Abstract
Drug Affinity Responsive Target Stability (DARTS) is a relatively quick and straightforward approach to identify potential protein targets for small molecules. It relies on the protection against proteolysis conferred on the target protein by interaction with a small molecule. The greatest advantage of this method is being able to use the native small molecule without having to immobilize or modify it (e.g. by incorporation of biotin, fluorescent, radioisotope, or photo-affinity labels). Here we describe in detail the protocol for performing unbiased DARTS with complex protein lysate to identify potential binding targets of small molecules and for using DARTS-Western blotting to test, screen, or validate potential small molecule targets. Although the ideas have mainly been developed from studying molecules in areas of biology that are currently of interest to us and our collaborators, the general principles should be applicable to the analysis of all molecules in nature.
Keywords: small molecules, drugs, target identification, metabolites, natural products, proteomics, mass spectrometry, immunoblotting
1. Introduction
Small molecule target identification is a critical aspect of chemical genetics, metabolomics, and drug discovery (1-4). A variety of methods have been developed for small molecule target identification, with affinity chromatography being the most commonly used approach (5-7). However, affinity chromatography and related approaches are limited by the need to derivatize each small molecule, and many compounds cannot be modified without loss of binding specificity or affinity. On the other hand, genetic/genomic methods are limited to particular classes of compounds (e.g. those that affect fitness, transcription, localization, etc.) and, because they rely on downstream readouts, do not necessarily pinpoint the direct targets (5, 8). These limitations have spurred the continual development of new and improved methods. Drug Affinity Responsive Target Stability (DARTS) is a paradigm-changing method developed to overcome these limitations. DARTS leverages the thermodynamic stabilization of the target protein that occurs upon small molecule binding by detecting the binding-induced increase in resistance to proteolysis (9). This is highly advantageous because it uses the native, unmodified small molecules and relies solely on the binding interaction but not downstream readouts to discover target proteins.
DARTS is a relatively simple technique that can easily be adopted by most labs. Unlike affinity chromatography, DARTS is not limited by the chemistry of the small molecule of interest and does not require derivatization or immobilization of the compound. Rather, DARTS is performed by simply treating aliquots of cell lysate with the compound of interest and either vehicle control or an inactive analog, followed by limited digestion of the proteins in the cell lysate with proteases. Subsequently, the samples are separated by SDS-PAGE and stained to identify protein bands that are protected from proteolysis by the small molecule. Mass spectrometry (MS) is then used to identify the proteins present in each band. This unbiased DARTS approach has been successfully utilized to identify novel protein targets for natural products and other bioactive small molecules; see (10) for a recent example identifying a novel protein target for disulfiram, an FDA-approved drug used to treat chronic alcoholism. Although this gel-based approach is the easiest to implement, more efficient gel-free proteomics approaches are also being used with DARTS to facilitate identification of the protected proteins (5, 11).
While DARTS has been successfully performed in an unbiased fashion as a discovery tool to identify unknown targets of natural products and drugs (see (9, 10, 12, 13) for some examples), it is also powerful as a means to screen or validate binding of compounds to proteins of interest. This targeted approach has been widely used, with recombinant and/or purified proteins using gel staining, endogenous proteins in lysates using western blotting, and epitope-tagged proteins expressed in cells or in vitro and detected with epitope-specific antibodies (9, 10, 14-19). Moreover, the targeted approach could be used for high-throughput screening for compounds that bind a specific protein. Here we describe examples using DARTS to assay additional small molecule-protein interactions, including two model drug-protein pairs, methotrexate-DHFR and olaparib-PARP (20), as well as omigapil (CGP3466B)-GAPDH, which has been suggested to be protective against motor neuron apoptosis (21).
2. Materials
2.1 DARTS Materials
Phosphate-buffered saline (PBS).
Protease inhibitor cocktail (20X): Dilute one tablet of protease inhibitor cocktail (Roche) with 525 μL of ultrapure water to make 20X concentration. Mix to fully dissolve tablet, and store at −20 °C. (Protease inhibitor cocktails from other vendors may also work, but the concentrations for each inhibitor vary. Cocktail can also be home assembled to customize specific concentrations if necessary.)
Lysis buffer: For 1 mL of mammalian protein extraction lysis buffer, mix 50 μL 20X protease inhibitor cocktail (Roche), 50 μL 1 M sodium fluoride, 100 μL 100 mM β-glycerophosphate, 100 μL 50 mM sodium pyrophosphate, and 10 μL 200 mM sodium orthovanadate with 690 μL M-PER reagent (M-PER, Thermo Scientific) (see Note 1). Once lysis buffer is made, keep on ice. Make fresh lysis buffer for every DARTS experiment.
TNC buffer (10X): For 1 mL of 10X TNC buffer, mix 500 μL 1 M Tris-HCl pH 8.0, 100 μL 5 M sodium chloride, and 100 μL 1 M calcium chloride with 300 μL ultrapure water (see Note 2). Once 10X TNC buffer is made, keep on ice. Store aliquots at −20 °C.
BCA protein concentration assay reagents (other protein concentration assays such as Bradford can be used instead). Bovine Serum Albumin can be used for the standard.
Small molecule: Dilute in appropriate solvent and store accordingly in glass vials (see Note 3).
Pronase (Roche): Prepare a 10 mg/mL stock solution in ultrapure water, aliquot, and store at −20 °C.
Thermolysin (Sigma): Prepare a 10 mg/mL stock solution in 1X TNC buffer, aliquot, and store at −20 °C. (Proteases from other suppliers should also work, but they may require different amounts than we describe herein.)
SDS-PAGE loading buffer (5X): For 50 mL of SDS-PAGE loading buffer, mix 12.5 mL 1 M Tris-HCl (pH 6.8), 25 mL 100% glycerol, 5 g sodium dodecyl sulfate, 0.25 g bromophenol blue, and 2.5 mL 14.3 M β-mercaptoethanol with 10 mL ultrapure water. Aliquot and store at −20 °C.
2.2 SDS-PAGE, Visualization, and Mass Spectrometry Materials
SDS-PAGE gel (see Note 4).
Silver staining kit (must be MS compatible, such as from Sigma Aldrich), SimplyBlue stain (Invitrogen), or SYPRO Ruby stain (Invitrogen) for visualization.
Mass spectrometry materials (see Note 5).
Western blotting materials.
Antibody for potential small molecule target and control protein.
3. Methods
3.1 DARTS with Complex Protein Lysate
Grow cells to approximately 80-85% confluence (see Note 6).
Aspirate media from plates. Wash the cells with ice-cold phosphate buffered saline (see Note 7).
Lyse cells with appropriate amount of lysis buffer (see Note 8).
Scrape cells off with cell scraper and collect.
Allow lysis of cells to occur on ice for 10 min (see Note 9).
Centrifuge for 10 min at 18,000 X g at 4 °C to pellet cellular debris and DNA.
Remove supernatant (cell lysate) and transfer to a new 1.5 mL tube. Keep on ice. 8. Add appropriate volume of 10X TNC buffer to make a final concentration of 1X TNC buffer in the lysate (see Note 10).
Perform BCA protein concentration assay to determine protein concentration of cell lysate (see Note 11).
Create 100X stock solutions of small molecule via serial dilutions (see Note 12).
Split cell lysate into identical aliquots of 99 μL (see Note 13).
Add 1 μL of vehicle control (solvent that the small molecule is dissolved in) and various 100X small molecule stock solutions to each aliquot of lysate. Incubate cell lysate with small molecule for 15-30 min at room temperature with shaking with a thermomixer (see Note 14 for information about the concentration, volume, solvent, etc. for the small molecule). Alternatively, samples can be rotated on a rotator.
Make protease dilutions in 1X TNC buffer (see Note 15 for protease choice and solution preparation). Be sure to use a fresh aliquot of protease in every experiment.
After incubation with the small molecule is complete, split each sample into 20 μL samples (see Note 16).
Add 2 μL of the range of protease solutions (see Note 15 for protease choice) prepared in Section 3.1 Step 13 to achieve the appropriate final ratio of total enzyme to total substrate in each sample. Add the protease solutions at specific intervals (e.g. every 30 seconds) to ensure that each sample is digested for the same amount of time. Be sure to include a sample that is not digested. For the non-digested sample, add 2 μL of 1X TNC buffer instead of protease.
Incubate at room temperature with protease of choice for appropriate time (see Note 17).
Stop each digestion reaction by adding 2 μL of 20X protease inhibitor cocktail (at the same specific intervals as used above) and incubate on ice for 10 min.
Add 6 μL of 5X SDS-PAGE loading buffer to the samples to achieve a final 1X SDS-PAGE loading buffer concentration.
Heat at 70 °C for 10 min.
Spin samples down briefly with a microfuge and proceed to analysis via SDS-PAGE in Section 3.2. At this point, samples may be stored at −20 °C for at least several weeks if SDS-PAGE will not be performed immediately.
3.2 Identification and Validation of Potential Small Molecule Targets
If samples were frozen, thaw the samples prepared in section 3.1 to room temperature.
For each sample, load 10-20 μg protein into each lane of a 10-well or 12-well minigel (1.0 mm thickness).
Perform electrophoresis at room temperature using a constant voltage of 100V until the dye front has reached the bottom of the gel (typically 1.5 - 2 hours).
Carefully remove the gel from the plastic or glass plates using clean gloves and transfer it into a clean staining tray containing distilled water.
Wash the gel for 5 minutes 3 times with distilled water by shaking on a flat rotator.
Stain the gel to visualize protein samples using a mass spectrometry-compatible silver staining kit, SimplyBlue stain, or SYPRO Ruby stain (see Note 18). Follow the manufacturer's instructions for staining.
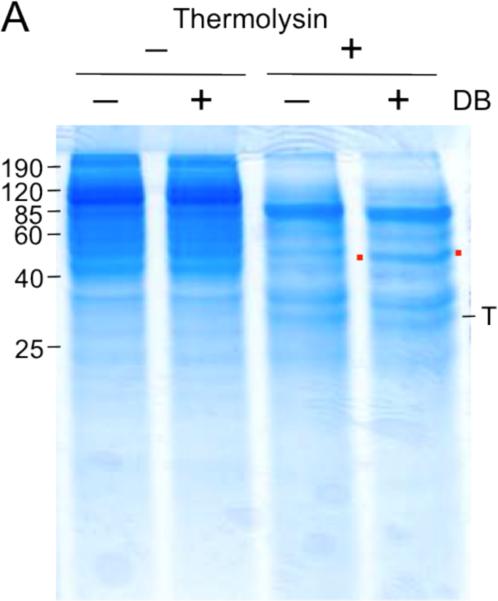
Look for bands that appear to be protected by incubation with the small molecule over vehicle control (see Note 19 and 20). For example, in Figure 1, incubation with the small molecule confers protection against proteolysis:
Excise gel bands corresponding to the protected proteins with a small, clean scalpel or razor blade and analyze via mass spectrometry using standard protein identification approaches. It is important to include the corresponding region from the vehicle control lane in the analysis because multiple proteins may be identified in the gel band. Quantitative mass spectrometry analysis (e.g. using spectral counting or extracted ion chromatography) is an appropriate approach to determine which protein is enriched in the drug-treated versus control sample. Once the protein(s) present in the protected band is identified, however, whether or not each is protected can be verified by immunoblotting.
Once potential small molecule targets are identified via mass spectrometry, the binding of the small molecule to candidate targets can be immediately validated by Western blotting of samples from the unbiased DARTS experiment as well as an independent DARTS experiment using antibodies specific to the candidate target proteins (see Note 21). If no antibodies are available, then candidate proteins can be produced by in vitro translation or by expression of epitope-tagged proteins in transfected cells (see (9, 10, 14) for examples). This DARTS-Western analysis is also extremely useful for validating any potential target proteins identified using other methods, such as omics profiling (14), pathway screening, in silico docking (19), or other computational predictions.
After DARTS, perform SDS-PAGE, transfer the proteins to a membrane suitable for immunoblotting, and blot the membrane with an antibody against the putative protein target as well as at least one control protein (see Note 22). For example, in Figure 2, we used three familiar small molecule-protein target pairs to illustrate the use of DARTS for candidate target validation:
Figure 1.
Example of SimplyBlue staining visualization of unbiased DARTS with the small molecule didemnin B (DB). Red dots flank the protected band; T, thermolysin. Reprinted from (9).
Figure 2.
(A) DARTS with methotrexate (Mtx) shows interaction with its known target dihydrofolate reductase (DHFR) but not eukaryotic elongation factor 1 alpha (eEF1A), which serves as a control protein. Jurkat lysates were incubated with varying concentrations of methotrexate or vehicle (in equal volume, with final 1% DMSO), followed by digestion with 1:900 Pronase:protein ratios for 15 min. The dissociation constant for purified recombinant DHFR is ~10 nM. Its IC50 for cell lines varies greatly, and some cells have nM IC50 values corresponding to its binding affinity. We found that with ~30 nM of Mtx, there is the same level of protection of DHFR against proteolysis as with ~100 μM of Mtx. (B) DARTS with olaparib (O) (IC50 ~1 nM) confirms its interaction with its known target poly(ADP-ribose) polymerase (PARP), but not DHFR, which is instead the target of Mtx. Performed as in A using varying concentrations of olaparib or vehicle (in equal volume, with final 1% DMSO). (C) DARTS with CGP 3466B confirms its interaction with GAPDH while eEF1A serves as a control protein. HEK293 cell lysates were incubated with 100 μM CGP 3466B or 1% DMSO, followed by digestion with 1:1600, 1:800, 1:400, and 1:200 Pronase:protein ratios for 15 min (see Note 23). Although CGP was reported to show strong neuroprotective effects at 1 nM (21), it is not clear that this is mediated by GAPDH.
Acknowledgments
Supported by the US National Institutes of Health grants R01 CA124974 (J.H.), R21 CA149774 (J.H.), U19 AI067769 (W.B.), R01 GM103479 (J.A.L.), R01 GM104610 (J.A.L.), and training grants to M.Y.P. (T32 GM007185) and B.L. (T32 CA009120).
Footnotes
Protease inhibitor cocktails from other vendors may also work, but the concentrations for each inhibitor vary. The cocktail can also be home assembled to customize specific concentrations if necessary.
Other lysis buffers with various detergents (e.g. Triton X-100 or NP-40) can be used with DARTS as long as they are non-denaturing.
If the lysis buffer used includes any type of buffering agent (e.g. Tris or HEPES) and sodium chloride or another salt (such as potassium chloride), 10X TNC buffer is not necessary.
Small molecules should be stored in glass vials to avoid loss due to potential absorption by plastic tubes. This may result in a drastically lower concentration of certain compounds than intended.
When performing unbiased DARTS, a 4-12% Bis-Tris gradient gel can first be used to separate the protein samples. Once potential protein targets are identified, depending on the molecular weight of those targets, a gel that best separates either small or large molecular weight proteins can be used if necessary.
For new users, it is highly advised to collaborate with researchers who have expertise in mass spectrometry and MS-based proteomics.
The number of cells needed for each DARTS experiment will vary based on how much protein can be extracted from various cell lines. In general, the protein concentration of the lysate used is between 2.5-5 μg/μL. In one DARTS experiment with DB where we tested Jurkat lysates at 1 μg/μL and at 5 μg/μL, the protection was more apparent in the 5 μg/μL DB-treated lysate. However, plenty of experiments by others have used lower concentrations, around 2-4 μg/μL, that work just as well for other compounds. We have not tested using substantially higher protein concentrations.
Make sure to remove all media, especially those that contain fetal bovine serum (FBS) as proteins in FBS may interfere with the protein concentration assay and downstream protease concentration calculations.
Use less lysis buffer for a more concentrated protein lysate. One 10 cm plate of HEK293 cells at 85-90% confluency lysed with 600 μL of lysis buffer typically results in a protein lysate of ~2.5 μg/μL.
Be sure not to vortex the protein lysate as this may disrupt the native conformation of some proteins and alter or abolish their ligand-binding activity.
In our experience, when the 10X TNC buffer is added to M-PER lysis buffer, the lysate will become slightly cloudy. Again, if an alternate lysis buffer that includes a buffer and salt is used, 10X TNC buffer is not needed (see Note 2).
Any sufficiently sensitive protein concentration assay (e.g. Bradford) can be used to determine the protein concentration of the lysate.
If the small molecule is stored at 4 °C or −20 °C, make sure to allow the vials to warm up to room temperature before opening to avoid condensation and ensure that the weighing of the compound is accurate. Weigh enough of the small molecule to make a beginning stock concentration of 100 mM (or lower depending on maximum solubility). From there, make serial dilutions from the beginning stock to create 100X stock solutions. When performing unbiased DARTS, one may begin with a higher concentration of the small molecule (5-10X the IC50 value) to ensure optimal binding, although this could also potentially increase the number of non-specific binders identified. Additionally, one may begin testing concentrations near the IC50 of the compound to minimize identification of off-targets, and only subsequently testing higher doses if necessary.
The number of aliquots of protein lysate needed depends on the number of small molecule concentrations that are going to be tested. When performing unbiased DARTS, begin with one or two concentrations of the small molecule (refer to Note 12 for choosing concentrations). Once a candidate target protein is determined, additional concentrations of the small molecule can be used to determine relative binding affinity. Remember to include a sample for vehicle control or inactive analog control.
The time required for small molecule-lysate incubation can vary. While most binding equilibria are reached in seconds, we generally incubate for at least 15 to 30 min to ensure optimal binding.
For DARTS, we recommend the proteases thermolysin and Pronase because they have been used successfully by us and others for numerous different compounds. See (5, 9, 11) for more information on choosing a protease to use. While other proteases may work equally well, we have not substantially explored alternatives as it has not been necessary. To begin, test a range of protease concentrations (e.g. from 1:100 to 1:1000 Pronase:protein ratio) to ensure the potential small molecule target is neither completely digested or not digested enough. Protease concentrations can be adjusted if the proteome is over- or under-digested. To calculate protease concentrations (example): 2.5 μg/μL protein concentration X 20 μL sample = 50 μg protein For a protease concentration of 1:100 protease:protein – 50 μg ÷ 100 ÷ 2μL = 0.25 μg/μL protease concentration needed 2.5 μL 10 μg/μL stock protease in 97.5 μL 1X TNC buffer
Just prior to this, if the salt from the TNC buffer has settled to the bottom of the tube, mix by tapping the tube to ensure the solution is homogenous.
Generally, begin with 20 min digestion times. This can be eventually tailored, if necessary, once potential small molecule targets are identified. In fact, we have found that some small molecules may provide better protection with shorter digestion times (e.g. 5-10 min).
Many staining methods are available for gels. Silver, SimplyBlue, or SYPRO Ruby stainings have all been used successfully with DARTS and LC-MS/MS analysis, although other methods may also work. When performing staining, be sure that gloves and containers used are clean to prevent contamination by keratin and other environmental proteins in downstream mass spectrometry analysis.
If after visualization the entire lane of sample treated with the small molecule seems to be darker than the entire lane of sample treated with vehicle control, either loading is inconsistent between lanes or the small molecule has an effect on the protease used. If the latter is the case, another protease can be used.
Once a potential protein target is identified, the protease concentrations used can be tailored (if necessary) to the sensitivity of the potential protein target. From the DARTS experiments with various small molecules that we have tested, we observed that larger proteins (e.g. mTOR, eIF4G, dynein heavy chain) tend to be more sensitive to proteolysis, which may be explained by the increased number of flexible regions across the full length of the protein and/or the increased number of peptide bonds (protease substrates). On the other hand, many small proteins (e.g. FKBP12, eIF4E, GAPDH), especially those consisting of a single domain, are relatively resistant to proteolysis and therefore require more protease or increased digestion time. Regardless of this variability in susceptibility to proteolysis among different proteins, protection of the target protein can be seen across a range of protease concentrations in which the target is partially or fully digested in the vehicle-treated control.
There are two types of validation during target identification: binding vs. functional, i.e. validation of the physical binding interaction between the small molecule and the potential target and validation of the potential target as a physiological target. While quantitative mass spectrometry should be able to determine which protein identified is protected from proteolysis, we suggest validating this protection by repeating the DARTS experiment and performing Western blotting, when possible. If a hit identified to be protected against proteolysis via mass spectrometry was not detected as such when analyzed via Western blotting with a specific antibody, it may be due to different sensitivities of mass spectrometry and Western blotting or the lack of appropriate epitope in the full-length or partially digested candidate target protein. In such a case, using a FLAG-tagged construct is recommended. Furthermore, it is important to functionally validate the protein target not only as interacting with the small molecule but also in some way modifying the protein's activity (e.g. in an in vitro biochemical assay and in vivo biological readout). The binding target identified may not necessarily be the target responsible for the bioactivity of interest of the small molecule, and functional tests must be performed to determine whether or not it is the target of interest. The functional tests used will depend on the bioactivity under study and the binding targets identified for the small molecule, a discussion of which is outside the scope of this chapter.
Probing for a control protein is required to show that binding is specific and that the small molecule does not have an inhibitory effect on the protease used. GAPDH, actin, and tubulin are often used as control proteins, although any protein with a similar sensitivity to proteolysis may be used. In addition, to further show that the interaction between the potential protein target and the small molecule is specific, other unrelated small molecules or inactive analogs can be used alongside the small molecule of interest when performing DARTS. If the small molecule interaction with the protein target is truly specific to the pair, then most other small molecules should not result in protection of the protein target from proteolysis.
The DARTS experiments in Figure 2 were done with both Jurkat and HEK293 cell lysates. Depending on the small molecule under study, the exact cells used for DARTS may be unimportant, as many target proteins are expressed ubiquitously (22-24). For example, DARTS with a generally cytotoxic drug that has effects in many diverse cell types could be performed with any cell line sensitive to its effects. However, if the small molecule exhibits bioactivity in a specific cell type or under specifically induced conditions (e.g. upon starvation or radiation), we recommend using those cells because the target protein may not be expressed or active in other cell types.
References
- 1.O'Connor CJ, Laraia L, Spring DR. Chemical genetics. Chem Soc Rev. 2011;40:4332–4345. doi: 10.1039/c1cs15053g. [DOI] [PubMed] [Google Scholar]
- 2.Yang GX, Li X, Snyder M. Investigating metabolite-protein interactions: an overview of available techniques. Methods. 2012;57:459–466. doi: 10.1016/j.ymeth.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McFedries A, Schwaid A, Saghatelian A. Methods for the elucidation of protein-small molecule interactions. Chem Biol. 2013;20:667–673. doi: 10.1016/j.chembiol.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Rask-Andersen M, Masuram S, Schioth HB. The druggable genome: Evaluation of drug targets in clinical trials suggests major shifts in molecular class and indication. Annu Rev Pharmacol Toxicol. 2014;54:9–26. doi: 10.1146/annurev-pharmtox-011613-135943. [DOI] [PubMed] [Google Scholar]
- 5.Lomenick B, Olsen RW, Huang J. Identification of direct protein targets of small molecules. ACS Chem Biol. 2011;6:34–46. doi: 10.1021/cb100294v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ong SE, Li X, Schenone M, Schreiber SL, Carr SA. Identifying cellular targets of small-molecule probes and drugs with biochemical enrichment and SILAC. Methods Mol Biol. 2012;803:129–140. doi: 10.1007/978-1-61779-364-6_9. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler S, Pries V, Hedberg C, Waldmann H. Target identification for small bioactive molecules: finding the needle in the haystack. Angew Chem Int Ed Engl. 2013;52:2744–2792. doi: 10.1002/anie.201208749. [DOI] [PubMed] [Google Scholar]
- 8.Futamura Y, Muroi M, Osada H. Target identification of small molecules based on chemical biology approaches. Mol Biosyst. 2013;9:897–914. doi: 10.1039/c2mb25468a. [DOI] [PubMed] [Google Scholar]
- 9.Lomenick B, Hao R, Jonai N, Chin RM, Aghajan M, Warburton S, Wang J, Wu RP, Gomez F, Loo JA, Wohlschlegel JA, Vondriska TM, Pelletier J, Herschman HR, Clardy J, Clarke CF, Huang J. Target identification using drug affinity responsive target stability (DARTS). Proc Natl Acad Sci U S A. 2009;106:21984–21989. doi: 10.1073/pnas.0910040106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson TJ, Pai M, Liu JC, Vizeacoumar F, Sun T, Egan SE, Datti A, Huang J, Zacksenhaus E. High-throughput screen identifies disulfiram as a potential therapeutic for triple-negative breast cancer cells: interaction with IQ motif-containing factors. Cell Cycle. 2013;12:3013–3024. doi: 10.4161/cc.26063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lomenick B, Jung G, Wohlschlegel JA, Huang J. Target identification using drug affinity responsive target stability (DARTS). Curr Protoc Chem Biol. 2011;3:163–180. doi: 10.1002/9780470559277.ch110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tohda C, Urano T, Umezaki M, Nemere I, Kuboyama T. Diosgenin is an exogenous activator of 1,25D(3)-MARRS/Pdia3/ERp57 and improves Alzheimer's disease pathologies in 5XFAD mice. Sci Rep. 2012;2:535. doi: 10.1038/srep00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun W, Tanaka TQ, Magle CT, Huang W, Southall N, Huang R, Dehdashti SJ, McKew JC, Williamson KC, Zheng W. Chemical signatures and new drug targets for gametocytocidal drug development. Sci Rep. 2014;4:3743. doi: 10.1038/srep03743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aghajan M, Jonai N, Flick K, Fu F, Luo M, Cai X, Ouni I, Pierce N, Tang X, Lomenick B, Damoiseaux R, Hao R, Del Moral PM, Verma R, Li Y, Li C, Houk KN, Jung ME, Zheng N, Huang L, Deshaies RJ, Kaiser P, Huang J. Chemical genetics screen for enhancers of rapamycin identifies a specific inhibitor of an SCF family E3 ubiquitin ligase. Nat Biotechnol. 2010;28:738–742. doi: 10.1038/nbt.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen T, Ozel D, Qiao Y, Harbinski F, Chen L, Denoyelle S, He X, Zvereva N, Supko JG, Chorev M, Halperin JA, Aktas BH. Chemical genetics identify eIF2alpha kinase heme-regulated inhibitor as an anticancer target. Nat Chem Biol. 2011;7:610–616. doi: 10.1038/nchembio.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao S, Wang Q, Wang G, Lomenick B, Liu J, Fan CW, Deng LW, Huang J, Lum L, Chen C. The Chemistry and Biology of Nakiterpiosin -C-nor-D Homosteroids. Synlett. 2012;16:2298–2310. doi: 10.1055/s-0031-1290460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu S, Oshima T, Imada T, Masuda M, Debnath B, Grande F, Garofalo A, Neamati N. Stabilization of MDA-7/IL-24 for colon cancer therapy. Cancer Lett. 2013;335:421–430. doi: 10.1016/j.canlet.2013.02.055. [DOI] [PubMed] [Google Scholar]
- 18.Lim M, Otto-Duessel M, He M, Su L, Nguyen D, Chin E, Alliston T, Jones JO. Ligand-independent and tissue-selective androgen receptor inhibition by pyrvinium. ACS Chem Biol. 2013 doi: 10.1021/cb400759d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Xiao H, Lin L, Jou D, Kumari V, Lin J, Li C. Drug Design Targeting Protein-Protein Interactions (PPIs) Using Multiple Ligand Simultaneous Docking (MLSD) and Drug Repositioning: Discovery of Raloxifene and Bazedoxifene as Novel Inhibitors of IL-6/GP130 Interface. J Med Chem. 2014;57:632–641. doi: 10.1021/jm401144z. [DOI] [PubMed] [Google Scholar]
- 20.Molina DM, Jafari R, Ignatushchenko M, Seki T, Larsson EA, Dan C, Sreekumar L, Cao Y, Nordlund P. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science. 2013;341:84–87. doi: 10.1126/science.1233606. [DOI] [PubMed] [Google Scholar]
- 21.Kragten E, Lalande I, Zimmermann K, Roggo S, Schindler P, Muller D, van Oostrum J, Waldmeier P, Furst P. Glyceraldehyde-3-phosphate dehydrogenase, the putative target of the antiapoptotic compounds CGP 3466 and R-(−)-deprenyl. J Biol Chem. 1998;273:5821–5828. doi: 10.1074/jbc.273.10.5821. [DOI] [PubMed] [Google Scholar]
- 22.Lundberg E, Fagerberg L, Klevebring D, Matic I, Geiger T, Cox J, Algenas C, Lundeberg J, Mann M, Uhlen M. Defining the transcriptome and proteome in three functionally different human cell lines. Mol Syst Biol. 2010;6:450. doi: 10.1038/msb.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geiger T, Wehner A, Schaab C, Cox J, Mann M. Comparative proteomic analysis of eleven common cell lines reveals ubiquitous but varying expression of most proteins. Mol Cell Proteomics. 2012;11 doi: 10.1074/mcp.M111.014050. M111 014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geiger T, Velic A, Macek B, Lundberg E, Kampf C, Nagaraj N, Uhlen M, Cox J, Mann M. Initial quantitative proteomic map of 28 mouse tissues using the SILAC mouse. Mol Cell Proteomics. 2013;12:1709–1722. doi: 10.1074/mcp.M112.024919. [DOI] [PMC free article] [PubMed] [Google Scholar]