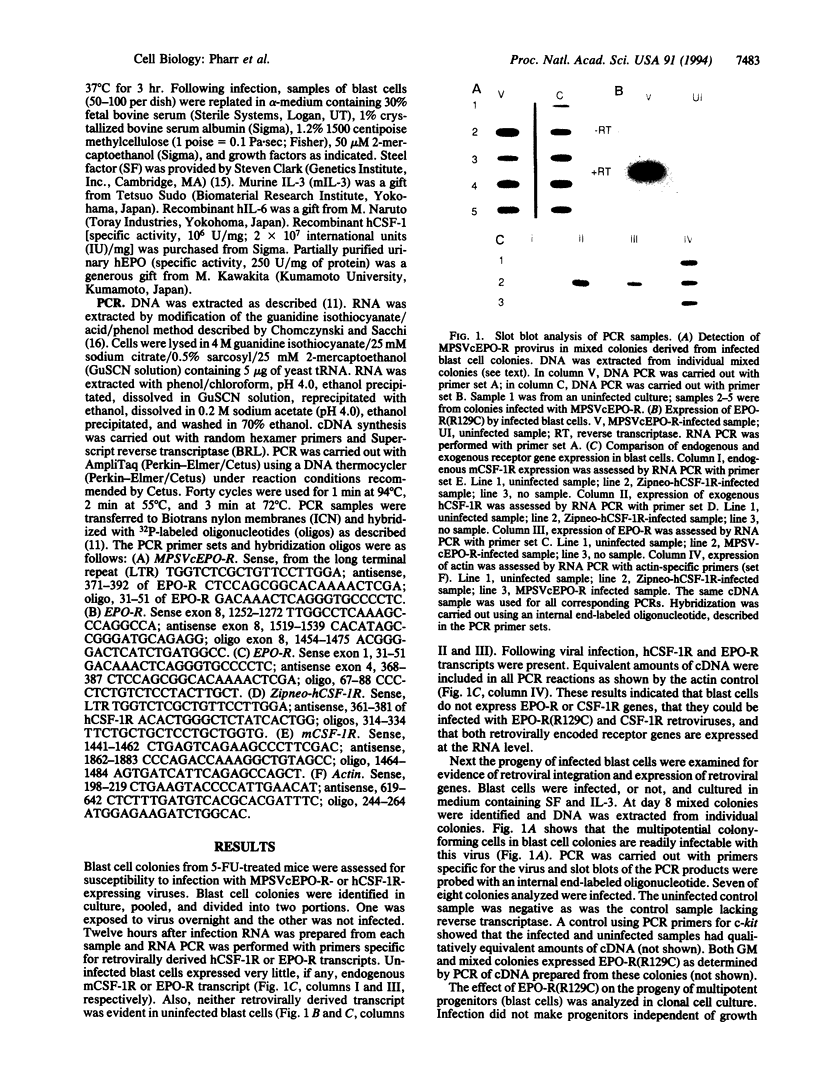
Abstract
Whether the presence of specific receptors on the surface of developing cells is the cause or consequence of lineage restriction is not known. If activation of specific receptors is the driving event in differentiation, the premature expression of specific receptors would promote differentiation along that pathway. In this study pluripotent progenitors, obtained from blast cell colonies (pooled or individual) of 5-fluorouracil-treated mice, were infected with retroviral vectors containing either an activated receptor for erythropoietin (EPO), an erythroid progenitor growth factor, or the receptor for colony-stimulating factor 1 (CSF-1), a macrophage growth factor. These receptors exhibit expression patterns restricted to committed progenitors. The developmental potential of infected pluripotent progenitors was not changed, although they expressed the exogenous genes, suggesting that in these cells activation of lineage-specific receptors does not induce differentiation. Acquisition of a constitutively activated EPO receptor allowed erythroid development in mixed colonies in the absence of EPO, as expected. Infection of progenitors with a virus containing the CSF-1 receptor promoted the development of granulocyte/macrophage (GM) colonies but did not alter the differentiation potential of either colony-forming unit (CFU)-GM or CFU-mix.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. M., Lyman S. D., Baird A., Wignall J. M., Eisenman J., Rauch C., March C. J., Boswell H. S., Gimpel S. D., Cosman D. Molecular cloning of mast cell growth factor, a hematopoietin that is active in both membrane bound and soluble forms. Cell. 1990 Oct 5;63(1):235–243. doi: 10.1016/0092-8674(90)90304-w. [DOI] [PubMed] [Google Scholar]
- BRUCE W. R., MCCULLOCH E. A. THE EFFECT OF ERYTHROPOIETIC STIMULATION ON THE HEMOPOIETIC COLONY-FORMING CELLS OF MICE. Blood. 1964 Feb;23:216–232. [PubMed] [Google Scholar]
- Borzillo G. V., Ashmun R. A., Sherr C. J. Macrophage lineage switching of murine early pre-B lymphoid cells expressing transduced fms genes. Mol Cell Biol. 1990 Jun;10(6):2703–2714. doi: 10.1128/mcb.10.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourette R. P., Mouchiroud G., Ouazana R., Morlé F., Godet J., Blanchet J. P. Expression of human colony-stimulating factor-1 (CSF-1) receptor in murine pluripotent hematopoietic NFS-60 cells induces long-term proliferation in response to CSF-1 without loss of erythroid differentiation potential. Blood. 1993 May 15;81(10):2511–2520. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cosman D., Lyman S. D., Idzerda R. L., Beckmann M. P., Park L. S., Goodwin R. G., March C. J. A new cytokine receptor superfamily. Trends Biochem Sci. 1990 Jul;15(7):265–270. doi: 10.1016/0968-0004(90)90051-c. [DOI] [PubMed] [Google Scholar]
- Gliniak B. C., Rohrschneider L. R. Expression of the M-CSF receptor is controlled posttranscriptionally by the dominant actions of GM-CSF or multi-CSF. Cell. 1990 Nov 30;63(5):1073–1083. doi: 10.1016/0092-8674(90)90510-l. [DOI] [PubMed] [Google Scholar]
- JACOBSON L. O., GOLDWASSER E., PLZAK L. F., FRIED W. Studies on erythropoiesis. IV. Reticulocyte response of hypophysectomized and polycythemic rodents to erythropoietin. Proc Soc Exp Biol Med. 1957 Feb;94(2):243–249. doi: 10.3181/00379727-94-22911. [DOI] [PubMed] [Google Scholar]
- Kinashi T., Lee K. H., Ogawa M., Tohyama K., Tashiro K., Fukunaga R., Nagata S., Honjo T. Premature expression of the macrophage colony-stimulating factor receptor on a multipotential stem cell line does not alter differentiation lineages controlled by stromal cells used for coculture. J Exp Med. 1991 May 1;173(5):1267–1279. doi: 10.1084/jem.173.5.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longmore G. D., Lodish H. F. An activating mutation in the murine erythropoietin receptor induces erythroleukemia in mice: a cytokine receptor superfamily oncogene. Cell. 1991 Dec 20;67(6):1089–1102. doi: 10.1016/0092-8674(91)90286-8. [DOI] [PubMed] [Google Scholar]
- Longmore G. D., Pharr P., Neumann D., Lodish H. F. Both megakaryocytopoiesis and erythropoiesis are induced in mice infected with a retrovirus expressing an oncogenic erythropoietin receptor. Blood. 1993 Oct 15;82(8):2386–2395. [PubMed] [Google Scholar]
- Mayani H., Dragowska W., Lansdorp P. M. Lineage commitment in human hemopoiesis involves asymmetric cell division of multipotent progenitors and does not appear to be influenced by cytokines. J Cell Physiol. 1993 Dec;157(3):579–586. doi: 10.1002/jcp.1041570318. [DOI] [PubMed] [Google Scholar]
- Metcalf D. Lineage commitment of hemopoietic progenitor cells in developing blast cell colonies: influence of colony-stimulating factors. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11310–11314. doi: 10.1073/pnas.88.24.11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio A. R., Migliaccio G., D'Andrea A., Baiocchi M., Crotta S., Nicolis S., Ottolenghi S., Adamson J. W. Response to erythropoietin in erythroid subclones of the factor-dependent cell line 32D is determined by translocation of the erythropoietin receptor to the cell surface. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11086–11090. doi: 10.1073/pnas.88.24.11086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharr P. N., Hankins D., Hofbauer A., Lodish H. F., Longmore G. D. Expression of a constitutively active erythropoietin receptor in primary hematopoietic progenitors abrogates erythropoietin dependence and enhances erythroid colony-forming unit, erythroid burst-forming unit, and granulocyte/macrophage progenitor growth. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):938–942. doi: 10.1073/pnas.90.3.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharr P. N., Ogawa M., Hankins W. D. In vitro retroviral transfer of ras genes to single hemopoietic progenitors. Exp Hematol. 1987 May;15(4):323–330. [PubMed] [Google Scholar]
- Pierce J. H., Di Marco E., Cox G. W., Lombardi D., Ruggiero M., Varesio L., Wang L. M., Choudhury G. G., Sakaguchi A. Y., Di Fiore P. P. Macrophage-colony-stimulating factor (CSF-1) induces proliferation, chemotaxis, and reversible monocytic differentiation in myeloid progenitor cells transfected with the human c-fms/CSF-1 receptor cDNA. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5613–5617. doi: 10.1073/pnas.87.15.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaldi A., Young D. C., Griffin J. D. Expression of the M-CSF (CSF-1) gene by human monocytes. Blood. 1987 May;69(5):1409–1413. [PubMed] [Google Scholar]
- Reissmann K. R., Samorapoompichit S. Effect of erythropoietin on proliferation of erythroid stem cells in the absence of transplantable colony-forming units. Blood. 1970 Sep;36(3):287–296. [PubMed] [Google Scholar]
- Reissmann K. R., Udupa K. B. Effect of erythropoietin on proliferation of erythropoietin-responsive cells. Cell Tissue Kinet. 1972 Nov;5(6):481–489. doi: 10.1111/j.1365-2184.1972.tb00386.x. [DOI] [PubMed] [Google Scholar]
- Roussel M. F., Downing J. R., Rettenmier C. W., Sherr C. J. A point mutation in the extracellular domain of the human CSF-1 receptor (c-fms proto-oncogene product) activates its transforming potential. Cell. 1988 Dec 23;55(6):979–988. doi: 10.1016/0092-8674(88)90243-7. [DOI] [PubMed] [Google Scholar]
- Roussel M. F., Dull T. J., Rettenmier C. W., Ralph P., Ullrich A., Sherr C. J. Transforming potential of the c-fms proto-oncogene (CSF-1 receptor). Nature. 1987 Feb 5;325(6104):549–552. doi: 10.1038/325549a0. [DOI] [PubMed] [Google Scholar]
- Stahl N., Boulton T. G., Farruggella T., Ip N. Y., Davis S., Witthuhn B. A., Quelle F. W., Silvennoinen O., Barbieri G., Pellegrini S. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science. 1994 Jan 7;263(5143):92–95. doi: 10.1126/science.8272873. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Guilbert L. J., Tushinski R. J., Bartelmez S. H. CSF-1--a mononuclear phagocyte lineage-specific hemopoietic growth factor. J Cell Biochem. 1983;21(2):151–159. doi: 10.1002/jcb.240210206. [DOI] [PubMed] [Google Scholar]
- Suda T., Suda J., Ogawa M. Disparate differentiation in mouse hemopoietic colonies derived from paired progenitors. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2520–2524. doi: 10.1073/pnas.81.8.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T., Suda J., Ogawa M. Proliferative kinetics and differentiation of murine blast cell colonies in culture: evidence for variable G0 periods and constant doubling rates of early pluripotent hemopoietic progenitors. J Cell Physiol. 1983 Dec;117(3):308–318. doi: 10.1002/jcp.1041170305. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W., Ratajczak M. Z., Ptasznik A., Sell K. W., Ahmed-Ansari A., Ostertag W. CSF-1 deficiency in the op/op mouse has differential effects on macrophage populations and differentiation stages. Exp Hematol. 1992 Sep;20(8):1004–1010. [PubMed] [Google Scholar]
- Witthuhn B. A., Quelle F. W., Silvennoinen O., Yi T., Tang B., Miura O., Ihle J. N. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993 Jul 30;74(2):227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- Yoshimura A., Longmore G., Lodish H. F. Point mutation in the exoplasmic domain of the erythropoietin receptor resulting in hormone-independent activation and tumorigenicity. Nature. 1990 Dec 13;348(6302):647–649. doi: 10.1038/348647a0. [DOI] [PubMed] [Google Scholar]