Abstract
Objective
Controversy persists regarding the use of the low-dose adrenocorticotropic hormone (ACTH) stimulation test (LDST) for the diagnosis of adrenal insufficiency (AI) and optimal test result interpretation. However, many centers are now using the LDST to assess cortisol secretion adequacy, and some only use a 30-minute cortisol level to determine adrenal sufficiency or AI. This study examined both 30- and 60-minute cortisol levels to assess whether the interpretation of the test was affected when both cortisol levels were taken into consideration.
Methods
Data were obtained by retrospective chart review from a single pediatric endocrinology unit over a 7-year period. We identified 82 patients who completed the LDST. Their mean age was 11.7 years, and 37% were female. Cortisol levels were evaluated at baseline and 30 and 60 minutes after cosyntropin administration. A cutoff value ≥18 μg/dL was used to define adrenal sufficiency.
Results
We found that 54% of patients reached peak cortisol levels at 60 minutes, and 11 patients who did not pass the test at 30 minutes did so at 60 minutes. The only predictive characteristic was weight status; overweight and obese individuals tended to peak at 30 minutes, and normal and underweight individuals tended to peak at 60 minutes.
Conclusion
Although further studies are necessary to confirm our findings, it appears that measuring cortisol both 30 and 60 minutes following synthetic ACTH administration may be necessary to avoid overdiagnosing AI.
INTRODUCTION
Despite controversy regarding the use of low-versus high-dose adrenocorticotrophic hormone (ACTH) stimulation testing for diagnosing adrenal insufficiency (AI), particularly for central AI, low-dose corticotropin stimulation tests (LDSTs) are now routinely used in many centers. Many studies that evaluated the LDST assessed cortisol levels 0, 30, and 60 minutes following synthetic ACTH administration reported that mean cortisol concentrations were highest at 30 minutes (1-3). Based on this finding, some recommend utilizing the 30-minute cortisol value as the test of choice for evaluating adrenal function using the LDST (2,4-6). Consequently, some centers now limit blood sampling to 0 and 30 minutes following synthetic ACTH administration.
However, many institutions (including ours) continue to assess cortisol levels 0, 30, and 60 minutes following synthetic ACTH administration for the LDST. Anecdotally, endocrinologists at our center have noted that in some patients, peak cortisol values occur at 60 rather than at 30 minutes. This raises concerns regarding potential AI overdiagnosis (if only the 30-minute cortisol level is taken into consideration) in patients who would have otherwise passed the test based on their 60-minute cortisol level.
Based on this concern, we investigated data from LDSTs at our center, with particular attention paid to patients whose cortisol levels peaked at 60 rather than 30 minutes, as well as the number of patients who would have been reclassified as adrenally sufficient if the test included a 60-minute cortisol value. In addition, to determine possible predictors of timing of peak cortisol, we evaluated demographic and clinical characteristics relative to peak cortisol value timing.
METHODS
Data were collected from the electronic medical record at Massachusetts General Hospital for Children from January 2007 through April 2013. Eighty-four patients had 97 LDSTs completed in the Pediatric Endocrinology Unit during this time. Approval was obtained from the Partners HealthCare Institutional Review Board, and data were retrospectively collected in compliance with the Health Insurance Portability and Accountability Act. Based on Institutional Review Board guidelines, informed consent from the parents/patients over 18 and assent from the children were not required. Of the 97 tests, 13 were excluded because they were repeat tests in the same patient. For the 13 patients with repeat testing, the result was preferentially excluded if data regarding height and weight were missing. If data were not missing, the older test was excluded. Two additional tests were excluded due to the highest cortisol value being at 0 minutes.
At the Massachusetts General Hospital for Children, the protocol for a low-dose ACTH stimulation test is as follows: orders are placed by the physician with the calculated dose of cosyntropin (synthetic ACTH) (1 mcg/m2) based on the most recent height and weight available, which is typically rounded up or down for ease of administration. Cosyntropin is diluted in 0.9% normal saline to a concentration of 1 mcg/mL. An intravenous catheter is placed, and blood drawn for 0-minute cortisol and ACTH levels. The dose of cosyntropin is administered over 2 minutes. Subsequently, cortisol levels are drawn at 30 and 60 minutes, and the intravenous catheter is removed after the 60-minute blood draw.
We obtained clinical information from a retrospective review of the electronic medical record and collected information regarding date of birth; sex; anthropometric data on which the cosyntropin dose was based; diagnosis prompting the test; exposure to pituitary irradiation and radiation dose (if available); presence of other pituitary deficiencies; test date and time; cosyntropin dose; the 0-minute ACTH level; 0-, 30-, and 60-minute cortisol levels; and data regarding whether or not the child was started on glucocorticoid replacement following the test. When available, we also collected data on brain magnetic resonance imaging (MRI) results (within 1 year of the test) and bone age (within 3 months of the test). Finally, we collected data regarding levels of thyroid-stimulating hormone, free thyroxine, insulin-like growth factor 1 (IGF-1), IGF-binding protein 3, prolactin, luteinizing hormone, follicle-stimulating hormone, testosterone, and estradiol when available within 1 month of the test. Body mass index (BMI) and BSA were calculated; body surface area (BSA) using the Mosteller equation, and zBMI (or BMI SDS) determined from the 2000 NCHS dataset, which provides BMI data for ages 2 to 20 for both sexes. To maximize our ability to calculate zBMI scores in our sample, a patient who was 23 months old and 3 patients who were between 20 and 20.5 years of age had their ages rounded up and down to the lower and upper limits of the NCHS data set, respectively, to determine their zBMIs. Four patients were too young for zBMI calculation.
Cortisol levels were assessed using a chemiluminescent microparticle immunoassay from Abbott Architect (Abbott Park, IL), and ACTH via a chemiluminescent immunometric assay from Siemens Immulite (Munich, Germany). For this study, we used a cortisol value ≥18 μg/dL (500 nmol/L) following cosyntropin administration to define adrenal sufficiency (1,7-9), and also utilized ≥16 μg/dL (10) and ≥20 μg (11) to compare various definitions for adrenal sufficiency found in the literature. In addition, zBMI≥1.64, which corresponds to the 85th percentile for BMI, was used to define overweight or obesity, and zBMI≤−1.64 to define underweight (11).
Statistical Analyses
Analyses were performed with SAS version 9.2 software (SAS Institute, Cary, NC). Statistical significance was based on a P value <.05. Analyses performed included the χ2 versus Fisher exact test when comparing proportions, the Student's t test when comparing means across groups, Pearson correlations to determine associations between variables, and logistic regression to determine whether the association between zBMI groups and peak cortisol timing persisted after controlling for age and the absolute cosyntropin dose.
RESULTS
Clinical Characteristics
Our subjects included 82 patients (mean age 11.7 ± 5.7 years) who presented to the Pediatric Endocrinology Outpatient Unit at Massachusetts General Hospital for Children between 2005 and 2012 for low-dose ACTH stimulation testing. Among our patients, 37% were female. Thirty-four had abnormal brain MRIs, of whom 13 had previously received radiation therapy. Fourteen patients had ≥1 other pituitary deficiencies, pubertal delay, or precocity but showed no structural anomalies on MRI. Three were assessed following a prolonged steroid wean, 3 had a history of traumatic brain injuries, and 9 had other autoimmune diseases and symptomatology concerning for AI. Seven had genetic syndromes associated with AI and 10 had symptoms concerning for AI. One patient had the test performed following surgery for Cushing disease, and another for a family history of panhypopituitarism and short stature. Overall, 16% had previous exposure to cranial irradiation, and 33% had ≥1 pituitary insufficiency. Eighty-seven percent of the tests were performed before noon. There was no significant difference in the clinical characteristics of patients who peaked at 30 versus 60 minutes or those who passed the test at 30 versus 60 minutes (Table 1).
Table 1.
| Variable | All patients | <18 at 30′ | ≥18 at 30′ | <18 at 60′ | ≥18 at 60′ |
|---|---|---|---|---|---|
| Number of patients | 82 | 50 | 32 | 45 | 37 |
| Age (y) | 11.6 ± 5.6 | 11.7 ± 5.8 | 11.4 ± 5.3 | 11.6 ± 5.5 | 11.6 ± 5.8 |
| Female, n (%) | 31 (38) | 18 (36) | 13 (41) | 16 (36) | 15 (41) |
| zBMI (n = 78) | 0.5 ± 1.3 | 0.4 ± 1.1 | 0.5 ± 1.4 | 0.8 ± 1.2 | 0.1 ± 1.4 |
| History of cranial irradiation, n (%) | 13 (16) | 9 (18) | 4 (13) | 7 (16) | 6 (16) |
| Patients with other pituitary insufficiencies, n (%) | 31 (38) | 20 (40) | 11 (34) | 20 (44) | 11 (30) |
| Tests done before noon, n (%) | 69 (84) | 42 (84) | 27 (84) | 38 (84) | 31 (84) |
| Patients started on glucocorticoids after LDST, n (%) | 27 (33) | 27 (54) | 0 (0) | 25 (56) | 2 (5) |
Abbreviations: LDST = low-dose suppression test; zBMI = body mass index z-score.
Data are presented as mean ± SD
Groups were < or ≥18 μg/dL) at 30 versus 60 minutes.
No significant differences were found between groups (all P>.05).
LDST
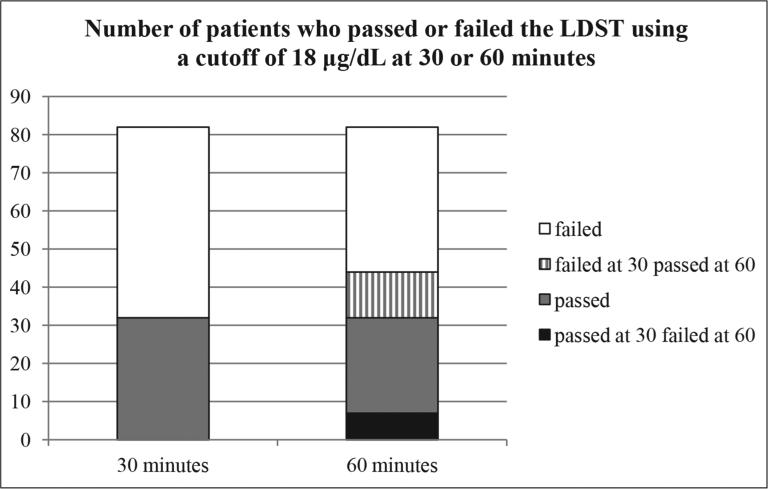
The mean cortisol levels at 0, 30, and 60 minutes were 7.4 ± 4.0, 17.5 ± 5.5, and 16.9 ± 7.1 μg/dL respectively. Forty-four patients (54%, confidence interval [CI] 43-64%) attained the peak cortisol level at 60 minutes. Of these patients who did not attain peak levels at 30 minutes (n = 44) and using a cortisol value ≥18 μg/dL to indicate adrenal sufficiency, continuing the test to 60 minutes would have resulted in 24% (11) of patients classified as AI based on the 30-minute cortisol level being reclassified as adrenally sufficient based on the 60-minute cortisol level. Conversely, 7 (9%) patients who passed the test at 30 minutes had a cortisol level <18 μg/dL at the 60-minute mark and would thus have been classified as AI if sampling had been performed at only 60 (and not 30) minutes. Three patients had a baseline cortisol ≥18 μg/dL (Fig. 1).
Fig. 1.
Participants who passed or failed the low-dose suppression test at 30 and 60 minutes using a cortisol level cutoff ≥18 μg/dL at either time point to indicate adrenal sufficiency. White = cortisol levels <18 μg/dL at both time points; striped = cortisol levels ≥18 μg/dL at 60 minutes only; gray = cortisol levels ≥18 μg/dL at both 30 and 60 minutes; black = cortisol levels ≥18 μg/dL at 30 minutes and <18 μg/dL at 60 minutes.
We also evaluated the cohort using cortisol values ≥16 μg/dL (4) and ≥20 μg/dL (10) to define AI. Using ≥16 μg/ dL, we found 45 (35%) patients failed at 30 minutes, 6 (7%) patients failed at 30 minutes but passed at 60, and 9 (10%) patients passed at 30 but failed at 60. The other 29 patients who failed at 30 minutes also failed at 60 minutes. With a cutoff ≥20 μg/dL, we found that 54 (63%) patients were classified as AI at 30 minutes, 8 (9%) patients failed at 30 minutes and passed at 60 minutes, and 6 (7%) patients passed at 30 minutes and failed at 60. The remaining 46 patients who failed at 30 minutes also failed at 60 minutes.
Predictors of Peak Cortisol Level Timing
There was no statistical significance between patients who peaked at 30 versus 60 minutes (Table 2) for sex (P = .28), presence of other pituitary insufficiencies (P = .45), previous exposure to cranial irradiation (P = .99), whether the test was done before or after noon (P = .99), and whether or not the patient was started on steroids (stress dose or replacement) after the test was performed (P = .24). However, those who were overweight or obese (zBMI≥1.64) tended to peak at 30 minutes, whereas those who were normal weight or underweight tended to peak at 60 minutes (P = .01). There was no difference between these zBMI groups for mean cortisol levels at 30 or 60 minutes or at the time of peak cortisol value regardless of peak timing. When we controlled for age and absolute cosyntropin dose, the significant association of overweight/obese versus normal/underweight status with timing of peak cortisol was maintained (P = .01).
Table 2.
Patient Characteristics by Peak Cortisol Timing
| Peaked at 30′ | Peaked at 60′ | P | |
|---|---|---|---|
| n (%) | 38 (46) | 44 (54) | |
| Sex, female | 12 (32) | 19 (43) | .28 |
| Weight status (n = 78) | .005 | ||
| Overweight/obese | 20 (56) | 9 (21) | |
| Normal weight | 14 (39) | 30 (68) | |
| Underweight | 2 (6) | 3 (7) | |
| Presence of other pituitary insufficiencies | 22 (58) | 29 (66) | .45 |
| Exposure to cranial irradiation | 6 (16) | 7 (16) | .99 |
| Presumed central vs. peripheral AI | |||
| Central | 31 (82) | 33 (75) | .36 |
| Unclear | 0 (0) | 3 (7) | |
| Peripheral | 7 (18) | 8 (18) | |
| am LDST | 32 (84) | 37 (84) | .99 |
| Patient on steroids after testing | 15 (40) | 12 (27) | .24 |
Abbreviations: AI = adrenal insufficiency; LDST = low-dose suppression test.
DISCUSSION
The LDST has been and still is hotly debated in the literature regarding its capacity to diagnose AI, especially central AI (4,12). In addition, studies differ in their recommendations regarding the criteria with which to evaluate LDST results (1-5). However, these studies have relied on the mean cortisol concentrations at specific time points following cosyntropin administration to determine these recommendations and have generally reported higher mean cortisol levels at 30 rather than 60 minutes. This in turn has led to terminating testing at 30 minutes at many centers, and the 30-minute cortisol level is used to determine whether or not the patient passes the LDST. Indeed, our own data show that mean cortisol concentrations are higher at 30 versus 60 minutes.
However, when examining the timing of peak cortisol levels following the LDST, as we have done in this study, it becomes clear that there is a significant proportion of individuals who peak at 60 rather than 30 minutes. This has implications for test interpretation, as many studies recommend using the peak cortisol level rather than that at 30 or 60 minutes after cosyntropin administration (1-3). Indeed, our findings demonstrate that continuing the LDST to 60 minutes (rather than terminating the test at 30 minutes) resulted in an additional 11 patients being classified as adrenally sufficient, as their cortisol levels peaked at 60 rather than 30 minutes. Thus, continuing the LDST to 60 minutes may reduce the number of individuals diagnosed with AI.
Interestingly, when evaluating predictors of peak cortisol level timing, the only predictive factor was weight status, with those in the overweight/obese and normal/underweight categories being more likely to peak at 30 and 60 minutes, respectively. While there is evidence of increased urinary free cortisol in children with metabolic syndrome (13) associated with waist circumference (14), their cortisol elevations appear to be related to visceral adiposity (15), and it is unclear why ACTH-stimulated cortisol levels should differ based on weight status. In our study, we found no significant difference in baseline serum cortisol levels based on weight status (zBMI); however, we did find a difference in peak cortisol timing. One may speculate that the overall tendency for higher cortisol levels in overweight/obese individuals also accounts for a steeper rise in cortisol levels following cosyntropin stimulation, reflecting an overdrive of the hypothalamic-pituitary-adrenal axis. However, we did not find a difference across groups based on weight status for cortisol levels at each time point after cosyntropin administration or in the peak cortisol level, regardless of the peak time.
Previous studies have reported that neither sex nor the time of day when the test is performed impacts the capacity of the adrenals to mount a reasonable response to the LDST (1,2). This would imply that these variables do not affect the timing of peak cortisol during the LDST, which is consistent with the present findings. We also examined factors that are typically associated with an increased risk of having central AI (history of previous radiation exposure to the pituitary and multiple pituitary insufficiencies) to determine whether this impacted peak cortisol timing, but we did not find any effects associated with these factors.
The limitations of this study include its retrospective design and lack of confirmatory testing with gold standard tests or long-term longitudinal data to evaluate the accuracy of the diagnosis of AI or sufficiency. It is particularly challenging to utilize longitudinal laboratory data, such as hyponatremia, hyperkalemia and hypoglycemia in assessments of definition of AI versus sufficiency in these children (16), given that (1) these laboratory findings are more likely in primary AI, whereas our cohort included patients with central as well as primary AI and (2) hypoglycemia from AI is mostly observed in infants and subjects on insulin (17). In addition, due to the retrospective design and desire to maximize the number of patients, this study included LDSTs ordered by multiple practitioners, all of whom have slightly different interpretations of LDSTs and thus started or did not start AI treatment based on their interpretation of the current literature. Also, it was not always clear whether the providers were concerned about central or peripheral AI. Furthermore, there was an insufficient number of infants (≤1 year of age) in the study population to evaluate peak cortisol timing in that age group, although our results held true when infants were removed from the analysis. There was also inadequate information to accurately determine the specific time of day each test was performed. It was, however, possible to determine whether the test began in the morning or afternoon, so an am versus pm categorization was possible. Further studies are necessary to comprehensively take into account these issues and questions.
CONCLUSION
In conclusion, while further study is necessary, especially regarding the impact of weight status on LDST results, our findings indicate that it may be necessary to evaluate cortisol levels both 30 and 60 minutes following cosyntropin administration to avoid overdiagnosing AI using the LDST.
ACKNOWLEDGMENT
This work was supported by NIH K24HD 071843 support to M.M. and with support and consultation from the Harvard Catalyst Statistical Consultation.
Abbreviations
- ACTH
adrenocorticotropic hormone
- AI
adrenal insufficiency
- BMI
body mass index
- LDST
low-dose ACTH stimulation test
- MRI
magnetic resonance imaging
Footnotes
DISCLOSURE
The authors have no multiplicity of interest to disclose.
REFERENCES
- 1.Tordjman K, Jaffe A, Grazas N, Apter C, Stern N. The role of the low dose (1 microgram) adrenocorticotropin test in the evaluation of patients with pituitary diseases. J Clin Endocrinol Metab. 1995;80:1301–1305. doi: 10.1210/jcem.80.4.7714104. [DOI] [PubMed] [Google Scholar]
- 2.Dickstein G, Shechner C, Nicholson WE, et al. Adrenocorticotropin stimulation test: effects of basal cortisol level, time of day, and suggested new sensitive low dose test. J Clin Endocrinol Metab. 1991;72:773–778. doi: 10.1210/jcem-72-4-773. [DOI] [PubMed] [Google Scholar]
- 3.Weintrob N, Sprecher E, Josefsberg Z, et al. Standard and low-dose short adrenocorticotropin test compared with insulin-induced hypoglycemia for assessment of the hypothalamic-pituitary-adrenal axis in children with idiopathic multiple pituitary hormone deficiencies. J Clin Endocrinol Metab. 1998;83:88–92. doi: 10.1210/jcem.83.1.4496. [DOI] [PubMed] [Google Scholar]
- 4.Kazlauskaite R, Evans AT, Villabona CV, et al. Corticotropin tests for hypothalamic-pituitary-adrenal insufficiency: a metaanalysis. J Clin Endocrinol Metab. 2008;93:4245–4253. doi: 10.1210/jc.2008-0710. [DOI] [PubMed] [Google Scholar]
- 5.Choi CH, Tiu SC, Shek CC, Choi KL, Chan FK, Kong PS. Use of the low-dose corticotropin stimulation test for the diagnosis of secondary adrenal insufficiency. Hong Kong Med J. 2002;8:427–434. [PubMed] [Google Scholar]
- 6.Rasmuson S, Olsson T, Hagg E. A low dose ACTH test to assess the function of the hypothalamic-pituitary-adrenal axis. Clin Endocrinol (Oxf) 1996;44:151–156. doi: 10.1046/j.1365-2265.1996.600482.x. [DOI] [PubMed] [Google Scholar]
- 7.Abdu TA, Elhadd TA, Neary R, Clayton RN. Comparison of the low dose short synacthen test (1 microg), the conventional dose short synacthen test (250 microg), and the insulin tolerance test for assessment of the hypothalamo-pituitary-adrenal axis in patients with pituitary disease. J Clin Endocrinol Metab. 1999;84:838–843. doi: 10.1210/jcem.84.3.5535. [DOI] [PubMed] [Google Scholar]
- 8.Suliman AM, Smith TP, Labib M, Fiad TM, McKenna TJ. The low-dose ACTH test does not provide a useful assessment of the hypothalamic-pituitary-adrenal axis in secondary adrenal insufficiency. Clin Endocrinol (Oxf) 2001;56:533–539. doi: 10.1046/j.1365-2265.2002.01509.x. [DOI] [PubMed] [Google Scholar]
- 9.Cho HY, Kim JH, Kim SW, et al. Different cut-off values of the insulin tolerance test, the high-dose short Synacthen test (250 μg) and the low-dose short Synacthen test (1 μg) in assessing central adrenal insufficiency. Clin Endocrinol (Oxf) 2014;81:77–84. doi: 10.1111/cen.12397. [DOI] [PubMed] [Google Scholar]
- 10.Maghnie M, Uga E, Temporini F, et al. evaluation of adrenal function in patients with growth hormone deficiency and hypothalamic-pituitary disorders: comparison between insulin-induced hypoglycemia, low-dose ACTH, standard ACTH and CRH stimulation tests. Eur J Endocrinol. 2005;152:735–741. doi: 10.1530/eje.1.01911. [DOI] [PubMed] [Google Scholar]
- 11.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;8:1–27. [PubMed] [Google Scholar]
- 12.Dorin RI, Qualls CR, Crapo LM. Diagnosis of adrenal insufficiency. Ann Intern Med. 2003;139:194–204. doi: 10.7326/0003-4819-139-3-200308050-00009. [DOI] [PubMed] [Google Scholar]
- 13.Reinehr T, Kulle A, Wolters B, et al. Relationships between 24-hour urinary free cortisol concentrations and metabolic syndrome in obese children. J Clin Endocrinol Metab. 2014;99:2391–2399. doi: 10.1210/jc.2013-4398. [DOI] [PubMed] [Google Scholar]
- 14.Hill EE, Eisenmann JC, Gentile D, Holmes ME, Walsh D. The association between morning cortisol and adiposity in children varies by weight status. J Pediatr Endocrinol Metab. 2011;24:709–713. doi: 10.1515/jpem.2011.267. [DOI] [PubMed] [Google Scholar]
- 15.Purnell JQ, Kahn SE, Samuels MH, Brandon D, Loriaux DL, Brunzell JD. Enhanced cortisol production rates, free cortisol, and 11beta-HSD-1 expression correlate with visceral fat and insulin resistance in men: effect of weight loss. Am J Physiol Endocrinol Metab. 2009;296:E351–E357. doi: 10.1152/ajpendo.90769.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsieh S, White PC. Presentation of primary adrenal insufficiency in childhood. J Clin Endocrinol Metab. 2011;96:E925–E928. doi: 10.1210/jc.2011-0015. [DOI] [PubMed] [Google Scholar]
- 17.Artavia-Loria E, Chaussah JL, Bougnères PF, Job JC. Frequency of hypoglycemia in children with adrenal insufficiency. Acta Endocrinol Suppl (Copenh) 1986;279:275–278. doi: 10.1530/acta.0.112s275. [DOI] [PubMed] [Google Scholar]