Abstract
Animals share an intimate and life-long partnership with a myriad of resident microbial species, collectively referred to as the microbiota. Symbiotic microbes have been shown to regulate nutrition and metabolism, and are critical for the development and function of the immune system. More recently, studies have suggested that gut bacteria can impact neurological outcomes – altering behavior and potentially affecting the onset and/or severity of nervous system disorders. In this review, we highlight emerging evidence that the microbiome extends its influence to the brain via various pathways connecting the gut to the central nervous system. While understanding and appreciation of a gut microbial impact on neurological function is nascent, unraveling gut-microbiome-brain connections holds the promise of transforming the neurosciences and revealing potentially novel etiologies for psychiatric and neurodegenerative disorders.
Introduction
Metazoans evolved in a world dominated by microbial life. Despite the long evolutionary history that has forged elaborate host-microbial symbioses over many millennia, it is only recently that science and society have begun to appreciate the inextricable connection between microbes and mammals. We are witnessing a groundswell of research that is describing and defining how gut bacteria (known as the microbiota) influence critical aspects of our physiology. The last decade of research has illuminated numerous complex interactions between the microbiota and the immune and metabolic systems, many of which have significant implications on human health. While the fascinating and profound mechanisms by which gut bacteria control immunity and metabolism has led to a modern renaissance in biomedical research, regulation of the nervous system by the microbiota had remained relatively unexplored until very recently (Mayer et al., 2014; Mayer et al., 2015; Stilling et al., 2014b). How could simple gut microbes influence a complex and distant organ such as the brain? This seemingly improbable concept that specific microbes influence the behavior and neurological function of their hosts had, in fact, already been established. One prime example of “microbial mind control” is the development of aggression and hydrophobia in mammals infected with the rabies virus (Driver, 2014). Another well-known example of behavior modification occurs by Toxoplasma gondii, which alters the host rodents’ fear response. Infected rodents lose their defensive behavior in the presence of feline predators, and instead actually become sexually attracted to feline odors (House et al., 2011). This results in infected rodents being preyed upon more readily by cats, and allows Toxoplasma to continue its lifecycle in the feline host (House et al., 2011). Further, a variety of parasitic microbes are capable of altering the locomotive behavior and environmental preferences of their hosts to the benefit of the microbe. For instance, the Spinochordodes tellinii parasite causes infected grasshopper hosts to not only jump more frequently, but also seek an aquatic environment where the parasite emerges to mate and produce eggs (Biron et al., 2005). Temperature preference of the host can even be altered, such as observed during infection of stickleback fish by Schistocephalus solidus, which changes the hosts’ preference from cooler waters to warmer waters where the parasite can grow more readily (Macnab and Barber, 2012). Other microbes can even alter host behavior to seek higher elevations, believed to allow the infected host to be noticed more easily by predators or to eventually fall and disperse onto susceptible hosts below (Maitland, 1994). More coercively still, microbes can influence the social behavior of their hosts, causing insects, such as ants, to become more or less social to the benefit of the parasite (Hughes, 2005). In fact, the sexually transmitted virus IIV-6/CrIV causes its cricket host (Gryllus texensis) to increase its desire to mate, causing its rate of mating to be significantly elevated and allowing for transmission between individual hosts (Adamo et al., 2014).
While all of the above examples most certainly represent pathogenic and/or parasitic relationships, they nonetheless raise the possibility that the indigenous microbes, which are in constant, life-long interaction with their human and animal hosts, could influence neurological function and behavior during development, or within health and disease states. It is becoming increasingly recognized that psychiatric and neurological illnesses are often co-morbid with gastrointestinal (GI) pathology (Vandvik et al., 2004), including schizophrenia, autism, neurodegenerative diseases and depression. Furthermore, recent observations have indicated that the commensal microbiota of the intestine do indeed alter aspects of their hosts’ neurological function, leading to effects on mood and behavior, including depression, anxiety, social behavior, and mate choice (Table 1) (Bravo et al., 2011; Desbonnet et al., 2010; Foster and McVey Neufeld, 2013; Hsiao et al., 2013; Neufeld et al., 2011; Sharon et al., 2010). The intestinal microbiota are, however, well established to have an profound impact in shaping the host immune system, which itself may subsequently influence host behavior (Dantzer et al., 2008), and indirectly have effects on neurodegeneration and repair during the process of aging, neurological trauma, and disease. The precise mechanisms of how the intestinal microbes impact neurological function and behavior remain largely unknown, but are likely vast, varied, and complex.
Table 1.
Selected phenotypic attributes influenced by gut microbes
| Category | Attribute | Effect | Citation(s) |
|---|---|---|---|
| Behavioral | Stress response | Increased response to restraint stress in germ-free (GF) mice | Sudo et al., 2004 |
| Behavioral | Anxiety-like behavior | Decreased anxiety-like behavior in GF mice (Swiss Webster, NIH Swiss, NMRI) | Clarke et al, 2013; Diaz Heijtz et al., 2011; Neufeld et al., 2011; Selkrig et al., 2014 |
| Behavioral | Anxiety-like behavior | Increased anxiety-like behavior in GF mice (BALB/c, C57Bl6) | Bercik et al., 2011; Selkrig et al., 2014 |
| Behavioral | Anxiety-like behavior | Reduced anxiety-like behavior in rodents treated with: Bifidobacterium breve 1205, B. longum 1714, B. longum R0175 Lactobacillus helveticus R0052, or L. rhamnosusJB-1 | Bravo et al., 2011; Messaoudi et al., 2011; Savignac et al., 2014 |
| Behavioral | Depression-like behavior | Decreased depression-like behavior in mice treated with B. infantis or L. rhamnosus JB-1 | Bravo et al., 2011; Desbonnet et al., 2010; Savignac et al., 2014; Savignac et al., 2015 |
| Behavioral | Emotional processing | Reduced activation following emotional stimulus in humans treated with probiotic milk product | Tillisch et al., 2013 |
| Behavioral | Depression | Reduced self-reported feelings of depression and aggression in humans treated with probiotics | Steenbergen et al., 2015 |
| Behavioral | Anxiety | Reduced self-reported anxiety in humans treated with L. helveticus R0052 and B. longum R0175 | Messaoudi et al., 2011 |
| Behavioral | Social recognition | Reduced novel and familiar social recognition in GF mice | Desbonnet et al., 2014 |
| Behavioral | Stereotyped behaviors, vocalizations | Restoration of social behaviors in Bacteroides fragilis treated MIA mice | Hsiao et al., 2012 |
| Hormonal | Corticosterone | Increased hypothalmic corticosterone in GF mice | Sudo et al., 2004 |
| Hormonal | Cortisol | Reduced urinary cortisol in humans treated with L. helveticus R0052 and B. longum R0175 | Messaoudi et al., 2011 |
| Neurochemical | Brain derived neurotrophic factor (BDNF) | Decreased hypothalmic BDNF in GF mice | Sudo et al., 2004 |
| Neurochemical | Serotonin and Serotonin receptor | Decreased serotonin and receptor (5HT1A) in the amygdala and hippocampus of GF mice | Bercik et al., 2011; Clarke et al., 2013; Diaz heijtz et al., 2011; Neufeld et al., 2011; Yano et al., 2015 |
| Neurochemical | Peripheral serotonin | Decreased peripheral and intestinal serotonin in GF mice, restored by colonization with spore forming bacteria | Wikoff et al., 2009; Yano et al., 2015 |
| Neurochemical | Dopamine, gamma-aminobutyric acid (GABA) | Decreased serum levels of dopamine and GABA in GF mice | Matsumoto et al., 2012; Velagapudi et al., 2010 |
| Neurochemical | Serotonin, Noradrenaline, Dopamine | Increased turnover of serotonin, noradrenaline, and dopamine in the striatum of GF mice | Diaz Heijtz et al., 2011 |
| Neurochemical | Granulocyte colony stimulating factor (G-CSF) | Reduced serum levels of G-CSF in GF mice | Deshmukh et al., 2014 |
| Neurochemical | Blood-brain barrier (BBB) | Decreased expression of tight junction proteins, and subsequent increase of BBB permeability | Braniste et al., 2014 |
Interactions between a host and the microbiome are decidedly intricate. Intestinal microbiota influence numerous aspects of metabolism, producing metabolic precursors to hormones and neurotransmitters, or directly producing the active metabolites themselves (Lyte, 2014; Sharon et al., 2014) (Figure 1). Symbiotic bacteria additionally have the capability to influence the status of the systemic immune system, which may alter how the immune system subsequently interacts with the nervous system (Belkaid and Hand, 2014; Hooper et al., 2012; Round and Mazmanian, 2009) (Figure 1). Furthermore, the enteric nervous system (ENS) is directly connected to the central nervous system (CNS) through the vagus nerve, providing a direct neurochemical pathway for microbial-promoted signaling in the GI tract to be propagated to the brain (Forsythe et al., 2014) (Figure 1). Herein, we review the current understanding of how the intestinal microbiota influence behavior and neurological function during both health and disease. First, we will focus on how indigenous microbes shape mood and cognitive behaviors, as well as social behaviors. We will next discuss the physiological aspects that are modulated by signals derived from the microbiota. In particular, we will focus on how the commensal microbes directly and indirectly shape neurochemical and immunologic responses that can subsequently affect behaviors and other neurological functions (Table 1). With well-documented evidence that the microbiota shape immunity and metabolism, the impact of gut microbes on the nervous system represents an exciting new frontier for research with vast translational implications.
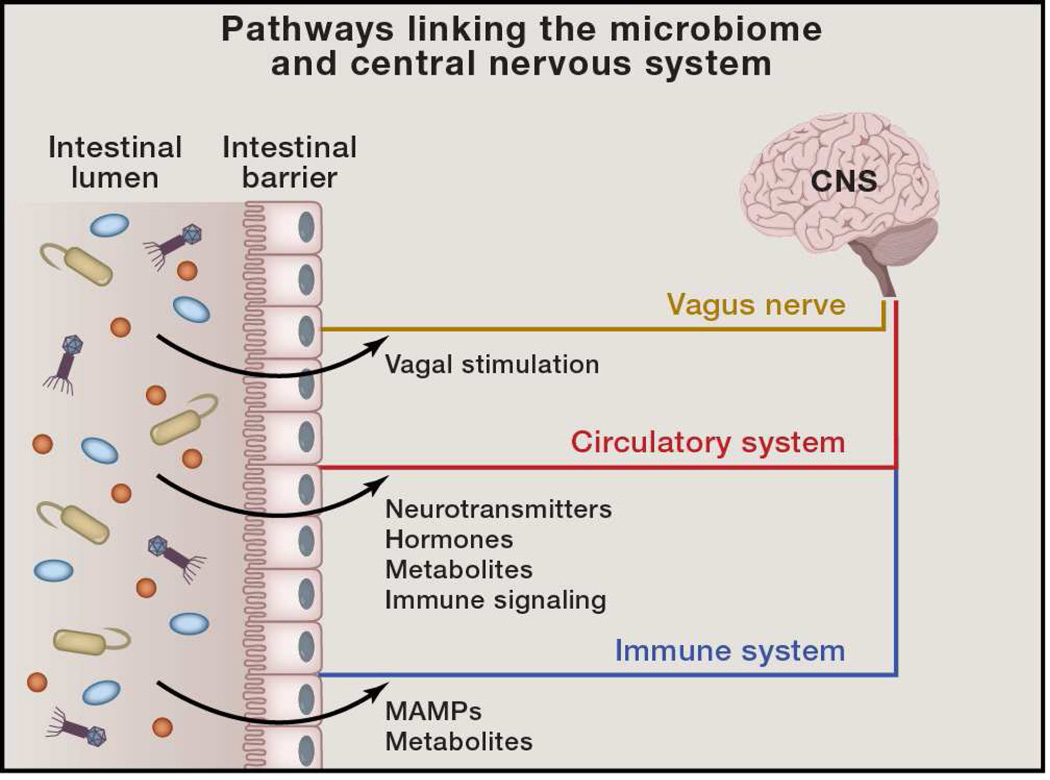
Figure 1. Pathways Linking the Microbiome and Central Nervous System.
Signals from the intestinal microbiome may potentially traffic to the central nervous system (CNS) via several mechanisms. 1) Direct activation of the vagus nerve from the enteric nervous system to the CNS. 2) Production of, or induction of, various metabolites that pass through the intestinal barrier and into the circulatory system, where they may cross the blood-brain barrier to regulate neurological function. 3) Microbial associated molecular patterns (MAMPs, such as LPS, BLP, and PSA) and metabolites produced by the microbiome can signal to the immune system. Immune cells (and particularly their cytokines) can influence neurophysiology.
Role of the Microbiota in Mood and Individual Behaviors
One of the seminal studies on the influence of the commensal microbiota on neurological function observed that germ-free (GF) mice display an elevated response to restraint stress, the stress that occurs during forced immobilization (Sudo et al., 2004). In the absence of the microbiota, mice have substantially higher concentrations of corticosterone, a stress hormone in the hypothalamus, as well as reduced levels of brain-derived neurotrophic factor (BDNF; a protein which stimulates neurogenesis and synaptic growth, and modulates synaptic plasticity and transmission) (Lu et al., 2013; Sudo et al., 2004). Interestingly, this phenomenon can be partially reversed by re-colonization with a diverse microbiota in adulthood, suggesting that active signals from the microbiota play a critical role in brain development. In fact, colonization with a specific bacterial species, Bifidobacterium infantis restores the defect (compared to monocolonization with Esherichia coli), demonstrating that rather than the general sensing of the bacteria within the population of indigenous microbes, signals from specific bacteria drive normal behavior (Sudo et al., 2004). This rigorous demonstration that the microbiota affects the hypothalamic–pituitary–adrenal axis (HPA axis) revealed bi-directional communication between the gut and the brain, with a significant effect on host behavior.
Conversely, in the absence of restraint stress, some strains of GF mice (Swiss Webster, NIH Swiss, NMRI) display decreased anxiety-like behavior, in the form of increased exploration compared to specific pathogen free (SPF) mice, which are colonized with a diverse microbial population. For instance, GF mice spend more time in the lighted section of a light-dark box, as well as the open arms of an elevated plus-maze than their colonized counterparts, evidence of decreased anxiety-like behavior, as mice typically desire to be in a closed, dark area (Clarke et al., 2013; Diaz Heijtz et al., 2011; Neufeld et al., 2011; Selkrig et al., 2014). Importantly, these behavioral effects can be restored to levels similar to conventionally raised mice, by recolonizing the GF mice with a complete SPF microbiota. The effect of the microbiota is not necessarily consistent across all strains of mice. For instance, SPF BALB/c and C57Bl6 mice display increased anxiety behavior compared to other strains, such as NIH Swiss mice (Bercik et al., 2011; Selkrig et al., 2014). The microbiome present in BALB/c mice is significantly different than that present in NIH Swiss mice. When fecal microbiota from either strain is transplanted into GF mice of the counterpart strain, the behavior of the parent strain is transferred (Bercik et al., 2011). This interesting finding implicates that signals derived from the microbiota can drive behaviors in the host. Further, phenotypic changes following fecal microbiota transplants in adult mice reveal that anxiety can be actively modulated by the microbial population, and are not necessarily developmental in origin. It is therefore interesting to contemplate that active modulation of the population or function of the gut microbiota, such as through probiotic supplementation, may act to modify host stress and anxiety behaviors as well. In fact, there is an active and growing line of observations of the effect of probiotic species on the behavior of the host.
In one such probiotic supplementation study, it was observed that mice given the probiotic bacterium Lactobacillus rhamnosus (JB-1) over a 28 day period displayed decreased anxiety-like behavior in the elevated plus maze and open field test (Bravo et al., 2011). Additionally, the effect of L. rhamnosus on anxiety behavior is ameliorated in mice that have been vagotomized, and lack connectivity of the vagus nerve between the ENS and the CNS (Bravo et al., 2011). This would suggest that microbial signals can be directed by the vagus nerve to alter CNS outputs (such as behavior), further implicating an active role by the microbiota in mediating neurological functions. In a similar fashion, treatment with B. longum 1714 and B. breve 1205 was observed to decrease anxiety-like behaviors to the same extent as the pharmaceutical anti-anxiety medication escitalopram (Savignac et al., 2014). Other studies in rats utilizing a probiotic cocktail of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 found that treatment with these microbes over a 30 day period resulted in decreased anxiety in an electric shock model, further suggesting that the functionality of the gut microbiota influences anxiety-like behaviors (Messaoudi et al., 2011). Together, these studies provide credence to the intriguing hypothesis that active modulation of the intestinal microbiota, through probiotic supplementation, can have drastic effects on behavior, perhaps with therapeutic potential.
Anxiety is not the only behavior that is modulated by signals from symbiotic bacteria. The same study that utilized the probiotic L. rhamnosus (JB-1) to treat mice also observed that these mice displayed decreased depression-like behaviors, particularly in the forced swim test (Bravo et al., 2011). Probiotic treatment resulted in animals spending less time immobilized when introduced into a water filled cylinder, rather than attempt to swim. In a similar fashion, a separate study utilized the maternal separation model of depression in rats, in which pups are removed from their mothers for a period of time each day until weaning (Vetulani, 2013). This study noted that the depression-like behaviors induced in this model could be reduced upon treatment with Bifidiobacterium infantis (Desbonnet et al., 2010). Strikingly, B. infantis treatment resulted in a similar reduction in depression-like behavior in the forced swim test as was observed upon treatment with the pharmaceutical antidepressant citalopram, a selective serotonin reuptake inhibitor (SSRI) (Desbonnet et al., 2010; Savignac et al., 2014; Savignac et al., 2015). These surprising findings further suggest that the composition and/or function of the microbiota actively modulates behavior in adult animals.
Intriguingly, probiotic supplementation, or even diet alone, are observed to have an impact on both anxiety and depressive behavior in mice, as well as on learning and memory (Li et al., 2009; Pyndt Jorgensen et al., 2014; Savignac et al., 2015). While some studies have also demonstrated that specific diets alter the composition of the microbiome and have effects on behaviors, whether the microbial composition itself is causative for the observed depressive-behavior and learning deficits observed in these studies is not clear (Li et al., 2009; Pyndt Jorgensen et al., 2014). Functional studies utilizing transplantation of diet-altered microbiomes would be essential to establish a microbial role. Nonetheless, such observations serve to pave the way toward understanding the role that the composition and functionality of indigenous microbes may play in shaping mood and behavior in the host.
While most studies have utilized animal models, work is being performed to understand whether the microbiota have similar roles in shaping human neurological function. A recent human study was performed to address whether the consumption of a fermented, probiotic milk product (containing Bifidobacterium animalis subsp Lactis, Streptococcus thermophilus, Lactobacillus bulgaricus, and Lactococcus lactis subsp Lactis.) could have an effect on the brain response to emotional stimuli. Using functional magnetic resonance imaging (fMRI), Mayer and colleagues found that probiotic consumption over the span of four weeks could affect the processing of emotion (Tillisch et al., 2013). Specifically, those regions of the brain involved in emotional processing, including the primary interoceptive and somatosensory regions were less activated following emotional stimulation (emotional faces task) in those individuals who had consumed this probiotic product (Tillisch et al., 2013). Similarly, a separate study observed that probiotic consumption reduced self-reported feelings of sadness and aggressive thoughts (Steenbergen et al., 2015). Importantly, while these particular studies were small and did not address exactly how the bacterial cocktail mediated changes in functional brain activity and mood, they are nonetheless strongly suggestive that the microbiota can actively alter some functional aspects of mood in humans. A larger human study of fifty-five individuals examined whether probiotic consumption could influence anxiety (through self-assessment questionnaires) (Messaoudi et al., 2011). Administration of L. helveticus R0052 and B. longum R0175 resulted in decreased self-reported anxiety, and notably, also resulted in decreased urinary cortisol (Messaoudi et al., 2011). Together these studies further the concept that the microbiota effects neurological function in humans, and ultimately influences mood and behavior. However, additional work with increased cohort sizes, using a crossover study design and clinical assessment, are needed to validate these seminal observations for a gut-microbiome-brain connection in humans.
Interestingly, it has been observed in both humans and mice that probiotic supplementation with fermented dairy products does not necessarily alter the composition of the gut microbiome (McNulty et al., 2011). Instead, the transcriptional state and metabolic activity of the microbiota is altered (McNulty et al., 2011). It is therefore interesting to consider that these behavioral and neurological changes may not necessarily be a direct function of the specific species of bacteria within the probiotic treatment; rather, microbial-mediated effects on emotion may be due to broader functionality of the symbiotic bacteria within the gut. Continued study of how the presence of specific bacterial species, particularly low abundance niche species, influences physiology but does not alter the overall population of gut microbes, will be critical to understanding the function of the microbiota and how they can be effectively harnessed and developed as a potential therapeutic modality for behavioral disorders.
One well-established physiological function of the microbiota is the generation of essential nutrients for host physiology, such as vitamins and other cofactors (Gordon et al., 2012). Therefore, it is tempting to speculate that perhaps these microbes could subsequently alter satiety, influencing how much food the host ingests. While there is no unequivocal evidence demonstrating that the microbiota directly influence appetite and satiety, there are intriguing indirect observations. Upon ingestion of fermentable, complex carbohydrates, the microbiota metabolize fiber into short-chain fatty acids (SCFAs) such as acetate, butyrate, and propionate (Miller and Wolin, 1979). Interestingly, SCFAs produced in the GI tract are trafficked not only into the serum, but also are capable of crossing the blood-brain barrier (BBB) (Conn et al., 1983; Mitchell et al., 2011). Once in the brain, one particular SCFA, acetate, has been observed to enact physiological changes in the hypothalamus. Here, acetate alters the level of the neurotransmitters glutamate, glutamine, and gamma-aminobutyric acid (GABA), as well as increases anorectic neuropeptide expression (Frost et al., 2014), which together act as hormone signals to reduce appetite (Sobrino Crespo et al., 2014). In total, production of acetate, leads to suppression of appetite. In fact, a small study in humans found that fermentation of complex carbohydrates to SCFAs by the microbiota was directly correlated with the sensation of satiety (Cani et al., 2009), providing a basis for the hypothesis that the symbiotic microbes may be capable of modulating host appetite. Indeed, changes to the microbiome, due to a specific genetic mutation in mice, induce a metabolic syndrome, likely through changes in feeding behaviors rather than alterations to metabolism (Vijay-Kumar et al., 2010).
Microbiota Shape Social Behaviors
Numerous facets of social behavior are also altered by the presence, composition, and functionality of the microbiota (Mayer et al., 2015; Stilling et al., 2014b). GF mice are significantly socially impaired compared to SPF colonized counterparts (Desbonnet et al., 2014). GF mice do not seek out other mice (both new and familiar) as readily as mice harboring a diverse microbial consortium (Desbonnet et al., 2014), suggesting that the microbiota affect these social behaviors. Surprisingly, social avoidance could be restored through recolonizing adult mice with a complete microbiota. Conversely, the defect in social cognition (that is, the ability to recognize familiar versus unfamiliar mice) was not restored following colonization. Thus, gut bacteria have differential effects on both developmental aspects, as well as active processes that occur in adulthood, which ultimately shape long-term behavioral traits (Desbonnet et al., 2014).
One particularly interesting example of microbiome-controlled social behavior is that of sexual mate preference in the fruit fly, Drosophila melanogaster. Flies that have been colonized with Lactobacillus plantarum prefer to mate only with similarly colonized flies, and not with flies colonized by other bacterial species (Sharon et al., 2010). This is due to the increased production of certain pheromones whose precursors are produced by L. plantarum within these flies. As such, this provides a strong, mechanistic example to begin to understand how the microbiome can influence the extremely complex social behavior of mate preference (Sharon et al., 2010). Building on this, an interesting study of social behaviors in hyenas found that individual social groups of these animals harbored distinct microbiota within their scent glands (Theis et al., 2012; Theis et al., 2013). Scent-gland dwelling symbiotic bacteria are known to produce volatile compounds, and each unique microbial community produces different ratios of these compounds (Theis et al., 2012; Theis et al., 2013). As distinct social groups had similar microbiomes, individuals likely are recognized as belonging to a particular social group directly due to the composition of the microbes within their glands. These studies provide support for the notion that symbiotic bacteria affect how a host interacts in social settings, modulating how the host perceives novel versus familiar individuals for both mate choice and social grouping. The ramifications of this microbial influence on social behaviors has not gone unnoticed by evolutionary biologists (Montiel et al., 2014; Rosenberg et al., 2010; Stilling et al., 2014a). If gut bacteria modulate neurological function leading to the choice of mate, the microbiome may therefore play a significant role in driving the evolution of their hosts. By influencing how individuals interact and undergo vertical genetic transfer, the microbiota could ultimately be a critical contributing factor in the evolution of metazoan species.
The Link between Gut Bacteria and Disorders Involving Social Impairment
Autism spectrum disorder (ASD) comprises a set of complex neurodevelopmental disabilities characterized by repetitive/stereotypic behaviors and deficits in communication and social interaction. Intriguingly, a significant subset of ASD children exhibit GI complications, including constipation, increased intestinal permeability and altered composition of the intestinal microbiome (Kang et al., 2013; Mulle et al., 2013; Rosenfeld, 2015). Mice displaying autism-like behaviors have a significantly altered microbiome compared to neurotypical controls, and have an increase in the permeability of the colon, and correlatively, display differences in serum metabolites (Hsiao et al., 2013). Surprisingly, treatment with a single organism, Bacteroides fragilis, was able to restore the intestinal permeability defects in a mouse model of ASD (Hsiao et al., 2013). While treatment with this probiotic did not restore the overall composition of the microbial population, B. fragilis treatment could restore levels of a small number of specific species (Hsiao et al., 2013). Notably, B. fragilis treatment rescued some behavioral defects, including stereotyped behavior (compulsive marble burying), communication deficits (ultrasonic vocalizations), and anxiety-behaviors (open-field exploration)(Hsiao et al., 2013).
B. fragilis has been shown to augment the development and function of the immune system (Mazmanian et al., 2008; Ochoa-Reparaz et al., 2010; Round and Mazmanian, 2010); however, treatment with B. fragilis did not restore several aspects of immune dysfunction in an animal model of autism (Hsiao et al., 2012; Hsiao et al., 2013). Instead, levels of serum metabolites found to be altered in mice with ASD-related behaviors were restored to normal levels. Administration to healthy animals of a specific serum metabolite that was elevated in mice with behavioral deficits, namely 4-ethylphenyl sulfate (4EPS), was sufficient to induce anxiety-like behavior. As 4EPS is predicted to be of microbial origin, this remarkably demonstrates that defined molecules from the microbiome can impact behavior in mammals. Similarly, another study demonstrated that mice with features of ASD display a significantly altered microbiome and increased intestinal inflammation (de Theije et al., 2014). Although still correlative, these data together suggest that certain neurodevelopmental disorders such as autism may have microbial etiologies, a hypothesis that will require further validation in both animal models and human trials. The notion that the gut microbiome constitutes an environmental risk factor for autism is supported by epidemiologic studies showing a rapid increase in ASD diagnoses over the past few decades, suggesting that genetics alone cannot explain many cases of disease. If true, targeted repair of an altered microbiome through interventions including probiotics, prebiotics or diet may represent natural, safe and effective treatments for neurological disorders such as autism.
Microbiome-Mediated Alterations to Neurophysiology
The observation that GF or probiotic-treated mice have altered behavior raises numerous interesting questions related to gut-brain communication. Do microbially-derived signals act directly on the nervous system, via the immune or metabolic or endocrine systems, and/or other pathways? Is the influence of the microbiome due to developmental effects, or is the microbiome-brain axis an actively modulated process? What are the neurophysiological changes in the brain that arise due to alterations to the microbiome, which may underlie behavioral effects? Mechanisms and consequences for long distance interoceptive communication between gut bacteria and the brain are likely to be context-specific and not mutually exclusive.
In some cases, there is evidence that microbiota-mediated outcomes are required to occur during a specific time frame of development, and subsequently have irreversible downstream neurological affects (Borre et al., 2014). In other examples, neurological function can be actively modulated by signals from the microbiome. For example, recolonizing adult GF mice with a complete microbiota restores their anxiety-like behavior to that of SPF mice, demonstrating that control of anxiety behavior can occur through an active and constant process between signals from the microbiome and the CNS (Clarke et al., 2013). However, GF mice also display lower levels of BDNF, serotonin (5-HT; 5-hydroxytryptamine), and specific 5-HT receptors (for instance, 5HT1A) in regions such as the amygdala and hippocampus (Bercik et al., 2011; Clarke et al., 2013; Diaz Heijtz et al., 2011; Neufeld et al., 2011). The levels of these host molecules are not restored upon re-colonization of adult mice, suggesting that certain phenotypes are likely programmed by the microbiota during fetal development or in adolescence (Clarke et al., 2013).
Other processes are more actively modulated by the microbiome. It has also been observed that GF mice display an increased rate of turnover of noradrenaline, dopamine, and 5-HT in the striatum region of the brain (Diaz Heijtz et al., 2011). A high rate of turnover may subsequently have an effect on steady-state levels of these neurotransmitters. Turnover of norepinephrine and dopamine specifically may be responsible for the increase in motor activity that is well documented in GF mice (Diaz Heijtz et al., 2011), as these neurotransmitters have roles in increasing blood flow to muscle and central motor control, respectively.
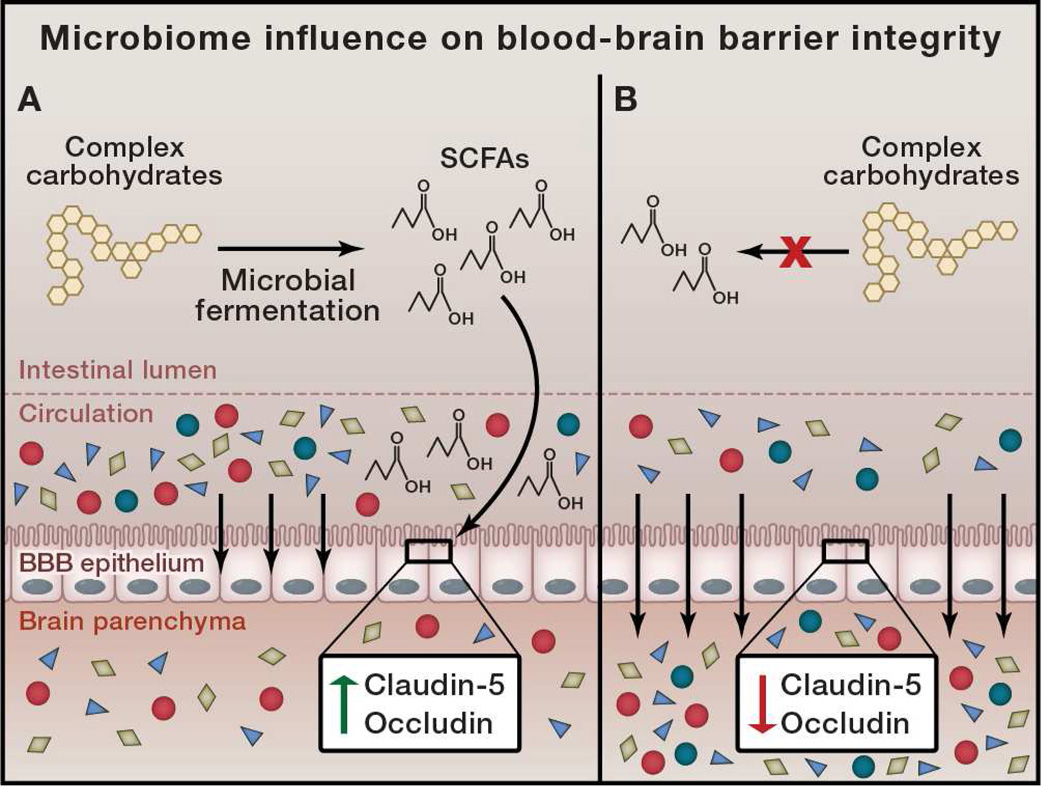
While alterations to the population or function of the microbiome may result in differential production of metabolites that enter the periphery, it is unknown if these molecules could cross the BBB and influence neurological function. Intriguingly, GF mice have significantly increased permeability of the BBB, both during fetal development and in adulthood (Braniste et al., 2014) (Figure 2). This increase in permeability is due to lowered expression of endothelial tight junction proteins, specifically occludin and claudin-5, in the absence of the microbiota. Interestingly, mono-colonization with either Clostridium tyrobutyricum or Bacteroides thetaiotaomicron could restore BBB integrity (and tight junction expression), even in adult mice. As these species produce SCFAs from fermentation of complex carbohydrates in the gut, it was subsequently observed that the SCFA butyrate was sufficient to restore BBB integrity (Braniste et al., 2014) (Figure 2). This suggests that metabolic signals that are derived from the gut microbiota actively and constantly influence the physiological status of the BBB, a site quite distant from their origin. However, it is unknown whether other SCFAs or microbial-derived signals, or even microbial species may also play a role in influencing BBB permeability. This finding has important implications in other physiological processes: it indicates that serum metabolites that normally do not cross into the brain parenchyma, may potentially cross the BBB based on the status of the microbiota, providing a mechanism for gut microbes to control concentrations of numerous metabolites that can act directly on neurological systems (Figure 2).
Figure 2. Microbiome Influence on Blood-Brain Barrier Integrity.
Intestinal microbes are capable of fermenting complex carbohydrates into short chain fatty acids (SCFAs). A) Microbially-produced SCFAs signal to epithelial cells that create the blood-brain barrier (BBB) and increase production of the tight junction proteins claudin-5 and occludin. This leads to a tight and selective barrier, preventing undesired metabolites from entering the brain parenchyma. B) In the absence of microbial fermentation, no SCFA signaling occurs, and tight junction proteins are repressed. This leads to increased permeability of the BBB, and a loss of the selective barrier to serum metabolites.
Regulation of Neurotransmitter Levels by the Gut Microbiota
While it has been observed that changes to neurophysiology can be mediated by the microbiome, the precise mechanism by which this influence occurs is still unclear. Although altering the permeability of the BBB would change the flux of serum metabolites into and out of the brain, there are potentially more direct ways in which the microbiome can alter neurological function. One of the most direct mechanisms could be through controlling the concentration of various neurotransmitters, both in the brain and in the periphery. For example, 5-HT levels in peripheral serum are decreased in the absence of the gut microbiota (Wikoff et al., 2009; Yano et al., 2015). This decrease corresponds to lower levels of 5-HT metabolites and precursors in the intestinal luminal contents and urine (Marcobal et al., 2013; Matsumoto et al., 2012). The vast majority of 5-HT in the body (~90%) is produced by enterochromaffin cells in the gut (Gershon, 2013). Release of 5-HT by enterochromaffin cells is necessary for modulating colonic motility (Fukumoto et al., 2003). It has recently been demonstrated that microbial-derived SCFAs are capable of inducing 5-HT production by enterochromaffin cells, in vitro and in animals (Reigstad et al., 2014; Yano et al., 2015). Additionally, 5-HT is not known to cross the blood-brain barrier, and therefore the microbiome control of 5-HT turnover in the brain may instead occur through alterations in 5-HT precursor levels (O'Mahony et al., 2015; Sharon et al., 2014).
In particular, the essential amino acid tryptophan is a central precursor to 5-HT synthesis. Tryptophan itself is generated by the intestinal microbiota, and tryptophan present in the periphery is capable of crossing the BBB where it can then participate in 5-HT synthesis (O'Mahony et al., 2015; Sharon et al., 2014). Nonetheless, even local stimulation and production of 5-HT in the GI tract would have important effects on host physiology, since 5-HT modulates gastrointestinal motility (Berger et al., 2009). Intriguingly, 5-HT levels in the gut can be restored in GF mice following colonization with a defined cocktail of spore forming gut bacteria (Yano et al., 2015), a process that regulates platelet aggregation and blood clotting. Non-SCFA metabolites produced by this microbial community stimulate gut enterochromafin cells to produce 5-HT, compensating for a defect in platelet activity and coagulation (a process known to be regulated by 5-HT). Consequences of 5-HT regulation by bacteria in the intestine on the concentration of 5-HT in the brain and on behavior in mice remain unknown; however, the prevalent usage of SSRIs, which increase the concentration of 5-HT (serotonin) at the synapse, warrants interest in the potential of developing probiotic therapies as an alternative treatment for major depressive disorder and anxiety.
5-HT is not the only neurotransmitter whose concentrations are influenced by the microbiome. Serum levels of other neurotransmitters are also decreased in the absence of the gut microbiota. Dopamine and GABA are decreased in the serum of GF mice, and specific precursors and metabolites of these are also altered in the intestine (Matsumoto et al., 2012; Velagapudi et al., 2010). Exactly how gut bacteria alter the levels of these neurotransmitters remains to be determined. Direct signals from the microbiota to neurotransmitter-producing cells, such as the enterochromaffin cells or even to enteric neurons and glia may trigger neurotransmitter production. While such signaling to neurotransmitter producing cells is one way in which the microbiota may influence neurotransmitter concentrations, gut dwelling bacteria also directly produce small molecules with potential to act as neurotransmitters. In turn, these may act as signals to gastrointestinal cells, or make their way to the periphery and potentially the brain, and ultimately influence neurological function.
It has been known for many years that specific species of gut bacteria are capable of producing small molecules such as serotonin, dopamine, norepinephrine, epinephrine, GABA and acetylcholine, possible bioactive neuropeptides (Wall et al., 2014). However, it is unknown whether the microbially-derived molecules can act directly on host receptors as neurotransmitters. Recently, it has been observed that the specific human gut microbes Clostridium sporogenes and Ruminococcus gnavus are capable of producing the neurotransmitter tryptamine through decarboxylation of tryptophan (Williams et al., 2014). In the brain, tryptamine plays a role in the inhibitory response to 5-HT, through its action on the trace amine-associated receptor, and may modulate mood and appetite (Zucchi et al., 2006). In the gut, tryptamine can also induce enterochromaffin cells to release 5-HT. While tryptamine can in fact cross the blood-brain barrier from serum, it is yet unknown whether gut microbiota-produced tryptamine is trafficked from the GI tract to the CNS, and subsequently influences neurological function.
Another potential neurotransmitter that is produced by bacteria present in the gut is tyramine. Multiple microorganisms, including Lactobacillus brevis and Enterococcus species, are capable of decarboxylating tyrosine to tyramine (Lucas et al., 2003). Since tyramine has been shown to modulate motor function in worms, as well as trace amine-associated receptors in mammals (Zucchi et al., 2006), it raises the possibility that gut microbiota-produced tyramine may act as a modulator of neurological function as well. Similarly, 5-HT is produced by a number of gut microbes in vitro, specifically many lactic acid bacteria (Ozogul et al., 2012), and other human intestinal bacteria have been shown to produce GABA (Minuk, 1986). While GABA and 5-HT are not known to cross the BBB, intestinally-derived (and therefore likely microbiota-derived) production of these molecules may instead act locally on the vagus nerve, or through signaling via the periphery (Barrett et al., 2012). In the intestine, GABA is important for modulating motility, emptying and secretion in the intestine; in the periphery, it controls aspects of stress and thermoregulation (Hyland and Cryan, 2010; Li et al., 2012; Paredes and Agmo, 1992), suggesting that signals from the microbiota may contribute to these diverse neurophysiological functions.
Contrary to conventional wisdom, most neurotransmitters are found in the gut at levels equal to or exceeding those in the brain. Furthermore, the proportion of total body levels of various neurotransmitters is greater in the gut than the brain. While most of the current data for microbiota-modulation of neurotransmitter production and/or levels is available for the gut and periphery, local neurotransmitter regulation by gut bacteria may have long distance effects on the brain. Neurotransmitters or other molecules derived from gut microbes have the potential to modulate activity of the vagus nerve, the primary nerve connecting the ENS to the CNS, and subsequently influence brain function (Bravo et al., 2011; Goehler et al., 2005). It is also possible that microbially-derived metabolites which can act as precursors to neurotransmitter production (such as tryptophan) and may cross through the intestinal barrier and the BBB, could subsequently influence both systemic and CNS neurotransmitter concentrations. It is important to note, however, that many of these studies describing neurotransmitter production by intestinal bacteria have been performed in vitro, and thus it is not known precisely whether these bacteria utilize such metabolic pathways in vivo, and if so, when these specific gut microbes produce these compounds. Nevertheless, it is an interesting prospect that the intestinal microbiota act to produce neurotransmitters and directly modulate the nervous system (Lyte, 2013). Such findings open up exciting possibilities in understanding how the microbiome may affect function of the nervous system, through the creation of bioactive metabolites that are capable of modulating CNS activity via several modes of gut-brain connection.
Microbial Control of Neurological Function by the Immune System
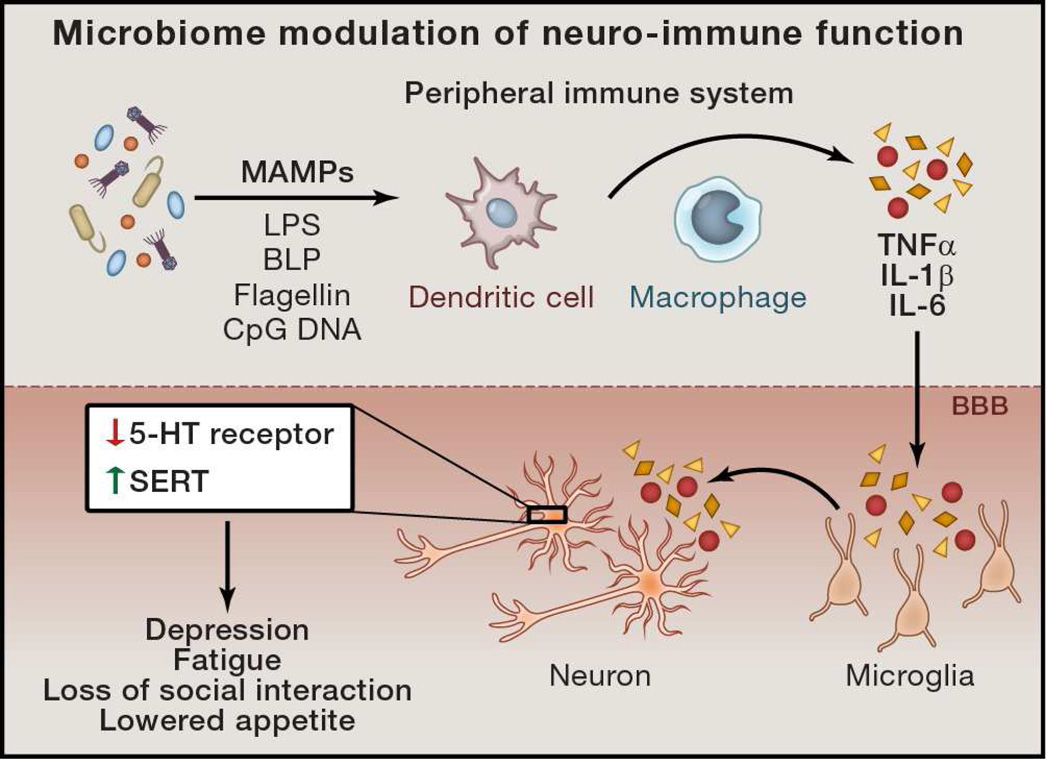
Interactions between the microbiota and the nervous system may be indirect. Growing data indicate that the peripheral immune system can influence neurological function and behavior. In fact, immune signaling has been shown to cause or allow progression of certain neurological disorders, including neurodegenerative diseases and psychological illness such as anxiety and depression. One of the better-known examples of immune-mediated effects on neurological function is sickness behavior (Figure 3). This behavior is characterized by appetite suppression, decreased motor activity, loss of social interaction and reduced cognition (Dantzer et al., 2008). Microbial-associated molecular patterns (MAMPs), such as lipopolysaccharide (LPS), bacterial lipoprotein (BLP), flagellin, CpG DNA, among others, activate various cells immune system, particularly innate immune cells such as macrophages, neutrophils, and dendritic cells. Once activated, these cells produce numerous pro-inflammatory cytokines, such as IL-1α, IL-1β, TNFα, and IL-6, which make their way to the brain by crossing the BBB via both diffusion and cytokine transporters. Once in the brain, these cytokines act on receptors expressed by neurons and glial cells, particularly microglia (brain-resident, innate immune phagocytes), altering their activation status and physiology (Dantzer et al., 2000) (Figure 3). In the periphery, these cytokines are capable of acting on receptors in afferent nerves, promoting alterations in the signals leading from distant, peripheral body sites to the CNS. Introduction of purified IL-1β and TNFα into the brain or the periphery, as well as systemic treatment with LPS (which induces pro-inflammatory gene expression in the brain and periphery) is sufficient to mediate sickness behavior, while IL-6 treatment is only capable of promoting fever, but not behavioral attributes (Breder et al., 1994; van Dam et al., 1992) (Figure 3). In further support of pro-inflammatory cytokines mediating the onset of this behavior, treatment with anti-inflammatory mediators, such as IGF-1 and IL-10, prevents sickness behavior (Bluthe et al., 1999; Bluthe et al., 2006). In fact, the role of pro-inflammatory cytokines in modulating human behavior is exemplified by the finding that one-third of patients who have been treated with IL-2 and IFNα (used as immunotherapy against cancer and certain viral infections) develop major depressive disorder (Raison et al., 2006).
Figure 3. Microbiome Modulation of Neuro-Immune Function.
Microbial associated molecular patterns (MAMPs) derived from the intestinal microbiome can drive various aspects of immune function in the periphery. Cytokine signals, such as TNFα, IL-1β, and IL-6 can cross the blood-brain barrier and trigger their production by the microglia. Interaction of these cytokines with neurons influences physiology and leads to sickness behavior and depression.
Additionally, immune dysregulation is observed in both human cases and animal-models of ASD (Ashwood et al., 2006; Hsiao, 2013; Hsiao et al., 2012; Mead and Ashwood, 2015). In the maternal immune activation (MIA) model of autism, a decrease in regulatory T cells and an increase in pro-inflammatory innate monocytes are observed in mice that display ASD symptoms. Bone marrow transplantation from healthy donors (and subsequent restoration of immune function) is capable of rescuing behavioral defects in this model (Hsiao et al., 2012). However, given the role of the microbiota in modulating behaviors in this model (Hsiao et al., 2013), it is interesting to consider that irradiation itself may have also restored behavioral functions due to an effect on the composition of the microbiome and/or by ‘resetting’ the immune system. Nonetheless, these data provide evidence for a link between immune dysfunction and complex social and behavioral disorders.
Exactly how pro-inflammatory cytokine signaling can mediate alterations in neurological functions that affect behavior and behavioral disorders is beginning to be revealed. Sickness behavior and depressive disorders, for instance, are linked to alterations in 5-HT signaling. In this vein, IL-1β or TNFα treatment stimulate 5-HT uptake through upregulation of the serotonin transporter (SERT), decreasing the concentration of 5-HT available to signal in the synapase (Zhu et al., 2006). At the same time, these cytokines decrease the level of 5-HT receptor (5-HT2A) present on neurons, escalating the loss of 5-HT signaling, and likely mediating the alterations to behavior (Cai et al., 2005) (Figure 3).
While pro-inflammatory cytokine signaling can be driven by pathogenic microbes, some microbiota-derived signals induce non-inflammatory cytokine pathways. For instance, in the absence of a complete microbiota, mice exhibit a lower plasma concentration of the cytokine granulocyte colony stimulating factor (G-CSF) (Deshmukh et al., 2014). Plasma G-CSF is capable of crossing the BBB and acts to stimulate neurogenesis in the brain (Zhao et al., 2007). Thus, through stimulation of G-CSF production, the microbiota may influence the rate of neurogenesis. This could have effects not only on normal neurodevelopmental processes where G-CSF signaling plays a role, but also in injury and neurodegenerative disease. G-CSF is a protective factor following ischemic injury (Shyu et al., 2004) as well as protective and therapeutic in certain models of both Parkinson’s and Alzheimer’s diseases (Meuer et al., 2006; Prakash et al., 2013). Therefore, it is exciting to think that this may be one way in which alterations to the microbiome could modulate the outcome of these neurodegenerative diseases. Or more intriguingly, introduction of microbes that specifically promote G-CSF production may represent a future avenue of microbiota-mediated therapy to combat neurodegenerative illnesses.
Future Directions and Conclusions
While the role of the microbiome in influencing numerous aspects of metabolic and immunologic aspects is well established, how indigenous microbes modulate neurological function during health and disease is only now becoming appreciated. Here, we have described emerging evidence for the behavioral and neurophysiological conditions in animal models and human studies that have been linked to the microbiome. Rapid and sustained growth of research on the gut-microbiome-brain connection may lead to discoveries that prompt a reconsideration of potential environmental influences on numerous neurological diseases whose cause(s) has remained enigmatic, and where treatment options are limited.
Several seminal reports now show that animals lacking microbiota have significantly altered brain development and behavior compared to colonized counterparts, highlighting the stark importance of host-microbial symbiosis. Germ-free animals provide a valuable model to determine not only the precise physiological processes that the microbiota influence, but also the extent of the effects. Along with probiotic or antibiotic treatment of colonized animals, germ-free models provide an experimental platform to reconstitute biological systems with defined communities or single microbial species, and from a variety of donor sources (e.g., genetically engineered mice, humans, etc.) allowing for the discovery and functional characterization of organisms and molecules that impact the nervous system. Identification of microbes (either single species or consortia) that modulate these systems will advance efforts to explore the nature of the microbial-derived signals and host pathways that influence specific neurophysiological function. Genomic and genetic approaches to study newly discovered organisms, alongside metabolomic analyses to identify products or compounds from symbiotic bacteria, may provide critical insight into how particular microbial molecules alter host neurophysiology. Accordingly, identification of bioactive microbial signals may serve as a tool for the discovery of currently unidentified host pathways or novel activities of known pathways that influence behavior and neurological function, similar to how the study of bacterial pathogenesis has uncovered the intricacies of host immune system pathways by first identifying those microbial signals that modulate them.
It will be critical to differentiate whether the influence of the microbiome on a host process is developmental or active in nature. That is, are the signals from the microbiota that alter neurological function important at a specific time during development (Borre et al., 2014), or can phenotypes be actively modulated in fully developed animals? Are certain aspects of behavior mediated by signals that are derived from the maternal microbiome, or instead, can fluctuations to the composition and function of an adult microbiome also contribute to neurological function? Given that the majority of brain development occurs in utero, maternal signals are likely to play a significant role. While the womb was believed to be sterile, recent controversial observations have called this into question, and suggest microbially-derived products may interact directly with the developing fetus (Aagaard et al., 2014; Borre et al., 2014; Funkhouser and Bordenstein, 2013; Jimenez et al., 2008). Furthermore, the physiological influence on maternal systems may have indirect effects on fetal development, such as through metabolic and/or immune pathways (PrabhuDas et al., 2015). Several studies have recently shed light on the relevance of the maternal-fetal interaction and the important contribution of microbiome dynamics in the first few years of life. There is likely not a single answer and specific processes will likely have distinct roles for the microbiome in different contexts.
Perhaps one of the most pressing areas of research in the field is understanding the physiological consequences of altered microbiome populations that correlate with certain disease states. Do these changed populations enact a physiological effect that drives the disease they are correlated with? In other words, are alterations to the microbiome causal to a given condition? Or instead, are changes to gut bacterial composition simply a consequence of the disease state? For many neurological diseases that lack a strong genetic component, environmental factors are thought to play a critical role. Further study may uncover the microbiome as an important environmental factor that may be an etiological agent of disease, instigating effects that have lasting consequences on the initiation and/or progression of neurological illness. Future studies addressing these questions will add critical relevance to correlative associations described to date between the microbiome and disease of the nervous system.
As we begin to understand how microbial-derived processes influence the brain and behavior during health and disease, we may perhaps begin to rationally design microbiota-based therapeutics for neurological disorders. For example, understanding the signals that drive neurotransmitter production by intestinal bacteria (either directly or indirectly through the production of precursors) may provide a foundation for therapies in diseases that are currently treated by pharmacological alteration of neurotransmitter levels. This could include a range of disorders, from neurodegenerative diseases like Parkinson’s disease, which is treated with oral L-DOPA to stimulate dopamine levels, to depression which is commonly treated with SSRIs, that increase the concentration of 5-HT available to signal in the synapse. Additionally, understanding how the microbiota modulate immune responses at distal sites may lead to understanding how we can specifically alter gut microbes to influence immune-mediated pathologies in the brain, such as those that occur during stroke, seizures and neurodegenerative diseases.
While microbiologists have understood for decades that microbes can alter the hosts’ behavior in various invertebrate and vertebrate systems, it is only in the last five years that other scientific communities have begun to appreciate the potential scale and depth by which commensal microbes affect complex neurological function in mammals. A handful of molecular mechanisms that control these interactions have been identified; however the field is poised to make great strides in understanding this new paradigm based on growing awareness of the gut-microbiome-brain axis. Multi-disciplinary collaborations between microbiologists, immunologists, neuroscientists and bioinformaticians will continue to drive discovery into how symbiotic microbes function to shape our brains and behaviors, and how we may exploit these organisms to combat neurological diseases. Neurodevelopmental, psychiatric and neurodegenerative disorders represent some of the most serious medical and societal burdens of our time; yet the etiology of most disorders of the brain remain unknown and therapies are largely either ineffective or have severe side effects. New concepts and therapeutic modalities are desperately needed to explain and address many neurological conditions. It appears that future discoveries in the neurosciences will not solely rely on studying the brain, but surprisingly through exploration of a forgotten organ with similar size and complexity—the human microbiome.
ACKNOWLEDGMENTS
We apologize to those authors whose work we have failed to mention due to space constraints. We would like to thank Hannah Ratner, Catherine Schretter, Dr. Gil Sharon, and Dr. Wei-Li Wu for helpful discussions and critical review of this manuscript. Research in the Mazmanian laboratory is funded by grants from the National Institutes of Health (MH100556, DK078938, NS085910), the Heritage Medical Research Institute, the Emerald Foundation, Autism Speaks and the Simons Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Science translational medicine. 2014;6:237ra265. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamo SA, Kovalko I, Easy RH, Stoltz D. A viral aphrodisiac in the cricket Gryllus texensis. The Journal of experimental biology. 2014;217:1970–1976. doi: 10.1242/jeb.103408. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Wills S, Van de Water J. The immune response in autism: a new frontier for autism research. Journal of leukocyte biology. 2006;80:1–15. doi: 10.1189/jlb.1205707. [DOI] [PubMed] [Google Scholar]
- Barrett E, Ross RP, O'Toole PW, Fitzgerald GF, Stanton C. gamma-Aminobutyric acid production by culturable bacteria from the human intestine. Journal of applied microbiology. 2012;113:411–417. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609. 609 e591–609 e593. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annual review of medicine. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron DG, Marche L, Ponton F, Loxdale HD, Galeotti N, Renault L, Joly C, Thomas F. Behavioural manipulation in a grasshopper harbouring hairworm: a proteomics approach. Proceedings Biological sciences / The Royal Society. 2005;272:2117–2126. doi: 10.1098/rspb.2005.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthe RM, Castanon N, Pousset F, Bristow A, Ball C, Lestage J, Michaud B, Kelley KW, Dantzer R. Central injection of IL-10 antagonizes the behavioural effects of lipopolysaccharide in rats. Psychoneuroendocrinology. 1999;24:301–311. doi: 10.1016/s0306-4530(98)00077-8. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Kelley KW, Dantzer R. Effects of insulin-like growth factor-I on cytokine-induced sickness behavior in mice. Brain, behavior, and immunity. 2006;20:57–63. doi: 10.1016/j.bbi.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borre YE, O'Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends in molecular medicine. 2014;20:509–518. doi: 10.1016/j.molmed.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, Korecka A, Bakocevic N, Guan NL, Kundu P, et al. The gut microbiota influences bloodbrain barrier permeability in mice. Science translational medicine. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breder CD, Hazuka C, Ghayur T, Klug C, Huginin M, Yasuda K, Teng M, Saper CB. Regional induction of tumor necrosis factor alpha expression in the mouse brain after systemic lipopolysaccharide administration. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:11393–11397. doi: 10.1073/pnas.91.24.11393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Khaoustov VI, Xie Q, Pan T, Le W, Yoffe B. Interferon-alpha-induced modulation of glucocorticoid and serotonin receptors as a mechanism of depression. Journal of hepatology. 2005;42:880–887. doi: 10.1016/j.jhep.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Cani PD, Lecourt E, Dewulf EM, Sohet FM, Pachikian BD, Naslain D, De Backer F, Neyrinck AM, Delzenne NM. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. The American journal of clinical nutrition. 2009;90:1236–1243. doi: 10.3945/ajcn.2009.28095. [DOI] [PubMed] [Google Scholar]
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Molecular psychiatry. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- Conn AR, Fell DI, Steele RD. Characterization of alpha-keto acid transport across blood-brain barrier in rats. The American journal of physiology. 1983;245:E253–E260. doi: 10.1152/ajpendo.1983.245.3.E253. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Konsman JP, Bluthe RM, Kelley KW. Neural and humoral pathways of communication from the immune system to the brain: parallel or convergent? Autonomic neuroscience : basic & clinical. 2000;85:60–65. doi: 10.1016/S1566-0702(00)00220-4. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Theije CG, Wopereis H, Ramadan M, van Eijndthoven T, Lambert J, Knol J, Garssen J, Kraneveld AD, Oozeer R. Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain, behavior, and immunity. 2014;37:197–206. doi: 10.1016/j.bbi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Molecular psychiatry. 2014;19:146–148. doi: 10.1038/mp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179–1188. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Deshmukh HS, Liu Y, Menkiti OR, Mei J, Dai N, O'Leary CE, Oliver PM, Kolls JK, Weiser JN, Worthen GS. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nature medicine. 2014;20:524–530. doi: 10.1038/nm.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver C. Rabies: risk, prognosis and prevention. Nursing times. 2014;110:16–18. [PubMed] [Google Scholar]
- Forsythe P, Bienenstock J, Kunze WA. Vagal pathways for microbiome-brain-gut axis communication. Advances in experimental medicine and biology. 2014;817:115–133. doi: 10.1007/978-1-4939-0897-4_5. [DOI] [PubMed] [Google Scholar]
- Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends in neurosciences. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, Anastasovska J, Ghourab S, Hankir M, Zhang S, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nature communications. 2014;5:3611. doi: 10.1038/ncomms4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, Eubanks S, Harris M, Pappas TN, Takahashi T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. American journal of physiology Regulatory, integrative and comparative physiology. 2003;284:R1269–R1276. doi: 10.1152/ajpregu.00442.2002. [DOI] [PubMed] [Google Scholar]
- Funkhouser LJ, Bordenstein SR. Mom knows best: the universality of maternal microbial transmission. PLoS biology. 2013;11:e1001631. doi: 10.1371/journal.pbio.1001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Current opinion in endocrinology, diabetes, and obesity. 2013;20:14–21. doi: 10.1097/MED.0b013e32835bc703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RP, Opitz N, Reddaway R, Badr N, Lyte M. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain, behavior, and immunity. 2005;19:334–344. doi: 10.1016/j.bbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Gordon JI, Dewey KG, Mills DA, Medzhitov RM. The human gut microbiota and undernutrition. Science translational medicine. 2012;4:137ps112. doi: 10.1126/scitranslmed.3004347. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House PK, Vyas A, Sapolsky R. Predator cat odors activate sexual arousal pathways in brains of Toxoplasma gondii infected rats. PloS one. 2011;6:e23277. doi: 10.1371/journal.pone.0023277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY. Immune dysregulation in autism spectrum disorder. International review of neurobiology. 2013;113:269–302. doi: 10.1016/B978-0-12-418700-9.00009-5. [DOI] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Chow J, Mazmanian SK, Patterson PH. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:12776–12781. doi: 10.1073/pnas.1202556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DP. Parasitic manipulation: a social context. Behavioural processes. 2005;68:263–266. doi: 10.1016/j.beproc.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Hyland NP, Cryan JF. A Gut Feeling about GABA: Focus on GABA(B) Receptors. Front Pharmacol. 2010;1:124. doi: 10.3389/fphar.2010.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez E, Marin ML, Martin R, Odriozola JM, Olivares M, Xaus J, Fernandez L, Rodriguez JM. Is meconium from healthy newborns actually sterile? Res Microbiol. 2008;159:187–193. doi: 10.1016/j.resmic.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB, Krajmalnik-Brown R. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PloS one. 2013;8:e68322. doi: 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Dowd SE, Scurlock B, Acosta-Martinez V, Lyte M. Memory and learning behavior in mice is temporally associated with diet-induced alterations in gut bacteria. Physiology & behavior. 2009;96:557–567. doi: 10.1016/j.physbeh.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Li Y, Xiang YY, Lu WY, Liu C, Li J. A novel role of intestine epithelial GABAergic signaling in regulating intestinal fluid secretion. American journal of physiology Gastrointestinal and liver physiology. 2012;303:G453–G460. doi: 10.1152/ajpgi.00497.2011. [DOI] [PubMed] [Google Scholar]
- Lu B, Nagappan G, Guan X, Nathan PJ, Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nature reviews Neuroscience. 2013;14:401–416. doi: 10.1038/nrn3505. [DOI] [PubMed] [Google Scholar]
- Lucas P, Landete J, Coton M, Coton E, Lonvaud-Funel A. The tyrosine decarboxylase operon of Lactobacillus brevis IOEB 9809: characterization and conservation in tyramine-producing bacteria. FEMS microbiology letters. 2003;229:65–71. doi: 10.1016/S0378-1097(03)00787-0. [DOI] [PubMed] [Google Scholar]
- Lyte M. Microbial endocrinology in the microbiome-gut-brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS pathogens. 2013;9:e1003726. doi: 10.1371/journal.ppat.1003726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyte M. Microbial endocrinology and the microbiota-gut-brain axis. Advances in experimental medicine and biology. 2014;817:3–24. doi: 10.1007/978-1-4939-0897-4_1. [DOI] [PubMed] [Google Scholar]
- Macnab V, Barber I. Some (worms) like it hot: fish parasites grow faster in warmer water, and alter host thermal preferences. Global Change Biol. 2012;18:1540–1548. [Google Scholar]
- Maitland DP. A Parasitic Fungus Infecting Yellow Dungflies Manipulates Host Perching Behavior. P Roy Soc B-Biol Sci. 1994;258:187–193. [Google Scholar]
- Marcobal A, Kashyap PC, Nelson TA, Aronov PA, Donia MS, Spormann A, Fischbach MA, Sonnenburg JL. A metabolomic view of how the human gut microbiota impacts the host metabolome using humanized and gnotobiotic mice. The ISME journal. 2013;7:1933–1943. doi: 10.1038/ismej.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Kibe R, Ooga T, Aiba Y, Kurihara S, Sawaki E, Koga Y, Benno Y. Impact of intestinal microbiota on intestinal luminal metabolome. Scientific reports. 2012;2:233. doi: 10.1038/srep00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K. Gut microbes and the brain: paradigm shift in neuroscience. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:15490–15496. doi: 10.1523/JNEUROSCI.3299-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. The Journal of clinical investigation. 2015;125:926–938. doi: 10.1172/JCI76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- McNulty NP, Yatsunenko T, Hsiao A, Faith JJ, Muegge BD, Goodman AL, Henrissat B, Oozeer R, Cools-Portier S, Gobert G, et al. The impact of a consortium of fermented milk strains on the gut microbiome of gnotobiotic mice and monozygotic twins. Science translational medicine. 2011;3:106ra106. doi: 10.1126/scitranslmed.3002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead J, Ashwood P. Evidence supporting an altered immune response in ASD. Immunology letters. 2015;163:49–55. doi: 10.1016/j.imlet.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, Bisson JF, Rougeot C, Pichelin M, Cazaubiel M, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. The British journal of nutrition. 2011;105:755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- Meuer K, Pitzer C, Teismann P, Kruger C, Goricke B, Laage R, Lingor P, Peters K, Schlachetzki JC, Kobayashi K, et al. Granulocyte-colony stimulating factor is neuroprotective in a model of Parkinson's disease. Journal of neurochemistry. 2006;97:675–686. doi: 10.1111/j.1471-4159.2006.03727.x. [DOI] [PubMed] [Google Scholar]
- Miller TL, Wolin MJ. Fermentations by saccharolytic intestinal bacteria. The American journal of clinical nutrition. 1979;32:164–172. doi: 10.1093/ajcn/32.1.164. [DOI] [PubMed] [Google Scholar]
- Minuk GY. Gamma-aminobutyric acid (GABA) production by eight common bacterial pathogens. Scandinavian journal of infectious diseases. 1986;18:465–467. doi: 10.3109/00365548609032366. [DOI] [PubMed] [Google Scholar]
- Mitchell RW, On NH, Del Bigio MR, Miller DW, Hatch GM. Fatty acid transport protein expression in human brain and potential role in fatty acid transport across human brain microvessel endothelial cells. Journal of neurochemistry. 2011;117:735–746. doi: 10.1111/j.1471-4159.2011.07245.x. [DOI] [PubMed] [Google Scholar]
- Montiel C, Augusto J, Baez Y, Mario G, Pacheco-Lopez G. Social neuroeconomics: the influence of microbiota in partner-choice and sociality. Current pharmaceutical design. 2014;20:4774–4783. doi: 10.2174/1381612820666140130210631. [DOI] [PubMed] [Google Scholar]
- Mulle JG, Sharp WG, Cubells JF. The gut microbiome: a new frontier in autism research. Current psychiatry reports. 2013;15:337. doi: 10.1007/s11920-012-0337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2011;23:255–264. e119. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- O'Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behavioural brain research. 2015;277C:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, Burroughs AR, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. Journal of immunology. 2010;185:4101–4108. doi: 10.4049/jimmunol.1001443. [DOI] [PubMed] [Google Scholar]
- Ozogul F, Kuley E, Ozogul Y, Ozogul I. The Function of Lactic Acid Bacteria on Biogenic Amines Production by Food-Borne Pathogens in Arginine Decarboxylase Broth. Food Sci Technol Res. 2012;18:795–804. [Google Scholar]
- Paredes RG, Agmo A. GABA and behavior: the role of receptor subtypes. Neuroscience and biobehavioral reviews. 1992;16:145–170. doi: 10.1016/s0149-7634(05)80177-0. [DOI] [PubMed] [Google Scholar]
- PrabhuDas M, Bonney E, Caron K, Dey S, Erlebacher A, Fazleabas A, Fisher S, Golos T, Matzuk M, McCune JM, et al. Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat Immunol. 2015;16:328–334. doi: 10.1038/ni.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash A, Medhi B, Chopra K. Granulocyte colony stimulating factor (GCSF) improves memory and neurobehavior in an amyloid-beta induced experimental model of Alzheimer's disease. Pharmacology, biochemistry, and behavior. 2013;110:46–57. doi: 10.1016/j.pbb.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Pyndt Jorgensen B, Hansen JT, Krych L, Larsen C, Klein AB, Nielsen DS, Josefsen K, Hansen AK, Sorensen DB. A possible link between food and mood: dietary impact on gut microbiota and behavior in BALB/c mice. PloS one. 2014;9:e103398. doi: 10.1371/journal.pone.0103398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reigstad CS, Salmonson CE, Rainey JF, 3rd, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, Kashyap PC. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014;29:1395–1403. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E, Sharon G, Atad I, Zilber-Rosenberg I. The evolution of animals and plants via symbiosis with microorganisms. Environmental microbiology reports. 2010;2:500–506. doi: 10.1111/j.1758-2229.2010.00177.x. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CS. Microbiome Disturbances and Autism Spectrum Disorders. Drug metabolism and disposition: the biological fate of chemicals. 2015 doi: 10.1124/dmd.115.063826. In press, [DOI] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nature reviews Immunology. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savignac HM, Kiely B, Dinan TG, Cryan JF. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2014;26:1615–1627. doi: 10.1111/nmo.12427. [DOI] [PubMed] [Google Scholar]
- Savignac HM, Tramullas M, Kiely B, Dinan TG, Cryan JF. Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behavioural brain research. 2015;287:59–72. doi: 10.1016/j.bbr.2015.02.044. [DOI] [PubMed] [Google Scholar]
- Selkrig J, Wong P, Zhang X, Pettersson S. Metabolic tinkering by the gut microbiome: Implications for brain development and function. Gut microbes. 2014;5:369–380. doi: 10.4161/gmic.28681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon G, Garg N, Debelius J, Knight R, Dorrestein PC, Mazmanian SK. Specialized metabolites from the microbiome in health and disease. Cell metabolism. 2014;20:719–730. doi: 10.1016/j.cmet.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon G, Segal D, Ringo JM, Hefetz A, Zilber-Rosenberg I, Rosenberg E. Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20051–20056. doi: 10.1073/pnas.1009906107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu WC, Lin SZ, Yang HI, Tzeng YS, Pang CY, Yen PS, Li H. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor-stimulated stem cells. Circulation. 2004;110:1847–1854. doi: 10.1161/01.CIR.0000142616.07367.66. [DOI] [PubMed] [Google Scholar]
- Sobrino Crespo C, Perianes Cachero A, Puebla Jimenez L, Barrios V, Arilla Ferreiro E. Peptides and food intake. Frontiers in endocrinology. 2014;5:58. doi: 10.3389/fendo.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen L, Sellaro R, van Hemert S, Bosch JA, Colzato LS. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain, behavior, and immunity. 2015 doi: 10.1016/j.bbi.2015.04.003. In press, [DOI] [PubMed] [Google Scholar]
- Stilling RM, Bordenstein SR, Dinan TG, Cryan JF. Friends with social benefits: host-microbe interactions as a driver of brain evolution and development? Frontiers in cellular and infection microbiology. 2014a;4:147. doi: 10.3389/fcimb.2014.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilling RM, Dinan TG, Cryan JF. Microbial genes, brain & behaviour - epigenetic regulation of the gut-brain axis. Genes, brain, and behavior. 2014b;13:69–86. doi: 10.1111/gbb.12109. [DOI] [PubMed] [Google Scholar]
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. The Journal of physiology. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis KR, Schmidt TM, Holekamp KE. Evidence for a bacterial mechanism for group-specific social odors among hyenas. Scientific reports. 2012;2:615. doi: 10.1038/srep00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis KR, Venkataraman A, Dycus JA, Koonter KD, Schmitt-Matzen EN, Wagner AP, Holekamp KE, Schmidt TM. Symbiotic bacteria appear to mediate hyena social odors. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:19832–19837. doi: 10.1073/pnas.1306477110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, Guyonnet D, Legrain-Raspaud S, Trotin B, Naliboff B, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394–1401. 1401, e1391–e1394. doi: 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam AM, Brouns M, Louisse S, Berkenbosch F. Appearance of interleukin-1 in macrophages and in ramified microglia in the brain of endotoxin-treated rats: a pathway for the induction of non-specific symptoms of sickness? Brain research. 1992;588:291–296. doi: 10.1016/0006-8993(92)91588-6. [DOI] [PubMed] [Google Scholar]
- Vandvik PO, Wilhelmsen I, Ihlebaek C, Farup PG. Comorbidity of irritable bowel syndrome in general practice: a striking feature with clinical implications. Alimentary pharmacology & therapeutics. 2004;20:1195–1203. doi: 10.1111/j.1365-2036.2004.02250.x. [DOI] [PubMed] [Google Scholar]
- Velagapudi VR, Hezaveh R, Reigstad CS, Gopalacharyulu P, Yetukuri L, Islam S, Felin J, Perkins R, Boren J, Oresic M, et al. The gut microbiota modulates host energy and lipid metabolism in mice. Journal of lipid research. 2010;51:1101–1112. doi: 10.1194/jlr.M002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetulani J. Early maternal separation: a rodent model of depression and a prevailing human condition. Pharmacological reports : PR. 2013;65:1451–1461. doi: 10.1016/s1734-1140(13)71505-6. [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R, Cryan JF, Ross RP, Fitzgerald GF, Dinan TG, Stanton C. Bacterial neuroactive compounds produced by psychobiotics. Advances in experimental medicine and biology. 2014;817:221–239. doi: 10.1007/978-1-4939-0897-4_10. [DOI] [PubMed] [Google Scholar]
- Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BB, Van Benschoten AH, Cimermancic P, Donia MS, Zimmermann M, Taketani M, Ishihara A, Kashyap PC, Fraser JS, Fischbach MA. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell host & microbe. 2014;16:495–503. doi: 10.1016/j.chom.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano JM, Yu K, Donaldson G, Shastri G, Ma L, Ann P, Nagler C, Ismagliov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LR, Navalitloha Y, Singhal S, Mehta J, Piao CS, Guo WP, Kessler JA, Groothuis DR. Hematopoietic growth factors pass through the blood-brain barrier in intact rats. Experimental neurology. 2007;204:569–573. doi: 10.1016/j.expneurol.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31:2121–2131. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]
- Zucchi R, Chiellini G, Scanlan TS, Grandy DK. Trace amine associated receptors and their ligands. Br J Pharmacol. 2006;149:967–978. doi: 10.1038/sj.bjp.0706948. [DOI] [PMC free article] [PubMed] [Google Scholar]