ABSTRACT
Epstein-Barr virus (EBV), an oncogenic herpesvirus, has the potential to immortalize primary B cells into lymphoblastoid cell lines (LCLs) in vitro. During immortalization, several EBV products induce cytokines or chemokines, and most of these are required for the proliferation of LCLs. Interleukin-32 (IL-32), a recently discovered proinflammatory cytokine, is upregulated after EBV infection, and this upregulation is detectable in all LCLs tested. EBV latent membrane protein 1 (LMP1) is responsible for inducing IL-32 expression at the mRNA and protein levels. Mechanistically, we showed that this LMP1 induction is provided by the p65 subunit of NF-κB, which binds to and activates the IL-32 promoter. Furthermore, the short hairpin RNA (shRNA)-mediated depletion of endogenous LMP1 and p65 in LCLs suppressed IL-32 expression, further suggesting that LMP1 is the key factor that stimulates IL-32 in LCLs via the NF-κB p65 pathway. Functionally, knockdown of IL-32 in LCLs elicits viral reactivation and affects cytokine expression, but it has no impact on cell proliferation and apoptosis. Of note, we reveal the mechanism whereby IL-32 is involved in the maintenance of EBV viral latency by inactivation of Zta promoter activity. This atypical cytoplasmic IL-32 hijacks the Zta activator protein kinase Cδ (PKCδ) and inhibits its translocation from the cytoplasm to the nucleus, where PKCδ binds to the Zta promoter and activates lytic cycle progression. These novel findings reveal that IL-32 is involved in the maintenance of EBV latency in LCLs. This finding may provide new information to explain how EBV maintains latency, in addition to viral chromatin structure and epigenetic modification.
IMPORTANCE EBV persists in two states, latency and lytic replication, which is a unique characteristic of human infections. So far, little is known about how herpesviruses maintain latency in particular tissues or cell types. EBV is an excellent model to study this question because more than 90% of people are latently infected. EBV can immortalize primary B cells into lymphoblastoid cell lines in vitro. Expression of IL-32, a novel atypical cytoplasmic proinflammatory cytokine, increased after infection. The expression of IL-32 was controlled by LMP1. In investigating the regulatory mechanism, we demonstrated that the p65 subunit of NF-κB is required for this upregulation. Of note, the important biological activity of IL-32 was to trap protein kinase Cδ in the cytoplasm and prevent it from binding to the Zta promoter, which is the key event for EBV reaction. So, the expression of LMP1-induced IL-32 plays a role in the maintenance of EBV latency.
INTRODUCTION
Epstein-Barr virus (EBV) is a ubiquitous human gammaherpesvirus: more than 90% of the human population are latently infected (1). Of note, EBV has been reported to be associated with Burkitt's lymphoma (BL), Hodgkin's lymphoma (HL), natural killer (NK)/T-cell lymphoma, AIDS-associated lymphoma, and posttransplantation lymphoproliferative disorder (PTLD) (2). The strongest evidence for EBV oncogenicity is that it can immortalize primary B cells into lymphoblastoid cell lines (LCLs) in vitro (3). The immortalized LCLs display unlimited proliferation, aberrant cytokine and chemokine production, and clumping morphology (1). These unusual characteristics may provide a suitable microenvironment for EBV immortalization (2).
In vivo, following primary infection, B cells encounter antigen migrate and become fully activated via interaction with CD40-CD40 ligand-expressing T helper cells, resulting in germinal center (GC) formation in peripheral lymphoid tissues (4). GCs are formed by aggregated proliferating B cells (also known as centroblasts) and nondividing B cells (also known as centrocytes). Finally, high-affinity antibody (Ab)-producing GC B cells are further selected to differentiate into either memory or plasma B cells (4). B-cell malignancies are believed to originate at the GC or post-GC differentiation stages (5). The EBV life cycle consists of latency and lytic replication. EBV infects B cells preferentially because B-cell transcriptional factors are involved in EBV gene expression and the switch from the latent to the lytic cycle. In the clinical setting, four types of EBV latency may be demonstrated in humans. In latency 0, EBV-encoded small RNAs (EBERs) and latent membrane protein 2A (LMP2A) are expressed on memory B cells. In latency I, EBERs and EBNA1 have been found in BL (originating from centroblasts). In latency II, EBERs, EBNA1, LMP1, and LMP2A are detected in HL (originating from centrocytes). In latency III, as found in tonsils and peripheral B cells isolated from PTLD, all latency products are expressed, including six EBNAs, three latent membrane proteins, EBERs, and BamHI A rightward transcripts (BARTs).
In vitro, EBV can immortalize peripheral B cells to establish LCLs with latency III. Morphologically, EBV-infected B cells begin to aggregate and clump together, which are traits of activated B cells in GCs (6). Early in infection, the B-cell transcription factor PAX5 binds and regulates the viral latency promoter Wp (7). After that, the first viral product, EBNA2, is expressed, and this interacts with RBPjκ and PU.1 to regulate the expression of many host and viral genes, such as those for LMP1 and LMP2A (8, 9). Meanwhile, the expression of lytic transactivator Zta can be repressed by host early B-cell factor 1 (EBF1), signal transducer and activator of transcription 3 (STAT3), and octamer-binding protein 2 (OCT2) (10–12). On the other hand, B-cell transcription factor XBP1 directly activates the BZLF1 promoter and then initiates lytic cycle progression (13, 14). In this study, we focused on the EBV-altered cytokines in establishment, maintenance, and regulation of the latency III program. EBV encodes several proteins which mimic or associate with host cellular factors, and these are particularly involved in modulating cell functions, such as proliferation, survival, differentiation, and antiapoptosis. The major factors are three viral proteins, LMP1, LMP2A, and Zta. LMP1 acts as a constitutively activated CD40 and provides signals for proliferation, survival, and differentiation in B cells. LMP1 has three C-terminal activating regions (CTARs)—CTAR1, -2, and -3—which associate with tumor necrosis factor receptor (TNFR)-associated factors, the TNFR-associated death domain protein, and Janus-activated kinase 3 and then constitutively activate the extracellular signal-regulated kinase (ERK), Jun N-terminal protein kinase (JNK), p38, and NF-κB pathways (15, 16). In LMP1 transgenic mice, constitutively expressed LMP1 drives B-cell lymphoma formation (17), suggesting that LMP1 acts as an oncogene and plays an important role in EBV-mediated lymphomagenesis. LMP2A is also a latent surface protein and mimics the functional B-cell receptor. It interacts with Syk and Lyn tyrosine kinases to deliver constitutively activated signals through the phosphoinositol 3-kinase (PI3K)/AKT and ERK pathways (15). Zta is a viral transactivator controlling the initiation of the lytic cycle switch and mimics a structural AP-1 protein (18). The production of the B-cell growth cytokine interleukin-13 (IL-13) is upregulated by Zta (19). In contrast to B-cell lymphoma, EBV immortalizes B cells into LCLs without any chromosome translocation or genetic mutation (3). According to a study of EBV immortalization by Sugimoto et al., the host chromosomes are maintained in a normal condition in the early stage of immortalization (3). So, we speculate that EBV may employ different strategies to create a favorable status for the unlimited proliferation of B cells harboring the latent virus. Previous studies demonstrated that native chromatin structure and epigenetic modification maintain viral latency, but the detailed mechanisms need further investigation (20, 21). Investigation of the cellular factors that are involved in viral latency will provide an alternative approach to this question. In this study, we systematically screened for the unusual expression of cytokines after EBV infection. We found that IL-32 is undetectable until day 7 postinfection and then is expressed strongly, which, to our knowledge, is distinct from other EBV-induced cytokines and chemokines.
IL-32, originally named natural killer cell transcript 4, is a newly defined proinflammatory cytokine (22). Several studies indicate that IL-32 is not a typical cytokine, and it is distributed predominantly in the cytoplasm (23). So far, it is not clear whether IL-32 has a receptor (23). Structurally, IL-32 comprises six splice variant isoforms, IL-32α, IL-32β, IL-32γ, IL-32δ, IL-32ε, and IL-32ζ (24). IL-32β is the most abundant form in activated T cells (25). Its function was not defined until Kim et al. found that it can act as a proinflammatory cytokine to trigger the production of tumor necrosis factor alpha (TNF-α), IL-1, and IL-6 (24). Knockdown of IL-32 decreased TNF-α, ΙL-1β, IL-6, and IL-8 expression in monocytic THP-1 cells and human umbilical cord vein endothelial cells (HUVECs) (26, 27). Impressively, upregulation of IL-6 can be enhanced by the interaction between IL-32α, protein kinase Cε (PKCε), and STAT3 protein in monocytic THP-1 cells (28). In neutrophils, IL-32 can interact with proteinase 3, which may result in the enhancement of IL-32 production to induce IL-8 (29). All these lines of evidence demonstrate a close association between IL-32 and cytokine expression. In clinical studies, IL-32 has been reported to be highly expressed in several autoimmune diseases, including rheumatoid arthritis, inflammatory bowel disease, and chronic obstructive pulmonary disease (22, 30, 31). Moreover, IL-32 expression is upregulated in several cancers, including gastric cancer, lung cancer, and pancreatic cancer (32–34).
Recently, the functions of this intracellular cytokine have been studied in other cell types (35). The distinctive α-helix and Arg-Gly-Asp structures of IL-32 have the capacity to interact with integrins or paxillin, suggesting that IL-32 may be a member of the family of focal adhesion proteins. Of note, this interaction may influence cell survival (35). Based on the impact of IL-32-mediated cytokine production and its association with inflammation and carcinogenesis, we sought to explore the potential role of IL-32 in EBV-infected B cells. We discovered that IL-32 expression is significantly increased after EBV infection. The first defined EBV oncogenic protein, LMP1, is responsible for IL-32 expression through the NF-κB pathway. This LMP1-mediated expression of IL-32 may be critical for the switch between the latent and lytic state. The regulatory and unusual biological functions of IL-32 should be investigated further.
MATERIALS AND METHODS
B-cell purification and EBV infection.
Human whole blood was obtained from anonymous donors (from Taipei Blood Service Foundation, Taipei, Taiwan), and its use has been approved by the Institutional Review Boards of National Taiwan University Hospital, Taipei, Taiwan. For EBV infection, purified B cells were infected with EBV as previously described (19).
Cell culture.
The EBV-negative BL cell lines Akata and Ramos and EBV-immortalized LCLs were maintained in RPMI medium. The human embryonic kidney cell line HEK 293T and human cervical adenocarcinoma cell line HeLa were maintained in Dulbecco modified Eagle medium (DMEM). All media were supplemented with 10% fetal bovine serum, 1 mM glutamine, 100 U/ml of penicillin, and 100 μg/ml of streptomycin at 37°C with 5% CO2.
Plasmids.
Plasmids expressing EBNA1, LMP1, LMP2A, Rta, Zta, lentivirus-based LMP1, and shLMP1 were described previously (19, 36, 37). shIL-32 and shp65 were purchased from National RNAi Core Facility Platform, Taipei, Taiwan. The targeted sequence of shIL-32-3 is 5′-GCTCTTCATGTCCTCTTTCCA-3′, that of shIL-32-5 is 5′-GCAGGCCCTCTGGAAACAGTT-3′, that of shp65-1 is 5′-CCCTGAGCACCATCAACTATG-3′, and that of shp65-4 is 5′-CACCATCAACTATGATGAGTT-3′. The series of luciferase reporter plasmids driven by the IL-32 promoter (nucleotides −548 to +495, −117 to +495, and +11 to +495) were inserted into the pGL2-basic vector (Promega) through the 5′ KpnI and 3′ NheI sites. To construct the IL-32 promoter (nucleotides −548 to +495) mutant reporter plasmid, the putative NF-κB site (GGGAGTTTCC) within the IL-32 promoter region (nucleotide −8 to +2) was mutated to GGGAGTTAGA by sited-directed mutagenesis. The lentivirus-based p65-expressing plasmid, pSIN-p65, was constructed by insertion of a full-length p65 fragment into the pSIN vector at the 5′ MluI site and 3′ NotI site. The IL-32β plasmids were constructed by insertion of a full-length IL-32 fragment into the pCDH vector at the 5′ XbaI site and 3′ EcoRI site. The IL-32β-Flag plasmids were constructed by insertion of a full-length IL-32β fragment into pCMV-tag-2B vector at the 5′ EcoRI site and 3′ XhoI site. The plasmids of pEGFP-N1-derived wild-type PKC (WT-PKCδ), the kinase-inactive form of PKCδ (DN-PKCδ), Zta promoter (Zp), and ZID deletion of Zp (ΔZID-Zp) were described in our previous study (38).
RNA extraction and RT-qPCR.
Extraction of total RNA, synthesis of cDNA, and reverse transcription-quantitative PCR (RT-qPCR) were described previously (36). IL-32 mRNAs were detected using the Roche universal probe (number 68), forward primer 5′-TCAAAGAGGGCTACCTGGAG-3′, and reverse primer 5′-TTTCAAGTAGAGGAGTGAGCTCTG-3′. IL-6 mRNAs were detected using the Roche universal probe (number 40), forward primer 5′-GATGAGTACAAAAGTCCTGATCCA-3′, and reverse primer 5′-CTGCAGCCACTGGTTCTGT-3′. Zta mRNAs were detected using the TaqMan primer/probe sets (Pre-Developed Assay Reagents; Applied Biosystems) as described previously (19). RT-PCR was performed using IL-32 forward primer 5′-ATGTGCTTCCCGAAGGTCCTC-3′ and reverse primer 5′-TCATTTTGAGGATTGGGGTTCAG-3′, β-actin forward primer 5′-TTCTACAATGAGCTGCGTGT-3′ and reverse primer 5′-GCCAGACAGCACTGTGTTGG-3′, IL-8 forward primer 5′-GTGCAGTTTTGCCAAGGAGT-3′ and reverse primer 5′-TTGTATTGCATCTGGCAACC-3′, and IL-10 forward primer 5′-TTACCTGGAGGAGGTGATGC-3′ and reverse primer 5′-TGGGGGTTGAGGTATCAGAG-3′.
Fractionation of cytosolic and nuclear proteins.
Cells were resuspended in buffer A (20 mM HEPES, 10 mM KCl, 0.1 mM EDTA, 0.1 mM, 0.1 mM EGTA, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride [PMSF], 1 mM Na3VO4, 1% NP-40), and the crude nuclei were spun down at low speed. The supernatant was the cytosolic fraction. Then the nuclei were lysed with hypotonic NE buffer (20 mM Tris-HCl, 400 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 25% glycerol, 0.5 mM PMSF, 1 mM Na3VO4). Collected cytosolic and nuclear proteins were analyzed by Western blotting.
Electroporation and lentivirus infection.
Cells were electroporated using a Neon kit (Invitrogen) according to the manufacturer's instructions (36). For lentivirus infection of B cells, 2 × 105 to 5 × 105 cells were infected with lentiviruses at multiplicities of infection (MOIs) of 0.0625 to 4.
Western blotting and antibodies.
Western blotting was performed as described previously (19). Antibodies against the following were used in this study: IL-32 (KU32-52; Biolegend), β-actin (AC-15; Sigma-Aldrich), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (6C5; Biodesign), LMP1 (CS1-4; Dako), p65 (sc-372; Santa Cruz), EBNA1 (NPC47), PARP (sc-8007; Santa Cruz), α-tubulin (DM1A; Calbiochem), hemagglutinin (HA) (16B12; Covance), phosphorylated IκB-α (9246; Cell Signaling), total IκB-α (sc-203; Santa Cruz), phosphorylated JNK (9251; Cell Signaling), total JNK (06-748; Upstate), phosphorylated Erk (9106; Cell Signaling), total Erk (sc-94; Santa Cruz), BGLF4 (39), LMP2A (40), Rta (41), Zta (4F10), Flag (3165; Sigma-Aldrich), PKCδ (sc-937; Santa Cruz), caspase-3 (9662, Cell Signaling), and green fluorescent protein (GFP) (sc-9996 [Santa Cruz] and 632592 [Clontech]).
[3H]thymidine incorporation assay for cell proliferation.
LCLs were plated at 1 × 104 cells/well for 54 h, 1 μCi [3H]thymidine was added, and the cells were incubated for another 18 h and then measured using a beta counter (LS6000 IC; Beckman).
Reporter assay.
HEK 293T cells were seeded at a density of 1 × 105 cells per 12-well plate and transfected with 0.4 μg of IL-32 promoter luciferase reporter plasmids combined with 0.4 μg of pSG5-LMP1 and 0.2 μg of pEGFP-C1 as a transfection control, using T-Pro Non-liposome Transfection Reagent II (NTRII; T-Pro Biotechnology) for a 48-h incubation. For assays of the activities of Zp and ΔZID-Zp, the cells were transfected with 0.5 μg of Zp or ΔZID-Zp combined with 0.25 μg of pSG5-Zta, 0.25 μg of IL-32β-Flag expression plasmids, and 0.05 μg of pEGFP-C1 as a transfection control, using NTRII for a 48-h incubation. For PKCδ-induced activation of Zp, the cells were transfected with 0.5 μg of Zp combined with 0.4 μg of pCDH-IL-32 and 0.25 μg of WT-PKCδ or DN-PKCδ, using NTRII for a 72-h incubation. The luciferase activity was detected using a Bright-Glo luciferase assay kit (Promega).
ChIP assay.
The binding of p65 complex and DNA was performed in a chromatin immunoprecipitation (ChIP) assay as described previously (36). Binding sites of p65 within IL-32 promoter were detected by forward primer 5′-CACACCTCATGCAAGGACAG-3′ and reverse primer 5′-AGTCTCTGAGCCCAGGAATG-3′.
Coimmunoprecipitation (co-IP) assay.
293T cells were transfected with IL-32β-Flag and WT-PKCδ or DN-PKCδ plasmids for 3 days. Lysates of 293T cells and LCLs were harvested using cell lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM sodium orthovanadate, 1 mM PMSF). For immunoprecipitation, precleared cell lysates were incubated with GFP antibody, Flag antibody, IL-32 antibody, or PKCδ antibody overnight at 4°C on a rotating rocker, and protein A-Sepharose beads were added to pull down the immunocomplexes. After intensive washing with phosphate-buffered saline (PBS), the immunoprecipitated complexes were added to 25 μl of SDS sample buffer at 95°C for 5 min and displayed in 10% SDS-PAGE for immunoblotting.
Confocal fluorescence microscopy.
Cells were fixed with 4% paraformaldehyde and then permeabilized with 0.5% Triton X-100. The fixed cells were blocked with 1% bovine serum albumin (BSA) and incubated with primary antibodies against IL-32. Rhodamine- or fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG (Chemicon) was added, and the cell nuclei were stained with Hoechst 33258. The fluorescent signals were observed by confocal microscopy (TCS SP2 laser scanning; Leica).
Analysis of Zta-positive LCL cells by flow cytometry.
shLuciferase (shLuc)- and shIL-32-5-expressing LCLs were harvested at day 5 postinfection. Intracellular staining of Zta expression was assayed using the protocol of the Foxp3/transcription factor staining buffer set (eBioscience) according to the manufacturer's instructions. Fixed cells were stained with mouse immunoglobulin (mIg) or anti-Zta Ab (clone 4F10) for 30 min at room temperature. The cells were washed and incubated with FITC-labeled anti-mouse IgG for the other 30 min, and then cells were washed again and resuspended in FACS buffer (PBS with 2% fetal calf serum [FCS]) and analyzed by LSRII flow cytometry (BD Biosciences).
RESULTS
Expression of IL-32 was detected during EBV-induced immortalization.
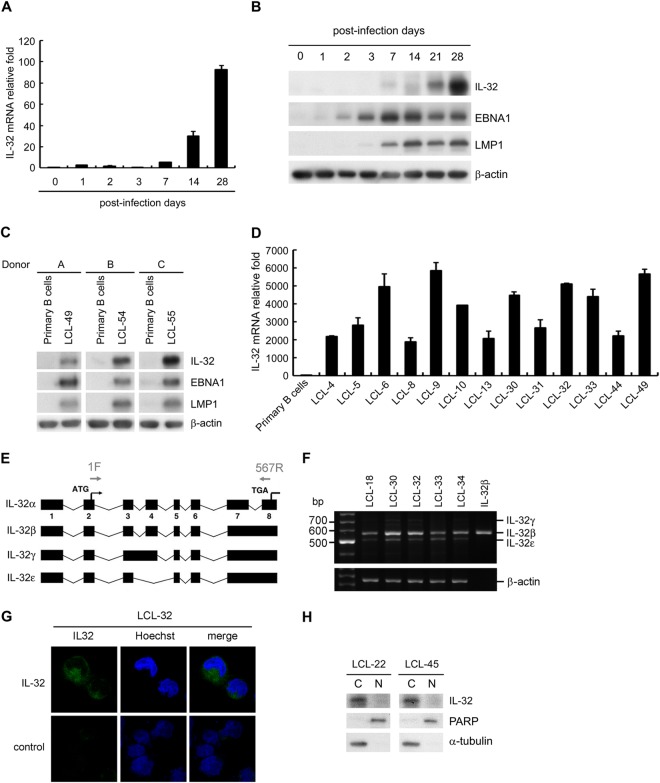
Previous studies have shown that IL-32 is expressed predominantly in NK cells, T cells, monocytes, and endothelial cells (27, 42, 43). EBV immortalizes primary B cells into LCLs which proliferate indefinitely by manipulating the expression of cellular factors (2). To explore whether EBV infection could induce IL-32 expression, human primary B cells were infected with B95.8 strain EBV. As shown in Fig. 1A and B, the IL-32 transcripts and proteins increased at day 7 after EBV infection. Expression of EBNA1 and LMP1 by Western blotting indicated that the infection was successful (Fig. 1B). Simultaneously, lysates of primary B cells and LCLs were prepared from three paired donors; the expression of IL-32 was significantly increased in all tested LCLs but not in uninfected primary B cells (Fig. 1C). To confirm the upregulation of IL-32 in EBV-immortalized LCLs, IL-32 transcripts were detected in all tested LCLs. Compared with those in uninfected primary B cells, IL-32 transcripts were significantly upregulated, from 1,860-fold to 5,864-fold, in LCLs (Fig. 1D).
FIG 1.
IL-32 is induced by EBV infection. (A) Expression of IL-32 transcripts was detected by RT-qPCR in human primary B cells on the indicated days after infection by EBV. The relative fold of IL-32 transcripts was normalized to the amounts of IL-32 transcripts from uninfected primary B cells. (B) Expression of IL-32, EBNA1, LMP1, and β-actin was analyzed by Western blotting in human primary B cells on the indicated days after infection with EBV. β-Actin served as the internal control. (C) The expression of IL-32, EBNA1, LMP1, and β-actin was analyzed in three paired sets of human primary B cells and LCLs. (D) The expression of IL-32 transcripts in various EBV-immortalized LCLs was detected by RT-qPCR, and the relative fold of IL-32 transcripts was normalized to the amounts of IL-32 transcripts from uninfected primary B cells. (E) Schematic illustration of the various IL-32 splicing isoforms. The arrows indicate the specific IL-32 primers (1F and 567R) which are designed in exon 2 and exon 8 and detect IL-32α, -β, -γ, and -ε. (F) Expression of IL-32 isoforms was detected in 5 LCLs by RT-PCR. IL-32β is a positive control of IL-32β mRNA. (G) LCLs were fixed and then stained with IL-32 antibody and the nuclear dye Hoechst. Green fluorescence indicates IL-32 expression, and blue fluorescence indicates cell nuclei. (H) Expression of IL-32 was detected in LCLs by Western blotting. PARP was the marker of the nuclear fraction (N), and α-tubulin was the marker of the cytosolic fraction (C).
Expression pattern and cellular distribution of IL-32 in LCLs.
Previous studies demonstrated that IL-32 comprises several isoforms, including IL-32α, IL-32β, IL-32γ, and IL-32ε, so we designed RT-PCR primers located at +1 of exon 2 and +567 of exon 8 (Fig. 1E). These primers can detect four different PCR products from IL-32α (396 bp), IL-32β (567 bp), IL-32γ (705 bp), and IL-32ε (507 bp). Using RT-PCR, we amplified 567-bp and 507-bp products from LCLs, suggesting that IL-32β is the most abundantly expressed isoform in LCLs (Fig. 1F). Because IL-32 is rarely secreted into the extracellular environment, the location of IL-32 was detected in whole cells by immunofluorescence assays and Western blotting (23). Results from the immunofluorescence assay showed that IL-32 was expressed in the cytoplasm of LCLs (Fig. 1G). Furthermore, a cellular compartment fractionation assay verified that IL-32 is mainly distributed in the cytosolic fraction and not in the nuclear portion (Fig. 1H). These results suggest that infection with EBV significantly induces IL-32β expression during the immortalization process and that this is constitutively maintained in the cytoplasm of LCLs.
The EB viral protein LMP1 is responsible for IL-32 upregulation.
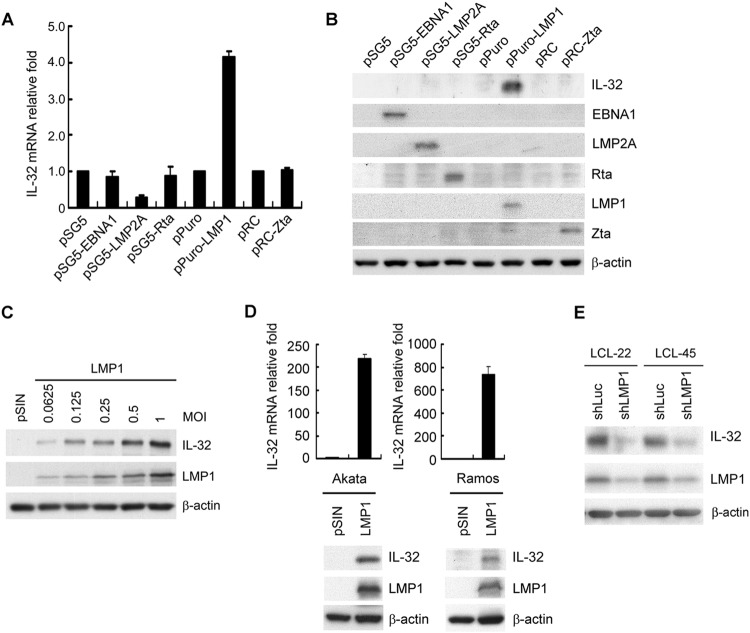
The EBV latent proteins EBNA1 and LMP1 are essential for EBV-mediated immortalization of B cells (1). LMP2A, which has a structure resembling that of the B-cell receptor, is an important regulator for B-cell survival (44). The lytic proteins Zta and Rta are critical transactivators that regulate the expression of many cellular and viral genes (1). To determine which EB viral gene product contributes predominantly to IL-32 expression, EBV-negative Akata cells were transfected with these important EB viral genes. The results in Fig. 2A and B show that LMP1 induces IL-32 expression in Akata cells at the transcriptional and translational levels. Furthermore, LMP1 stimulated IL-32 expression in a dose-dependent manner (Fig. 2C). LMP1-mediated IL-32 induction was observed in the BL cell lines Akata and Ramos (Fig. 2D). To confirm that LMP1 upregulates IL-32 expression in EBV-immortalized LCLs, LMP1 was depleted in LCLs using a short hairpin RNA (shRNA) approach. The results indicated that knockdown of LMP1 in LCLs caused a significant decrease of IL-32 expression, suggesting that LMP1 is indeed responsible for IL-32 expression in LCLs (Fig. 2E). All these lines of evidence indicate that LMP1 stimulates IL-32 expression in EBV-immortalized LCLs.
FIG 2.
EBV LMP1 induces IL-32 expression. (A and B) EBV-negative Akata cells were electroporated with the plasmids indicated. RNA and proteins were harvested from each transfectant at 72 h posttransfection. (A) IL-32 transcripts were measured by RT-qPCR, and the relative fold was normalized to the amounts of IL-32 transcripts from vector control cells. (B) Expression of IL-32, EBNA1, LMP2A, Rta, LMP1, Zta, and β-actin was detected by Western blotting. β-Actin served as the internal control. (C) Akata cells were infected with the pSIN vector- or LMP1-expressing lentiviruses at the indicated MOI for 5 days. Expression of IL-32, LMP1, and β-actin was detected by Western blotting. β-Actin served as the internal control. (D) Akata and Ramos cells were infected with the pSIN vector- or LMP1-expressing lentiviruses at an MOI of 4 for 5 days. IL-32 transcripts were measured by RT-qPCR, and the relative fold was normalized to the amounts of IL-32 transcripts from vector control cells (upper portion). Expression of IL-32, LMP1, and β-actin was detected by Western blotting. β-Actin served as the internal control (lower portion). (E) LCLs were infected with shLuciferase (shLuc) or shLMP1 lentiviruses at an MOI of 2 for 5 days. Expression of IL-32, LMP1, and β-actin was measured by Western blotting. β-Actin served as the internal control.
Dissecting the signaling pathway for IL-32 induction by LMP1.
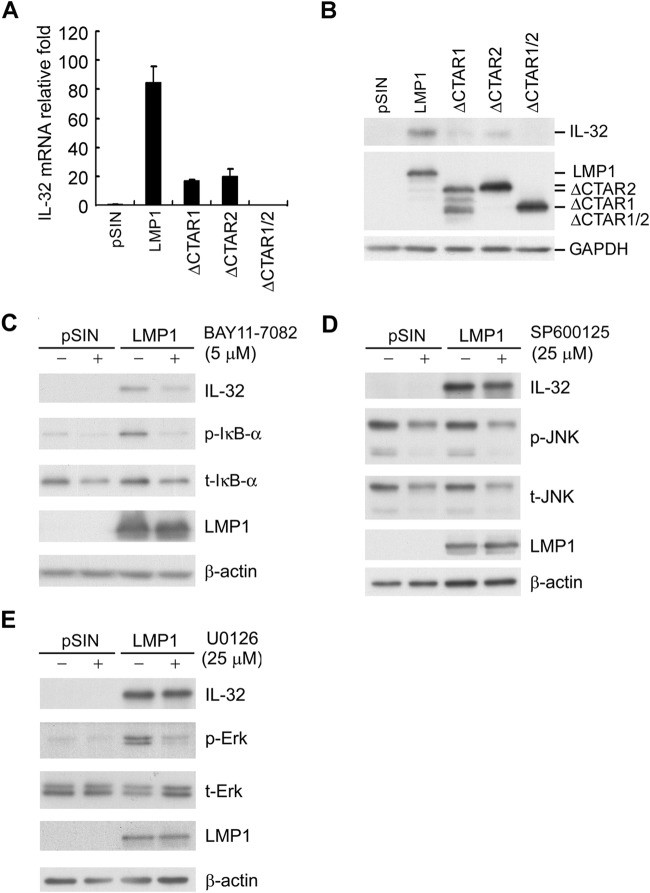
Structurally, LMP1 mimics a constitutively active CD40 ligand (45). LMP1 comprises a short amino terminus, six transmembrane domains, and a long carboxyl terminus with two major carboxyl-terminal activation region 1 (CTAR1) and CTAR2 domains, which mediate activation of JNK, extracellular signal-related kinase (ERK), and NF-κB pathways (45). To dissect the functional domain of LMP1 required for IL-32 expression, Akata cells were transduced with lentiviruses expressing pSIN, LMP1, ΔCTAR1, ΔCTAR2, or ΔCTAR1/2. As shown in Fig. 3A and B, deletion of CTAR1, CTAR2, or CTAR1/2 significantly reduced LMP1-mediated IL-32 expression. These results suggested that LMP1 induced IL-32 expression via its CTAR1 and CTAR2 domains. To confirm the regulatory mechanism of LMP1-induced IL-32 expression, LMP1-expressing Akata cells were treated with NF-κB inhibitor (BAY11-7082), JNK inhibitor (SP600125), or MEK inhibitor (U0126). As shown in Fig. 3C to E, LMP1-mediated IL-32 expression was significantly decreased in NF-κB inhibitor-treated cells but not in JNK inhibitor- or MEK inhibitor-treated cells, suggesting that the NF-κB pathway is critical for LMP1-mediated upregulation of IL-32.
FIG 3.
LMP1-induced IL-32 expression is mediated by its CTAR1 and CTAR2 domains. (A and B) Akata cells were infected with pSIN vector-, LMP1-, ΔCTAR1-, ΔCTAR2-, or ΔCTAR1/2-expressing lentiviruses at an MOI of 2 for 5 days. (A) The IL-32 transcripts were detected by RT-qPCR, and the relative fold expression of IL-32 was normalized to the amounts of IL-32 transcripts in pSIN vector control cells. (B) Expression of IL-32, full-length LMP1, LMP1 with CTAR domain deletions, and GAPDH was measured by Western blotting. GAPDH served as an internal control. (C to E) Akata cells were infected with pSIN vector- or LMP1-expressing lentiviruses at MOI of 1 for 5 days. Infected cells were treated with BAY11-7082 (C), SP600125 (D), or U0126 (E) for 24 h. The protein expression of IL-32, LMP1, phosphorylated IκB-α (p-IκB-α), total IκBα (t-IκBα), phosphorylated JNK (p-JNK), total JNK (t-JNK), phosphorylated Erk (p-Erk), total Erk (t-Erk), and β-actin was measured by Western blotting. β-Actin served as the internal control.
NF-κB p65 binding to the IL-32 promoter is essential for LMP1-mediated IL-32 expression.
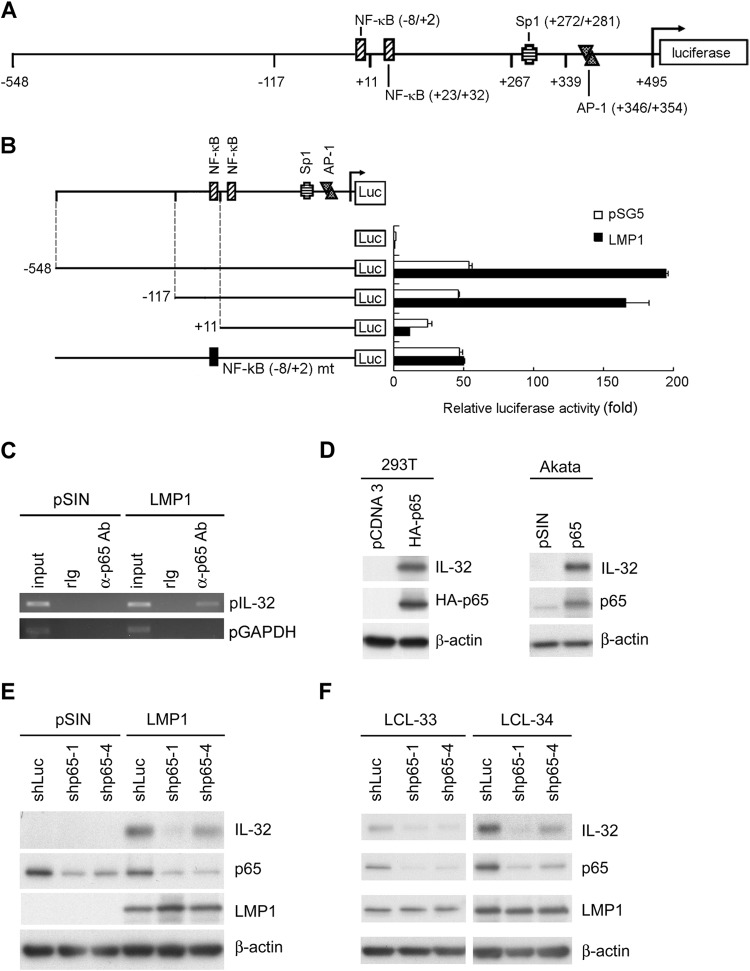
According to PROMO transcription factor prediction (http://alggen.lsi.upc.es/), there are several transcription factor binding sites on the IL-32 promoter (Fig. 4A). Among them, several LMP1-regulated transcription factors are included: two NF-κB, one AP-1, and one Sp1 binding site. To identify which transcription factor is involved in IL-32 expression, serial deletions and particular mutations of IL-32 promoter constructs were analyzed by reporter assay. LMP1 activated the IL-32 promoter approximately 4-fold compared to the vector control. Deletion of the region from −548 to −117 did not affect the reporter activity, while deletion of the region from −548 to +11 significantly abolished the reporter activity. Particular mutation of the NF-κB (−8/+2) binding sites also showed similar results (Fig. 4B). A ChIP assay was performed to determine whether NF-κB binds directly to the IL-32 promoter. As shown in Fig. 4C, NF-κB p65 bound to the IL-32 promoter only when cells were transduced with LMP1. Moreover, overexpression of NF-κB p65 induced IL-32 expression in Akata and 293T cells (Fig. 4D). Of note, knockdown of NF-κB p65 prohibited LMP1-stimulated IL-32 expression (Fig. 4E). Furthermore, in EBV-immortalized LCLs, depletion of NF-κB p65 using an shRNA approach decreased IL-32 expression (Fig. 4F). All these results indicate that LMP1 stimulates IL-32 expression mainly via NF-κB p65.
FIG 4.
LMP1 regulates IL-32 expression through p65 activation. (A) Schematic illustration of the reporter plasmids of the IL-32 promoter. Predicted binding sites of transcription factors on the promoter region are indicated. (B) 293T cells were transfected with LMP1-expressing plasmids, 5′ deletion IL-32 reporter plasmids, and pEGFP-C1 as a transfection control. After 72 h, the relative luciferase activity of each transfectant was normalized to its GFP intensity and standardized to the vector control cells. (C) Akata cells were infected with pSIN- and LMP1-expressing lentiviruses at an MOI of 1 for 5 days. Complexes of DNA and p65 were immunoprecipitated from the cells using anti-p65 antibody or rabbit IgG. DNA of IL-32 promoters (pIL-32) and GAPDH promoters (pGAPDH) were detected in the immunoprecipitates by PCR. Total DNA was harvested from vector- or LMP1-expressing cells and used as the input control. (D) 293T cells were transfected with p65-expressing plasmids for 3 days, and Akata cells were infected with p65 lentiviruses at an MOI of 1 for 5 days. Expression of IL-32, p65, and β-actin was measured by Western blotting. β-Actin served as the internal control. (E) Akata cells were coinfected with pSIN- or LMP1-expressing lentiviruses at an MOI of 1 and shLuciferase, shp65-1, or shp65-4 lentiviruses at an MOI of 4 for 5 days. Expression of IL-32, p65, LMP1, and β-actin was measured by Western blotting. β-Actin served as the internal control. (F) LCLs were infected with shLuciferase, shp65-1, or shp65-4 lentiviruses at an MOI of 4 for 5 days, and infected cells were further selected with 2 μg/ml of puromycin for another 2 days. Expression of IL-32, p65, LMP1, and β-actin was measured by Western blotting. β-Actin served as the internal control.
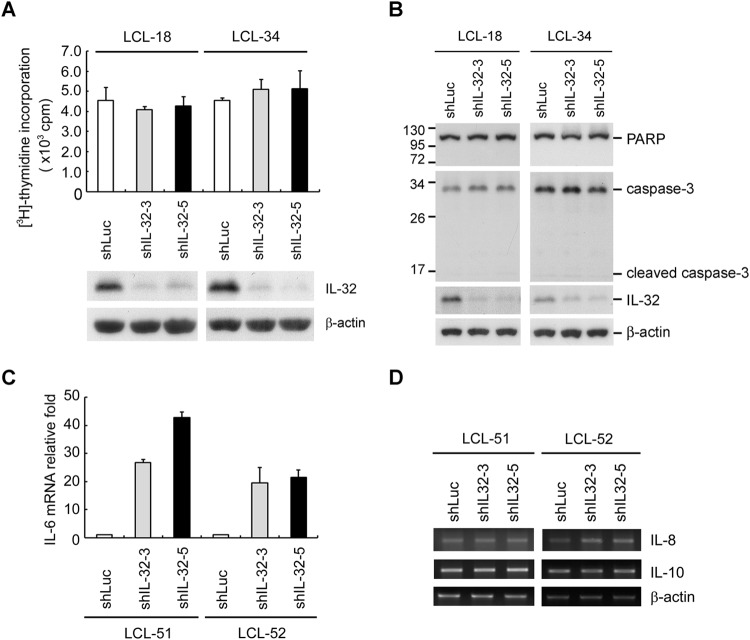
Knockdown of IL-32 increases IL-6 expression.
To investigate further the biological function of IL-32 in LCLs, the cell proliferation status was evaluated using a [3H]thymidine incorporation assay. As shown in Fig. 5A, knockdown of IL-32 did not affect cell proliferation. Data from Fig. 5B show that knockdown of IL-32 did not induce PARP or caspase-3 cleavage, suggesting that depletion of IL-32 does not affect apoptosis. Previous studies demonstrated that IL-32 enhances IL-6, IL-8, and IL-10 (29, 46, 47). In addition, these cytokines have been induced by LMP1 (48–50). To explore the effects of IL-32-induced cytokine expression, we depleted IL-32 expression in LCLs. As shown in Fig. 5C and D, knockdown of IL-32 expression increased IL-6 but not IL-8 or IL-10, suggesting that the effect of IL-32 on cytokine expression in LCLs differs from those in other cell types.
FIG 5.
Knockdown of IL-32 increases IL-6 expression. (A to D) LCLs were infected with shLuciferase, shIL-32-3, or shIL-32-5 lentiviruses for 5 days, and infected cells were further selected with 2 μg/ml of puromycin for another 2 days. (A) Cells were reseeded at a concentration of 1 × 104 cells per well in 96-well plates. After 54 h of incubation, 1 μCi of [3H]thymidine was added and incubation was continued for another 18 h. The amount of incorporated [3H]thymidine was measured using a beta counter (upper portion). The protein expression of IL-32 and β-actin was measured by Western blotting. β-Actin served as the internal control (lower portion). (B) Expression of PARP, caspase-3, IL-32, and β-actin was measured by Western blotting. β-Actin served as the internal control. (C) IL-6 transcripts were detected by RT-qPCR, and the relative fold expression of IL-6 was normalized to the amounts of IL-32 transcripts in shLuciferase lentivirus-infected cells. (D) Transcripts of IL-8 and IL-10 were detected by RT-PCR. β-Actin served as the internal control.
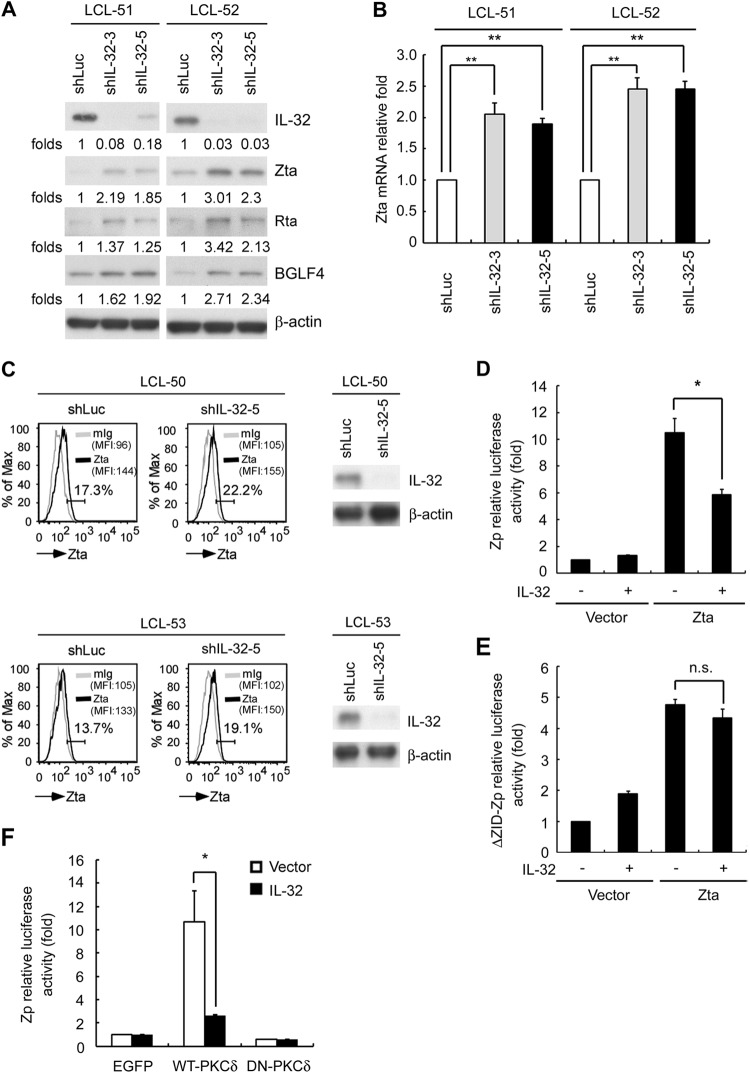
IL-32 inhibited Zp activation through PKCδ to maintain viral latency.
Of interest, knockdown of IL-32 triggered viral lytic cycle progression in LCLs via expression of lytic proteins, including Zta, Rta, and BGLF4 (Fig. 6A). Furthermore, the Zta transcripts and the percentage of Zta-positive LCLs were increased in cells in which IL-32 had been knocked down (Fig. 6B and C). We explored the molecular mechanism by which IL-32 regulated lytic cycle progression. EBV lytic cycle progression is triggered by the expression of the immediate early genes Zta and Rta to activate the Zta promoter (Zp) and Rta promoter (Rp) (1). As shown in Fig. 6D, overexpression of IL-32 inhibited Zta transactivation of Zp activity by 50%. Several domains are present in Zp and are crucial for Zp activities. ZID is one of these domains. Previous studies have shown that Sp1, Sp3, and MEF2D can potentially bind to the ZID element (51–53). Furthermore, our previous report showed that the ZID region of Zp is essential for PKCδ-mediated Zp transactivation via Sp1 (38). To investigate further whether IL-32 repressed Zp activation through the PKCδ-responsible ZID domain, a reporter assay was performed with ZID deletion of Zp (ΔZID-Zp). As shown in Fig. 6E, IL-32 expression did not influence Zta-activated ΔZID-Zp activity, suggesting that IL-32 inhibition of Zp activation is probably mediated via the PKCδ-triggered ZID domain. To determine further whether IL-32 affects PKCδ-mediated Zp activation, we coexpressed IL-32 and enhanced GFP (EGFP)-derived wild-type PKCδ (WT-PKCδ) or a kinase-inactive form of PKCδ (DN-PKCδ) and determined the Zp activity. The results were similar to those in our previous work: overexpression of WT-PKCδ, but not DN-PKCδ, induced Zp activity (38). Of note, IL-32 expression decreased WT-PKCδ-mediated Zp activation (Fig. 6F). Together, our data suggest that IL-32 suppressed Zp activity via PKCδ.
FIG 6.
IL-32 maintains viral latency and inhibits Zp activation. (A and B) LCL-51 and LCL-52 were infected with shLuciferase, shIL-32-3, or shIL-32-5 lentiviruses for 5 days, and the cells were further selected with 2 μg/ml of puromycin for another 2 days. (A) Expression of IL-32, Zta, Rta, BGLF4, and β-actin was measured by Western blotting. β-Actin served as the internal control. The relative folds of Zta protein were determined by normalizing the level of each group to the corresponding β-actin intensity and then standardized with the shLuciferase control in cells. (B) Expression of Zta transcripts was detected by RT-qPCR, and the relative folds of Zta transcripts were normalized the level of each shIL-32 group to the corresponding β-actin intensity and then standardized with the shLuciferase control in cells. (C) Zta expression levels were compared in cells infected with lentiviruses containing shLuc or shIL-32-5 at day 5 postinfection. The percentage of Zta-positive cells is shown on the left side, and efficiency of knockdown of IL-32 was verified by Western blotting as shown on the right side. (MFI, mean fluorescence intensity.) (D) 293T cells were transfected with Zp plasmids, IL-32 expression plasmids, and pEGFP-C1. After 72 h, the relative luciferase activity of each transfectant was normalized to its GFP intensity and standardized to the vector control cells. (E) 293T cells were transfected with ΔZID-Zp plasmids, IL-32 expression plasmids, and pEGFP-C1. After 72 h, the relative luciferase activity of each transfectant was normalized to its GFP intensity and standardized to the vector control cells. (F) 293T cells were transfected with Zp plasmids, IL-32 expression plasmids, and EGFP-derived wild-type PKCδ (WT-PKCδ) or the kinase-inactive form of PKCδ (DN-PKCδ). After 72 h, the relative luciferase activity of each transfectant was normalized to its GFP intensity and standardized to the vector control cells. (Statistical analysis was performed by Student's t test. *, P < 0.05; **, P < 0.01; n.s., no significance.)
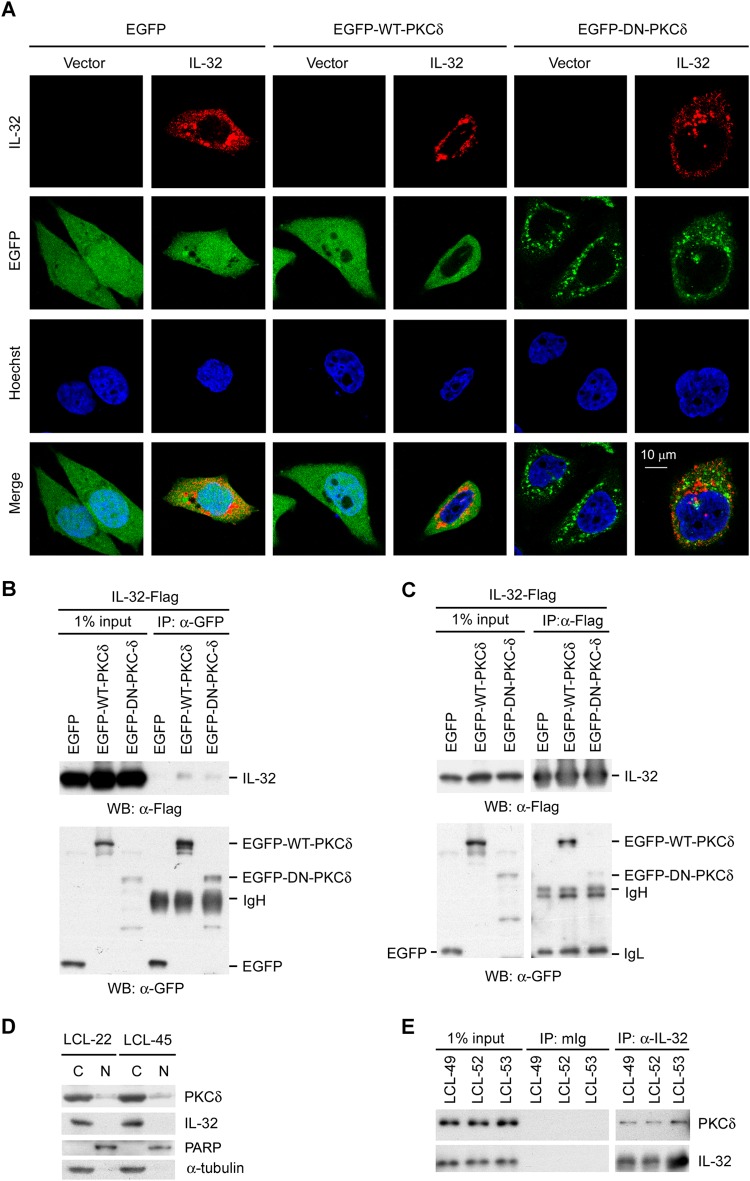
IL-32 hijacks PKCδ in the cytoplasm to limit EBV lytic cycle progression.
It is well documented that PKCδ activation may be detected by its translocation from the cytoplasm to the nucleus, phosphorylation at specific sites, and cleavage to a catalytic fragment (54–57). Confocal microscopy was used to investigate whether IL-32 affected the localization of PKCδ. Figure 7A revealed that WT-PKCδ is expressed in both the cytoplasm and nucleus, while DN-PKCδ is expressed only in the cytosol. Of note, coexpression of IL-32 prohibited the nuclear translocation of WT-PKCδ, WT-PKCδ being retained in the cytosol. In ectopic 293 cell transfection, data from a co-IP assay indicated that IL-32 interacts with PKCδ (Fig. 7B and C). To investigate further the cellular localization of PKCδ and IL-32, a fractionation assay was performed on EBV-immortalized LCLs. As shown in Fig. 7D, PKCδ was mainly expressed in the cytosolic fraction, with little in the nuclear portion of LCLs. IL-32 was expressed only in the cytosolic fraction. To confirm the endogenous interaction of IL-32 and PKCδ in LCLs, a co-IP assay was performed on LCLs. Figure 7E shows that IL-32 interacts with PKCδ in LCLs. These results suggest that IL-32 interferes with Zp activation by interacting with PKCδ to block its nuclear translocation and trap it in the cytosol.
FIG 7.
IL-32 interacts with cytosolic PKCδ. (A) HeLa cells were transfected with IL-32 expression plasmids and WT-PKCδ or DN-PKCδ. Cells were fixed and then stained with IL-32 antibody and the nuclear dye Hoechst. Red fluorescence indicates the IL-32 expression signal. Green fluorescence indicates EGFP, WT-PKCδ, or DN-PKCδ. Blue fluorescence indicates the cell nucleus. The colocalization of IL-32 and PKCδ is shown in the merge panel. (B and C) 293T cells were transfected with IL-32 expression plasmids and WT-PKCδ or DN-PKCδ. The cell lysates were immunoprecipitated with anti-GFP antibody (B) or anti-Flag antibody (C). The interaction was detected by Western blotting (WB) using anti-Flag antibody (B) or anti-GFP antibody (C). IgL, immunoglobulin light chain; IgH, immunoglobulin heavy chain. (D) The subcellular localization of IL-32 and PKCδ was detected in LCLs by fractionation and Western blotting. PARP served as the marker for the nuclear fraction (N), and α-tubulin served as the marker for the cytosolic fraction (C). (E) The endogenous interaction between IL-32 and PKCδ was demonstrated in LCLs. LCL lysates were immunoprecipitated with anti-IL-32 antibody. The interaction was detected by Western blotting using anti-PKCδ antibody. The mouse immunoglobulin (mIg) was the negative control.
DISCUSSION
In general, EBV escapes immune surveillance by expressing a restricted set of viral proteins. In addition to viral mimicry of immune modulators, EBV-encoded latent proteins play important roles in hijacking cellular factors to control further its progression to lytic replication. However, how gamma herpesviruses maintain a latent infection in the host remains obscure (58, 59). Previous studies demonstrated that LMP1 maintains EBV latency by NF-κB activation (60, 61). In this study, we revealed that LMP1-mediated upregulation of IL-32 may be another way EBV maintains latency.
Several studies have shown that viruses modulate IL-32 expression through a variety of mechanisms. Influenza virus infection of human lung epithelial cells, A549 cells, stimulates IL-32 expression via the cyclooxygenase-2 (COX-2) and RIG-I/MAVS/IKK pathways to activate NF-κB (62). In addition, human papillomavirus (HPV) stimulates IL-32 expression in cervical cancer cells through COX-2 (63). The hepatitis B virus (HBV)-encoded HBx protein stimulates NF-κB to activate IL-32 promoter activity in Huh7 cells (64). Our results showed that LMP1 stimulates IL-32 expression through its CTAR1 and CTAR2 domains (Fig. 3A and B), which is consistent with a previous study (65). Furthermore, a reporter assay indicated that the NF-κB sites of IL-32 promoter play an important role in LMP1-induced expression of IL-32 (Fig. 4B). A ChIP assay demonstrated LMP1-induced NF-κB binding to the IL-32 promoter, leading to IL-32 transcription (Fig. 4C). A previous study has shown that activation of NF-κB inhibits the lytic replication of a gamma herpesvirus (66). Our study is consistent with these reports and suggests that LMP1-induced NF-κB activation to upregulate IL-32 expression may play an important role in the maintenance of virus latency.
Moreover, IL-32 promotes the survival of hepatoma cells through the NF-κB pathway (67). However, expression of IL-32 induces activation-induced death of T cells (25). IL-32 may be involved in the pathogenesis of atopic dermatitis, because knockdown of IL-32 decreases TNF-α- and IFN-γ-induced apoptosis (68). In our study, knockdown of IL-32 in LCLs did not affect cell proliferation or apoptosis (Fig. 5A and B). This may be because IL-32 plays various roles in different cell types. Taken together, our results showed that knockdown of IL-32 increased IL-6 expression and promoted virus lytic replication, suggesting that LMP1-induced IL-32 plays a major role in maintaining viral latency, as well as affecting cytokine expression.
We wondered how IL-32, as an atypical cytoplasmic cytokine, represses the Zta promoter. Recently, Kang et al. demonstrated that IL-32 interacts with PKCε and STAT3 to induce IL-6 expression in monocytic THP-1 cells (28). The interaction of PKCδ, IL-32, and C/EBPα suppressed the inhibitory effects of C/EBPα on the IL-10 promoter in myeloid cells (47). These studies suggested that IL-32 may modulate various functions by interacting with different proteins in different cell types. In this work, we uncovered a novel mechanism of a cellular factor which modulates the EBV life cycle. Our previous studies indicated that PKCδ is an important modulator of EBV lytic cycle progression and that overexpression of PKCδ significantly stimulates Zp activity (38, 54). In this study, we clearly showed that IL-32 downregulates Zp activation by interacting with PKCδ and inhibiting the nuclear translocation of PKCδ to maintain viral latency. These results suggest that IL-32 may be one of the key regulators that maintain EBV in persistent latent infection in the host. Our finding may provide new evidence of how EBV uses cellular proteins to inhibit its replication cycle.
ACKNOWLEDGMENTS
We thank Tim J. Harrison of UCL Medical School (London, United Kingdom) for reviewing the manuscript critically.
This work was supported by the Ministry of Science and Technology (MOST 103-2320-B-002-038-MY3), the National Health Research Institute (NHRI-EX103-10306BI), funds from Excellent Translational Medicine Research Projects of National Taiwan University College of Medicine and National Taiwan University Hospital (103C-101-A1) to Ching-Hwa Tsai, and a grant from the Ministry of Science and Technology (MOST 103-2320-B-182-028) to Sue-Jane Lin.
K.-Y.L. performed experiments and analyzed data; Y.-C.C. performed experiments, analyzed data, and cowrote the manuscript; J.-H.L. provided materials; Y.L. performed experiments and analyzed data; S.-L.D. provided materials; M.-R.C. provided materials; T.-H.Y. provided materials; S.-J.L. guided experimental design and cowrote the manuscript; and C.-H.T. guided experimental design and cowrote the manuscript.
We declare no competing financial interests.
REFERENCES
- 1.Rickinson AB, Kieff E. 2007. Epstein-Barr virus-associated malignancies of the immunocompromised host, p 2668–2700. In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 5th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 2.Thorley-Lawson DA, Allday MJ. 2008. The curious case of the tumour virus: 50 years of Burkitt's lymphoma. Nat Rev Microbiol 6:913–924. doi: 10.1038/nrmicro2015. [DOI] [PubMed] [Google Scholar]
- 3.Sugimoto M, Tahara H, Ide T, Furuichi Y. 2004. Steps involved in immortalization and tumorigenesis in human B-lymphoblastoid cell lines transformed by Epstein-Barr virus. Cancer Res 64:3361–3364. doi: 10.1158/0008-5472.CAN-04-0079. [DOI] [PubMed] [Google Scholar]
- 4.Klein U, Dalla-Favera R. 2008. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol 8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- 5.Shaffer AL, Rosenwald A, Staudt LM. 2002. Lymphoid malignancies: the dark side of B-cell differentiation. Nat Rev Immunol 2:920–932. doi: 10.1038/nri953. [DOI] [PubMed] [Google Scholar]
- 6.Thorley-Lawson DA. 2001. Epstein-Barr virus: exploiting the immune system. Nat Rev Immunol 1:75–82. doi: 10.1038/35095584. [DOI] [PubMed] [Google Scholar]
- 7.Tierney R, Nagra J, Hutchings I, Shannon-Lowe C, Altmann M, Hammerschmidt W, Rickinson A, Bell A. 2007. Epstein-Barr virus exploits BSAP/Pax5 to achieve the B-cell specificity of its growth-transforming program. J Virol 81:10092–10100. doi: 10.1128/JVI.00358-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henkel T, Ling PD, Hayward SD, Peterson MG. 1994. Mediation of Epstein-Barr virus EBNA2 transactivation by recombination signal-binding protein J kappa. Science 265:92–95. doi: 10.1126/science.8016657. [DOI] [PubMed] [Google Scholar]
- 9.Johannsen E, Koh E, Mosialos G, Tong X, Kieff E, Grossman SR. 1995. Epstein-Barr virus nuclear protein 2 transactivation of the latent membrane protein 1 promoter is mediated by J kappa and PU.1. J Virol 69:253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies ML, Xu S, Lyons-Weiler J, Rosendorff A, Webber SA, Wasil LR, Metes D, Rowe DT. 2010. Cellular factors associated with latency and spontaneous Epstein-Barr virus reactivation in B-lymphoblastoid cell lines. Virology 400:53–67. doi: 10.1016/j.virol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Daigle D, Megyola C, El-Guindy A, Gradoville L, Tuck D, Miller G, Bhaduri-McIntosh S. 2010. Upregulation of STAT3 marks Burkitt lymphoma cells refractory to Epstein-Barr virus lytic cycle induction by HDAC inhibitors. J Virol 84:993–1004. doi: 10.1128/JVI.01745-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson AR, Kwek SS, Kenney SC. 2012. The B-cell specific transcription factor, Oct-2, promotes Epstein-Barr virus latency by inhibiting the viral immediate-early protein, BZLF1. PLoS Pathog 8:e1002516. doi: 10.1371/journal.ppat.1002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang X, Collins CM, Mendel JB, Iwakoshi NN, Speck SH. 2009. Gammaherpesvirus-driven plasma cell differentiation regulates virus reactivation from latently infected B lymphocytes. PLoS Pathog 5:e1000677. doi: 10.1371/journal.ppat.1000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun CC, Thorley-Lawson DA. 2007. Plasma cell-specific transcription factor XBP-1s binds to and transactivates the Epstein-Barr virus BZLF1 promoter. J Virol 81:13566–13577. doi: 10.1128/JVI.01055-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young LS, Rickinson AB. 2004. Epstein-Barr virus: 40 years on. Nat Rev Cancer 4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 16.Li HP, Chang YS. 2003. Epstein-Barr virus latent membrane protein 1: structure and functions. J Biomed Sci 10:490–504. doi: 10.1007/BF02256110. [DOI] [PubMed] [Google Scholar]
- 17.Zhang B, Kracker S, Yasuda T, Casola S, Vanneman M, Homig-Holzel C, Wang Z, Derudder E, Li S, Chakraborty T, Cotter SE, Koyama S, Currie T, Freeman GJ, Kutok JL, Rodig SJ, Dranoff G, Rajewsky K. 2012. Immune surveillance and therapy of lymphomas driven by Epstein-Barr virus protein LMP1 in a mouse model. Cell 148:739–751. doi: 10.1016/j.cell.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinclair AJ. 2006. Unexpected structure of Epstein-Barr virus lytic cycle activator Zta. Trends Microbiol 14:289–291. doi: 10.1016/j.tim.2006.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai SC, Lin SJ, Chen PW, Luo WY, Yeh TH, Wang HW, Chen CJ, Tsai CH. 2009. EBV Zta protein induces the expression of interleukin-13, promoting the proliferation of EBV-infected B cells and lymphoblastoid cell lines. Blood 114:109–118. doi: 10.1182/blood-2008-12-193375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lieberman PM. 2013. Keeping it quiet: chromatin control of gammaherpesvirus latency. Nat Rev Microbiol 11:863–875. doi: 10.1038/nrmicro3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barton E, Mandal P, Speck SH. 2011. Pathogenesis and host control of gammaherpesviruses: lessons from the mouse. Annu Rev Immunol 29:351–397. doi: 10.1146/annurev-immunol-072710-081639. [DOI] [PubMed] [Google Scholar]
- 22.Joosten LA, Netea MG, Kim SH, Yoon DY, Oppers-Walgreen B, Radstake TR, Barrera P, van de Loo FA, Dinarello CA, van den Berg WB. 2006. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc Natl Acad Sci U S A 103:3298–3303. doi: 10.1073/pnas.0511233103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dinarello CA, Kim SH. 2006. IL-32, a novel cytokine with a possible role in disease. Ann Rheum Dis 65(Suppl 3):iii61–iii64. doi: 10.1136/ard.2006.058511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. 2005. Interleukin-32: a cytokine and inducer of TNFalpha. Immunity 22:131–142. doi: 10.1016/j.immuni.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Goda C, Kanaji T, Kanaji S, Tanaka G, Arima K, Ohno S, Izuhara K. 2006. Involvement of IL-32 in activation-induced cell death in T cells. Int Immunol 18:233–240. doi: 10.1093/intimm/dxh339. [DOI] [PubMed] [Google Scholar]
- 26.Hong J, Bae S, Kang Y, Yoon D, Bai X, Chan ED, Azam T, Dinarello CA, Lee S, Her E, Rho G, Kim S. 2010. Suppressing IL-32 in monocytes impairs the induction of the proinflammatory cytokines TNFalpha and IL-1beta. Cytokine 49:171–176. doi: 10.1016/j.cyto.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Nold-Petry CA, Nold MF, Zepp JA, Kim SH, Voelkel NF, Dinarello CA. 2009. IL-32-dependent effects of IL-1beta on endothelial cell functions. Proc Natl Acad Sci U S A 106:3883–3888. doi: 10.1073/pnas.0813334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang JW, Park YS, Lee DH, Kim JH, Kim MS, Bak Y, Hong J, Yoon DY. 2012. Intracellular interaction of interleukin (IL)-32alpha with protein kinase Cepsilon (PKCepsilon) and STAT3 protein augments IL-6 production in THP-1 promonocytic cells. J Biol Chem 287:35556–35564. doi: 10.1074/jbc.M112.400911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novick D, Rubinstein M, Azam T, Rabinkov A, Dinarello CA, Kim SH. 2006. Proteinase 3 is an IL-32 binding protein. Proc Natl Acad Sci U S A 103:3316–3321. doi: 10.1073/pnas.0511206103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shioya M, Nishida A, Yagi Y, Ogawa A, Tsujikawa T, Kim-Mitsuyama S, Takayanagi A, Shimizu N, Fujiyama Y, Andoh A. 2007. Epithelial overexpression of interleukin-32alpha in inflammatory bowel disease. Clin Exp Immunol 149:480–486. doi: 10.1111/j.1365-2249.2007.03439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calabrese F, Baraldo S, Bazzan E, Lunardi F, Rea F, Maestrelli P, Turato G, Lokar-Oliani K, Papi A, Zuin R, Sfriso P, Balestro E, Dinarello CA, Saetta M. 2008. IL-32, a novel proinflammatory cytokine in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 178:894–901. doi: 10.1164/rccm.200804-646OC. [DOI] [PubMed] [Google Scholar]
- 32.Kim KH, Shim JH, Seo EH, Cho MC, Kang JW, Kim SH, Yu DY, Song EY, Lee HG, Sohn JH, Kim J, Dinarello CA, Yoon DY. 2008. Interleukin-32 monoclonal antibodies for immunohistochemistry, Western blotting, and ELISA. J Immunol Methods 333:38–50. doi: 10.1016/j.jim.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 33.Sorrentino C, Di Carlo E. 2009. Expression of IL-32 in human lung cancer is related to the histotype and metastatic phenotype. Am J Respir Crit Care Med 180:769–779. doi: 10.1164/rccm.200903-0400OC. [DOI] [PubMed] [Google Scholar]
- 34.Nishida A, Andoh A, Inatomi O, Fujiyama Y. 2009. Interleukin-32 expression in the pancreas. J Biol Chem 284:17868–17876. doi: 10.1074/jbc.M900368200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heinhuis B, Koenders MI, van den Berg WB, Netea MG, Dinarello CA, Joosten LA. 2012. Interleukin 32 (IL-32) contains a typical alpha-helix bundle structure that resembles focal adhesion targeting region of focal adhesion kinase-1. J Biol Chem 287:5733–5743. doi: 10.1074/jbc.M111.288290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chou YC, Lin SJ, Lu J, Yeh TH, Chen CL, Weng PL, Lin JH, Yao M, Tsai CH. 2011. Requirement for LMP1-induced RON receptor tyrosine kinase in Epstein-Barr virus-mediated B-cell proliferation. Blood 118:1340–1349. doi: 10.1182/blood-2011-02-335448. [DOI] [PubMed] [Google Scholar]
- 37.Tsai SC, Lin SJ, Lin CJ, Chou YC, Lin JH, Yeh TH, Chen MR, Huang LM, Lu MY, Huang YC, Chen HY, Tsai CH. 2013. Autocrine CCL3 and CCL4 induced by the oncoprotein LMP1 promote Epstein-Barr virus-triggered B cell proliferation. J Virol 87:9041–9052. doi: 10.1128/JVI.00541-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai PF, Lin SJ, Weng PL, Tsai SC, Lin JH, Chou YC, Tsai CH. 2011. Interplay between PKCdelta and Sp1 on histone deacetylase inhibitor-mediated Epstein-Barr virus reactivation. J Virol 85:2373–2385. doi: 10.1128/JVI.01602-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang JT, Yang PW, Lee CP, Han CH, Tsai CH, Chen MR. 2005. Detection of Epstein-Barr virus BGLF4 protein kinase in virus replication compartments and virus particles. J Gen Virol 86:3215–3225. doi: 10.1099/vir.0.81313-0. [DOI] [PubMed] [Google Scholar]
- 40.Chen SY, Lu J, Shih YC, Tsai CH. 2002. Epstein-Barr virus latent membrane protein 2A regulates c-Jun protein through extracellular signal-regulated kinase. J Virol 76:9556–9561. doi: 10.1128/JVI.76.18.9556-9561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang Y, Lee HH, Chang SS, Hsu TY, Wang PW, Chang YS, Takada K, Tsai CH. 2004. Induction of Epstein-Barr virus latent membrane protein 1 by a lytic transactivator Rta. J Virol 78:13028–13036. doi: 10.1128/JVI.78.23.13028-13036.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dahl CA, Schall RP, He HL, Cairns JS. 1992. Identification of a novel gene expressed in activated natural killer cells and T cells. J Immunol 148:597–603. [PubMed] [Google Scholar]
- 43.Shoda H, Fujio K, Yamaguchi Y, Okamoto A, Sawada T, Kochi Y, Yamamoto K. 2006. Interactions between IL-32 and tumor necrosis factor alpha contribute to the exacerbation of immune-inflammatory diseases. Arthritis Res Ther 8:R166. doi: 10.1186/ar2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bieging KT, Swanson-Mungerson M, Amick AC, Longnecker R. 2010. Epstein-Barr virus in Burkitt's lymphoma: a role for latent membrane protein 2A. Cell Cycle 9:901–908. doi: 10.4161/cc.9.5.10840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morris MA, Dawson CW, Young LS. 2009. Role of the Epstein-Barr virus-encoded latent membrane protein-1, LMP1, in the pathogenesis of nasopharyngeal carcinoma. Future Oncol 5:811–825. doi: 10.2217/fon.09.53. [DOI] [PubMed] [Google Scholar]
- 46.Netea MG, Azam T, Ferwerda G, Girardin SE, Walsh M, Park JS, Abraham E, Kim JM, Yoon DY, Dinarello CA, Kim SH. 2005. IL-32 synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2 ligands for IL-1beta and IL-6 production through a caspase 1-dependent mechanism. Proc Natl Acad Sci U S A 102:16309–16314. doi: 10.1073/pnas.0508237102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang JW, Park YS, Kim MS, Lee DH, Bak Y, Ham SY, Park SH, Hong JT, Yoon DY. 2013. Interleukin (IL)-32beta-mediated CCAAT/enhancer-binding protein alpha (C/EBPalpha) phosphorylation by protein kinase Cdelta (PKCdelta) abrogates the inhibitory effect of C/EBPalpha on IL-10 production. J Biol Chem 288:23650–23658. doi: 10.1074/jbc.M113.465575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eliopoulos AG, Stack M, Dawson CW, Kaye KM, Hodgkin L, Sihota S, Rowe M, Young LS. 1997. Epstein-Barr virus-encoded LMP1 and CD40 mediate IL-6 production in epithelial cells via an NF-kappaB pathway involving TNF receptor-associated factors. Oncogene 14:2899–2916. doi: 10.1038/sj.onc.1201258. [DOI] [PubMed] [Google Scholar]
- 49.Yoshizaki T, Horikawa T, Qing-Chun R, Wakisaka N, Takeshita H, Sheen TS, Lee SY, Sato H, Furukawa M. 2001. Induction of interleukin-8 by Epstein-Barr virus latent membrane protein-1 and its correlation to angiogenesis in nasopharyngeal carcinoma. Clin Cancer Res 7:1946–1951. [PubMed] [Google Scholar]
- 50.Lambert SL, Martinez OM. 2007. Latent membrane protein 1 of EBV activates phosphatidylinositol 3-kinase to induce production of IL-10. J Immunol 179:8225–8234. doi: 10.4049/jimmunol.179.12.8225. [DOI] [PubMed] [Google Scholar]
- 51.Liu S, Borras AM, Liu P, Suske G, Speck SH. 1997. Binding of the ubiquitous cellular transcription factors Sp1 and Sp3 to the ZI domains in the Epstein-Barr virus lytic switch BZLF1 gene promoter. Virology 228:11–18. doi: 10.1006/viro.1996.8371. [DOI] [PubMed] [Google Scholar]
- 52.Liu S, Liu P, Borras A, Chatila T, Speck SH. 1997. Cyclosporin A-sensitive induction of the Epstein-Barr virus lytic switch is mediated via a novel pathway involving a MEF2 family member. EMBO J 16:143–153. doi: 10.1093/emboj/16.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Speck SH, Chatila T, Flemington E. 1997. Reactivation of Epstein-Barr virus: regulation and function of the BZLF1 gene. Trends Microbiol 5:399–405. doi: 10.1016/S0966-842X(97)01129-3. [DOI] [PubMed] [Google Scholar]
- 54.Lee HH, Chang SS, Lin SJ, Chua HH, Tsai TJ, Tsai K, Lo YC, Chen HC, Tsai CH. 2008. Essential role of PKCdelta in histone deacetylase inhibitor-induced Epstein-Barr virus reactivation in nasopharyngeal carcinoma cells. J Gen Virol 89:878–883. doi: 10.1099/vir.0.83533-0. [DOI] [PubMed] [Google Scholar]
- 55.Shirai Y, Saito N. 2002. Activation mechanisms of protein kinase C: maturation, catalytic activation, and targeting. J Biochem 132:663–668. doi: 10.1093/oxfordjournals.jbchem.a003271. [DOI] [PubMed] [Google Scholar]
- 56.Steinberg SF. 2004. Distinctive activation mechanisms and functions for protein kinase Cdelta. Biochem J 384:449–459. doi: 10.1042/BJ20040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamamoto T, Matsuzaki H, Kamada S, Ono Y, Kikkawa U. 2007. Biochemical assays for multiple activation states of protein kinase C Nat Protoc 1:2791–2795. doi: 10.1038/nprot.2006.420. [DOI] [PubMed] [Google Scholar]
- 58.Thorley-Lawson DA, Duca KA, Shapiro M. 2008. Epstein-Barr virus: a paradigm for persistent infection—for real and in virtual reality. Trends Immunol 29:195–201. doi: 10.1016/j.it.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 59.Ye FC, Zhou FC, Xie JP, Kang T, Greene W, Kuhne K, Lei XF, Li QH, Gao SJ. 2008. Kaposi's sarcoma-associated herpesvirus latent gene vFLIP inhibits viral lytic replication through NF-kappaB-mediated suppression of the AP-1 pathway: a novel mechanism of virus control of latency. J Virol 82:4235–4249. doi: 10.1128/JVI.02370-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adler B, Schaadt E, Kempkes B, Zimber-Strobl U, Baier B, Bornkamm GW. 2002. Control of Epstein-Barr virus reactivation by activated CD40 and viral latent membrane protein 1. Proc Natl Acad Sci U S A 99:437–442. doi: 10.1073/pnas.221439999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prince S, Keating S, Fielding C, Brennan P, Floettmann E, Rowe M. 2003. Latent membrane protein 1 inhibits Epstein-Barr virus lytic cycle induction and progress via different mechanisms. J Virol 77:5000–5007. doi: 10.1128/JVI.77.8.5000-5007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li W, Liu Y, Mukhtar MM, Gong R, Pan Y, Rasool ST, Gao Y, Kang L, Hao Q, Peng G, Chen Y, Chen X, Wu J, Zhu Y. 2008. Activation of interleukin-32 pro-inflammatory pathway in response to influenza A virus infection. PLoS One 3:e1985. doi: 10.1371/journal.pone.0001985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee S, Kim JH, Kim H, Kang JW, Kim SH, Yang Y, Kim J, Park J, Park S, Hong J, Yoon DY. 2011. Activation of the interleukin-32 pro-inflammatory pathway in response to human papillomavirus infection and over-expression of interleukin-32 controls the expression of the human papillomavirus oncogene. Immunology 132:410–420. doi: 10.1111/j.1365-2567.2010.03377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pan X, Cao H, Lu J, Shu X, Xiong X, Hong X, Xu Q, Zhu H, Li G, Shen G. 2011. Interleukin-32 expression induced by hepatitis B virus protein X is mediated through activation of NF-kappaB. Mol Immunol 48:1573–1577. doi: 10.1016/j.molimm.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 65.Gewurz BE, Mar JC, Padi M, Zhao B, Shinners NP, Takasaki K, Bedoya E, Zou JY, Cahir-McFarland E, Quackenbush J, Kieff E. 2011. Canonical NF-kappaB activation is essential for Epstein-Barr virus latent membrane protein 1 TES2/CTAR2 gene regulation. J Virol 85:6764–6773. doi: 10.1128/JVI.00422-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown HJ, Song MJ, Deng H, Wu TT, Cheng G, Sun R. 2003. NF-kappaB inhibits gammaherpesvirus lytic replication. J Virol 77:8532–8540. doi: 10.1128/JVI.77.15.8532-8540.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kang YH, Park MY, Yoon DY, Han SR, Lee CI, Ji NY, Myung PK, Lee HG, Kim JW, Yeom YI, Jang YJ, Ahn DK, Song EY. 2012. Dysregulation of overexpressed IL-32alpha in hepatocellular carcinoma suppresses cell growth and induces apoptosis through inactivation of NF-kappaB and Bcl-2. Cancer Lett 318:226–233. doi: 10.1016/j.canlet.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 68.Meyer N, Zimmermann M, Burgler S, Bassin C, Woehrl S, Moritz K, Rhyner C, Indermitte P, Schmid-Grendelmeier P, Akdis M, Menz G, Akdis CA. 2010. IL-32 is expressed by human primary keratinocytes and modulates keratinocyte apoptosis in atopic dermatitis. J Allergy Clin Immunol 125:858–865.e810. doi: 10.1016/j.jaci.2010.01.016. [DOI] [PubMed] [Google Scholar]