Abstract
Curcumin is a naturally occurring compound which has been used in traditional medicine in India for a long time. This study investigated the ability of curcumin to inhibit the contractility of isolated caprine (goat) detrusor muscle. The ability of three concentrations of curcumin (30, 100 and 300 µM) to inhibit the 100 µM acetylcholine-induced contractility of the isolated caprine urinary bladder detrusor muscle was investigated. The effect of raising the concentration of acetylcholine from 100, 200 and 400 µM to overcome the curcumin-induced inhibition of detrusor contractility and the effects of the reversal agents tetraethylammonium, a potassium channel blocker (100 µM), glibenclamide, an ATP-sensitive potassium channel blocker (10 µM), and propranolol, a beta adrenergic receptor blocker (1 µM), on the inhibitory effect of detrusor contractility was also studied. Curcumin caused a concentration-dependent inhibition of acetylcholine-induced contractility of the isolated detrusor muscle which was statistically significant at all three concentrations of curcumin used. This inhibition was partially overcome by raising the concentration of ACh to 200 and 400 µM. The inhibition was overcome by the concurrent administration of tetraethylammonium. Glibenclamide reversed the inhibitory effect of 100 µM curcumin, but not that of 300 µM curcumin. Propranolol reversed the inhibitory effect of 100 µM curcumin but not that of 300 µM curcumin. These results suggest that curcumin inhibited the contractions of the isolated detrusor muscle. The results further suggest that the inhibitory effect is mediated by various mechanisms: stimulation of beta adrenergic receptors; an anticholinergic effect; and the opening of ATP-sensitive potassium channels.
Keywords: Contractions, curcumin, goat, isolated detrusor
Curcumin (diferuloylmethane) is obtained from the plant Curcuma longa, but is also available in the synthetic form. It is an orange colored powder with a molecular weight of 368.38. It is insoluble in water but soluble in a number of solvents like ethanol, acetone, and methanol. Curcumin has been used in traditional medicine in India for hundreds of years for the treatment of ailments like diarrhoea and asthma. Indeed, it has a wide range of pharmacological effects in animals and humans. One of the pharmacological actions of curcumin is to inhibit the contractility of smooth muscles. It has been shown to inhibit the contractions of isolated rat aorta[1], guinea pig ileum[2], rat uterus[2], rabbit jejunum[3] and rabbit trachea[3]. Curcumin has also been shown to inhibit the contractility of isolated urinary bladder, but in only two studies[4,5]. Overactive bladder (OAB) is an increasingly common clinical problem which is treated with drugs that relax the detrusor muscle. At present the drug therapy of OAB mainly comprises anticholinergics[6]. The presently used drugs for OAB are not always effective and frequently produce adverse effects[6]. Hence, new drugs that could be used to treat OAB with good efficacy and safety will be useful in clinical practice. In this context, we studied the inhibitory effect of curcumin on isolated caprine (goat) detrusor using a methodology reported earlier for studying the effect of drugs on the isolated detrusor[7,8,9]. Caprine detrusor muscle was used in this study because of easy availability and similarity in sizes of the goat and human urinary bladders.
Ten fresh goat bladders were obtained from the local slaughter house and transported to the pharmacology laboratory in Krebs solution. The composition of Krebs solution was in mM, NaCl: 118, KCl: 4.7, CaCl2 : 2.5, MgSO4 : 1.2, NaHCO3 : 25, KH2 PO4 : 1.2, and glucose: 5.55. In the laboratory, ten strips of detrusor muscle measuring 10×3 mm were cut from the urinary bladder. The detrusor strips were mounted vertically in a 20 ml organ bath containing adequately oxygenated Krebs solution maintained at 37°. A tension of 2 g was applied and an equilibration period of 90 min was allowed. The study was approved by the Institutional Animal Ethics Committee (file number: 7110, dated 10 March 2010).
Acetylcholine (ACh; Sigma Aldrich, St Louis, MO, USA) was dissolved in distilled water to obtain a stock solution of 7 mg/ml. Curcumin (Sigma Aldrich, St Louis, MO, USA) was dissolved in ethanol to produce stock solutions of 3.75 mg/ml and 6.33 mg/ml. Propranolol (Samarth Life Sciences, Mumbai, India) was dissolved in distilled water to make a stock solution of 0.1 mg/ml. Glibenclamide (Sigma Aldrich, St Louis, MO, USA) was dissolved in distilled water to make a stock solution of 1 mg/ml. Methylene blue (Fisher Scientific, Mumbai, India) was dissolved in distilled water to make a stock solution of 5 mg/ml.
After an equilibration period of 90 min, the tension was readjusted to 2 g. The response of the detrusor to the administration of 100 µM ACh was then studied followed by the response after the administration of 100 µM ACh and the test drug curcumin. This concentration of ACh was based on previous studies that had used the same concentration[8,9]. Three concentrations of curcumin were used, 30, 100, and 300 µM. Next, the experiments were repeated usingfirst 200 µM ACh, and then 400 µM ACh. During each tracing, after the drug administration, a contact time of 90 s was given after which the tissue was washed till the baseline was reached.
In order to determine the mechanism of inhibition of curcumin on ACh-induced detrusor contractility, the following inhibitory agents were added along with 100 µM and 300 µM curcumin after the addition of 100 µM and 300 µM ACh: 100 µM TEA, 1 µM propranolol, and 10 µM glibenclamide. The concentrations of these antagonists used were those that have been used in previous in vitro studies[9,10,11].
Contractility was quantified by the maximum height of contraction and the area under the contractile curve (AUCC), a method which has been standardized in our laboratory[7,8,9]. These parameters were determined by scanning the tracings after each experiment. The scanned tracings were analyzed with the software Image Tool (University of Texas Health Sciences Center at San Antanio, Texas, USA). Statistical evaluation was made by comparing the values of these parameters of the control data (after the administration of ACh alone) and the values of the test data (after the administration of the test drug with ACh). The nonparametric test, Wilcoxon sign rank test, was employed because the sample size used was 10 and hence, the study data might not have had a normal (Gaussian) distribution.
The results of the effect of curcumin on ACh-induced contractility of isolated caprine detrusor muscle are shown in Table 1. As shown, curcumin produced a concentration-dependent inhibitory effect of ACh-induced detrusor contractility which was statistically significant at all 3 concentrations of curcumin used. This inhibitory effect of curcumin on ACh-induced detrusor contractility was partially overcome by raising the concentration of ACh. Table 2 shows the effects of the reversal agents on the inhibition by curcumin of ACh-induced detrusor contractility. As shown, TEA reversed the inhibition of 100 and 300 µM curcumin. Propranolol reversed the inhibition due to 100 µM curcumin but not that due to 300 µM curcumin. Glibenclamide partially reversed the inhibition due to 100 and 300 µM curcumin. Sample tracings of the inhibitory effect of curcumin on ACh-induced contractility of the detrusor and the reversal of the inhibitory effect by TEA are shown in fig 1.
TABLE 1.
PERCENT INHIBITION OF ACETYLCHOLINE (ACH)-INDUCED CONTRACTILITY OF ISOLATED CAPRINE DETRUSOR MUSCLE BY CURCUMIN
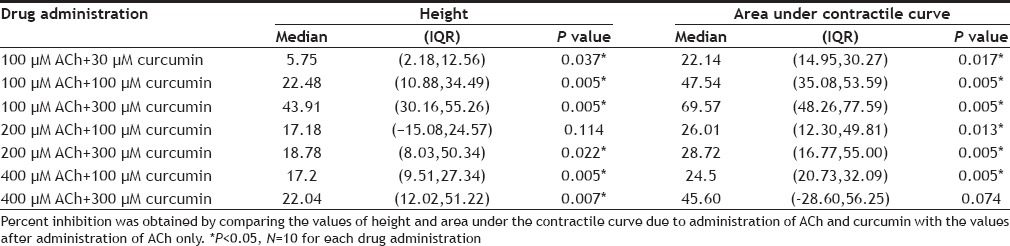
TABLE 2.
PERCENT INHIBITION OF ACH-INDUCED CONTRACTILITY OF ISOLATED CAPRINE DETRUSOR AFTER ADMINISTRATION OF REVERSAL AGENTS WITH CURCUMIN
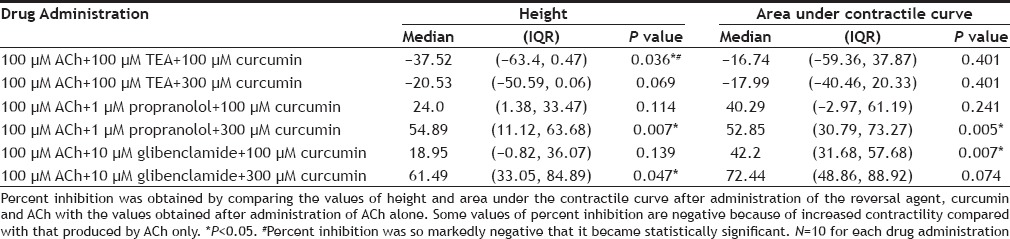
Fig. 1.
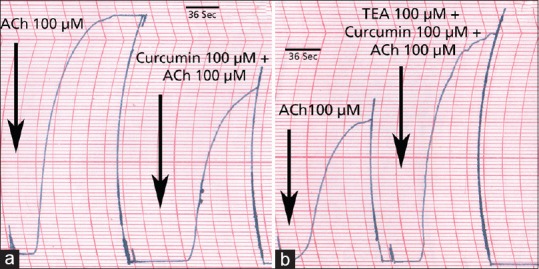
Effect of curcumin on ACh-induced contractility under various experimental conditions.
Examples of traces showing the effect of curcumin on ACh-induced contractility of isolated caprine detrusor. (a) Contractile effect of 100 μM ACh before (left side) and after (right side) addition of 100 μM curcumin. (b) Contractile effect of 100 μM ACh before (left side) and after (right side) addition of 100 μM tetraethylammonium (TEA) and 100 μM curcumin.
This study found that curcumin produced a concentration-dependent inhibition of ACh-induced detrusor contractility (Tables 1 and 2; fig. 1). These results suggest that curcumin relaxes the isolated detrusor and support the study of Patacchini et al.[4] which found that in isolated rat urinary bladder curcumin at concentrations of 10 to 300 µM was effective in desensitizing the bladder to the contractile effects of capsaicin although in that study the antagonistic effect could have at least partly been due to a mechanical effect of undissolved curcumin on the rat urinary bladder muscle. Our results also support studies that have reported a relaxant effect of curcumin on other types of isolated smooth muscle[1,2,3]. Since the inhibitory effect of curcumin on detrusor contractility was reversed by glibenclamide, our results also suggest that curcumin relaxes the isolated detrusor muscle by opening ATP-sensitive potassium channels. Glibenclamide, a second generation sulphonylurea oral hypoglycaemic agent, is well known to act by blocking ATP-sensitive potassium channels. Although the ability of curcumin to open ATP-sensitive channels has not been shown in smooth muscle, curcumin has been shown to exert an antinociceptive effect in Wistar rats by opening ATP-sensitive channels[12]. The inhibitory effect of curcumin on ACh-induced contractility of the isolated detrusor could be partially overcome by raising the concentration of ACh (Table 1). Hence, curcumin could have exerted an anticholinergic effect leading to detrusor muscle relaxation. The inhibitory effect of 100 µM curcumin could also be reversed by the beta adrenergic receptor blocker propranolol (Table 2). Hence, curcumin could have also had an agonistic effect at beta adrenergic receptors in the detrusor muscle. There are close similarities between the structure of curcumin and the structure of noradrenaline[13], and a previous study on cheek pouch tissue exteriorized in anaesthetized male hamsters found that curcumin mediated both dilation and constriction of peripheral arterioles by stimulating alpha and beta adrenergic receptors[14]. Unlike in our study, the study by Cheng et al.[5] found that curcumin in a concentration-dependent manner caused increased contractility of the isolated rat urinary bladder. However, their methodology was different from that of our study in that they measured isometric tension in muscle strips using strain gauges and chart software. Moreover, they had anaesthetized the rats with injection pentobarbital at a dose of 50 mg/Kg. It must also be noted that Cheng et al.[5] found that curcumin at the same concentrations that they used on rat urinary bladder, did not modify the muscle tone of urinary bladder isolated from mice.
In conclusion, the present study has shown that curcumin inhibits the contractility of the isolated caprine detrusor. The results suggest that this effect is due to an anticholinergic effect, an agonistic effect on beta adrenergic receptors, as well as the opening of ATP-sensitive potassium channels. The results suggest that further studies on the effect of curcumin on the detrusor muscle are warranted. If the inhibitory effect of curcumin on the detrusor is confirmed, it could be evaluated for the management of clinical conditions like OAB which could benefit from the relaxant effect on the detrusor muscle.
ACKNOWLEDGEMENTS
This study was funded by an intramural research grant. The authors thank Dr. Jacob Peedicayil for his help in the experimental work as well as in the preparation of the manuscript. The authors also thank Dr. Girish Naik for help with statistical analysis.
Footnotes
Manvizhi, et al.: Effect of Curcumin on Detrusor Muscle
REFERENCES
- 1.Sasaki Y, Goto H, Tohda C, Hatanaka F, Shibahara N, Shimada Y, et al. Effect of Curcuma drugs on vasomotion in isolated rat aorta. Biol Pharm Bull. 2003;26:1135–43. doi: 10.1248/bpb.26.1135. [DOI] [PubMed] [Google Scholar]
- 2.Itthipanichpong C, Ruangrungsi N, Kemsri W, Sawasdipanich A. Antispasmodic effects of curcuminoids on isolated guinea-pig ileum and rat uterus. J Med Assoc Thai. 2003;86(Suppl 2):S299–309. [PubMed] [Google Scholar]
- 3.Gilani AH, Shah AJ, Ghayur MN, Majeed K. Pharmacological basis for the use of turmeric in gastrointestinal and respiratory disorders. Life Sci. 2005;76:3089–105. doi: 10.1016/j.lfs.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Patacchini R, Maggi CA, Meli A. Capsaicin-like activity of some natural pungent substances on peripheral endings of visceral primary afferents. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:72–7. doi: 10.1007/BF00178975. [DOI] [PubMed] [Google Scholar]
- 5.Cheng TC, Lu CC, Chung HH, Hsu CC, Kakizawa N, Yamada S, et al. Activation of muscarinic M-1 cholinoceptors by curcumin to increase contractility in urinary bladder isolated from Wistar rats. Neurosci Lett. 2010;473:107–9. doi: 10.1016/j.neulet.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 6.Hood B, Andersson KE. Common theme for drugs effective in overactive bladder: Inhibition of afferent signaling from the bladder. Int J Urol. 2013;20:21–7. doi: 10.1111/j.1442-2042.2012.03196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faruqui AR, Mathai J, George J, Peedicayil L, Ernest K, Neelakanthan N. Inhibitory effect of nicorandil on the contractility of isolated human urinary bladder detrusor muscle. Methods Find Exp Clin Pharmacol. 2008;30:363–6. doi: 10.1358/mf.2008.30.5.1186078. [DOI] [PubMed] [Google Scholar]
- 8.George N, Shiny PJ, Miriam J, Nancy CA, Dhanasekar KR, Peedicayil J. Inhibitory effect of anticholinergics on the contraction of isolated caprine urinary bladder detrusor muscle. Auton Autacoid Pharmacol. 2010;30:173–7. doi: 10.1111/j.1474-8673.2009.00447.x. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Prabha R, Paul T, Shanthi M, George J, Peedicayil J, et al. Inhibitory effect of tramadol on isolated caprine detrsuor muscle. Auton Autacoid Pharmacol. 2012;32:15–22. doi: 10.1111/j.1474-8673.2012.00470.x. [DOI] [PubMed] [Google Scholar]
- 10.Heepe P, Starke K. Alpha-adrenoceptor antagonists and the release of nor-adrenaline in rabbit cerebral cortex slices: Support for the alpha-autoreceptor hypothesis. Br J Pharmacol. 1985;84:147–55. [PMC free article] [PubMed] [Google Scholar]
- 11.Prabhakaran SS, Dhanasekhar KR, Thomas E, Jose R, Peedicayil J, Samuel P. Inhibition of isolated human myometrium by minoxidil and reversal by glibenclamide. Methods Find Exp Clin Pharmacol. 2010;32:97–100. doi: 10.1358/mf.2010.32.2.1434163. [DOI] [PubMed] [Google Scholar]
- 12.De Paz Campas MA, Chavez Pina AE, Ortiz MI, Castaneda Hernandez G. Evidence for the participation of ATP-sensitive potassium channels in the antinociceptive effect of curcumin. Korean J Pain. 2012;25:221–7. doi: 10.3344/kjp.2012.25.4.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: An age-old spice with modern targets. Trends Pharmacol Sci. 2009;30:85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Dewar AM, Clark RA, Singer AJ, Frame MD. Curcumin mediates both dilation and constriction of peripheral arterioles via adrenergic receptors. J Invest Dermatol. 2011;131:1754–60. doi: 10.1038/jid.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]


