Abstract
Simian immunodeficiency virus SIVsab infection is completely controlled in rhesus macaques (RMs) through functional immune responses. We report that in SIVsab-infected RMs, (i) viral replication is controlled to <0 to 3 copies/ml, (ii) about one-third of the virus strains in reservoirs are replication incompetent, and (iii) rebounding virus after CD8+ cell depletion is replication competent and genetically similar to the original virus stock, suggesting early reservoir seeding. This model permits assessment of strategies aimed at depleting the reservoir without multidrug antiretroviral therapy.
TEXT
The report of one patient that was cured of human immunodeficiency virus (HIV) infection (1) renewed enthusiasm for cure research aimed at understanding the mechanisms of HIV persistence and developing therapeutic strategies to reduce/eliminate viral reservoirs (2). However, virus rebound in the Mississippi baby (3) and the Boston patients (4) pointed to the difficulty of achieving a cure/functional cure of HIV infection and the need to develop new strategies to reach this goal. Multiple limitations to the cure have been identified, including (i) rapid establishment of latently infected cells, (ii) residual viral replication in patients receiving combination antiretroviral therapy (cART), which prevents proper reservoir characterization, and (iii) the existence of anatomic reservoirs (privileged sites of latency insufficiently penetrated by drugs) (5, 6). Due to these limitations, it is generally agreed that a more feasible alternative to an HIV infection cure (i.e., complete eradication of HIV and HIV-infected cells from the body) may be a functional cure (i.e., control of HIV infection without complete HIV eradication: undetectable viremia without ART, no disease progression, no CD4+ T-cell loss, and lack of HIV transmission) (6). This concept is supported by the observation that functional cure has been achieved in a fraction of patients that received long-term ART initiated during acute HIV infection (7).
Aside from the general barriers to a cure, there are specific limitations to cure research: (i) ethical problems (therapy cannot be stopped in patients without the risks of virus rebound and the development of viral resistance and increased virus transmission), (ii) technical problems (there is no acceptable biomarker for latently infected cells), and (iii) limited availability of invasive samples from the multiple potential reservoir sites (8). These limitations make it imperative that cure research be performed in analogous and tractable animal models. Currently available models need to be improved for such studies. For example, SIVmac infection of rhesus macaques (RMs) (the most widely used animal model for AIDS research) is more difficult to control with ART than HIV-1 infection in humans, requiring complex combination therapies (9, 10). Furthermore, infection with molecular clones (e.g., simian-human immunodeficiency viruses carrying the reverse transcriptase gene [RT-SHIVs]) does not permit tracking of viral spread or detailed characterization of the reservoirs. Although the development of humanized mice (11, 12) may lead to major progress in cure research, critical size limitations and insufficient repopulation of mucosal sites prevent a detailed assessment of viral reservoirs in this model.
We developed an animal model of complete immunological suppression with persistent reservoirs by infecting RMs with SIVsab92018 (13, 14). In this model, complete immune control of SIVsab infection is achieved in 100% of RMs in the absence of ART through effective cellular immune responses (14). While it can be argued that this model does not reproduce the complexity of chronically infected patients receiving ART, its main strength is that it allows for the rapid, low-cost screening of new therapeutic strategies aimed at depleting viral reservoirs in vivo without the need to boost cellular immune responses or the complexity of multidrug ART. Furthermore, this model reproduces key features of HIV infection, namely, robust acute infection accompanied by massive depletion of memory cells in the gut and infection of CD4+ T cells expressing CCR5 (14). In this model, an acute increase in T-cell immune activation and proliferation are observed and systemic inflammation is maintained during the initial stages of chronic infection, long after virus control (as monitored using conventional viral load [VL] quantification assays) (14).
Our goal here was to further characterize this model. We report that SIVsab infection is truly latent in RMs, that, similar to what has been observed in HIV-infected patients, the virus persists in memory CD4+ T cells, and that the controlled virus is replication competent in vivo.
Thirteen male RMs were included in this study. Eleven RMs were inoculated intravenously with plasma samples equivalent to 300 tissue culture infectious doses at 50% (TCID50) of SIVsab92018. The same virus strain induces a persistent nonprogressive infection in the natural African green monkey (AGM) host (13, 15) and a pathogenic progressive infection in pigtailed macaques (16–18). The remaining two RMs were inoculated with plasma samples collected from SIVsab92018 controllers collected at 4 years postinfection (p.i.), during virus rebound following in vivo CD8+ cell depletion (14). All animals were housed and maintained at the RIDC Park animal facility of the University of Pittsburgh according to the standards of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC), and experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee (IACUC; protocol no. 09039, approved in 2009). The animals were fed and housed according to regulations set forth by the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act (19). All the RMs included in this study were socially housed (paired) indoors in stainless steel cages, were exposed to a light-dark cycle of 12 h of light and 12 h of dark, were fed twice daily, had access to water ad libitum, and were given various toys and feeding enrichment. At the end of the study, the RMs were euthanized by following the euthanasia procedures approved in the IACUC protocol.
We first documented the lack of residual viral replication by quantifying SIVsab viral loads in controller RMs (14) using a reverse transcriptase quantitative PCR (RT-qPCR) assay with single-copy sensitivity (SCA) targeting the gag region of the SIVsab genome (20, 21), using the primers and probe of our conventional RT-qPCR (14). With this method, we tested samples from 11 SIVsab-infected RM controllers collected at 180 days p.i. and from 3 RMs that controlled infection for nearly 4 years. As shown in Fig. 1, SCA identified residual levels of viral replication (average ± standard deviation, 38.15 ± 16.33 viral RNA [vRNA] copies/ml; range, 0.5 to 181 vRNA copies/ml) below the levels of conventional real-time PCR assays (13–15, 22, 23) in the samples collected during the early stages of controlled SIVsab infection. These results support our published data indicating that low levels of virus production persist in selected tissues (lymph nodes [LNs] and intestine) collected at the same time point during necropsy (14). These low levels of residual viral replication most likely explain the residual immune activation that persists after the virus is controlled below 30 copies/ml (14). Conversely, in samples collected after nearly 4 years (1,440 days p.i.) of undetectable viral replication and 2 years after the RMs seroreverted and normalized the levels of immune activation on circulating T cells (14), the virus was completely controlled (<1 copy/ml) (Fig. 1a). These results agree with those reported for HIV-infected patients and show that once a functional cure is established, a progressive decrease in virus burden occurs even in the absence of ART (7).
FIG 1.
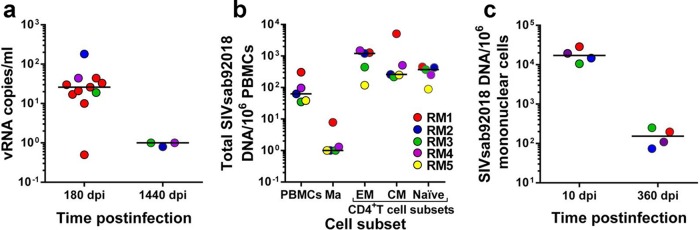
Virological characterization of the reservoir in SIVsab-infected RM controllers. (a) Plasma viral load testing with a single-copy assay (SCA) (sensitivity, 1 copy/ml) demonstrates that in RM controllers with viral loads undetectable by conventional assays, virus control is consolidated during the follow-up, with the infection eventually becoming latent. dpi, days p.i. (b) During the latent stage of rhesus macaque infection, SIVsab mainly persists in memory CD4+ T-cell subsets. Ma, monocytes; EM, effector memory; CM, central memory; TM, transitional memory. (c) In the lymph nodes, similar to peripheral blood, virus control is consolidated during the follow-up.
We then measured the total viral DNA (vDNA) burden in peripheral blood mononuclear cells (PBMCs) and in different CD4+ T-cell subsets. To this end, we collected 250-ml blood samples from five SIVsab-infected RMs at the stage of viral control (360 days p.i.). Using a panel of monoclonal antibodies consisting of CD3-V450 (clone SP34-2), CD4-allophycocyanin (APC) (L200), CD28-phycoerythrin (PE)-Cy7 (CD28.2), CD95-fluorescein isothiocyanate (FITC) (DX2), CCR5-PE (3A9), CCR7-Biotin (3D12), and CD14-peridinin chlorophyll protein (PerCP)-Cy5.5 (M5E2), we sorted naive (CD28+ CD95−), central memory (CD28+ CCR7+ CCR5+), and effector memory (CD28− CCR7− CCR5dim; where “dim” means low) CD4+ T cells as well as monocytes (CD14+) and measured their vDNA content by qPCR (23). We report that, during the controlled stage of infection, SIVsab persists in all CD4+ T-cell subsets, with memory cells (both effector and central memory) harboring the highest level of vDNA content (Fig. 1b). SIVsab could also be detected in naive cells (Fig. 1b). Using LNs serially collected from SIVsab-infected RMs, we monitored the DNA burden at different time points of infection (acute infection [10 days p.i.] and chronic, controlled infection [360 days p.i.]). As expected, the overall vDNA content in SIVsab-infected RMs was significantly lower during the controlled stage of infection than during acute infection (P < 0.0035) (Fig. 1c).
Since SIVsab is a cross-species-transmitted virus and the progressive consolidation of viral control detected by SCA may be attributed to the accumulation of hypermutations through the action of host restriction factors, we next investigated whether or not controlled SIVsab is replication competent in RMs. The previous CD8+ cell depletion experiment suggested that this is indeed the case (14). However, the CD8+ depletion study could not discriminate between virus replication following ablation of cell-mediated immune control and massive homeostatic proliferation of virus strains from the reservoir that are unable to complete full cycles of replication due to the massive immune activation of the CD4+ T cells induced by the CD8+ cell-depleting antibody (24). However, the latter scenario is unlikely, as the levels of viral replication following CD8+ cell depletion were >104 vRNA copies/ml (14), higher than those characteristic of homeostatic proliferation of CD4+ cells (25–27).
Due to the sample limitation that precluded application of conventional virus outgrowth assay to assess the replicative potential of the rebounding virus and the fact that this method has limitations in identifying the inducible virus (28), we opted for an in vivo assessment of the replication competence of SIVsab in latently infected RMs. To this end, we pooled plasma samples collected from RM controllers at the peak of virus rebound following CD8+ cell depletion. We then infected two naive, adult RMs with the pooled plasma. Viral replication was monitored using the conventional quantification method (23) with plasma samples collected at 3, 7, 10, 14, 21, 28, 42, 72, and 100 days p.i. One RM (RM88) was euthanized at 42 days p.i., prior to virus control. RM infection with the rebounding virus was indistinguishable from infection with the original SIVsab swarm, with similar peak VLs and similar amounts of time to the control of viremia, which became undetectable by 72 days p.i. (Fig. 2a). Furthermore, RM infection with the rebounding virus resulted in similar magnitudes of CD4+ T-cell depletion from circulation and mucosal sites (Fig. 2b and c), with the memory cell pool being preferentially depleted (Fig. 2d and e) and T-cell immune activation (Fig. 2f and g) and proliferation (Fig. 2 h and i), which were similar to those observed in RMs infected with the original viral stock. The replication pattern of the rebounding virus in naive RMs clearly demonstrates that the controlled virus is replication competent. Furthermore, this experiment validated a method for assessment of in vivo virus replicative competence after virus reactivation experiments.
FIG 2.
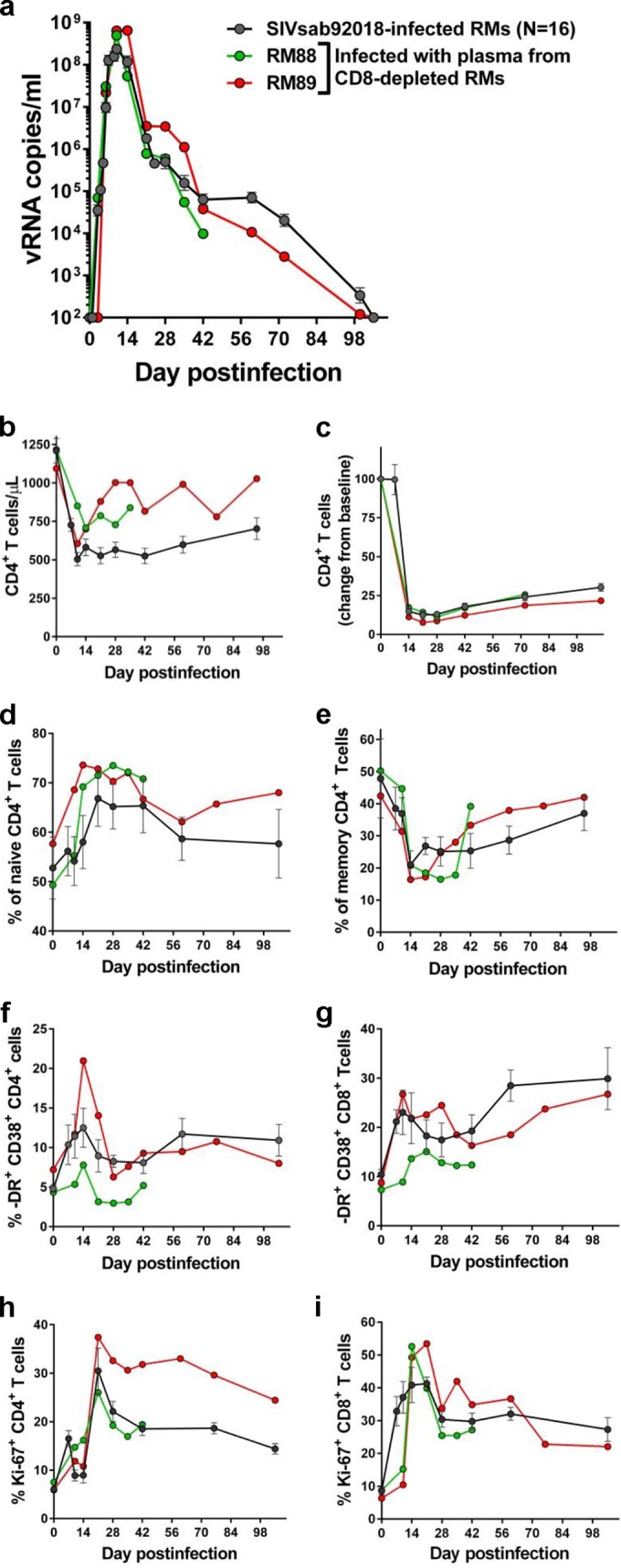
Rebounding SIVsab after CD8+ cell depletion in RM controllers is replication competent. Serial passage to naive RMs of pooled plasma collected from CD8+ cell-depleted RMs resulted in a productive infection which was indistinguishable from the infection with the original viral stock in terms of viral replication (a), peripheral (b) and mucosal (c) CD4+ T-cell depletion, changes in the frequency of naive (d) and memory (e) CD4+ T cells in the periphery, and immune activation (f, g) and proliferation (h, i) levels of CD4+ and CD8+ T cells, respectively.
Finally, we compared the diversity of the passaged virus to that of the virus reactivated in CD8+ cell-depleted RMs using single-genome amplification (SGA) and sequencing (15, 29).
Sequence analysis revealed that the reactivated virus exhibited relatively high diversity in the plasma samples from three CD8+ cell-depleted RMs, suggesting that a relatively large proportion of infected cells was at the origin of the virus rebound (Fig. 3). However, sequence analysis showed that up to 30% of the rebounding strains had stop codons or deletions (Fig. 3), similar to the HIV-1 strains induced in the viral outgrowth assay (30). Conversely, the RMs infected with pooled plasma from the CD8-depleted RMs harbored a mixture of two more homogenous lineages, confirming that only a fraction of the rebounding strains were replication competent (Fig. 3). Both reactivated and transmitted strains were relatively closely related to strains in the original viral stock, suggesting an early seeding of the reservoir, in agreement with other studies (21).
FIG 3.
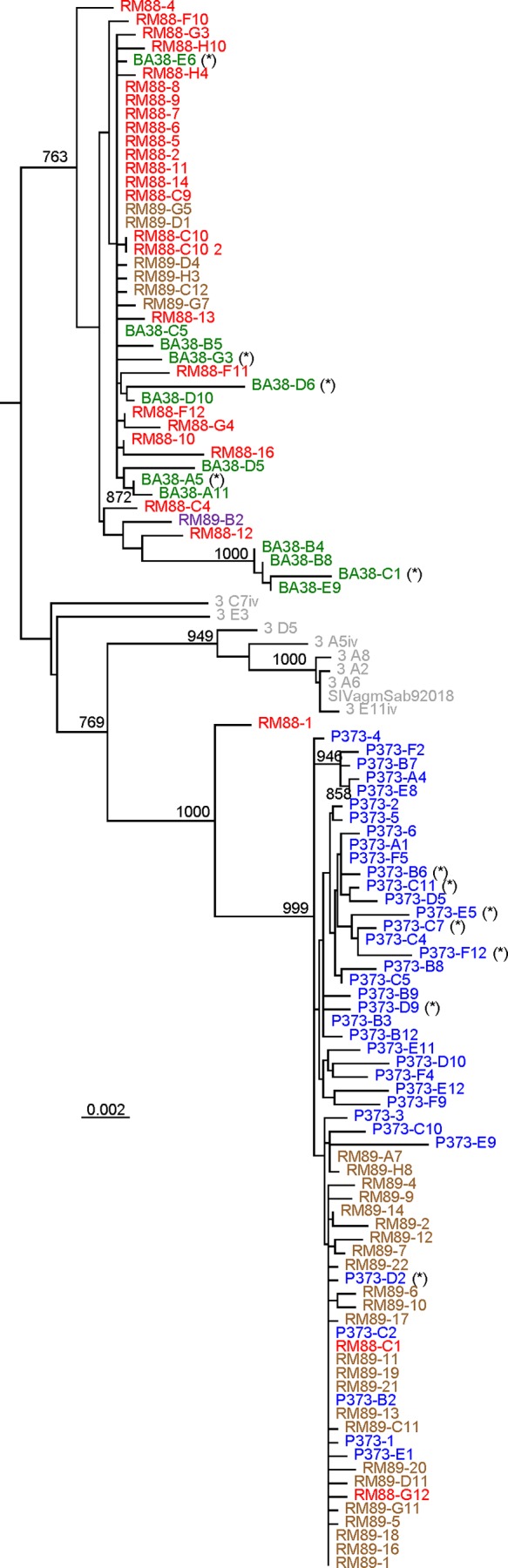
Phylogenetic evolutionary relationships of the plasma SIV strains (env sequences) from CD8-depleted RMs and from naive RMs that received pooled plasma containing the rebounding virus. Sequences from the same animal are color coded. RM88 (red) and RM89 (violet) are strains collected during the acute stage of infection from macaques infected with pooled plasma containing the rebounding virus after CD8 depletion. P373 (blue) and BA38 (green) are strains collected from CD8-depleted RM controllers (14) at the peak of viral rebound after CD8+ cell depletion. Original viral stock collected from an acutely infected AGM (13) is shown in gray. Defective sequences (stop codons or deletions) are marked with an asterisk (*). Numbers on nodes indicate bootstrap values of ≥80%; the scale bar represents genetic distance between strains.
Altogether, our results validate RM-SIVsab as an animal model of functionally cured lentiviral infection in which control of a replication-competent virus occurs in an immunologically competent host.
One of the current limitations of reactivation strategies is that in HIV-1-infected patients and in RMs infected with highly pathogenic simian immunodeficiency virus (SIV) strains, immune dysfunction prevents assessment of the efficacy of virus reactivation strategies. Current research is trying to identify strategies to improve cytotoxic T-lymphocyte (CTL) clearing of reactivated virus (31), as without improved CTL killing, the ability of “flush-and-kill” approaches (32, 33) to reduce the size of viral reservoirs cannot be properly assessed. In our model, immune cell dysfunction appears to be averted, and as such, this new model may be ideal for assessing different reactivation strategies with minimal confounding factors.
Our animal model of complete immunologic suppression of SIV infection in which latent infection occurs in all infected RMs in the absence of ART, but in the presence of a functional immune system, addresses a major limitation in the field, permitting the assessment of latency-reversing agents (LRAs) without the interference of confounding factors, such as incomplete control of replication by ART or the need to boost the immune responses to clear reactivated virus.
As we show here, this new model shares key features of viral persistence with pathogenic HIV and SIV infections, namely, rapid seeding of the viral reservoir, similar to that in both HIV (34) and SIV (21) infections and as suggested by our SGA data. Coreceptor usage and target cells infected during SIVagm infections in RMs are similar to those in other pathogenic and nonpathogenic HIV and SIV infections (14), suggesting that early seeding of the reservoir during SIVsab infection of RMs is similar to that in pathogenic HIV and SIV infection. Target cell similarity between SIVsab-infected RMs and other pathogenic HIV and SIV infections suggests a similar mechanism of viral persistence, i.e., the long decay of the central memory cells, the major reservoir of HIV and SIV. Finally, since this model employs RMs, it is expected that the decay of latently infected cells will be similar to that observed in macaques infected with pathogenic viruses.
Previous studies have shown that HIV and SIV share the general key features of virus persistence: (i) both HIV and SIV DNAs are integrated in the target cell genome (35, 36), and in similar ways (37), (ii) response to interferons results in transcriptional control of long terminal repeats (LTRs) through a bias of histone acetylation favoring HIV/SIV DNA persistence (38), (iii) costimulatory signals can induce latent HIV/SIV without coengagement of T-cell receptors (39), and (iv) distributions of cells containing HIV and SIV DNA and RNA sequences in peripheral blood, in lymph nodes (LNs), and at mucosal sites are similar in humans and macaques (40–43). In addition to the specific features described here for SIVsab infection in RMs, these general characteristics underlining the similar reservoir dynamics in HIV- and SIV-infected humans and macaques, respectively, strongly support the use of this new macaque model for various aspects of cure research. This new system has the advantage of being able to significantly reduce the costs and limitations associated with prolonged cART administration to nonhuman primates (NHPs), allows access to large volumes of tissues (compared to those of humanized mice), and is relatively simple. It can be used for a rapid in vivo screening of new LRAs. Once a new therapy demonstrates success in this unconventional model, it may be advanced in conventional ART-treated-RM models. As such, findings in this NHP model are relevant to clinical practice and may be used to improve the management of HIV-infected patients.
Nucleotide sequence accession numbers.
The env sequences analyzed here were deposited in GenBank under accession numbers KP896161 to KP896285.
ACKNOWLEDGMENTS
We thank Alan Landay and Daniel Douek for helpful discussions.
This work was supported by National Institutes of Health/National Center for Research Resources/National Heart, Lung and Blood Institute/National Institute of Allergy and Infectious Diseases grants R01 RR025781 (to C.A. and I.P.) and RO1 HL117715 (to I.P.). J.K. is supported by NIH training grant T32AI065380-10.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Allers K, Hutter G, Hofmann J, Loddenkemper C, Rieger K, Thiel E, Schneider T. 2011. Evidence for the cure of HIV infection by CCR5Delta32/Delta32 stem cell transplantation. Blood 117:2791–2799. doi: 10.1182/blood-2010-09-309591. [DOI] [PubMed] [Google Scholar]
- 2.Lewin SR, Evans VA, Elliott JH, Spire B, Chomont N. 2011. Finding a cure for HIV: will it ever be achievable? J Int AIDS Soc 14:4. doi: 10.1186/1758-2652-14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luzuriaga K, Gay H, Ziemniak C, Sanborn KB, Somasundaran M, Rainwater-Lovett K, Mellors JW, Rosenbloom D, Persaud D. 2015. Viremic relapse after HIV-1 remission in a perinatally infected child. N Engl J Med 372:786–788. doi: 10.1056/NEJMc1413931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henrich TJ, Hanhauser E, Hu Z, Stellbrink HJ, Noah C, Martin JN, Deeks SG, Kuritzkes DR, Pereyra F. 2015. Viremic control and viral coreceptor usage in two HIV-1-infected persons homozygous for CCR5 Δ32. AIDS doi: 10.1097/QAD.0000000000000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deeks SG, Barre-Sinoussi F. 2012. Public health: towards a cure for HIV. Nature 487:293–294. doi: 10.1038/487293a. [DOI] [PubMed] [Google Scholar]
- 6.International AIDS Society Scientific Working Group on HIV Cure, Deeks SG, Autran B, Berkhout B, Benkirane M, Cairns S, Chomont N, Chun TW, Churchill M, Di Mascio M, Katlama C, Lafeuillade A, Landay A, Lederman M, Lewin SR, Maldarelli F, Margolis D, Markowitz M, Martinez-Picado J, Mullins JI, Mellors J, Moreno S, O'Doherty U, Palmer S, Penicaud MC, Peterlin M, Poli G, Routy JP, Rouzioux C, Silvestri G, Stevenson M, Telenti A, Van Lint C, Verdin E, Woolfrey A, Zaia J, Barre-Sinoussi F. 2012. Towards an HIV cure: a global scientific strategy. Nat Rev Immunol 12:607–614. doi: 10.1038/nri3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saez-Cirion A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, Potard V, Versmisse P, Melard A, Prazuck T, Descours B, Guergnon J, Viard JP, Boufassa F, Lambotte O, Goujard C, Meyer L, Costagliola D, Venet A, Pancino G, Autran B, Rouzioux C, ANRS VISCONTI Study Group. 2013. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI study. PLoS Pathog 9:e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apetrei C, Pandrea I, Mellors JW. 2012. Nonhuman primate models for HIV cure research. PLoS Pathog 8:e1002892. doi: 10.1371/journal.ppat.1002892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shytaj IL, Norelli S, Chirullo B, Della Corte A, Collins M, Yalley-Ogunro J, Greenhouse J, Iraci N, Acosta EP, Barreca ML, Lewis MG, Savarino A. 2012. A highly intensified ART regimen induces long-term viral suppression and restriction of the viral reservoir in a simian AIDS model. PLoS Pathog 8:e1002774. doi: 10.1371/journal.ppat.1002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Prete GQ, Shoemaker R, Oswald K, Lara A, Trubey CM, Fast R, Schneider DK, Kiser R, Coalter V, Wiles A, Wiles R, Freemire B, Keele BF, Estes JD, Quinones OA, Smedley J, Macallister R, Sanchez RI, Wai JS, Tan CM, Alvord WG, Hazuda DJ, Piatak M Jr, Lifson JD. 2014. Effect of suberoylanilide hydroxamic acid (SAHA) administration on the residual virus pool in a model of combination antiretroviral therapy-mediated suppression in SIVmac239-infected Indian rhesus macaques. Antimicrob Agents Chemother 58:6790–6806. doi: 10.1128/AAC.03746-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denton PW, Olesen R, Choudhary SK, Archin NM, Wahl A, Swanson MD, Chateau M, Nochi T, Krisko JF, Spagnuolo RA, Margolis DM, Garcia JV. 2012. Generation of HIV latency in humanized BLT mice. J Virol 86:630–634. doi: 10.1128/JVI.06120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choudhary SK, Archin NM, Cheema M, Dahl NP, Garcia JV, Margolis DM. 2012. Latent HIV-1 infection of resting CD4+ T cells in the humanized Rag2−/− γc−/− mouse. J Virol 86:114–120. doi: 10.1128/JVI.05590-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandrea I, Apetrei C, Dufour J, Dillon N, Barbercheck J, Metzger M, Jacquelin B, Bohm R, Marx PA, Barre-Sinoussi F, Hirsch VM, Muller-Trutwin MC, Lackner AA, Veazey RS. 2006. Simian immunodeficiency virus SIVagm.sab infection of Caribbean African green monkeys: a new model for the study of SIV pathogenesis in natural hosts. J Virol 80:4858–4867. doi: 10.1128/JVI.80.10.4858-4867.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandrea I, Gaufin T, Gautam R, Kristoff J, Mandell D, Montefiori D, Keele BF, Ribeiro RM, Veazey RS, Apetrei C. 2011. Functional cure of SIVagm infection in rhesus macaques results in complete recovery of CD4+ T cells and is reverted by CD8+ cell depletion. PLoS Pathog 7:e1002170. doi: 10.1371/journal.ppat.1002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandrea I, Parrish NF, Raehtz K, Gaufin T, Barbian HJ, Ma D, Kristoff J, Gautam R, Zhong F, Haret-Richter GS, Trichel A, Shaw GM, Hahn BH, Apetrei C. 2012. Mucosal simian immunodeficiency virus transmission in African green monkeys: susceptibility to infection is proportional to target cell availability at mucosal sites. J Virol 86:4158–4168. doi: 10.1128/JVI.07141-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandell DT, Kristoff J, Gaufin T, Gautam R, Ma D, Sandler N, Haret-Richter G, Xu C, Aamer H, Dufour J, Trichel A, Douek DC, Keele BF, Apetrei C, Pandrea I. 2014. Pathogenic features associated with increased virulence upon Simian immunodeficiency virus cross-species transmission from natural hosts. J Virol 88:6778–6792. doi: 10.1128/JVI.03785-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kristoff J, Haret-Richter G, Ma D, Ribeiro RM, Xu C, Cornell E, Stock JL, He T, Mobley AD, Ross S, Trichel A, Wilson C, Tracy R, Landay A, Apetrei C, Pandrea I. 2014. Early microbial translocation blockade reduces SIV-mediated inflammation and viral replication. J Clin Invest 124:2802–2806. doi: 10.1172/JCI75090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wijewardana V, Kristoff J, Xu C, Ma D, Haret-Richter G, Stock JL, Policicchio BB, Mobley AD, Nusbaum R, Aamer H, Trichel A, Ribeiro RM, Apetrei C, Pandrea I. 2013. Kinetics of myeloid dendritic cell trafficking and activation: impact on progressive, nonprogressive and controlled SIV infections. PLoS Pathog 9:e1003600. doi: 10.1371/journal.ppat.1003600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC. [Google Scholar]
- 20.Hilldorfer BB, Cillo AR, Besson GJ, Bedison MA, Mellors JW. 2012. New tools for quantifying HIV-1 reservoirs: plasma RNA single copy assays and beyond. Curr HIV/AIDS Rep 9:91–100. doi: 10.1007/s11904-011-0104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitney JB, Hill AL, Sanisetty S, Penaloza-MacMaster P, Liu J, Shetty M, Parenteau L, Cabral C, Shields J, Blackmore S, Smith JY, Brinkman AL, Peter LE, Mathew SI, Smith KM, Borducchi EN, Rosenbloom DI, Lewis MG, Hattersley J, Li B, Hesselgesser J, Geleziunas R, Robb ML, Kim JH, Michael NL, Barouch DH. 2014. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature 512:74–77. doi: 10.1038/nature13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pandrea I, Gautam R, Ribeiro R, Brenchley JM, Butler IF, Pattison M, Rasmussen T, Marx PA, Silvestri G, Lackner AA, Perelson AS, Douek DC, Veazey RS, Apetrei C. 2007. Acute loss of intestinal CD4+ T cells is not predictive of SIV virulence. J Immunol 179:3035–3046. doi: 10.4049/jimmunol.179.5.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandrea I, Kornfeld C, Ploquin MJ, Apetrei C, Faye A, Rouquet P, Roques P, Simon F, Barre-Sinoussi F, Muller-Trutwin MC, Diop OM. 2005. Impact of viral factors on very early in vivo replication profiles in simian immunodeficiency virus SIVagm-infected African green monkeys. J Virol 79:6249–6259. doi: 10.1128/JVI.79.10.6249-6259.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okoye A, Park H, Rohankhedkar M, Coyne-Johnson L, Lum R, Walker JM, Planer SL, Legasse AW, Sylwester AW, Piatak M Jr, Lifson JD, Sodora DL, Villinger F, Axthelm MK, Schmitz JE, Picker LJ. 2009. Profound CD4+/CCR5+ T cell expansion is induced by CD8+ lymphocyte depletion but does not account for accelerated SIV pathogenesis. J Exp Med 206:1575–1588. doi: 10.1084/jem.20090356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stockhausen MT, Kristoffersen K, Stobbe L, Poulsen HS. 2014. Differentiation of glioblastoma multiforme stem-like cells leads to downregulation of EGFR and EGFRvIII and decreased tumorigenic and stem-like cell potential. Cancer Biol Ther 15:216–224. doi: 10.4161/cbt.26736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy JP, Haddad EK, Sekaly RP. 2009. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chomont N, DaFonseca S, Vandergeeten C, Ancuta P, Sekaly RP. 2011. Maintenance of CD4+ T-cell memory and HIV persistence: keeping memory, keeping HIV. Curr Opin HIV AIDS 6:30–36. doi: 10.1097/COH.0b013e3283413775. [DOI] [PubMed] [Google Scholar]
- 28.Ho YC, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DI, Lai J, Blankson JN, Siliciano JD, Siliciano RF. 2013. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 155:540–551. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, Wei X, Jiang C, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY, Wood N, Seoighe C, Perelson AS, Bhattacharya T, Korber BT, Hahn BH, Shaw GM. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A 105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisele E, Siliciano RF. 2012. Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity 37:377–388. doi: 10.1016/j.immuni.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katlama C, Deeks SG, Autran B, Martinez-Picado J, van Lunzen J, Rouzioux C, Miller M, Vella S, Schmitz JE, Ahlers J, Richman DD, Sekaly RP. 2013. Barriers to a cure for HIV: new ways to target and eradicate HIV-1 reservoirs. Lancet 381:2109–2117. doi: 10.1016/S0140-6736(13)60104-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Margolis DM. 2011. Eradication therapies for HIV infection: time to begin again. AIDS Res Hum Retroviruses 27:347–353. doi: 10.1089/aid.2011.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Margolis DM. 2011. Histone deacetylase inhibitors and HIV latency. Curr Opin HIV AIDS 6:25–29. doi: 10.1097/COH.0b013e328341242d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. 1998. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci U S A 95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen A, Zink MC, Mankowski JL, Chadwick K, Margolick JB, Carruth LM, Li M, Clements JE, Siliciano RF. 2003. Resting CD4+ T lymphocytes but not thymocytes provide a latent viral reservoir in a simian immunodeficiency virus-Macaca nemestrina model of human immunodeficiency virus type 1-infected patients on highly active antiretroviral therapy. J Virol 77:4938–4949. doi: 10.1128/JVI.77.8.4938-4949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishimura Y, Sadjadpour R, Mattapallil JJ, Igarashi T, Lee W, Buckler-White A, Roederer M, Chun TW, Martin MA. 2009. High frequencies of resting CD4+ T cells containing integrated viral DNA are found in rhesus macaques during acute lentivirus infections. Proc Natl Acad Sci U S A 106:8015–8020. doi: 10.1073/pnas.0903022106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crise B, Li Y, Yuan C, Morcock DR, Whitby D, Munroe DJ, Arthur LO, Wu X. 2005. Simian immunodeficiency virus integration preference is similar to that of human immunodeficiency virus type 1. J Virol 79:12199–12204. doi: 10.1128/JVI.79.19.12199-12204.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barber SA, Gama L, Dudaronek JM, Voelker T, Tarwater PM, Clements JE. 2006. Mechanism for the establishment of transcriptional HIV latency in the brain in a simian immunodeficiency virus-macaque model. J Infect Dis 193:963–970. doi: 10.1086/500983. [DOI] [PubMed] [Google Scholar]
- 39.Shen A, Yang HC, Zhou Y, Chase AJ, Boyer JD, Zhang H, Margolick JB, Zink MC, Clements JE, Siliciano RF. 2007. Novel pathway for induction of latent virus from resting CD4+ T cells in the simian immunodeficiency virus/macaque model of human immunodeficiency virus type 1 latency. J Virol 81:1660–1670. doi: 10.1128/JVI.01396-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bourry O, Mannioui A, Sellier P, Roucairol C, Durand-Gasselin L, Dereuddre-Bosquet N, Benech H, Roques P, Le Grand R. 2010. Effect of a short-term HAART on SIV load in macaque tissues is dependent on time of initiation and antiviral diffusion. Retrovirology 7:78. doi: 10.1186/1742-4690-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mannioui A, Bourry O, Sellier P, Delache B, Brochard P, Andrieu T, Vaslin B, Karlsson I, Roques P, Le Grand R. 2009. Dynamics of viral replication in blood and lymphoid tissues during SIVmac251 infection of macaques. Retrovirology 6:106. doi: 10.1186/1742-4690-6-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sellier P, Mannioui A, Bourry O, Dereuddre-Bosquet N, Delache B, Brochard P, Calvo J, Prevot S, Roques P. 2010. Antiretroviral treatment start-time during primary SIV(mac) infection in macaques exerts a different impact on early viral replication and dissemination. PLoS One 5:e10570. doi: 10.1371/journal.pone.0010570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kearney M, Spindler J, Shao W, Maldarelli F, Palmer S, Hu SL, Lifson JD, KewalRamani VN, Mellors JW, Coffin JM, Ambrose Z. 2011. Genetic diversity of simian immunodeficiency virus encoding HIV-1 reverse transcriptase persists in macaques despite antiretroviral therapy. J Virol 85:1067–1076. doi: 10.1128/JVI.01701-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


