ABSTRACT
Identification and characterization of CD8+ T cells effectively controlling HIV-1 variants are necessary for the development of AIDS vaccines and for studies of AIDS pathogenesis, although such CD8+ T cells have been only partially identified. In this study, we sought to identify CD8+ T cells controlling HIV-1 variants in 401 Japanese individuals chronically infected with HIV-1 subtype B, in which protective alleles HLA-B*57 and HLA-B*27 are very rare, by using comprehensive and exhaustive methods. We identified 13 epitope-specific CD8+ T cells controlling HIV-1 in Japanese individuals, though 9 of these epitopes were not previously reported. The breadths of the T cell responses to the 13 epitopes were inversely associated with plasma viral load (P = 2.2 × 10−11) and positively associated with CD4 count (P = 1.2 × 10−11), indicating strong synergistic effects of these T cells on HIV-1 control in vivo. Nine of these epitopes were conserved among HIV-1 subtype B-infected individuals, whereas three out of four nonconserved epitopes were cross-recognized by the specific T cells. These findings indicate that these 12 epitopes are strong candidates for antigens for an AIDS vaccine. The present study highlighted a strategy to identify CD8+ T cells controlling HIV-1 and demonstrated effective control of HIV-1 by those specific for 12 conserved or cross-reactive epitopes.
IMPORTANCE HLA-B*27-restricted and HLA-B*57-restricted cytotoxic T lymphocytes (CTLs) play a key role in controlling HIV-1 in Caucasians and Africans, whereas it is unclear which CTLs control HIV-1 in Asian countries, where HLA-B*57 and HLA-B*27 are very rare. A recent study showed that HLA-B*67:01 and HLA-B*52:01-C*12:02 haplotypes were protective alleles in Japanese individuals, but it is unknown whether CTLs restricted by these alleles control HIV-1. In this study, we identified 13 CTLs controlling HIV-1 in Japan by using comprehensive and exhaustive methods. They included 5 HLA-B*52:01-restricted and 3 HLA-B*67:01-restricted CTLs, suggesting that these CTLs play a predominant role in HIV-1 control. The 13 CTLs showed synergistic effects on HIV-1 control. Twelve out of these 13 epitopes were recognized as conserved or cross-recognized ones. These findings strongly suggest that these 12 epitopes are candidates for antigens for AIDS vaccines.
INTRODUCTION
Development of effective vaccines against HIV-1 is of importance for controlling the HIV-1 epidemic. Several extensive clinical trials have been performed, but only the RV144 vaccine, tested in a trial in Thailand, showed weak protection against HIV-1, most likely through generation of nonneutralizing antibodies (1, 2). A recent clinical trial showed no protection against HIV-1 acquisition, although vaccine-induced HIV-1-specific CD8+ T cell responses were detected in 64% of the vaccinees (3). This result implied that induction of low-quality HIV-1-specific CD8+ T cells may not be adequate for protection against HIV-1 infection. Therefore, vaccines stimulating the production of high-quality CD8+ T cells might be worth exploring. The quality of CD8+ T cells has been assessed in terms of several indicators, such as polyfunctionality (4), antigen sensitivity (5), proliferative capacity (6), immunoregulation (7), and properties of the interaction among the T cell receptor (TCR), viral peptide, and major histocompatibility complex (MHC) (8, 9). The ability of CD8+ T cells to suppress HIV-1 replication in vitro may be a better indicator of CD8+ T cell efficacy than the above indicators, since this ability in HIV-1 controllers is significantly higher than that in noncontrollers (10, 11).
It is well known that HLA-B*27-restricted and HLA-B*57-restricted cytotoxic T lymphocytes (CTLs) play a key role in the control of HIV-1 in Caucasians and Africans carrying these alleles (12–14). However, the emergence of the R264K mutation within an HLA-B*27-restricted KK10 immunodominant epitope (KRWIILGLNK) leads to increased viral replication and progression to AIDS in HLA-B*27-positive HIV-1-infected individuals (13), suggesting that the emergence of the mutation allowing escape from CTLs results in the loss of HIV-1 control in vivo. In contrast, HLA-B*57-positive individuals still have a low plasma viral load (pVL) after the emergence of the T242N escape mutant selected by TW10-specific CTLs, since this mutant has a high viral fitness cost (15). These individuals eventually show an increased pVL due to rescue from the reduction in the replication capacity by compensatory mutations (16, 17). HLA-B*27 and HLA-B*57 are common alleles in Caucasians and Africans but very rare ones in Japan and other Asian countries, indicating that HIV-1 is not controlled by these immunodominant epitope-specific CTLs in HIV-1-infected individuals in Japan and some Asian countries. So far, there has been no report of epitope-specific CTLs controlling HIV-1 in countries where HLA-B*57 and HLA-B*27 are absent or very rare, such as in Japan. Studies to identify such CTLs controlling HIV-1 will contribute to vaccine development in countries where HLA-B*27 and HLA-B*57 are rare and even in countries where these alleles are frequently found.
In the present study, we sought to identify HIV-1-specific CD8+ T cells controlling HIV-1 in chronically HIV-1-infected Japanese individuals by employing exhaustive and comprehensive strategies. We first analyzed CD8+ T cell responses to 842 11-mer overlapping HIV-1 Gag, Pol, and Nef peptides in 401 chronically HIV-1 clade B-infected, antiretroviral therapy (ART)-naive Japanese individuals. We then selected the candidates for CD8+ T cell responses controlling HIV-1. Following reevaluation for the role of the identified specific CTLs in the control of HIV-1, we characterized the cross-reactivity of their escape mutants.
MATERIALS AND METHODS
Subjects.
Four hundred one treatment-naive Japanese individuals with chronic HIV-1 clade B infection were enrolled in the National Center for Global Health and Medicine from 2008 to 2011. To minimize the influence of time lag postinfection among recruited patients on pVL and CD4 count, blood samples were collected from the recruited individuals at the first visit. In addition, patients with clinical AIDS were excluded. Informed consent was obtained from all individuals according to the Declaration of Helsinki. This study was approved by the ethics committees of the National Center for Global Health and Medicine and Kumamoto University. Peripheral blood mononuclear cells (PBMCs) were separated from whole blood. HLA types of HIV-infected individuals were determined by standard sequence-based genotyping. The median virus load and CD4 count were 25,000 copies/ml (interquartile range [IQR], 6,700 to 94,000 copies/ml) and 324 cells/μl (IQR, 195 to 443 cells/μl), respectively.
Peptides.
We previously designed overlapping peptides consisting of 11-mer amino acids, spanning Gag, Pol, and Nef of HIV-1 clade B consensus sequences (18). Each 11-mer peptide was overlapped by 9 amino acids. These 11-mer peptides and truncated peptides were synthesized by utilizing an automated multiple-peptide synthesizer and purified by high-performance liquid chromatography (HPLC). The purity was examined by HPLC and mass spectrometry. Peptides with more than 90% purity were used in the present study.
Enzyme-linked immunosorbent spot (ELISPOT) assay.
CD8+ T cells were sorted from cryopreserved PBMCs from chronically HIV-1 clade B-infected Japanese individuals by using CD8 magnetic beads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). Peptide cocktails including 10 11-mer overlapping peptides at a concentration of 1 μM and sorted cells at 1 × 105 cells/well were added to 96-well polyvinylidene plates (Millipore, Bedford, MA) that had been precoated with 5 mg/ml of anti-gamma interferon (anti-IFN-γ) monoclonal antibody (MAb) 1-D1K (Mabtech, Stockholm, Sweden). The plates were then incubated for 16 h at 37°C in 5% CO2 and subsequently washed with phosphate-buffered saline (PBS) before the addition of biotinylated anti-IFN-γ MAb (Mabtech) at 1 mg/ml. After the plates had been incubated at room temperature for 90 min, they were washed with PBS and then incubated with streptavidin-conjugated alkaline phosphatase (Mabtech) for 60 min at room temperature. After a washing with PBS, individual cytokine-producing cells were detected as dark spots after a 20-min reaction with 5-bromo-4-chloro-3-idolylphosphate and nitroblue tetrazolium by using an alkaline phosphatase-conjugated substrate (Bio-Rad, Richmond, CA). The spots were counted with an Eliphoto-Counter (Minerva Teck, Tokyo, Japan). The number of spots was standardized to that of spots/106 CD8+ T cells by measuring the frequency of CD8+ T cells using flow cytometry. A mean + 3 standard deviations (SDs of the spot number of samples from 13 HIV-1 naive individuals for these peptides was 162 spots/106 CD8+ T cells. Therefore, we defined >200 spots/106 CD8+ T cells as a positive response.
Cells.
721.221-CD4 cells expressing HLA-B*40:06, -B*67:01, -C*03:04, -C*04:01, -C*07:02, or -C*08:01 were generated by transfecting both the human CD4 gene and one of these HLA class I genes into 721.221 cells. These cells were maintained in RPMI medium containing 10% fetal calf serum (FCS) and 0.15 mg/ml of hygromycin B or 0.2 mg/ml of neomycin. C1R cells expressing HLA-B*40:06 and those expressing HLA-B*67:01 were generated by transfecting C1R cells with HLA-B*40:06 and -B*67:01, respectively, and they were maintained in RPMI medium containing 10% FCS and 0.2 mg/ml of neomycin. C1R and 721.221 cells expressing other HLAs used in this study were previously generated (18–25) and maintained in RPMI medium with 10% FCS and 0.15 mg/ml of hygromycin B or 0.2 mg/ml of neomycin.
Generation of epitope-specific CTL clones.
Epitope-specific CTL clones were generated from epitope-specific bulk T cells by limiting dilution in 96-U plates, together with 200 μl of cloning mixture (5 × 105 irradiated allogeneic PBMCs from healthy donors, 1 × 105 irradiated C1R cells expressing each HLA molecule, and epitope peptides at a concentration of 100 nM in RPMI medium containing FCS, 200 U/ml of recombinant interleukin 2 [rIL-2], and 2.5% phytohemagglutinin [PHA]).
Intracellular cytokine staining (ICS) assay.
After 721.221 cells or C1R cells had been incubated for 60 min with each peptide, they were washed twice with RPMI medium containing 10% FCS. These peptide-pulsed 721.221 cells (1 × 105 cells per well) and bulk-cultured cells (2 × 104 cells per well) were added to wells of a 96-well round-bottomed plate, and then the cells were incubated for 2 h at 37°C. Brefeldin A (10 μg/ml) was subsequently added, after which the cells were incubated for a further 4 h. After having been stained with allophycocyanin (APC)-labeled anti-CD8 MAb (Dako, Glostrup, Denmark), the cells were fixed with 4% paraformaldehyde and then made permeable with permeabilizing buffer (0.1% saponin and 5% FCS in PBS). Thereafter the cells were stained with fluorescein isothiocyanate (FITC)-labeled anti-IFN-γ MAb (BD Bioscience, CA). The percentage of IFN-γ+ CD8+ cells was determined by flow cytometry.
HIV-1 mutant clones.
NL4-3 mutants (NL4-3GagRI8-6S, NL4-3GagRI8-6V, and NL4-3GagRI8-6A) were generated by introducing the respective Gag-T280S, -T280V, and -T280A mutations into NL4-3 by use of a site-directed mutagenesis system (Invitrogen).
Sequence of autologous virus.
Viral RNA was extracted from plasma samples from HIV-1-infected patients by the use of a QIAamp MinElute virus spin kit (Qiagen). cDNA was synthesized from the RNA by using the SuperScript III first-strand synthesis system for reverse transcription-PCR (RT-PCR) and random hexamers (Invitrogen). Nef, Gag, and Pol regions were amplified by nested PCR using Taq DNA polymerase (Promega). The PCR products were purified with ExoSAP-IT (GE). All DNA sequencing was performed with a BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems) and an ABI 3500 genetic analyzer.
Determination of HLA alleles associated with a low pVL and high CD4 count in response to each cocktail.
We statistically analyzed differences in pVL or CD4 count between responders to each cocktail in individuals carrying a given HLA and the other individuals by using the two-tailed Mann-Whitney test. We then selected HLA alleles associated with a low pVL and high CD4 count in the responders to each cocktail according to the following 2 criteria: (i) the HLA alleles were associated with both a low pVL and a high CD4 count (P values for pVL and CD4 count were less than 0.1 and 0.05, respectively, or less than 0.05 and 0.1, respectively) or associated with a low pVL (P < 0.005), and (ii) the frequency of responders was more than 2% (more than 9 out of 401 subjects).
Statistical analysis.
For comparison of two groups in this study, two-tailed Mann-Whitney test was performed. Correlations between the breadths or the magnitudes and pVL or CD4 count were statistically analyzed using Pearson's correlation coefficient test and Spearman rank test, respectively. The frequency of the mutation between HLA+ and HLA− individuals was statistically analyzed using Fisher's exact test. P values of <0.05 were considered to be statistically significant.
RESULTS
Control of HIV-1 by Gag- or Pol-specific CD8+ T cells.
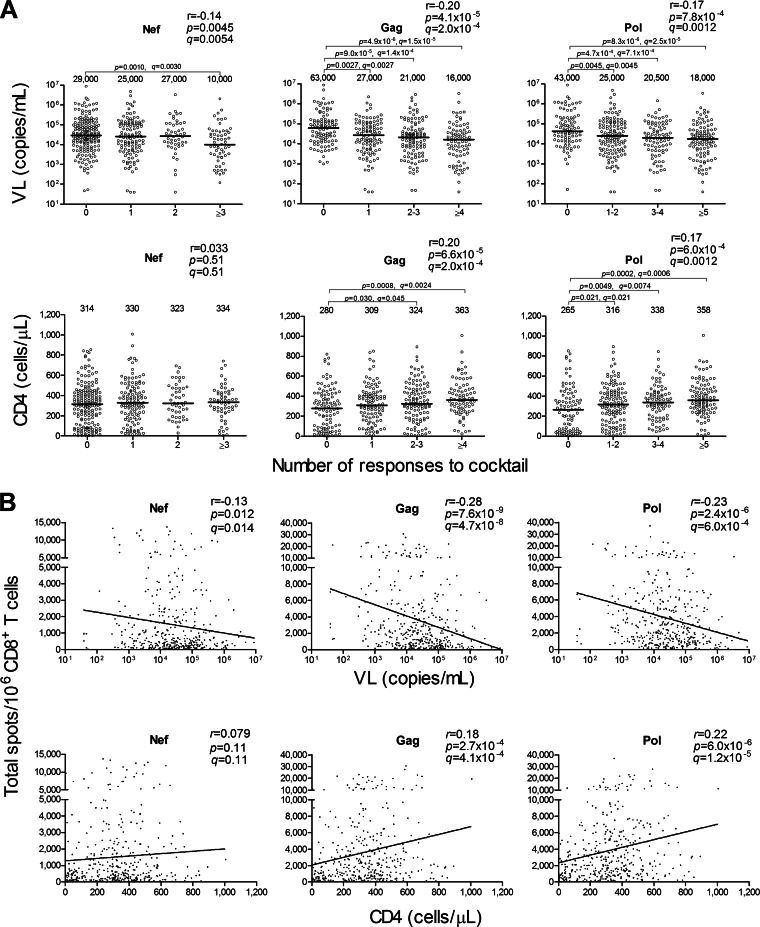
We recruited 401 chronically HIV-1-infected treatment-naive Japanese individuals from April 2008 to May 2011. We tested the CD8+ T cell responses to 10 Nef, 25 Gag, and 50 Pol peptide cocktails (one cocktail included 10 11-mer single overlapping HIV-1 clade B peptides [total of 842 peptides]) in these individuals and then analyzed the correlations between the CD8+ T cell responses to Nef, Gag, or Pol peptide cocktails and HIV-1 plasma viral load (pVL) or CD4 count. The breadths of the CTL responses to Gag and Pol peptides correlated positively with CD4 count (r = 0.20 and P = 6.6 × 10−5 and r = 0.17 and P = 6.0 × 10−4, respectively) and inversely with pVL (r = −0.20 and P = 4.1 × 10−5 and r = −0.17 and 7.8 × 10−4, respectively), whereas those to Nef peptides very weakly correlated negatively with pVL (r = −0.14 and P = 0.0045) but not with CD4 count (Fig. 1A). In addition, total magnitudes of the CD8+ T cell responses to Gag and Pol peptides correlated positively with CD4 count (r = 0.18 and P = 2.7 × 10−4 and r = 0.22 and P = 6.0 × 10−6, respectively) and inversely with pVL (r = −0.28 and P = 7.6 × 10−9 and r = −0.23 and P = 2.4 × 10−6, respectively [Fig. 1B]). Those to Nef peptides weakly correlated negatively with pVL (r = −0.13 and P = 0.012) but not with CD4 count. These results together indicate that CTL responses to both Gag and Pol epitopes played an important role in controlling HIV-1 replication in these chronically HIV-1-infected Japanese individuals.
FIG 1.
Correlation between CTL responses to HIV-1 peptide cocktails and pVL or CD4 count. The CD8+ T cell responses to 10 Nef, 25 Gag, and 50 Pol peptide cocktails including 10 11-mer single overlapping HIV-1 clade B peptides at a concentration of 1 μM in 401 chronically HIV-1-infected Japanese individuals were analyzed by using the ELISPOT assay. (A) Correlation between breadths of the CD8+ T cell responses to the peptide cocktails and pVL or CD4 count. The breadth was evaluated by calculating the number of cocktails recognized by the specific CD8+ T cells. The values and the lines in each graph represent medians of pVL and CD4 count (top and bottom, respectively). Statistical analysis was performed by use of Pearson's correlation coefficient test. Differences in pVL or CD4 count between nonresponders and responders were statistically analyzed by using the Mann-Whitney test. (B) Correlation between total magnitudes of the responses to the cocktails and pVL or CD4 count. The total magnitude was evaluated by calculating total spot numbers in the responses to Nef, Gag, or Pol peptide cocktails. Correlation coefficients (r) and P values were determined by using the Spearman rank correlation test. The line is the regression line. Multiple tests were performed by using the q value, a measure of significance in terms of the false-discovery rate (46). A significance threshold for q of <0.2 was employed. Each dot represents 1 individual. The limit of detection for pVL in this study is <40.
Identification of HIV-1-specific CTL responses associated with low pVL and high CD4 count.
We sought to identify HIV-1-specific CTL responses associated with low pVL and high CD4 count in this cohort as follows. First, we determined HLA alleles significantly associated with a low pVL and high CD4 count in response to each peptide cocktail (see Materials and Methods). Second, we identified single 11-mer peptide-specific CD8+ T cell responses restricted by these HLA alleles in the responses. Finally, we determined optimal peptides recognized by the specific CD8+ T cells.
We found 14 HLA alleles significantly associated with a low pVL and high CD4 count in the responses to 23 peptide cocktails (see Table S1 in the supplemental material) and then sought to identify the responses to single 11-mer peptides restricted by these HLA alleles, except the HLA-B*40:02-restricted T cell responses in Pol cocktail 46, since we had previously identified PolGI8 and PolTL8 epitopes by using this cocktail (20). We identified T cell responses to 53 11-mer single peptides from those to 22 peptide cocktails by using the ELISPOT assay or ICS assay (see Table S2). HLA restrictions of these 53 responses were determined by analyzing HLA restriction of the bulk CD8+ T cell response to each 11-mer peptide by using C1R or 721.221 cells expressing a given HLA allele. We found that CD8+ T cell responses to 23 11-mer peptides were restricted by 8 HLA alleles significantly associated with a low pVL and high CD4 count (see Table S3), but the responses to the other 11-mer peptides were restricted by other HLA alleles not significantly associated with a low pVL and high CD4 count (see Table S4). Finally, to identify optimal epitopes included in these 11-mer peptides, we analyzed the responses to truncated peptides by using the ICS assay. We identified 17 optimal epitopes restricted by the 8 HLA alleles, though 6 of these epitopes were included in 2 overlapping peptides (Table 1; see also Fig. S1 in the supplemental material). Thus, CD8+ T cell responses to these 17 epitopes and HLA-B*40:02-restricted PolGI8 and PolTL8 may control HIV-1 in the Japanese individuals.
TABLE 1.
Association of CD8+ T cell responses to 19 epitopes with pVL and CD4 count in chronically HIV-1-infected Japanese individuals
| Epitope | Sequence | Location | HLA restriction | Frequency |
Median pVL (copies/ml) |
Median CD4 (cells/μl) |
P valuea |
q valueb |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Responders | Nonresponders | Responders | Nonresponders | Responders | Nonresponders | pVL | CD4 | pVL | CD4 | ||||
| NefTY11 | TQGYFPDWQNY | Nef117-127 | B*15:01 | 3 | 390 | 3,400 | 25,000 | 386 | 321 | 0.22 | 0.38 | 0.25 | 0.4 |
| GagEM11 | EGATPQDLNTM | Gag177-187 | B*67:01 | 7 | 386 | 2,200 | 25,000 | 440 | 321 | 0.011 | 0.025 | 0.024 | 0.044 |
| C*08:01 | 2 | 391 | 29,000 | 25,000 | 451 | 322 | 0.86 | 0.36 | 0.86 | 0.4 | |||
| GagTL9 | TPQDLNTML | Gag180-188 | B*67:01 | 15 | 378 | 8,900 | 25,000 | 440 | 319 | 0.11 | 6.5 × 10−3 | 0.14 | 0.015 |
| GagMI8 | MQMLKETI | Gag198-205 | B*52:01 | 42 | 351 | 5,350 | 27,000 | 437 | 313 | 3.3 × 10−4 | 4.9 × 10−4 | 1.6 × 10−3 | 2.3 × 10−3 |
| GagQA11 | QMLKETINEEA | Gag199-209 | B*52:01 | 11 | 382 | 3,100 | 26,500 | 432 | 321 | 4.2 × 10−5 | 0.044 | 2.7 × 10−4 | 0.07 |
| GagRI8 | RMYSPTSI | Gag275-282 | B*52:01 | 60 | 333 | 10,650 | 27,000 | 446 | 309 | 2.1 × 10−3 | 3.2 × 10−7 | 6.6 × 10−3 | 6.1 × 10−6 |
| GagYL9 | YVDRFYKTL | Gag296-304 | C*03:04 | 2 | 391 | 2,885 | 25,000 | 303 | 322 | 0.079 | 0.79 | 0.12 | 0.79 |
| GagWV8 | WMTETLLV | Gag316-323 | B*52:01 | 43 | 350 | 4,700 | 28,000 | 446 | 309 | 1.0 × 10−6 | 8.7 × 10−7 | 1.9 × 10−5 | 8.3 × 10−6 |
| GagNL11 | NPDCKTILKAL | Gag327-337 | B*67:01 | 9 | 384 | 2,200 | 25,500 | 440 | 321 | 2.4 × 10−3 | 0.02 | 6.6 × 10−3 | 0.038 |
| GagGM9 | GPAATLEEM | Gag338-346 | B*67:01 | 1 | 392 | 780 | 25,000 | 693 | 322 | 0.14 | 0.11 | 0.16 | 0.15 |
| GagAA9 | ATLEEMMTA | Gag341-349 | A*02:06 | 30 | 363 | 8,650 | 27,000 | 437 | 314 | 0.019 | 6.6 × 10−4 | 0.036 | 2.5 × 10−3 |
| GagKL9 | KELYPLASL | Gag481-489 | B*40:02 | 12 | 381 | 20,000 | 25,000 | 480 | 321 | 0.68 | 0.017 | 0.72 | 0.035 |
| PolSV9 | SQIYAGIKV | Pol423-431 | A*02:06 | 48 | 345 | 15,000 | 27,000 | 392 | 313 | 0.04 | 4.6 × 10−3 | 0.064 | 0.015 |
| PolSI8 | SQYALGII | Pol654-661 | B*52:01 | 43 | 350 | 5,000 | 28,000 | 456 | 310 | 1.3 × 10−5 | 2.5 × 10−6 | 1.3 × 10−4 | 1.6 × 10−5 |
| PolLA9 | LEGKIILVA | Pol783-791 | B*40:06 | 16 | 377 | 4,150 | 27,000 | 437 | 321 | 5.6 × 10−4 | 0.078 | 2.1 × 10−3 | 0.11 |
| PolIT10 | IEAEVIPAET | Pol799-808 | B*40:06 | 25 | 368 | 13,000 | 26,500 | 353 | 321 | 4.5 × 10−3 | 0.19 | 0.011 | 0.22 |
| PolGI8 | GERIVDII | Pol912-919 | B*40:02 | 39 | 354 | 14,000 | 28,000 | 405 | 317 | 0.021 | 5.5 × 10−3 | 0.036 | 0.015 |
| PolTL8 | TDIQTKEL | Pol921-928 | B*40:02 | 5 | 388 | 7,200 | 25,000 | 478 | 322 | 0.11 | 0.17 | 0.14 | 0.22 |
The statistical analyses of differences in pVL or CD4 count between responders to each epitope and nonresponders were conducted by using the two-tailed Mann-Whitney test. Bold indicates that differences were statistically significant.
Multiple tests were performed by using the q value, a measure of significance in terms of the false-discovery rate (46). A significance threshold for q of <0.2 was employed.
Effective control of HIV-1 by HIV-1-specific CD8+ T cells specific for 13 epitope peptides.
To clarify the role of the CD8+ T cell responses to the 19 identified epitopes in HIV-1 control, we investigated the responses to these epitope peptides in our cohort (393 individuals) by using the ELISPOT assay. The responders to 10 epitopes had a significantly lower pVL and higher CD4 count than nonresponders, whereas those to 4 other epitopes had a significantly lower pVL or higher CD4 counts than the nonresponders (bold type in Table 1). To select HIV-1-specific CD8+ T cells having a strong effect in control of HIV-1 from those 19 specific epitopes, we used the following criteria: (i) both the pVLs and CD4 counts of the responders had to be significantly lower and higher than those of nonresponders, respectively (P < 0.05), (ii) the pVLs of the responders had to be much lower than those of the nonresponders (P < 0.01), or (iii) CD4 counts of the responders needed to be much higher than those of the nonresponders (P < 0.01). The responders to 13 epitope peptides satisfied one of these criteria (Table 1).
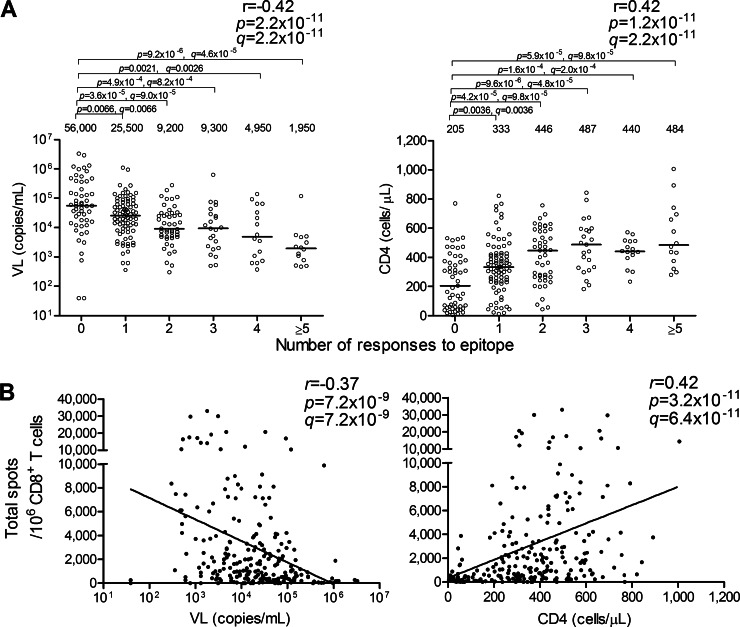
To investigate the effect of CD8+ T cells specific for these 13 epitopes on the control of HIV-1, we analyzed correlations between the total magnitude or the breadth of CD8+ T cell responses to the epitopes and pVL or CD4 count in 235 HIV-1-infected individuals carrying at least one of the restricting HLA alleles. The breadth and the total magnitude of these responses correlated inversely with pVL (breadth, r = −0.42 and P = 2.2 × 10−11; total magnitude, r = −0.37 and P = 7.2 × 10−9) and positively with CD4 count (breadth, r = 0.42 and P = 1.2 × 10−11; total magnitude, r = 0.42 and P = 3.2 × 10−11) in these individuals (Fig. 2). They also showed strong inverse and positive correlation with pVL (breadth, r = −0.34 and P = 5.7 × 10−12; total magnitude, r = −0.28 and P = 1.5 × 10−8) and CD4 count (breadth, r = 0.40 and P = 4.0 × 10−16; total magnitude, r = 0.35 and P = 8.9 × 10−13), respectively, in all 393 individuals tested (see Fig. S2 in the supplemental material). These findings taken together indicated strong synergistic effects of CD8+ T cells specific for these 13 epitopes on HIV-1 control.
FIG 2.
Correlation between multiple CD8+ T cell responses and pVL or CD4 count. Epitope-specific CD8+ T cell responses at a peptide concentration of 100 nM were analyzed by using the ELISPOT assay. (A) Correlation between the breadth of 13 epitope-specific CD8+ T cell responses and pVL or CD4 count in Japanese individuals carrying at least one of the restricting HLA alleles (n = 235). The values and the lines in each graph represent medians of pVL and CD4 counts (left and right graphs, respectively). Statistical analysis was performed by use of Pearson's correlation coefficient test. Differences in pVL or CD4 count between nonresponders and responders were statistically analyzed by using the Mann-Whitney test. (B) Correlation between the total magnitude of these responses and pVL or CD4 count in the Japanese individuals. The lines are regression lines. Correlation coefficients and P values were determined by using the Spearman rank correlation test. Multiple tests were performed by using the q value. A significance threshold for q of <0.2 was employed.
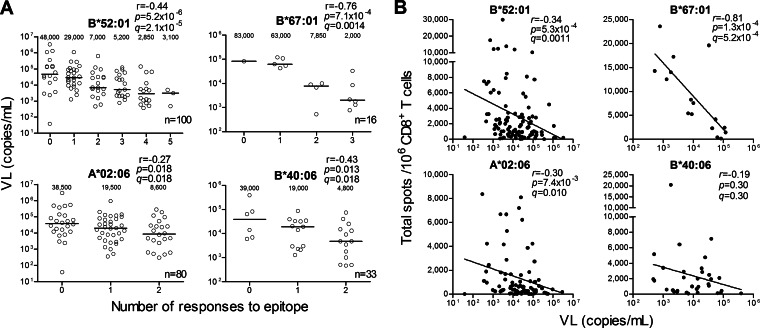
HLA-B*52:01 and HLA-B*67:01 were significantly associated with a low pVL and high CD4 count in chronically HIV-infected treatment-naive Japanese individuals (26). We therefore speculated that HLA-B*52:01-restricted and HLA-B*67:01-restricted CD8+ T cells would have stronger abilities to control HIV-1 than other CTLs. Indeed, the breadths of the HLA-B*52:01-restricted and the HLA-B*67:01-restricted CD8+ T cell responses were inversely correlated with pVL (r = −0.44 and P = 5.2 × 10−6 and r = −0.76 and P = 7.1 × 10−4, respectively [Fig. 3A]). In addition, the total magnitudes of the HLA-B*52:01-restricted and HLA-B*67:01-restricted CD8+ T cell responses were inversely correlated with pVL (r = −0.34 and P = 5.3 × 10−4 and r = −0.81 and P = 1.3 × 10−4, respectively [Fig. 3B]). On the other hand, the breadths of 2 HLA-A*02:06-restricted and 2 HLA-B*40:06-restricted CD8+ T cell responses showed a weak inverse correlation with pVL (r = −0.27 and P = 0.018 and r = −0.43 and P = 0.013, respectively [Fig. 3A]), whereas the total magnitude of HLA-A*02:06-restricted T cell responses showed a weak negative association with pVL (r = −0.30 and P = 7.4 × 10−3 [Fig. 3B]). These results together indicate that the HLA-B*52:01-restricted and HLA-B*67:01-restricted CD8+ T cells played a predominant role in the control of HIV-1 in these chronically HIV-1-infected Japanese individuals and support the previous finding that HLA-B*52:01 or HLA-B*67:01 are significantly associated with good clinical outcomes in Japanese individuals (26).
FIG 3.
Correlation between CD8+ T cell responses restricted by each HLA and pVL or CD4 count. Epitope-specific CD8+ T cell responses at a peptide concentration of 100 nM were analyzed by using the ELISPOT assay. (A) Correlations between breadths of HLA-B*52:01-, HLA-B*67:01-, HLA-A*02:06-, or HLA-B*40:06-restricted CD8+ T cell responses and pVL in the individuals carrying each HLA. Five HLA-B*52:01-restricted (GagMI8/QA11/RI8/WV8/PolSI8), 3 HLA-B*67:01-restricted (GagEM11/TL9/NL11), 2 HLA-A*02:06-restricted (GagAA9/PolSV9), and 2 HLA-B*40:06-restricted (PolLA9/IT10) CD8+ T cell responses were analyzed. The values in each graph represent medians of pVL. (B) Correlations between total magnitudes of these HLA allele-restricted CD8+ T cell responses and pVL in individuals carrying the corresponding HLA. Correlations between the breadths or the magnitudes and pVL or CD4 count were statistically analyzed using Pearson's correlation coefficient test and the Spearman rank correlation test, respectively. Multiple tests were performed by using the q value. A significance threshold for q of <0.2 was employed.
T cell recognition of 12 conserved or cross-reactive epitopes.
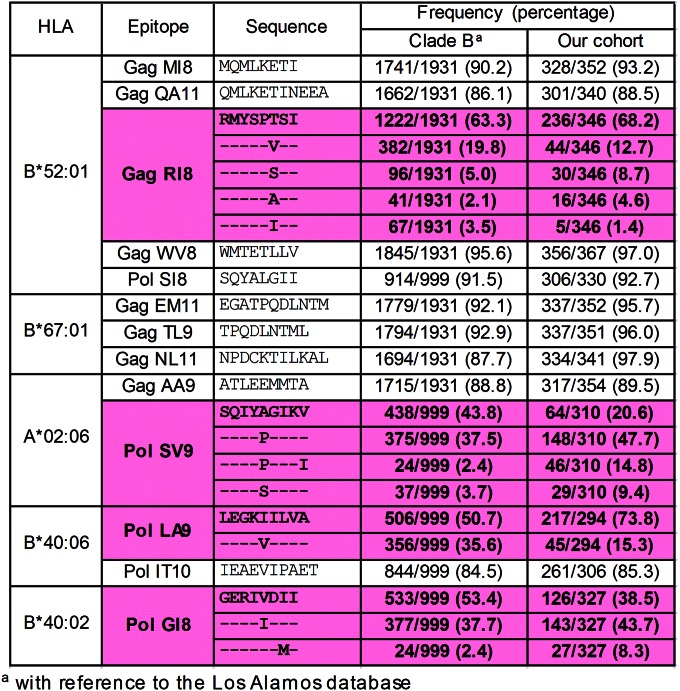
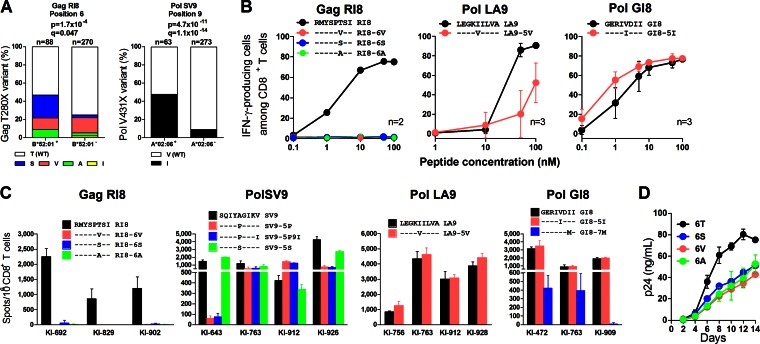
We analyzed the sequences of the 13 epitopes in this cohort. The data for reported clade B sequences in the Los Alamos database and our cohort are shown in Fig. 4. Nine of the 13 epitopes were conserved among approximately 85% or more of HIV-1 clade B-infected individuals, whereas 3 Pol epitopes (PolSV9, PolLA9, and PolGI8) and 1 Gag one (GagRI8) had some substitutions in approximately 20 to 60% of the individuals. We analyzed HLA-associated polymorphism in these epitopes to clarify the accumulation of CTL escape mutations as previously shown (27). Only 2 HLA-associated polymorphisms were found, at position 6 in GagRI8 and at position 9 in PolSV9 (Fig. 5A; see also Table S5 in the supplemental material). Therefore, we next analyzed cross-recognition of specific CTLs for these mutants. GagRI8-specific CTL clones failed to recognize GagRI8-6S, -6V, and -6A mutant peptides (Fig. 5B; see also Fig. S3 in the supplemental material), suggesting that these mutations had been selected by GagRI8-specific CTLs. Indeed, T cells specific for these 3 mutants were not detected in 3 HLA-B*52:01+ individuals (Fig. 5C). These mutations reduced viral fitness (Fig. 5D), suggesting that the emergence of these mutations may have resulted in a low pVL. On the other hand, PolSV9-5P and SV9-5P9I were cross-recognized in 3 of 4 HLA-A*02:06+ individuals (Fig. 5C), though these patients had 5P or 5S mutations (see Table S6). These results together suggest that these mutations were selected by PolSV9-specific CTLs and that CTLs cross-recognizing the 5P mutant were elicited after the emergence of the mutant.
FIG 4.
Frequencies of amino acid sequences for the 13 epitope regions with reference to clade B. The sequences of the 13 epitopes in this cohort were analyzed. The data for reported clade B sequences in the Los Alamos database and our cohort are shown. Pink shading and boldface indicate nonconserved epitopes.
FIG 5.
Recognition of conserved and cross-reactive epitopes. (A) Association of HLA-B*52:01 or HLA-A*02:06 with the GagT280X or the PolV431X mutation, respectively, in our cohort. The frequency of the mutation between HLA+ and HLA− individuals was statistically analyzed by using Fisher's exact test. Multiple tests were performed by using the q value, a measure of significance in terms of the false-discovery rate (46). In the analyses identifying HLA-associated polymorphisms, a significance threshold for q of <0.2 was employed. (B) Recognition of mutant peptides or wild-type peptide by epitope-specific CD8+ T cells. The epitope-specific CTL clones were stimulated with mutant or wild-type peptide-prepulsed C1R cells expressing the corresponding HLA allele, and then IFN-γ production from these CTL clones was detected by performing the ICS assay. The results are shown as means and SDs (n = 2 or 3). (C) Recognition of the mutant peptides or the wild-type peptide by specific T cells in HIV-1-infected individuals carrying the corresponding HLA. Peptide-specific CD8+ T cell responses at a peptide concentration of 100 nM were analyzed by using the ELISPOT assay. The results are shown as means and SDs (n = 3). (D) Fitness of the 3 GagRI8 mutant or wild-type viruses. CD4+ T cells from an HLA-B*52:01+ donor were infected with wild-type virus (NL4-3) or one of the 3 mutants (NL4-3GagRI8-6S, NL4-3GagRI8-6V, or NL4-3GagRI8-6A). The concentration of p24 antigen in the culture supernatant was determined by using an enzyme immunoassay. The results are presented as means and SDs (n = 3).
Regarding PolLA9 and GI8, these CTL clones cross-recognized LA9-5V and GI8-5I mutant peptides, respectively (Fig. 5B; see also Fig. S3 in the supplemental material). In addition, PolLA9-5V and GI8-5I were cross-recognized in 4 HLA-B*40:06+ and 3 HLA B*40:02+ individuals, respectively (Fig. 5C). These results indicate that PolLA9 and PolGI8 were functionally cross-recognized by specific CTLs. Thus, 12 epitopes were well recognized by specific CD8+ T cells, whereas escape mutants did not accumulate in 11 of 13 epitopes. Moreover, the breadth and the total magnitude of the T cell responses to these 12 epitopes correlated inversely with pVL (breadth, r = −0.44 and P = 1.8 × 10−12; total magnitude, r = −0.38 and P = 2.3 × 10−9) and positively with CD4 count (breadth, r = 0.39 and P = 3.7 × 10−10; total magnitude, r = 0.41 and P = 5.8 × 10−11) in the Japanese individuals (see Fig. S4A and B). These findings suggest that these T cells controlled HIV-1 in Japanese individuals, in whom protective alleles HLA-B*57 and B*27 are absent.
DISCUSSION
Analysis of clade C-infected Africans at a large population level demonstrated that the breadth of responses to Gag peptides is inversely associated with pVL, but not in the case of that to peptides in other protein regions (12), indicating that Gag-specific CTLs predominantly control HIV-1 in African individuals. On the other hand, only small-scale analyses of the CTL responses in clade B-infected individuals have been performed, and they show controversial results (28–32). The present study demonstrated that the breadth and total magnitude of CTL responses to Gag peptides were inversely associated with pVL and positively associated with CD4 count in approximately 400 clade B-infected individuals. In addition, we showed that those of CTL responses to Pol peptides were significantly associated with a low pVL and high CD4 count. These findings together indicated that both Gag and Pol epitope-specific CTLs played a critical role in the control of HIV-1 in the Japanese population studied. Indeed, we identified T cell responses to 5 Pol and 8 Gag epitopes significantly associated with a low pVL and high CD4 count. Thus, Pol-specific CTLs also play a critical role in HIV-1 control in Japanese individuals. A recent phase I clinical trial vaccine study using a conserved immunogen showed that CD8+ T cells specific for Pol peptides had a stronger ability to suppress HIV-1 replication in vitro than those specific for Gag, Env, and Vif peptides in healthy volunteers immunized with the vaccine (33), suggesting that Pol epitope-specific CTLs can effectively suppress HIV-1 replication in vivo. Thus, both studies suggest that Pol epitopes are also strong candidates as antigens for an AIDS vaccine.
Many previous studies investigated CD8+ T cell responses to overlapping HIV-1 peptides in order to clarify the correlation between the T cell responses and clinical outcome (12, 28–32, 34–36). However, they did not identify T cells effectively suppressing HIV-1 replication in vivo. A previous analysis of T cell responses to 18-mer overlapping peptides in the individuals infected with clade B or C demonstrated that the responses to the approximately 50 overlapping peptides were significantly associated with low pVL and the specific CTLs had strong antiviral activities in vitro (37). However, this study did not show minimum lengths of epitopes. The use of the 18-mer peptides may have a disadvantage in detection of specific CTL responses since such longer peptides hardly induce the specific CTLs. It is therefore essential to identify minimal epitopes to precisely clarify the ability of HIV-1-specific CTLs to control of HIV-1 in vivo. In the present study, we employed an exhaustive strategy involving the analysis of T cell responses to overlapping peptides significantly associated with low pVL and high CD4 count followed by identification of the responses to single peptides and HLA restrictions to them with subsequent determination of the optimal epitopes. Thereafter, we reanalyzed the responses to these optimal epitope peptides in our cohort and then reevaluated the correlation between these epitope-specific CTLs and the clinical outcome. By using this strategy, we could finally identify 8 Gag and 5 Pol epitope-specific CTLs controlling HIV-1. In addition, we analyzed the sequences for the epitopes in our cohort and then demonstrated that the CTLs specific for 12 conserved or cross-reactive epitopes controlled HIV-1. Thus, the present study using this exhaustive and comprehensive strategy was shown to be greatly advantageous for identifying HIV-1 epitope-specific CTLs clinically controlling HIV-1 in vivo.
The breadths of the HLA-B*52:01-restricted or HLA-B*67:01-restricted CD8+ T cell responses showed significant inverse associations with pVL. In addition, the breadths of these HLA-restricted T cell responses showed stronger effects on pVL than those of HLA-A*02:06-restricted or HLA-B*40:06-restricted T cell responses. These findings indicate that the responses of HLA-B*52:01-restricted or HLA-B*67:01-restricted CD8+ T cells controlling HIV-1 are critical factors for HIV-1 control in vivo.
A recent study investigated CD8+ T cell responses to 286 defined epitopes in 620 mainly Caucasian individuals with primary HIV-1 infection and showed that the specificity of the initial HIV-specific CD8 T cell response is a critical determinant of antiviral function rather than the restricting HLA class I molecule alone (38). In contrast, we here demonstrated that the breadths and the total magnitude of the T cell responses restricted by HLA-B*52:01 or HLA-B*67:01 were negatively correlated with pVL, suggesting that the multiple CTLs restricted by 2 protective alleles synergistically controlled HIV-1 in the Japanese individuals. Another recent study of 341 HIV-1-infected individuals in North America demonstrated that CD8+ T cell responses to 8 reported epitopes, including 3 well-established HLA-B*57-restricted ones, were associated with HIV-1 control (39). Since this study analyzed the T cell responses to only reported epitopes, it may identify only a part of the T cells controlling HIV-1.
T cells specific for 9 conserved and 3 cross-reactive epitopes controlled HIV-1 in the Japanese individuals examined in the present study. Previous studies demonstrated that conserved regions of HIV-1 are strong candidates for vaccine antigens (33, 40–45). Studies on HIV conserved vaccine demonstrated that polyfunctional and broad CTL responses were detected in macaques and humans that had received the vaccine (33, 40, 43–45). Since 6 out of the 12 conserved epitopes in this study (GagEM11/TL9/MI8/QA11/PolLA9/IT10) were included in the HIV conserved vaccine, vaccines may contribute to HIV-1 control in individuals carrying the corresponding HLAs. The addition of other epitopes identified in this study may improve the effect of the vaccine in clinical trials. Further analysis of these epitopes in vaccinated individuals will clarify the role of T cells specific for these epitopes in HIV-1 vaccine.
In the present study, we identified 8 Gag and 5 Pol epitope-specific CTLs controlling HIV-1 in HIV-1-infected Japanese individuals, in whom HLA-B*57 and HLA-B*27 are very rare. Twelve out of these 13 epitopes were recognized by CD8+ T cells as conserved or cross-reactive epitopes, suggesting that AIDS vaccines inducing CTLs specific for these 12 epitopes would be effective for protection against HIV-1. The comprehensive and exhaustive analysis of the CTLs shown here has been demonstrated to be a very useful strategy for identification of CTLs controlling HIV-1, and such analysis provides new insights into the studies of AIDS pathogenesis and the development of effective AIDS vaccines. This is the first study that identified HIV-1-specific T cells clinically controlling HIV-1 in the population, in whom HLA-B*57 and HLA-B*27 are very rare.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sachiko Sakai for secretarial assistance.
This research was supported by the Global COE program Global Education and Research Center Aiming at the Control of AIDS, launched as a project commissioned by the Ministry of Education, Science, Sports, and Culture, Japan, by a grant-in-aid for AIDS Research (H24-AIDS-007) from the Ministry of Health, Labor, and Welfare, and by a grant-in-aid (26293240, 25870550) for scientific research from the Ministry of Education, Science, Sports and Culture, Japan.
We have no financial conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.00020-15.
REFERENCES
- 1.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 2.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammer SM, Sobieszczyk ME, Janes H, Karuna ST, Mulligan MJ, Grove D, Koblin BA, Buchbinder SP, Keefer MC, Tomaras GD, Frahm N, Hural J, Anude C, Graham BS, Enama ME, Adams E, DeJesus E, Novak RM, Frank I, Bentley C, Ramirez S, Fu R, Koup RA, Mascola JR, Nabel GJ, Montefiori DC, Kublin J, McElrath MJ, Corey L, Gilbert PB. 2013. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med 369:2083–2092. doi: 10.1056/NEJMoa1310566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almeida JR, Sauce D, Price DA, Papagno L, Shin SY, Moris A, Larsen M, Pancino G, Douek DC, Autran B, Saez-Cirion A, Appay V. 2009. Antigen sensitivity is a major determinant of CD8+ T-cell polyfunctionality and HIV-suppressive activity. Blood 113:6351–6360. doi: 10.1182/blood-2009-02-206557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Migueles SA, Laborico AC, Shupert WL, Sabbaghian MS, Rabin R, Hallahan CW, Van Baarle D, Kostense S, Miedema F, McLaughlin M, Ehler L, Metcalf J, Liu S, Connors M. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol 3:1061–1068. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 7.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 8.Iglesias MC, Almeida JR, Fastenackels S, van Bockel DJ, Hashimoto M, Venturi V, Gostick E, Urrutia A, Wooldridge L, Clement M, Gras S, Wilmann PG, Autran B, Moris A, Rossjohn J, Davenport MP, Takiguchi M, Brander C, Douek DC, Kelleher AD, Price DA, Appay V. 2011. Escape from highly effective public CD8+ T-cell clonotypes by HIV. Blood 118:2138–2149. doi: 10.1182/blood-2011-01-328781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ladell K, Hashimoto M, Iglesias MC, Wilmann PG, McLaren JE, Gras S, Chikata T, Kuse N, Fastenackels S, Gostick E, Bridgeman JS, Venturi V, Arkoub ZA, Agut H, van Bockel DJ, Almeida JR, Douek DC, Meyer L, Venet A, Takiguchi M, Rossjohn J, Price DA, Appay V. 2013. A molecular basis for the control of preimmune escape variants by HIV-specific CD8+ T cells. Immunity 38:425–436. doi: 10.1016/j.immuni.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Sáez-Cirión A, Lacabaratz C, Lambotte O, Versmisse P, Urrutia A, Boufassa F, Barre-Sinoussi F, Delfraissy JF, Sinet M, Pancino G, Venet A. 2007. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci U S A 104:6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang H, Wu H, Hancock G, Clutton G, Sande N, Xu X, Yan H, Huang X, Angus B, Kuldanek K, Fidler S, Denny TN, Birks J, McMichael A, Dorrell L. 2012. Antiviral inhibitory capacity of CD8+ T cells predicts the rate of CD4+ T-cell decline in HIV-1 infection. J Infect Dis 206:552–561. doi: 10.1093/infdis/jis379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, Reddy S, de Pierres C, Mncube Z, Mkhwanazi N, Bishop K, van der Stok M, Nair K, Khan N, Crawford H, Payne R, Leslie A, Prado J, Prendergast A, Frater J, McCarthy N, Brander C, Learn GH, Nickle D, Rousseau C, Coovadia H, Mullins JI, Heckerman D, Walker BD, Goulder P. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med 13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- 13.Goulder PJ, Phillips RE, Colbert RA, McAdam S, Ogg G, Nowak MA, Giangrande P, Luzzi G, Morgan B, Edwards A, McMichael AJ, Rowland-Jones S. 1997. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat Med 3:212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- 14.Almeida JR, Price DA, Papagno L, Arkoub ZA, Sauce D, Bornstein E, Asher TE, Samri A, Schnuriger A, Theodorou I, Costagliola D, Rouzioux C, Agut H, Marcelin AG, Douek D, Autran B, Appay V. 2007. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med 204:2473–2485. doi: 10.1084/jem.20070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leslie AJ, Pfafferott KJ, Chetty P, Draenert R, Addo MM, Feeney M, Tang Y, Holmes EC, Allen T, Prado JG, Altfeld M, Brander C, Dixon C, Ramduth D, Jeena P, Thomas SA, St John A, Roach TA, Kupfer B, Luzzi G, Edwards A, Taylor G, Lyall H, Tudor-Williams G, Novelli V, Martinez-Picado J, Kiepiela P, Walker BD, Goulder PJ. 2004. HIV evolution: CTL escape mutation and reversion after transmission. Nat Med 10:282–289. doi: 10.1038/nm992. [DOI] [PubMed] [Google Scholar]
- 16.Crawford H, Lumm W, Leslie A, Schaefer M, Boeras D, Prado JG, Tang J, Farmer P, Ndung'u T, Lakhi S, Gilmour J, Goepfert P, Walker BD, Kaslow R, Mulenga J, Allen S, Goulder PJ, Hunter E. 2009. Evolution of HLA-B*5703 HIV-1 escape mutations in HLA-B*5703-positive individuals and their transmission recipients. J Exp Med 206:909–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brockman MA, Schneidewind A, Lahaie M, Schmidt A, Miura T, Desouza I, Ryvkin F, Derdeyn CA, Allen S, Hunter E, Mulenga J, Goepfert PA, Walker BD, Allen TM. 2007. Escape and compensation from early HLA-B57-mediated cytotoxic T-lymphocyte pressure on human immunodeficiency virus type 1 Gag alter capsid interactions with cyclophilin A. J Virol 81:12608–12618. doi: 10.1128/JVI.01369-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watanabe K, Murakoshi H, Tamura Y, Koyanagi M, Chikata T, Gatanaga H, Oka S, Takiguchi M. 2013. Identification of cross-clade CTL epitopes in HIV-1 clade A/E-infected individuals by using the clade B overlapping peptides. Microbes Infect 15:874–886. doi: 10.1016/j.micinf.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Yagita Y, Kuse N, Kuroki K, Gatanaga H, Carlson JM, Chikata T, Brumme ZL, Murakoshi H, Akahoshi T, Pfeifer N, Mallal S, John M, Ose T, Matsubara H, Kanda R, Fukunaga Y, Honda K, Kawashima Y, Ariumi Y, Oka S, Maenaka K, Takiguchi M. 2013. Distinct HIV-1 escape patterns selected by cytotoxic T cells with identical epitope specificity. J Virol 87:2253–2263. doi: 10.1128/JVI.02572-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe T, Murakoshi H, Gatanaga H, Koyanagi M, Oka S, Takiguchi M. 2011. Effective recognition of HIV-1-infected cells by HIV-1 integrase-specific HLA-B *4002-restricted T cells. Microbes Infect 13:160–166. doi: 10.1016/j.micinf.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Fujiwara M, Tanuma J, Koizumi H, Kawashima Y, Honda K, Mastuoka-Aizawa S, Dohki S, Oka S, Takiguchi M. 2008. Different abilities of escape mutant-specific cytotoxic T cells to suppress replication of escape mutant and wild-type human immunodeficiency virus type 1 in new hosts. J Virol 82:138–147. doi: 10.1128/JVI.01452-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borghan MA, Oka S, Takiguchi M. 2005. Identification of HLA-A*3101-restricted cytotoxic T-lymphocyte response to human immunodeficiency virus type 1 (HIV-1) in patients with chronic HIV-1 infection. Tissue Antigens 66:305–313. doi: 10.1111/j.1399-0039.2005.00489.x. [DOI] [PubMed] [Google Scholar]
- 23.Honda K, Zheng N, Murakoshi H, Hashimoto M, Sakai K, Borghan MA, Chikata T, Koyanagi M, Tamura Y, Gatanaga H, Oka S, Takiguchi M. 2011. Selection of escape mutant by HLA-C-restricted HIV-1 Pol-specific cytotoxic T lymphocytes carrying strong ability to suppress HIV-1 replication. Eur J Immunol 41:97–106. doi: 10.1002/eji.201040841. [DOI] [PubMed] [Google Scholar]
- 24.Koizumi H, Hashimoto M, Fujiwara M, Murakoshi H, Chikata T, Borghan MA, Hachiya A, Kawashima Y, Takata H, Ueno T, Oka S, Takiguchi M. 2010. Different in vivo effects of HIV-1 immunodominant epitope-specific cytotoxic T lymphocytes on selection of escape mutant viruses. J Virol 84:5508–5519. doi: 10.1128/JVI.02483-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falk K, Rotzschke O, Takiguchi M, Gnau V, Stevanovic S, Jung G, Rammensee HG. 1995. Peptide motifs of HLA-B58, B60, B61, and B62 molecules. Immunogenetics 41:165–168. doi: 10.1007/BF00182333. [DOI] [PubMed] [Google Scholar]
- 26.Naruto T, Gatanaga H, Nelson G, Sakai K, Carrington M, Oka S, Takiguchi M. 2012. HLA class I-mediated control of HIV-1 in the Japanese population, in which the protective HLA-B*57 and HLA-B*27 alleles are absent. J Virol 86:10870–10872. doi: 10.1128/JVI.00689-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chikata T, Carlson JM, Tamura Y, Borghan MA, Naruto T, Hashimoto M, Murakoshi H, Le AQ, Mallal S, John M, Gatanaga H, Oka S, Brumme ZL, Takiguchi M. 2014. Host-specific adaptation of HIV-1 subtype B in the Japanese population. J Virol 88:4764–4775. doi: 10.1128/JVI.00147-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edwards BH, Bansal A, Sabbaj S, Bakari J, Mulligan MJ, Goepfert PA. 2002. Magnitude of functional CD8+ T-cell responses to the gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J Virol 76:2298–2305. doi: 10.1128/jvi.76.5.2298-2305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuñiga R, Lucchetti A, Galvan P, Sanchez S, Sanchez C, Hernandez A, Sanchez H, Frahm N, Linde CH, Hewitt HS, Hildebrand W, Altfeld M, Allen TM, Walker BD, Korber BT, Leitner T, Sanchez J, Brander C. 2006. Relative dominance of Gag p24-specific cytotoxic T lymphocytes is associated with human immunodeficiency virus control. J Virol 80:3122–3125. doi: 10.1128/JVI.80.6.3122-3125.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Addo MM, Yu XG, Rathod A, Cohen D, Eldridge RL, Strick D, Johnston MN, Corcoran C, Wurcel AG, Fitzpatrick CA, Feeney ME, Rodriguez WR, Basgoz N, Draenert R, Stone DR, Brander C, Goulder PJ, Rosenberg ES, Altfeld M, Walker BD. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J Virol 77:2081–2092. doi: 10.1128/JVI.77.3.2081-2092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frahm N, Korber BT, Adams CM, Szinger JJ, Draenert R, Addo MM, Feeney ME, Yusim K, Sango K, Brown NV, Sen Gupta D, Piechocka-Trocha A, Simonis T, Marincola FM, Wurcel AG, Stone DR, Russell CJ, Adolf P, Cohen D, Roach T, St John A, Khatri A, Davis K, Mullins J, Goulder PJ, Walker BD, Brander C. 2004. Consistent cytotoxic-T-lymphocyte targeting of immunodominant regions in human immunodeficiency virus across multiple ethnicities. J Virol 78:2187–2200. doi: 10.1128/JVI.78.5.2187-2200.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia M, Hong K, Chen J, Ruan Y, Wang Z, Su B, Ren G, Zhang X, Liu Z, Zhao Q, Li D, Peng H, Altfeld M, Walker BD, Yu XG, Shao Y. 2012. Preferential CTL targeting of Gag is associated with relative viral control in long-term surviving HIV-1 infected former plasma donors from China. Cell Res 22:903–914. doi: 10.1038/cr.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borthwick N, Ahmed T, Ondondo B, Hayes P, Rose A, Ebrahimsa U, Hayton EJ, Black A, Bridgeman A, Rosario M, Hill AV, Berrie E, Moyle S, Frahm N, Cox J, Colloca S, Nicosia A, Gilmour J, McMichael AJ, Dorrell L, Hanke T. 2014. Vaccine-elicited human T cells recognizing conserved protein regions inhibit HIV-1. Mol Ther 22:464–475. doi: 10.1038/mt.2013.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Novitsky V, Gilbert P, Peter T, McLane MF, Gaolekwe S, Rybak N, Thior I, Ndung'u T, Marlink R, Lee TH, Essex M. 2003. Association between virus-specific T-cell responses and plasma viral load in human immunodeficiency virus type 1 subtype C infection. J Virol 77:882–890. doi: 10.1128/JVI.77.2.882-890.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masemola A, Mashishi T, Khoury G, Mohube P, Mokgotho P, Vardas E, Colvin M, Zijenah L, Katzenstein D, Musonda R, Allen S, Kumwenda N, Taha T, Gray G, McIntyre J, Karim SA, Sheppard HW, Gray CM. 2004. Hierarchical targeting of subtype C human immunodeficiency virus type 1 proteins by CD8+ T cells: correlation with viral load. J Virol 78:3233–3243. doi: 10.1128/JVI.78.7.3233-3243.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geldmacher C, Currier JR, Herrmann E, Haule A, Kuta E, McCutchan F, Njovu L, Geis S, Hoffmann O, Maboko L, Williamson C, Birx D, Meyerhans A, Cox J, Hoelscher M. 2007. CD8 T-cell recognition of multiple epitopes within specific Gag regions is associated with maintenance of a low steady-state viremia in human immunodeficiency virus type 1-seropositive patients. J Virol 81:2440–2448. doi: 10.1128/JVI.01847-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mothe B, Llano A, Ibarrondo J, Daniels M, Miranda C, Zamarreno J, Bach V, Zuniga R, Perez-Alvarez S, Berger CT, Puertas MC, Martinez-Picado J, Rolland M, Farfan M, Szinger JJ, Hildebrand WH, Yang OO, Sanchez-Merino V, Brumme CJ, Brumme ZL, Heckerman D, Allen TM, Mullins JI, Gomez G, Goulder PJ, Walker BD, Gatell JM, Clotet B, Korber BT, Sanchez J, Brander C. 2011. Definition of the viral targets of protective HIV-1-specific T cell responses. J Transl Med 9:208. doi: 10.1186/1479-5876-9-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Streeck H, Lu R, Beckwith N, Milazzo M, Liu M, Routy JP, Little S, Jessen H, Kelleher AD, Hecht F, Sekaly RP, Alter G, Heckerman D, Carrington M, Rosenberg ES, Altfeld M. 2014. Emergence of individual HIV-specific CD8 T cell responses during primary HIV-1 infection can determine long-term disease outcome. J Virol 88:12793–12801. doi: 10.1128/JVI.02016-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pereyra F, Heckerman D, Carlson JM, Kadie C, Soghoian DZ, Karel D, Goldenthal A, Davis OB, DeZiel CE, Lin T, Peng J, Piechocka A, Carrington M, Walker BD. 2014. HIV control is mediated in part by CD8+ T-cell targeting of specific epitopes. J Virol 88:12937–12948. doi: 10.1128/JVI.01004-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Létourneau S, Im EJ, Mashishi T, Brereton C, Bridgeman A, Yang H, Dorrell L, Dong T, Korber B, McMichael AJ, Hanke T. 2007. Design and pre-clinical evaluation of a universal HIV-1 vaccine. PLoS One 2:e984. doi: 10.1371/journal.pone.0000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rolland M, Nickle DC, Mullins JI. 2007. HIV-1 group M conserved elements vaccine. PLoS Pathog 3:e157. doi: 10.1371/journal.ppat.0030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rowland-Jones SL, Dong T, Fowke KR, Kimani J, Krausa P, Newell H, Blanchard T, Ariyoshi K, Oyugi J, Ngugi E, Bwayo J, MacDonald KS, McMichael AJ, Plummer FA. 1998. Cytotoxic T cell responses to multiple conserved HIV epitopes in HIV-resistant prostitutes in Nairobi. J Clin Invest 102:1758–1765. doi: 10.1172/JCI4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knudsen ML, Mbewe-Mvula A, Rosario M, Johansson DX, Kakoulidou M, Bridgeman A, Reyes-Sandoval A, Nicosia A, Ljungberg K, Hanke T, Liljestrom P. 2012. Superior induction of T cell responses to conserved HIV-1 regions by electroporated alphavirus replicon DNA compared to that with conventional plasmid DNA vaccine. J Virol 86:4082–4090. doi: 10.1128/JVI.06535-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosario M, Borthwick N, Stewart-Jones GB, Mbewe-Mvula A, Bridgeman A, Colloca S, Montefiori D, McMichael AJ, Nicosia A, Quakkelaar ED, Drijfhout JW, Melief CJ, Hanke T. 2012. Prime-boost regimens with adjuvanted synthetic long peptides elicit T cells and antibodies to conserved regions of HIV-1 in macaques. AIDS 26:275–284. doi: 10.1097/QAD.0b013e32834ed9b2. [DOI] [PubMed] [Google Scholar]
- 45.Rosario M, Bridgeman A, Quakkelaar ED, Quigley MF, Hill BJ, Knudsen ML, Ammendola V, Ljungberg K, Borthwick N, Im EJ, McMichael AJ, Drijfhout JW, Greenaway HY, Venturi V, Douek DC, Colloca S, Liljestrom P, Nicosia A, Price DA, Melief CJ, Hanke T. 2010. Long peptides induce polyfunctional T cells against conserved regions of HIV-1 with superior breadth to single-gene vaccines in macaques. Eur J Immunol 40:1973–1984. doi: 10.1002/eji.201040344. [DOI] [PubMed] [Google Scholar]
- 46.Storey JD, Tibshirani R. 2003. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A 100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.