 2014. Live simian immunodeficiency virus vaccine correlate of protection: local antibody production and concentration on the path of virus entry. J Immunol 193:3113–3125. doi: 10.4049/jimmunol.1400820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Julien JP, Cupo A, Sok D, Stanfield RL, Lyumkis D, Deller MC, Klasse PJ, Burton DR, Sanders RW, Moore JP, Ward AB, Wilson IA. 2013. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science 342:1477–1483. doi: 10.1126/science.1245625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lyumkis D, Julien JP, de Val N, Cupo A, Potter CS, Klasse PJ, Burton DR, Sanders RW, Moore JP, Carragher B, Wilson IA, Ward AB. 2013. Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer. Science 342:1484–1490. doi: 10.1126/science.1245627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Banerjee K, Klasse PJ, Sanders RW, Pereyra F, Michael E, Lu M, Walker BD, Moore JP. 2010. IgG subclass profiles in infected HIV type 1 controllers and chronic progressors and in uninfected recipients of Env vaccines. AIDS Res Hum Retroviruses 26:445–458. doi: 10.1089/aid.2009.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moore JP, Jarrett RF. 1988. Sensitive ELISA for the gp120 and gp160 surface glycoproteins of HIV-1. AIDS Res Hum Retroviruses 4:369–379. doi: 10.1089/aid.1988.4.369. [DOI] [PubMed] [Google Scholar]
- 71.Underwood PA. 1993. Problems and pitfalls with measurement of antibody affinity using solid phase binding in the ELISA. J Immunol Methods 164:119–130. doi: 10.1016/0022-1759(93)90282-C. [DOI] [PubMed] [Google Scholar]
- 72.Dimitrov JD, Lacroix-Desmazes S, Kaveri SV. 2011. Important parameters for evaluation of antibody avidity by immunosorbent assay. Anal Biochem 418:149–151. doi: 10.1016/j.ab.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 73.Gassmann C, Bauer G. 1997. Avidity determination of IgG directed against tick-borne encephalitis virus improves detection of current infections. J Med Virol 51:242–251. doi:. [DOI] [PubMed] [Google Scholar]
- 74.Hedman K, Vaheri A, Brummer-Korvenkontio M. 1991. Rapid diagnosis of hantavirus disease with an IgG-avidity assay. Lancet 338:1353–1356. doi: 10.1016/0140-6736(91)92235-T. [DOI] [PubMed] [Google Scholar]
- 75.Soderlund M, Brown CS, Cohen BJ, Hedman K. 1995. Accurate serodiagnosis of B19 parvovirus infections by measurement of IgG avidity. J Infect Dis 171:710–713. doi: 10.1093/infdis/171.3.710. [DOI] [PubMed] [Google Scholar]
- 76.Ward KN, Gray JJ, Joslin ME, Sheldon MJ. 1993. Avidity of IgG antibodies to human herpesvirus-6 distinguishes primary from recurrent infection in organ transplant recipients and excludes cross-reactivity with other herpesviruses. J Med Virol 39:44–49. doi: 10.1002/jmv.1890390109. [DOI] [PubMed] [Google Scholar]
- 77.Curtis KA, Kennedy MS, Luckay A, Cong ME, Youngpairoj AS, Zheng Q, Smith J, Hanson D, Heneine W, Owen SM, Garcia-Lerma JG. 2011. Delayed maturation of antibody avidity but not seroconversion in rhesus macaques infected with simian HIV during oral pre-exposure prophylaxis. J Acquir Immune Defic Syndr 57:355–362. doi: 10.1097/QAI.0b013e3182234a51. [DOI] [PubMed] [Google Scholar]
- 78.Duong YT, Qiu M, De AK, Jackson K, Dobbs T, Kim AA, Nkengasong JN, Parekh BS. 2012. Detection of recent HIV-1 infection using a new limiting-antigen avidity assay: potential for HIV-1 incidence estimates and avidity maturation studies. PLoS One 7:e33328. doi: 10.1371/journal.pone.0033328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lutz E, Ward KN, Gray JJ. 1994. Maturation of antibody avidity after primary human cytomegalovirus infection is delayed in immunosuppressed solid organ transplant patients. J Med Virol 44:317–322. doi: 10.1002/jmv.1890440402. [DOI] [PubMed] [Google Scholar]
- 80.Monsalvo AC, Batalle JP, Lopez MF, Krause JC, Klemenc J, Hernandez JZ, Maskin B, Bugna J, Rubinstein C, Aguilar L, Dalurzo L, Libster R, Savy V, Baumeister E, Cabral G, Font J, Solari L, Weller KP, Johnson J, Echavarria M, Edwards KM, Chappell JD, Crowe JE Jr, Williams JV, Melendi GA, Polack FP. 2011. Severe pandemic 2009 H1N1 influenza disease due to pathogenic immune complexes. Nat Med 17:195–199. doi: 10.1038/nm.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Polack FP, Hoffman SJ, Crujeiras G, Griffin DE. 2003. A role for nonprotective complement-fixing antibodies with low avidity for measles virus in atypical measles. Nat Med 9:1209–1213. doi: 10.1038/nm918. [DOI] [PubMed] [Google Scholar]
- 82.Ward KN, Dhaliwal W, Ashworth KL, Clutterbuck EJ, Teo CG. 1994. Measurement of antibody avidity for hepatitis C virus distinguishes primary antibody responses from passively acquired antibody. J Med Virol 43:367–372. doi: 10.1002/jmv.1890430409. [DOI] [PubMed] [Google Scholar]
- 83.Wei X, Liu X, Dobbs T, Kuehl D, Nkengasong JN, Hu DJ, Parekh BS. 2010. Development of two avidity-based assays to detect recent HIV type 1 seroconversion using a multisubtype gp41 recombinant protein. AIDS Res Hum Retroviruses 26:61–71. doi: 10.1089/aid.2009.0133. [DOI] [PubMed] [Google Scholar]
- 84.Wreghitt TG, Gray JJ, Aloyisus S, Contreras M, Barbara JA. 1990. Antibody avidity test for recent infection with hepatitis C virus. Lancet 335:789. doi: 10.1016/0140-6736(90)90902-H. [DOI] [PubMed] [Google Scholar]
- 85.Hartley O, Klasse PJ, Sattentau QJ, Moore JP. 2005. V3: HIV's switch-hitter. AIDS Res Hum Retroviruses 21:171–189. doi: 10.1089/aid.2005.21.171. [DOI] [PubMed] [Google Scholar]
- 86.Javaherian K, Langlois AJ, McDanal C, Ross KL, Eckler LI, Jellis CL, Profy AT, Rusche JR, Bolognesi DP, Putney SD, Matthews TJ. 1989. Principal neutralizing domain of the human immunodeficiency virus type 1 envelope protein. Proc Natl Acad Sci U S A 86:6768–6772. doi: 10.1073/pnas.86.17.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.LaRosa GJ, Davide JP, Weinhold K, Waterbury JA, Profy AT, Lewis JA, Langlois AJ, Dreesman GR, Boswell RN, Shadduck P, Holley LH, Karplus M, Bolognesi DP, Matthews TJ, Emini EA, Putney SD. 1990. Conserved sequence and structural elements in the HIV-1 principal neutralizing determinant. Science 249:932–935. doi: 10.1126/science.2392685. [DOI] [PubMed] [Google Scholar]
- 88.Sather DN, Armann J, Ching LK, Mavrantoni A, Sellhorn G, Caldwell Z, Yu X, Wood B, Self S, Kalams S, Stamatatos L. 2009. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J Virol 83:757–769. doi: 10.1128/JVI.02036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kwong PD, Wyatt R, Sattentau QJ, Sodroski J, Hendrickson WA. 2000. Oligomeric modeling and electrostatic analysis of the gp120 envelope glycoprotein of human immunodeficiency virus. J Virol 74:1961–1972. doi: 10.1128/JVI.74.4.1961-1972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moore JP. 1993. The reactivities of HIV-1+ human sera with solid-phase V3 loop peptides can be poor predictors of their reactivities with V3 loops on native gp120 molecules. AIDS Res Hum Retroviruses 9:209–219. doi: 10.1089/aid.1993.9.209. [DOI] [PubMed] [Google Scholar]
- 91.Moore JP, Cao Y, Conley AJ, Wyatt R, Robinson J, Gorny MK, Zolla-Pazner S, Ho DD, Koup RA. 1994. Studies with monoclonal antibodies to the V3 region of HIV-1 gp120 reveal limitations to the utility of solid-phase peptide binding assays. J Acquir Immune Defic Syndr 7:332–339. [PubMed] [Google Scholar]
- 92.Matsushita S, Robert-Guroff M, Rusche J, Koito A, Hattori T, Hoshino H, Javaherian K, Takatsuki K, Putney S. 1988. Characterization of a human immunodeficiency virus neutralizing monoclonal antibody and mapping of the neutralizing epitope. J Virol 62:2107–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Benjamin DC, Berzofsky JA, East IJ, Gurd FR, Hannum C, Leach SJ, Margoliash E, Michael JG, Miller A, Prager EM, Reichlin M, Sercarz EE, Smith-Gill SJ, Todd PE, Wilson AC. 1984. The antigenic structure of proteins: a reappraisal. Annu Rev Immunol 2:67–101. doi: 10.1146/annurev.iy.02.040184.000435. [DOI] [PubMed] [Google Scholar]
- 94.Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, Hurley A, Myung S, Boulad F, Poignard P, Burton DR, Pereyra F, Ho DD, Walker BD, Seaman MS, Bjorkman PJ, Chait BT, Nussenzweig MC. 2011. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Labrijn AF, Poignard P, Raja A, Zwick MB, Delgado K, Franti M, Binley J, Vivona V, Grundner C, Huang CC, Venturi M, Petropoulos CJ, Wrin T, Dimitrov DS, Robinson J, Kwong PD, Wyatt RT, Sodroski J, Burton DR. 2003. Access of antibody molecules to the conserved coreceptor binding site on glycoprotein gp120 is sterically restricted on primary human immunodeficiency virus type 1. J Virol 77:10557–10565. doi: 10.1128/JVI.77.19.10557-10565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ferrari G, Pollara J, Kozink D, Harms T, Drinker M, Freel S, Moody MA, Alam SM, Tomaras GD, Ochsenbauer C, Kappes JC, Shaw GM, Hoxie JA, Robinson JE, Haynes BF. 2011. An HIV-1 gp120 envelope human monoclonal antibody that recognizes a C1 conformational epitope mediates potent antibody-dependent cellular cytotoxicity (ADCC) activity and defines a common ADCC epitope in human HIV-1 serum. J Virol 85:7029–7036. doi: 10.1128/JVI.00171-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Acharya P, Tolbert WD, Gohain N, Wu X, Yu L, Liu T, Huang W, Huang CC, Kwon YD, Louder RK, Luongo TS, McLellan JS, Pancera M, Yang Y, Zhang B, Flinko R, Foulke JS Jr, Sajadi MM, Kamin-Lewis R, Robinson JE, Martin L, Kwong PD, Guan Y, DeVico AL, Lewis GK, Pazgier M. 2014. Structural definition of an antibody-dependent cellular cytotoxicity response implicated in reduced risk for HIV-1 infection. J Virol 88:12895–12906. doi: 10.1128/JVI.02194-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kong L, Lee JH, Doores KJ, Murin CD, Julien JP, McBride R, Liu Y, Marozsan A, Cupo A, Klasse PJ, Hoffenberg S, Caulfield M, King CR, Hua Y, Le KM, Khayat R, Deller MC, Clayton T, Tien H, Feizi T, Sanders RW, Paulson JC, Moore JP, Stanfield RL, Burton DR, Ward AB, Wilson IA. 2013. Supersite of immune vulnerability on the glycosylated face of HIV-1 envelope glycoprotein gp120. Nat Struct Mol Biol 20:796–803. doi: 10.1038/nsmb.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Witvrouw M, Fikkert V, Hantson A, Pannecouque C, O'Keefe BR, McMahon J, Stamatatos L, de Clercq E, Bolmstedt A. 2005. Resistance of human immunodeficiency virus type 1 to the high-mannose binding agents cyanovirin N and concanavalin A. J Virol 79:7777–7784. doi: 10.1128/JVI.79.12.7777-7784.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hester G, Kaku H, Goldstein IJ, Wright CS. 1995. Structure of mannose-specific snowdrop (Galanthus nivalis) lectin is representative of a new plant lectin family. Nat Struct Biol 2:472–479. doi: 10.1038/nsb0695-472. [DOI] [PubMed] [Google Scholar]
- 101.Gupta S, Clark ES, Termini JM, Boucher J, Kanagavelu S, LeBranche CC, Abraham S, Montefiori DC, Khan WN, Stone GW. 2015. DNA vaccine molecular adjuvants SP-D-BAFF and SP-D-APRIL enhance anti-gp120 immune response and increase HIV-1 neutralizing antibody titers. J Virol 89:4158–4169. doi: 10.1128/JVI.02904-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Richmond JF, Lu S, Santoro JC, Weng J, Hu SL, Montefiori DC, Robinson HL. 1998. Studies of the neutralizing activity and avidity of anti-human immunodeficiency virus type 1 Env antibody elicited by DNA priming and protein boosting. J Virol 72:9092–9100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jungwirth P, Cremer PS. 2014. Beyond Hofmeister. Nat Chem 6:261–263. doi: 10.1038/nchem.1899. [DOI] [PubMed] [Google Scholar]
- 104.Schell JB, Bahl K, Folta-Stogniew E, Rose N, Buonocore L, Marx PA, Gambhira R, Rose JK. 2015. Antigenic requirement for Gag in a vaccine that protects against high-dose mucosal challenge with simian immunodeficiency virus. Virology 476:405–412. doi: 10.1016/j.virol.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bonsignori M, Pollara J, Moody MA, Alpert MD, Chen X, Hwang KK, Gilbert PB, Huang Y, Gurley TC, Kozink DM, Marshall DJ, Whitesides JF, Tsao CY, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Kim JH, Michael NL, Tomaras GD, Montefiori DC, Lewis GK, DeVico A, Evans DT, Ferrari G, Liao HX, Haynes BF. 2012. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J Virol 86:11521–11532. doi: 10.1128/JVI.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dugast AS, Chan Y, Hoffner M, Licht A, Nkolola J, Li H, Streeck H, Suscovich TJ, Ghebremichael M, Ackerman ME, Barouch DH, Alter G. 2014. Lack of protection following passive transfer of polyclonal highly functional low-dose non-neutralizing antibodies. PLoS One 9:e97229. doi: 10.1371/journal.pone.0097229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]