ABSTRACT
Swine are susceptible to infection by both avian and human influenza viruses, and this feature is thought to contribute to novel reassortant influenza viruses. In this study, the influenza virus reassortment rate in swine and human cells was determined. Coinfection of swine cells with 2009 pandemic H1N1 virus (huH1N1) and an endemic swine H1N2 (A/swine/Illinois/02860/09) virus (swH1N2) resulted in a 23% reassortment rate that was independent of α2,3- or α2,6-sialic acid distribution on the cells. The reassortants had altered pathogenic phenotypes linked to introduction of the swine virus PA and neuraminidase (NA) into huH1N1. In mice, the huH1N1 PA and NA mediated increased MIP-2 expression early postinfection, resulting in substantial pulmonary neutrophilia with enhanced lung pathology and disease. The findings support the notion that swine are a mixing vessel for influenza virus reassortants independent of sialic acid distribution. These results show the potential for continued reassortment of the 2009 pandemic H1N1 virus with endemic swine viruses and for reassortants to have increased pathogenicity linked to the swine virus NA and PA genes which are associated with increased pulmonary neutrophil trafficking that is related to MIP-2 expression.
IMPORTANCE Influenza A viruses can change rapidly via reassortment to create a novel virus, and reassortment can result in possible pandemics. Reassortments among subtypes from avian and human viruses led to the 1957 (H2N2 subtype) and 1968 (H3N2 subtype) human influenza pandemics. Recent analyses of circulating isolates have shown that multiple genes can be recombined from human, avian, and swine influenza viruses, leading to triple reassortants. Understanding the factors that can affect influenza A virus reassortment is needed for the establishment of disease intervention strategies that may reduce or preclude pandemics. The findings from this study show that swine cells provide a mixing vessel for influenza virus reassortment independent of differential sialic acid distribution. The findings also establish that circulating neuraminidase (NA) and PA genes could alter the pathogenic phenotype of the pandemic H1N1 virus, resulting in enhanced disease. The identification of such factors provides a framework for pandemic modeling and surveillance.
INTRODUCTION
Each year, influenza A viruses (IAVs) cause epidemics with high morbidity and some mortality in humans (1). In avian species, IAV infection generally is associated with mild disease, and it is only when the virus crosses species barriers that pathogenic traits are attributed to infection (2). IAV is frequently associated with spillover events, as numerous species are susceptible to IAV. Often, reassortment of IAV leads to a subtype or variant that has not previously infected humans, and as the virus adapts and is transmitted more easily, a pandemic event may loom. IAV is the cause of >30,000 deaths in the United States and >500,000 deaths worldwide annually (3). In the past century, four IAV pandemics have occurred, i.e., 1918, 1956, 1968, and 2009 (4), and the last three events resulted from reassortment events occurring between IAVs of different origins (5). The 2009 pandemic resulted from triple reassortment of human, avian, and North American and Eurasian swine-origin viruses (6), and through competitive exclusion (4), this pandemic strain has become the dominant circulating virus, replacing the previous seasonal H1N1 strains of IAV.
Since the emergence of 2009 pandemic pH1N1 virus, reverse zoonosis to swine has been globally observed (7–14). North American classical swine viruses, European or Eurasian avian virus-like swine viruses, and triple reassortment of internal gene (TRIG) swine viruses cocirculate in swine populations today (15). TRIG viruses emerged in the U.S. swine population in 1998 and quickly became the predominant circulating strains in the United States (16). The TRIG cassette exhibits high genetic stability and has been shown to provide an efficient platform for glycoprotein exchange between viruses to subvert herd immunity (17). Reverse zoonosis of the pH1N1 virus into swine and the propensity to generate novel reassortant viruses with increased pathogenicity and pandemic potential are of concern (18). Recently, 320 confirmed cases and an estimated 2,000 total cases of swine influenza virus zoonosis involving a swine H3N2 variant (H3N2v) were observed in Iowa (19, 20). H3N2v viruses resulted from reassortment of H3N2 TRIG viruses with inclusion of the pH1N1 M gene. The M gene from pH1N1 is linked with human-to-human transmission through modulation of neuraminidase (NA) activity and morphology (21). Given the apparent role of the M gene, it is important to understand how the individual genetic components of IAV affect the pathogenic phenotype and pandemic potential of IAV. It is important to note that additional genetic reassortments have been identified with H3N2v, including inclusion of the pandemic H1N1 virus acidic protein (PA) (22).
The nonstructural proteins, particularly NS1, are regulators of the host response to infection and subsequent pathogenicity (23, 24). Recent evidence shows that protein subunits of the polymerase complex substantially contribute to the pathological outcome of IAV infection, but the overall role in immunity and disease pathogenesis is less well defined than that for NS1. The polymerases from the 1957, 1968, and 2009 pandemics were derived from reassortants (5). Protein components of the RNP complex have been implicated in various aspects of influenza virus virulence and pathogenicity in a variety of mammalian models, including mice, ferrets, and swine (25–29). For example, the well-described amino acid K627 in the PB2 protein enhances polymerase activity, leading to increased transmission, replication, and pathogenicity in mammals (30).
PA modulates replication kinetics, species specificity, and differential pathogenicity of IAV (31). Differences in PA proteins have been directly linked with increased and decreased levels of viral transcription and replication (25, 32). PA from highly pathogenic avian influenza (HPAI) virus isolates contributes to increased cytokine production levels while also increasing pathogenicity in mice (33). PA has been shown to induce differential levels of interferon (IFN) production and apoptosis early in infection along with also contributing to increased defective interfering genome production, leading to a more robust cellular innate response via activation of the RIG-I pathway (34, 35). Studies investigating the pH1N1 PA have shown that a single amino acid change, at position 552, can lower the species barrier for infection in mammals (31). Resulting from a frameshift, the PA gene encodes a second small protein, called PA-X, which hijacks the host immune response through host protein shutdown mechanisms, thereby modulating the antiviral and apoptotic pathways (36).
The pathogenic role of the neuraminidase (NA) protein is not well understood, and NA is not often associated with viral pathogenicity in mammalian hosts. NA facilitates viral release from infected cells by cleavage of sialic acids between the host cell and the HA protein (37). Recently, NA was identified as mediating dissolution of the mucus layer of the epithelium through cleavage of mucosal sugars during infection, potentially implicating different NA subtypes in enhancing IAV pathogenicity (38, 39). Genetic reassortment of PA and NA could play a significant role in mediating the pathogenic outcome of emerging IAVs.
In this study, the TRIG cassette was identified as supporting species-specific reassortment of A/swine/Illinois/02860/09 (swH1N2) and A/California/04/09 (huH1N1) in swine through screening of the hemagglutinin (HA), NA, and PB2 genes that resulted from coinfection. Interestingly, preferential inclusion of the swH1N2 NA gene into huH1N1 was observed. IAV reassortants resulting from coinfection of swine cells showed increased replicative fitness and increased pathogenicity attributed to reassortment of the swH1N2 HA, NA, and PA genes. The swine virus HA, NA, and PA mediate increased pathogenicity in ferrets and mice early postinfection (p.i.) with evidence of enhanced necrosis and lymphocytic infiltration resulting in acute lung injury. Intranasal infection of BALB/c mice with the triple reassortant (rILL346) was associated with pulmonary recruitment of neutrophils followed by natural killer (NK) cells early during infection and induction of a robust proinflammatory environment characterized by interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), and IL-6 expression. Subsequently, using reverse genetics-derived viruses, it was independently determined that the swH1N2 NA and PA increase viral pathogenesis by enhancement of neutrophil and NK cell recruitment into the lungs of mice postinfection. These studies showed that viruses harboring the swH1N2 NA and PA recruit neutrophils early during infection, which promotes stimulation of a robust proinflammatory (IL-6, IL-1β, and TNF-α) cytokine response, increasing overall pathology in the lungs of mice. Neutrophils exhibit extensive cross talk with innate effector cell types modulating NK cell maintenance, survival, and activation (40). Here, it is shown that swH1N2 PA contributes to NK cell recruitment and enhanced expression of gamma interferon (IFN-γ). Finally, swine virus PA and NA enhance MIP-2 expression, resulting in the observed increases in neutrophil recruitment to the lung.
MATERIALS AND METHODS
Ethics statement.
All animal experiments were performed in accordance with the national guidelines provided by the Guide for the Care and Use of Laboratory Animals (41) and the University of Georgia Institutional Animal Care and Use Committee (IACUC). The animal use protocol A2012 06-025-Y2-A2 was approved by the University of Georgia IACUC.
Cells and viruses.
Madin-Darby canine kidney (MDCK) cells (ATCC CCL-34) were cultured in Dulbecco's modified Eagle's medium (DMEM) containing high glucose (HyClone) supplemented with 5% heat-inactivated fetal bovine serum (FBS) and were maintained at 37°C with 5% CO2. LLC-PK1 cells (ATCC CL-101) were cultured in Dulbecco's modified Eagle's medium containing high glucose (HyClone) supplemented with 5% heat-inactivated fetal bovine serum (FBS) and were cultured at 37°C with 5% CO2. Calu-3 cells (ATCC HTB-55) were cultured in DMEM containing high glucose supplemented with 20% FBS, 1 mM l-glutamine, 1 mM HEPES, and 1× nonessential amino acids and were cultured at 37°C with 5% CO2. A/California/04/09 (huH1N1) was kindly provided by the CDC, and A/swine/Illinois/02860/09 (swH1N2) was kindly provided by Marie Culhane. Viral stocks were cultured in MDCK cells using infection medium (DMEM containing high glucose supplemented with 1 mM l-glutamine with 1-μg/ml tosylsulfonyl phenylalanyl chloromethyl ketone [TPCK]-treated trypsin). Viral stocks and samples were never introduced to embryonic chicken eggs in order to eliminate potential egg-derived mutations. Viruses were received as single-passage cell preparations in MDCK cells. Neither virus was introduced into eggs for amplification. All viral stocks used were used from a C3 stock which is representative of 3 passages in MDCK cells, including the initial preparation generated from the clinical sample. Viral stocks were confirmed by sequencing with the BigDye Terminator v3.1 cycle sequencing kit (Life Technologies). Stock viral titers were quantified using plaque assays as previously described (42). In vitro viral infections were performed in infection medium.
In vitro coinfections and plaque purification.
Coinfections of LLC-PK1 and Calu-3 cells were performed in infection medium. Each infection was performed at a multiplicity of infection (MOI) of 3 with each independent virus, therefore resulting in a total MOI of 6 during coinfection with 2 viruses. At 24 h postinfection (hpi), supernatants were harvested and stored at −80°C. Coinfection supernatants from LLC-PK1 and Calu-3 cells were plaque purified. Supernatants were serially diluted from 10−1 to 10−6 on MDCK cells. The supernatants were allowed to absorb to the cell monolayer for 1 h. Cells were rinsed three times with phosphate-buffered saline (PBS) and overlaid with a 1:1 mixture of 2% agarose and 2× overlay medium: 2× minimum essential medium (MEM) (Invitrogen) supplemented with, 0.3% NaHCO3, 4 mM l-glutamine, and 0.4 M HEPES. The plates were incubated for 72 h at 37°C with 5% CO2. After 72 hpi, plaques were isolated. Agar plugs from individual plaques were applied to MDCK cells and grown for 72 h in infection medium. At 72 hpi, RNA was isolated from supernatants using the RNeasy mini-RNA isolation kit (Qiagen). Residual DNA was eliminated via treatment with DNase I (Invitrogen). cDNA was synthesized using the Verso cDNA synthesis kit (Thermo Scientific) using random hexamers for nonspecific amplification and 200 ng RNA. Residual RNA was removed from newly synthesized cDNA using standard RNA salt precipitation methods as previously described (43). RNA-free cDNA samples were further purified using the QIAquick PCR purification kit (Qiagen).
Multiplex qPCR.
Quantitative PCRs (qPCRs) were first optimized in monospecific PCRs using previously described TaqMan primers and probes specific for the HA, NA, and PB2 genes of huH1N1 and swH1N2 (44). PCRs were performed using the QuantiFast multiplex qPCR kit (Qiagen) and carried out on a Stratagene MX3500p real-time PCR system. Primer/probe pairs were optimized for specificity against the parental and nonparental virus. Two triplex qPCRs were used to screen for influenza virus recombinants between the huH1N1 and swH1N2 HA, NA, and PB2 gene segments. Triplex qPCRs were performed using the QuantiFast multiplex qPCR kit (Qiagen), and 0.5 μM forward and reverse primers and 0.2 μM probes were used for each reaction. Multiplex reactions were performed on a Stratagene MX3500p real-time PCR system. Cycling conditions included an initial denaturation step of 95°C for 5 min, and amplification was performed for 40 cycles, including denaturation at 95°C for 30 s and annealing of 55°C for 30 s.
Sequencing.
Stocks of validated viral reassortants derived from coinfection LLC-PK1 cells were propagated in MDCK cells in infection medium. Viral reassortant titers were determined using a standard influenza virus plaque assay protocol as described above (42). RNA was isolated from viral stock samples using the RNeasy kit (Qiagen) according to the manufacturer's protocol. One-step reverse transcription-PCR (RT-PCR) was performed on viral RNA using virus-specific amplification primers. Amplified products were gel purified using the QIAquick gel purification kit (Qiagen) according to the manufacture's protocol. Full-genome sequencing using primers encompassing all 8 genes from huH1N1 and swH1N2 was performed. Samples were sequenced using the Sanger method with the BigDye Terminator v3.1 cycle sequencing kit (Life Technologies) on an ABI 3130-XL genetic analyzer. Sequences of reassortants were aligned with the parent strains using Clustal W analysis.
Quantitative PCR.
Total RNA was isolated from infected Calu-3 cells using RNeasy minikits (Qiagen). Reverse transcription was performed using a Verso cDNA synthesis kit (Thermo Scientific). Validated human-specific TaqMan gene expression primer/probe sets and master mix (Life Technologies) were used to amplify and quantify IFN-α, IFN-β, and IFN-λ, according to the manufacturer's protocols. Hypoxanthine-guanine phosphoribosyltransferase (HPRT) was used as a housekeeping gene to normalize gene expression. All qPCRs were performed on a Stratagene Mx3005P PCR instrument.
In vitro viral kinetics.
Calu-3 cells were infected with huH1N1, swH1N2, and reassortant viruses at an MOI of 0.01. Virus was diluted in infection medium. Supernatant from individual wells was collected at 6, 12, 18, 24, 48, and 72 hpi. Viral titers were determined on each sample collected using 50% tissue culture infective dose (TCID50) (45).
Mouse studies.
Specific-pathogen-free, 6- to 8-week-old female BALB/c mice (National Cancer Institute [NCI]) were used in all experiments. Mice were housed in microisolator cages and were provided food and water ad libitum. Mice were anesthetized by intraperitoneal injection of tribromoethanol (Avertin) (180 to 250 mg/kg of body weight; Sigma). Mice were intranasally challenged with 5 × 105 PFU of huH1N1, swH1N2, and viral reassortants diluted in PBS. Five mice (n = 5) were used for each experiment, and three independent experiments were conducted for all mouse experiments.
Lung viral titers.
Influenza A viral burden in lungs of mice was determined by TCID50 on whole-lung supernatants. Lungs were aseptically removed from infected and control mice at 0.5, 1, 2, 4, 6, 8, and 10 days postinfection (p.i.). Lungs were collected and homogenized at 4°C in 1 ml of DMEM containing high glucose (HyClone) using a gentleMACS dissociator (Miltenyi Biotec). The lung homogenates were pelleted, and the supernatant was collected. Viral titers were determined by TCID50 on MDCK cells as previously described (45).
Flow cytometry.
Bronchoalveolar lavage (BAL) fluid samples from mice were collected with 1 ml PBS at days 2, 4, and 10 p.i. For flow cytometry, BAL fluid cells were incubated in fluorescence-activated cell sorting (FACS) staining buffer (PBS containing 1% bovine serum albumin [BSA]) with FcγIII/II receptor antibody (BD) and with one of the following antibody groups: group 1, conjugated anti-Gr-1 and conjugated anti-Ly6G; group 2, conjugated anti-CD3, conjugated anti-DX5, and conjugated anti-NKp46; group 3, conjugated anti-Siglec-F, conjugated anti-CD11c, conjugated anti-CD11b, conjugated anti CD45, and conjugated anti-CD69; group 4, conjugated anti-CD4, conjugated anti-CD8, conjugated anti-CD44, and conjugated anti-CD69 (BD Biosciences). Cells were acquired on an LSRII flow cytometer (BD Biosciences), and data were analyzed using FlowJo software (v7.6.5).
Mouse pathology.
Mice were euthanized, and the lungs were collected and inflated through the trachea with 10% neutral buffered formalin (NBF) on days 2 and 4 p.i. Lungs were immersed in 10% NBF, fixed overnight, trimmed, embedded in paraffin, sectioned at 4-μm thickness, and stained with hematoxylin and eosin. Histopathological evaluation of the lungs was performed by a board-certified veterinary pathologist. Lesion severity was scored on a 0-to-4 scale.
Luminex bioassay and indirect ELISA.
Bronchoalveolar lavage (BAL) fluid samples from mice were collected with 1 ml PBS at days 2, 4, and 10 p.i. The cellular fraction was removed by centrifugation. Expression levels of cytokines, including IFN-γ, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p40, IL-15, IL-17, and TNF-α, were analyzed in BAL fluid supernatant samples using the Milliplex MAP kit (Millipore) per the manufacturer's instructions, and samples were analyzed using a Luminex 200 system. Mouse bronchoalveolar lavage (BAL) fluid wash was also evaluated for the presence of MIP-2 at 12 and 24 hpi by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (eBioscience).
Ferret studies.
Healthy, 16- to 18-week-old, castrated outbred male Fitch ferrets weighing between 0.8 and 1.0 kg (Triple F Farms) were used for all ferret experiments. Ferrets were housed individually in microisolator cages and provided food and water ad libitum. All ferrets were microchipped with subcutaneous implantable temperature transponders (IPPT300; Biomedic Datasystems). All ferrets were tested prior to infection for seroconversion against huH1N1 and swH1N2 using a hemagglutination inhibition (HI) assay (46). Ferrets were anesthetized using a ketamine, glycopyrrolate, and dexmedetomidine mixture (KGD) composed of 8 mg/kg ketamine, 0.01 mg/kg glycopyrrolate, and 0.04 mg/kg dexmedetomidine intramuscularly. KGD offers a deeper plane of sedation and allows for implementation of infections without the induction of sneezing during intranasal inoculation. The described method for sedation is part of our animal use protocol (AUP) required by the University of Georgia Animal Resources and IACUC. Ferrets were intranasally challenged with 106 PFU of huH1N1, swH1N2, or viral reassortants in 500 μl PBS. In order to facilitate recovery from sedation, ferrets were provided 0.2 mg/kg atipamezole (Antisedan; Zoetis) recovery agent intramuscularly (47). Body weight and temperatures were evaluated every other day p.i., and clinical signs of infection, including nasal and ocular discharge, sneezing, loose stool, and trouble breathing, were evaluated daily. Nasal washes were collected on days 2, 4, 6, 8, 10, and 12 p.i. using 2 ml PBS under anesthesia with 30 mg/kg ketamine. The amount of ketamine used allows for a more mild sedation that ensures that artificial induction of sneezing can occur to ensure proper nasal washing. The described method for sedation is part of our AUP required by the University of Georgia Animal Resources and IACUC. Cloanal swabbing was conducted at days 2 and 4 p.i. Nasal washes and cloanal swabs were transferred to 1.5-ml tubes and centrifuged for 1 min at 10,000 × g. Viral titers from nasal wash samples were determined by TCID50. Ferrets were anesthetized at day 14 p.i. with KGD and bled by intracardiac (i.c.) puncture (48). Following blood collection, ferrets were humanely euthanized with 0.5 ml Beuthanasia-D (Merck). Ferret serum was evaluated for the presence of influenza virus-specific IgG antibodies using standard microneutralization and hemagglutination inhibition assays (49). Three ferrets (n = 3) were used for each experiment, and 2 independent experiments were conducted.
Ferret pathology and immunohistochemistry.
Cranial and caudal lungs, trachea, and nasal turbinates were collected at days 2 and 4 p.i. Two ferrets per group were sacrificed for analysis (n = 2). All tissues were preserved in 10% neutral buffered formalin. Bone was decalcified 14 days prior to nasal turbinate analysis. Tissues were routinely processed and stained with hematoxylin and eosin (H&E). Immunohistochemical (IHC) analysis was performed using a mouse anti-NP influenza virus monoclonal antibody (ATCC; H16-L10-4R5) on lung and nasal tissues infected with huH1N1, swH1N2, and reassortant viruses. Tissues were deparaffinized and subsequently blocked with a protein-blocking agent (Dako Cytomaton) and labeled using a streptavidin-biotin immunoperoxidase system. The reaction was visualized with a 3,3′-diaminobenzidine substrate (Dako).
Reverse genetics.
Recombinant viruses rhuH1N1 HA, NA, PA, and HNP were generated by amplification of the genomes using RT-PCR and cloning into modified reverse genetics plasmids (50). Sequence-verified reverse genetics plasmids representing each of the 8 gene segments were cotransfected into HEK-293T/MDCK cocultured monolayers as described previously (50, 51). Briefly, 0.6 μg of plasmid for each gene segment was mixed and incubated with 15 μl of Lipofectamine 2000 (Invitrogen) at 20°C for 20 min. The Lipofectamine-DNA mixture was transferred to 90% confluent 293T/MDCK cell monolayers in a 35-mm tissue culture dish and incubated at 33°C with 5% CO2 for 8 h. Transfection supernatant was replaced with 3 ml of Opti-MEM I medium (Invitrogen) supplemented with 0.3% bovine serum albumin (BSA) fraction V (Invitrogen), 3 μg/ml TPCK-trypsin (Worthington), and 1% antibiotic-antimycotic (Invitrogen). Three days posttransfection, supernatant was collected and viruses were propagated in MDCK cells at 33°C.
Neuraminidase activity assay.
Total protein concentrations of viral stocks were determined using a bicinchoninic acid (BCA) assay (Pierce). A total of 0.1 mg protein from each viral stock was used to measure the NA activity of each sample. The 0.1-mg amount of protein was treated with 10 μg MUNANA [2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid] substrate and incubated at 37°C for 30 min. The reaction was stopped using 1 M glycine, pH 10.7. The cleavage of MUNANA by neuraminidase present in each sample was determined using a fluorimeter with a 365-nm excitation wavelength and a 450-nm emission wavelength.
Statistics.
All statistical analyses were performed using GraphPad software (San Diego, CA). Statistical significance was determined using one-way analysis of variance (ANOVA) followed by a post hoc Bonferroni comparison test. A P value of <0.05 was considered significant.
RESULTS
The triple reassortment internal gene cassette (TRIG) influences influenza A virus reassortment in a species-dependent manner.
Zoonotic transmission of the 2009 pandemic H1N1 virus ensued from a triple reassortment of avian, swine, and human origin (3). Swine have receptors to which swine, avian, and mammalian influenza viruses bind, which increases the potential for viruses to exchange genetic sequences and produce new reassortants (52–54), a feature increasing the potential for pandemic viruses. However, the sialic acid distribution profile in the swine respiratory tract does not solely support the reassortment potential (55–57). Current endemic swine IAVs in the United States are composed of the classical swine H1N1 viruses and reassortant TRIG viruses (18). The genotypic components of the TRIG cassette exhibit high genetic stability, resulting in a backbone that can support different glycoprotein combinations, providing a potential mechanism for reassortment resulting in evasion of herd immunity (20). These findings suggest that the TRIG cassette may provide a selective advantage for influenza virus reassortment in a species-dependent manner.
To determine if there were differential reassortment rates between human and swine cells, a human lung epithelial (Calu-3) cell line and swine kidney epithelial (LLC-PK1) cell line were evaluated for their susceptibility to infection. Calu-3 and LLC-PK1 cells were infected with A/California/04/09 (huH1N1), and A/swine/Illinois/02860/09 (swH1N2), an endemic swine TRIG IAV. swH1N2 and huH1N1 exhibited similar infectivities and had similar replication kinetics at 24 hpi. However, Calu-3 cells supported a higher level of virus replication for both huH1N1 and swH1N2 at 24 hpi (5.7 to 6.1 log10 PFU/ml) than did LLC-PK1 cells (4.3 to 5.2 log10 PFU/ml) (data not shown). Calu-3 and LLC-PK1 cells were coinfected with a 1:1 ratio of huH1N1 and swH1N2 at an MOI of 3 with each virus, and supernatants were collected 24 hpi. Individual viral isolates that resulted from virus coinfection were purified and amplified in MDCK cells for 72 h. Each individual plaque isolate was screened using a previously described multiplex qPCR approach directed against the HA, NA, and PB2 genes of each respective virus (44). One hundred individual plaques derived from coinfections of both Calu-3 and LLC-PK1 cells were screened. Influenza virus reassortants were not identified from coinfection of Calu-3 cells based on the HA, NA, and PB2 screen (Fig. 1B). The distribution of wild-type viruses after coinfection was greatly skewed, with 76% of the viruses harboring the HA, NA, and PB2 gene constellation derived from the swH1N2 virus (Fig. 1B). Interestingly, coinfection of Calu-3 cells resulted in preferential replication of the swH1N2 virus independent of similar replication kinetics between huH1N1 and swH1N2. In contrast, coinfection of LLC-PK1 cells resulted in a 23% reassortment prevalence based on the genetic rearrangement of the HA, NA, and PB2 genes (Fig. 1A). These data represent reassortment as it relates to the HA, NA, and PB2 genes, but this cannot preclude additional reassortments that may have occurred in human epithelial cells. All reassortants were validated by full-genome sequence analysis. Additional rearrangement of internal genes was observed but was limited, providing evidence for stability and high genetic compatibility of the TRIG cassette. Nucleotide differences were observed through sequence analysis with the parental strains; however, amino acid substitutions were not observed compared to the parental strains; therefore, all mutations were synonymous. Percentages are represented as introduction of swH1N2 genes into the huH1N1 virus as confirmed by full-genome sequencing. In this case, 91% of the reassortants isolated from coinfection of LLC-PK1 cells resulted in the introduction of swH1N2 glycoprotein genes into the huH1N1 virus, and in particular, 65% of the total reassortants resulted from inclusion of the swH1N2 NA (Fig. 1C).
FIG 1.
Comparison of influenza virus reassortment outcomes in swine and human cells following coinfection with huH1N1 and swH1N2. Coinfections were performed in LLC-PK-1 cells (A) and Calu-3 cells (B) with huH1N1 and swH1N2. Primers and probes specific to parental strain huH1N1 and huH1N2 HA, NA, and PB2 gene segments were used to screen the reassortment prevalence in both cell lines. One hundred individual plaque-purified isolates from each cell line were screened. Coinfection of LLC-PK1 cells resulted in a reassortment prevalence of 23% with a large percentage of reassortants resulting in glycoprotein gene exchange (C). The total number of reassortment profiles isolated is displayed in the profile matrix, and the genotype is profiled in gray with the prevalent reassortment genotype highlighted in yellow (C).
Coinfection in swine cells results in novel reassortants with altered phenotypes.
It has been previously shown that IAV reassortants having genetic exchange of regulatory genes such as NS, or replication machinery such as PA and PB2, can alter the viral kinetics and immune modulatory phenotype of IAVs (26–30). Thus, replication kinetics were evaluated for reassortants having genetic exchange between structural and nonstructural genes among the parental viruses, swH1N2 and huH1N1. rILL346 encodes the swH1N2 HA, NA, and PA, and the remaining genes are comprised of segments derived from the huH1N1 virus, while rILL18 has the swine-origin PB2 and NS genes, and the remaining genes were derived from the huH1N1 virus (Fig. 2A). The permissibility and susceptibility of Calu-3 cells were evaluated with the described reassortants. The supernatants from infected (MOI of 0.01) Calu-3 cells were collected 24, 48, and 72 hpi. Viral titers were evaluated by TCID50, and endpoint titers were expressed as TCID50/ml. Interestingly, rILL346 showed a significant increase in virus replication at 24, 48, and 72 hpi compared to the wild-type viruses. Most interesting were the differences observed at 24 hpi with rILL346 replicating to an ∼100-fold-higher mean viral titer than the parental viruses (107.4 versus 105.7 and 105.25 TCID50/ml, respectively) (Fig. 2B). rILL18 replicated efficiently in Calu-3 cells, but no substantial differences from the parental strains were evident. However, infection with rILL346 and rILL18 resulted in differential plaque formation. Plaque morphology is often associated with cell-to-cell spread that is linked to viral fitness (58). Plaques generated in MDCK cells following infection with rILL346 were large and uniform compared to moderate-size plaques made by the parental viruses. Plaques from rILL18 exhibited an attenuated phenotype compared to the parental viruses (Fig. 2C). These findings indicate that rILL18 may replicate to similar levels in vitro, and the addition of the swH1N2 NS or PB2 to the genome does not appear to alter viral replication. The increase in replication kinetics and morphological differences in plaque formation may suggest increased viral fitness of rILL346.
FIG 2.
Influenza virus reassortants derived from huH1N1 and swH1N2 viruses exhibit differential phenotypes in vitro. (A) Diagram of reassortment. (B) Viral replication in Calu-3 cells was determined. Calu-3 cells were infected (MOI of 0.01), and supernatants were collected at 0, 24, 48, and 72 hpi to determine viral titers. Endpoint titers are expressed as mean log10 TCID50/ml ± standard deviation. (C) The plaque morphologies of each viral reassortant and parental virus were compared in MDCK cells and scanned for comparison. (D) In vitro induction of the type III IFN response elicited by infection with rILL346, rILL18, and the parental viruses was evaluated by qPCR. IL-29 (IFN-λ)-specific primers and probes were used to evaluate the relative type III IFN expression in Calu-3 cells at 12 and 24 hpi (MOI of 1.0). UV-inactivated viral samples were used as replication controls. Fold changes and standard deviations were calculated based on triplicate threshold cycle (CT) values relative to mock-infected cells and the housekeeping gene HPRT. All data are representative of three individual experiments. Asterisks denote significance related to the pH1N1 parental virus while number signs (#) represent P values significant compared to the swH1N2 parental virus. Results were considered significant with P values of ≤0.05 (*/#), ≤0.01 (**/##), and ≤0.001 (***/###).
Differences in viral replication often correlate with differences in the antiviral response, in particular the type I and III IFN responses, which are dependent on many host and viral factors. It has been shown that viral components of replication such as the polymerase complex and the interferon (IFN) antagonist activity of the NS gene can affect the antiviral response (26–30). The type I and III IFN responses elicited by rILL346, rILL18, swH1N2, and huH1N1 infection of Calu-3 cells were investigated. Calu-3 cells were infected at an MOI of 1.0, and cell lysates were collected 12 and 24 hpi. RNA purified from cell lysates was evaluated using one-step qPCR with TaqMan primers and probes directed against IFN-α, IFN-β, and IFN-λ (IL-29) and normalized against the housekeeping gene, HPRT. Comparison of the type I IFN responses (IFN-α/IFN-β) showed modest expression levels, but no significant differences were observed between viruses (data not shown). However, infection with rILL346 and swH1N2 resulted in robust expression of IFN-λ at 12 and 24 hpi. Infection with rILL346 significantly (P < 0.001) increased (∼30-fold) IFN-λ mRNA expression at 12 hpi compared to huH1N1 and rILL18 (Fig. 2D). At 24 hpi, infection with rILL346 and swH1N2 induced significantly (P < 0.001) higher (100-fold) IFN-λ mRNA expression than did infection with huH1N1 and rILL18 (Fig. 2D). UV-inactivated viruses were used as controls to determine if virus replication was required for induction of the responses observed in gene expression. Although infection with rILL346 and swH1N2 induces a substantial antiviral state, the viruses appear to subvert the antiviral activity of IFN-λ, a feature that potentially may contribute to increased cell pathogenesis.
swH1N2 HA, NA, and PA are associated with increased pathogenicity in ferrets.
With evidence for increased replicative fitness (Fig. 2B and C) and implications for modulation of the antiviral type III IFN response in Calu-3 cells (Fig. 2D), the ferret model was used to assess potential pathogenicity of rILL346 and rILL18. Ferrets were intranasally infected with rILL346, rILL18, swH1N2, or huH1N1, and those infected with rILL346 exhibited increased morbidity (Fig. 3). Infection of ferrets with rILL346 resulted in substantial clinical signs characterized by a high degree of sneezing, lethargy, difficulty breathing, and nasal discharge throughout the course of infection. Only mild nasal discharge was observed with infection with huH1N1, and no clinical signs were observed with swH1N2 and rILL18. However, significant (P > 0.05) changes in weight loss were not observed between groups, but infection with rILL346 and swH1N2 resulted in the most weight loss during the course of infection (Fig. 3A). There were no appreciable differences in viral shedding at day 2, 4, or 6 p.i., with undetectable levels of virus at day 8 p.i. (Fig. 3B). Virus replication was not detectable from cloanal samples. Since there were no detectable differences in virus replication, it is likely that changes in pathogenicity were attributed to modulation of the host immune response to infection. This is supported by the levels of influenza virus-specific antibody neutralization titers elicited in response to infection. In this case, rILL346 and swH1N2 induced higher influenza virus-specific antibody titers than did rILL18 and huH1N1 (Fig. 3C). Antibody production is dependent on CD4+ T cell activation, which is partially dependent on the host innate immune response to infection (59). Increased influenza virus-specific antibody titers can directly correlate with robust induction of the innate immune response to infection (60).
FIG 3.
rILL346 mediates increased viral pathogenicity in ferrets—an effect associated with swine virus HA, NA, and PA genes. (A) Ferrets were intranasally infected with 106 PFU of virus, and weight loss was evaluated on a daily basis for n = 3 ferrets per group and is represented as the average percentage of the preinfection weight of the ferret. (B) Viral titers were determined from nasal washes at days 2, 4, and 6 p.i. Endpoint titers are expressed as mean log10 TCID50/ml ± standard deviation. The limit of virus detection was 10 TCID50/ml. (C) Influenza virus-specific antibody responses were measured based on microneutralization assays of serum collected from infected ferrets at day 14 p.i. Neutralization titers are presented as the log10 serum dilution at which virus could no longer be detected. (D) Histopathology was performed on lungs and nasal turbinates of infected ferrets. Nasal turbinates and lung tissues were collected at days 2 and 4 postinfection from n = 2 ferrets per time point per virus, and lesion severity was scored on a 4-point scale relative to the overall pathology induced during infection. (E) Representative H&E-stained sections of ferret nasal turbinates and lung tissues 2 days p.i. All data are representative of two independent experiments.
Histopathological and immunohistochemical (IHC) analyses were performed on lung and nasal turbinates of intranasally infected ferrets. No discernible differences were observed in the distribution of virus throughout the respiratory tract of infected ferrets by IHC staining (data not shown). The main lesions observed in the lungs occurred at days 2 and 4 p.i. and were characterized by multifocal necrotizing bronchiolitis and alveolitis along with a level of bronchial gland necrosis. rILL346 and swH1N2 induced the highest level of pulmonary lymphocytic infiltration, which occurred at days 2 and 4 p.i. rILL18 and huH1N1 infection was associated with minimal pulmonary lymphocyte trafficking at days 2 and 4 p.i., and at day 4 p.i., pulmonary lymphocyte levels were more substantial than those with rILL346 or rILL18. Infection with rILL346 and swH1N2 also resulted in higher levels of type II hyperplasia and bronchiolar epithelial hyperplasia than did infection with rILL18 and huH1N1 (Fig. 3E). Overall, rILL346 and swH1N2 exacerbated lung pathology at days 2 and 4 p.i. with no overt changes in viral shedding (Fig. 3D).
Infection of the upper respiratory tract is often credited with increasing transmissibility of influenza A viruses (61). Infection with rILL346 and swH1N2 exhibited the most severe pathology in the nasal turbinates at day 2 p.i. rILL346 infection had extensive mucosal involvement of the nasal cavity, loss of the epithelium, severe congestion, hemorrhage in the lamina propria, mild edema, necrotic debris, and lymphocytic infiltration. Interestingly, at day 4 p.i., huH1N1 infection had pathological outcomes similar to those observed with rILL346 at day 2 p.i., while rILL346-associated disease severity decreased over time. Ferrets infected with rILL18 had less severe pathology in the nasal turbinates and olfactory type epithelium at day 2 p.i. than with infections with the other viruses. Specifically, rILL18 infection was associated with very minor epithelial damage, mild edema, and very few lymphocytes in the lamina propria. Overall, rILL346 and swH1N2 infection induced the highest degree of pathology in the nasal turbinates of ferrets early and throughout infection (Fig. 3D).
rILL346 is associated with pulmonary neutrophil and NK cell recruitment.
rILL346 is a reassortant virus derived from three swH1N2 gene segments (HA, NA, and PA) with the remaining genes derived from huH1N1. The evidence of enhanced lung pathology in ferrets following infection with rILL346 and swH1N2 (Fig. 3D) suggested that swH1N2 HA, NA, and PA genes affected viral replication (Fig. 3B) and likely immune-mediated pathogenesis. To elucidate a mechanism, mice were similarly infected with rILL346, rILL18, swH1N2, and huH1N1 so that the pulmonary immune response (Fig. 4A) and lung histopathology (Fig. 4B and C) could be evaluated. Mice were infected with 5 × 105 PFU of virus, and lung viral titers were determined at days 2, 4, 6, 8, and 10 p.i. All viruses replicated to similar titers at day 2 p.i., but clearance of rILL346 and swH1N2 was delayed and these viruses replicated to substantially higher lung virus titers at days 4 and 6 p.i. (Fig. 4A), whereas rILL18 and huH1N1 were cleared by day 6 p.i. (Fig. 4A). Lung histopathological analysis revealed that at early time points of infection, e.g., day 2 p.i., rILL346 infection was associated with enhanced lung pathology compared to rILL18, swH1N2, and huH1N1 infection (Fig. 4B) that was characterized by numerous focal points of necrotic bronchiolar epithelial cells either in the lining of the epithelium or desquamated in the bronchiolar lumen (Fig. 4C). The pathologies associated with rILL346, rILL18, and swH1N2 infection were similar at day 4 p.i., and huH1N1 induced mild pathology throughout the course of infection (Fig. 4B).
FIG 4.
rILL346 infection is associated with exacerbated pathogenicity in BALB/c mice compared to the parental viruses. BALB/c mice (n = 5) were intranasally infected with 5 × 105 PFU of rILL346, rILL18, huH1N1, or swH1N2. (A) Lungs were collected and homogenized at days 2 and 4 p.i., and lung virus titers were determined. Endpoint titers are expressed as the mean log10 TCID50/ml ± standard deviation. The limit of virus detection is 10 TCID50/ml. (B) Histopathological analysis was performed on lungs of infected mice. (C) Arrows presented in representative H&E-stained sections of lung tissue isolated from mice at 2 and 4 days p.i. show sites of leukocyte infiltration and alveolar damage. All data are representative of three individual experiments. Asterisks (*) denote significance related to the pH1N1 parental virus while number signs (#) represent P values significant compared to the rILL18 reassortant virus. Results were considered significant with P values of ≤0.05 (*/#), ≤0.01 (**/##), and ≤0.001 (***/###).
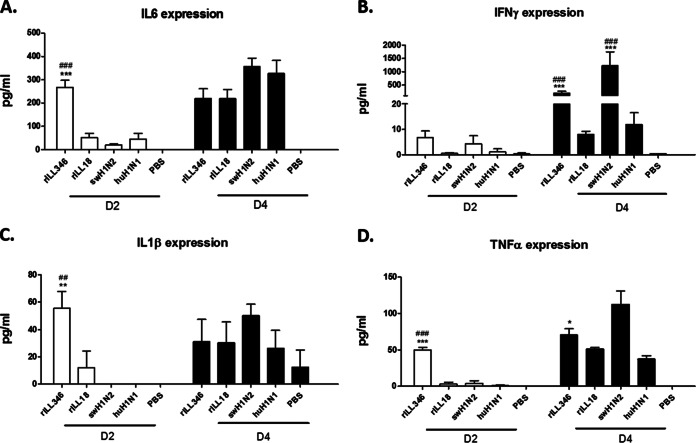
As there is a threshold at which the proinflammatory response facilitates influenza virus clearance and/or mediates disease, the cytokine levels for a panel of proinflammatory cytokines were evaluated in mice. Bronchoalveolar lavage (BAL) fluid samples harvested at days 2 and 4 p.i. were evaluated for expression of IFN-γ, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p40, IL-15, IL-17, and TNF-α. Infection with rILL346 was associated with increased protein levels of IL-6 (Fig. 5A), IL-1β (Fig. 5C), and TNF-α (Fig. 5D) at day 2 p.i. Infection with swH1N2 resulted in significant (P < 0.001) but delayed expression of IL-6 (Fig. 5A), IL-1β (Fig. 5C), and TNF-α (Fig. 5D) with trends showing increased levels of expression as well at day 4 p.i. At day 4 p.i., IFN-γ expression was significantly (P < 0.001) upregulated following infection with rILL346 and swH1N2 compared to that with huH1N1 and rILL18; however, only rILL346 infection caused an exuberant proinflammatory response in the lung early after infection (Fig. 5B).
FIG 5.
Early expression of proinflammatory cytokines linked to rILL346 infection. Proinflammatory cytokine expression levels in the BAL fluid of mice infected with rILL346, rILL18, huH1N1, and swH1N2 were evaluated at days 2 and 4 p.i. BALB/c mice (n = 5) were intranasally infected with 5 × 105 PFU of virus. At days 2 and 4 p.i., bronchoalveolar lavage (BAL) fluid was collected from infected mice. The BAL fluid was examined for expression of IL-6 (A), IFN-γ (B), IL-1β (C), and TNF-α (D) by a Luminex Bio-Plex 200. All data are representative of three individual experiments. Asterisks denote significance related to the pH1N1 parental virus while number signs (#) represent P values significant compared to the rILL18 reassortant virus. Results were considered significant with P values of ≤0.05 (*/#), ≤0.01 (**/##), and ≤0.001 (***/###).
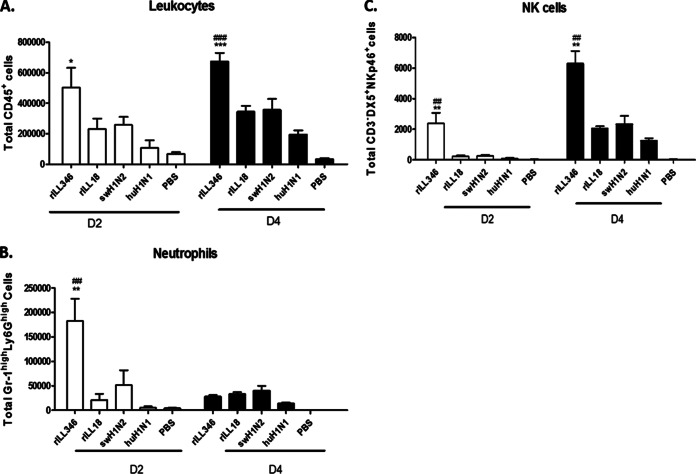
As expected from the cytokine profiles (Fig. 5), pulmonary infiltration by neutrophils and NK cells (Fig. 6) correlated with increased proinflammatory cytokine expression levels, suggesting that these cells contributed to a role in disease outcome. Infection with rILL346 resulted in substantially higher recruitment of leukocytes at day 2 p.i. in mice (Fig. 6A). BAL fluid-derived neutrophils (Gr-1hi Ly6Ghi) expressed high levels of proinflammatory cytokines at various times p.i., including IL-1β, IL-6, and TNF-α. At 24 hpi, significant (P < 0.01) neutrophil (Fig. 6B) recruitment following rILL346 infection correlated with elevated levels of IL-1β, IL-6, and TNF-α in the BAL fluid specimens (Fig. 5). This is consistent with the finding that neutrophils are involved in the activation and maintenance of NK cells during influenza virus infection (62) and with neutrophil cytokines such as IL-15 promoting survival and trafficking to sites of infection (63–65). Moreover, activated NK cells express high concentrations of IFN-γ after interaction with NKp46 and sugar residues expressed on the surface of the HA protein (66). Infection with rILL346 resulted in considerable NK cell recruitment at day 4 p.i. (Fig. 6C), reflecting high levels of IFN-γ expression p.i. (Fig. 5B).
FIG 6.
Infection with rILL346 and rILL18 affects pulmonary innate cell trafficking. Pulmonary cellular infiltration in mice infected with rILL346, rILL18, huH1N1, and swH1N2 at days 2 and 4 p.i. BALB/c mice (n = 5) were intranasally infected with 5 × 105 PFU of virus, and BAL fluid was collected on days 2 and 4 p.i. Pan-leukocyte (CD45+) (A), neutrophil (Gr-11hi Ly6Ghi) (B), and NK cell (CD3− DX5+ NK46p+) (C) infiltration was determined by flow cytometry. All data are representative of three individual experiments. Asterisks denote significance related to the pH1N1 parental virus while number signs (#) represent P values significant compared to the swH1N2 parental virus. Results were considered significant where P values were ≤0.05 (*/#), ≤0.01 (**/##), and ≤0.001 (***/###).
Swine H1N2 NA and PA affect neutrophil infiltration, while PA modulates NK cell trafficking.
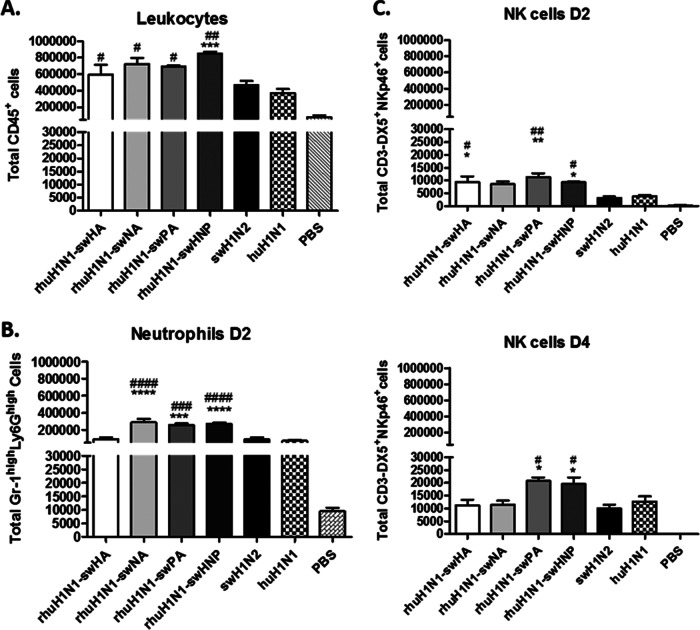
In order to elucidate the genetic components from swH1N2 that contribute to the increased immune pathology in the lung following rILL346 infection (Fig. 4), reverse genetics viruses were constructed as single-gene reassortants (rhuH1N1-swHA, -NA, and -PA) in the huH1N1 backbone. An additional reverse genetics virus was developed to mimic the naturally derived rILL346 that contains the swine virus HA, NA, and PA (rhuH1N1-swHNP). Pulmonary leukocyte (CD45+) recruitment in the BAL fluid was determined in mice infected with rhuH1N1-swHA, rhuH1N1-swNA, rhuH1N1-swPA, and the triple reassortant rhuH1N1-swHNP. At day 2 p.i., CD45+ leukocyte numbers in the BAL fluid were significantly (P < 0.05) enhanced compared to infection with the parental huH1N1 virus (Fig. 7A). Only infection with rhuH1N1-swHNP resulted in significant (P < 0.001) recruitment of BAL fluid-derived leukocytes (CD45+) compared to both parental viruses (Fig. 7A) with results comparable to that observed with rILL346 at day 2 p.i. (Fig. 6A). At 48 hpi, infection with rhuH1N1-swNA, rhuH1N1-swPA, and rhuH1N1-swHNP resulted in significant (P < 0.05) enhancement of GR-1hi Ly6Ghi neutrophils in the BAL fluid (Fig. 7B). At day 4 p.i., significant (P < 0.05) levels of NK cells (CD3− DX5+ NKp46+) are observed in the lungs of mice infected with rhuH1N1-swPA and rhuH1N1-swHNP (Fig. 7C).
FIG 7.
swH1N2 PA and NA contribute to increased levels of neutrophil and NK cell recruitment. To elucidate the genetic components that may be contributing to neutrophil and NK cell pulmonary recruitment, the reassortant viruses were rescued to assess the contribution of the swine virus HA, NA, and PA genes. Swine virus PA, HA, and NA genes were introduced into the huH1N1 backbone individually (HA, NA, and PA) or together (HNP). Mice (n = 5) were intranasally infected with 5 × 105 PFU of virus, and BAL fluid was collected at days 2 and 4 p.i. to assess the recruitment of leukocytes (CD45+) (A), neutrophils (Gr-1hi Ly6Ghi) (B), and NK cells (CD3− DX5+ NKp46+) (C) into lung. All data are representative of three individual experiments. Asterisks (*) denote significance related to the pH1N1 parental virus while number signs (#) represent P values significant compared to the swH1N2 parental virus. Results were considered significant with P values of ≤0.05 (*/#), ≤0.01 (**/##), and ≤0.001 (***/###).
Alteration of the innate immune response by the swH1N2 HA, NA, and PA has downstream implications for T cell activation.
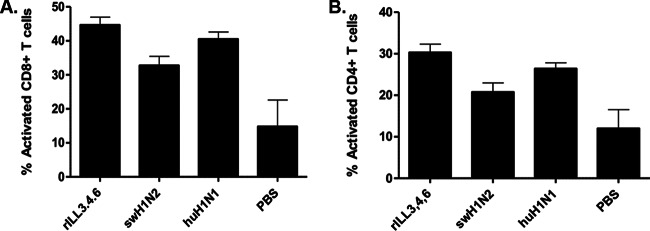
T cell development and activation are in part dependent on the quality of the innate response reflected by the proinflammatory environment (67). The levels of CD8+ (CD3+ CD8+ CD44+ CD69+) and CD4+ (CD3+ CD8+ CD44+ CD69+) T cell activation were determined in the BAL fluid at day 10 p.i. as a percentage of the total CD8+ (CD3+ CD8+) and CD4+ (CD3+ CD4+) T cells. Mice infected with rILL346 had the highest levels of CD8+ (CD3+ CD8+) and CD4+ (CD3+ CD4+) T cell activation (CD44+ CD69+) (Fig. 8), compared to mice infected with rILL18 and the parental viruses. Antibody titers were evaluated, and the results were correlated with the findings observed in ferrets (Fig. 3C). rILL346 and swH1N2 infection was associated with a higher level of influenza virus-specific IgG antibodies, but there were no differences in the IgG subtypes (IgG1 and IgG2a) expressed (data not shown). These findings are relevant as NK cell activation and expression of IFN-γ have been implicated in regulating CD8+ T cell priming and affecting differences in T cell activation (68).
FIG 8.
Modulation of the CD4 and CD8 T cell response following rILL346 infection. T cell recruitment to the lungs of infected mice was determined following infection with rILL346, rILL18, huH1N1, and swH1N2 at day 10 p.i. BALB/c mice were intranasally infected with 5 × 105 PFU of virus. BAL fluid was collected at day 10 p.i., and the percentages of activated CD4+ (CD3+ CD4+ CD44+ CCD69+) and CD8+ (CD3+ CD8+ CD44+ CD69+) T cells from the total CD4+ (CD3+ CD4+) and CD8+ (CD3+ CD8+) populations were determined. All data are representative of three individual experiments.
swH1N2 NA and PA affect levels of MIP-2 expression linked to neutrophil recruitment to the lungs.
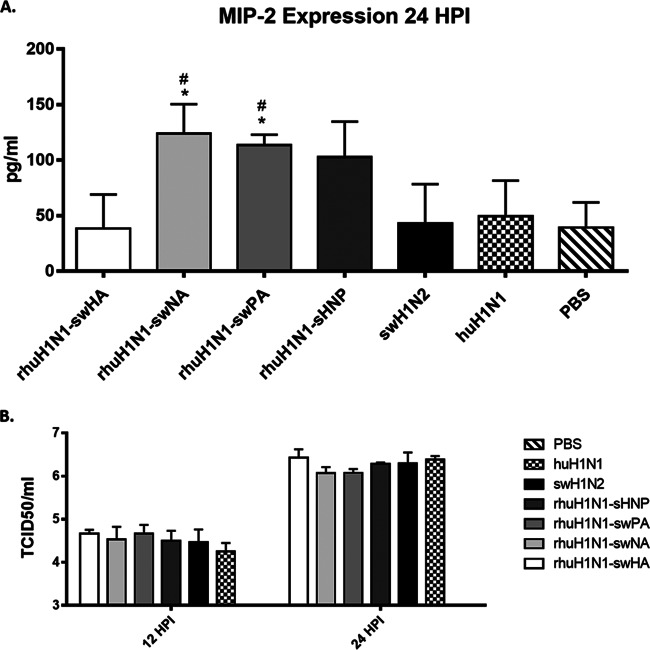
Neutrophil recruitment is generally mediated by the chemoattractant MIP-2 (69), which is expressed in part by virus-infected epithelial cells. In this study, infection with rhuH1N1-swNA or rhuH1N1-swPA resulted in significant (P < 0.05) MIP-2 expression in the BAL fluid at 24 hpi (Fig. 9A). Infection with rhuH1N1-swHNP did not result in significant (P < 0.05) MIP-2 expression above that expressed in response to the parental viruses but did increase MIP-2 expression, and differences in MIP-2 expression were not observed at 12 hpi (data not shown). These findings indicate that MIP-2 expression is associated with swH1N2 NA and PA genes, a feature that appears to recruit neutrophils early during infection. As expression of MIP-2 could be affected by the virus replication rate, the differences in virus replication kinetics were examined at 12 and 24 hpi between the reverse genetics-derived and parental viruses. There were no detectable differences in replication between viruses at 12 and 24 hpi Fig. 9B).
FIG 9.
swH1N2 NA and PA genes affect early MIP-2 expression. MIP-2 expression in the BAL fluid was measured by ELISA at 24 hpi (A). Mice were intranasally infected with 5 × 105 PFU of reverse genetics-derived viruses where the swH1N2 HA, NA, PA, and HNP were introduced into the huH1N1 virus along with the parental viruses. BAL fluid was collected at 24 hpi. Lung viral load was measured by TCID50 on BALB/c mice infected with reverse genetics-derived viruses or the parental swH1N2 and huH1N1 viruses. Viral titers were determined at 12 and 24 h postinfection (B). All data are representative of three individual experiments. Asterisks (*) denote significance related to the pH1N1 parental virus while number signs (#) represent P values significant compared to the swH1N2 parental virus. Results were considered significant with P values of ≤0.05 (*/#), ≤0.01 (**/##), and ≤0.001 (***/###).
Other factors that may contribute to the differences observed were investigated. Total protein was normalized from rILL346, rILL18, huH1N1, and swH1N2 viral stocks derived from cellular supernatants and tested for cleavage activity based on the cleavage of the fluorescent substrate MUNANA. The Clostridium perfringens NA protein was used as a positive control. The NA protein of rILL346 and swH1N2 is shared, while rILL18 and huH1N1 share NA proteins. Results were compared relative to the cleavage activity of protein isolated from huH1N1. Therefore, it was evident that both rILL346 and swH1N2 exhibited ∼50% more activity based on the cleavage of the substrate MUNANA compared to huH1N1 NA (Fig. 10A). The cleavage activity observed with rILL18 mimicked that detected from huH1N1. As both rILL346 and swH1N2 share the same NA protein, it is feasible to conclude that the swH1N2 NA is substantially more enzymatically active than the huH1N1 NA. These findings indicate a potential role for NA-mediated differences. The influenza virus PA gene encodes a second protein, PA-X, which is expressed, based on a ribosomal frameshift (70). PA-X has been shown to have a role in modulation of the innate immune response through upregulation of antiviral cytokine and chemokine expression through a host protein shutdown mechanism (20, 70, 71). Clustal W analysis of the amino acid sequences from the N-terminal and C-terminal regions of the PA from huH1N1 and swH1N2 shows extensive amino acid changes (Fig. 10B). Alterations in the protein sequence of PA-X could directly affect the mechanistic role of PA-X, resulting in direct modulation of the antiviral state of infected cells.
FIG 10.
Potential virus-associated factors contributing to NA- and PA-mediated pathogenicity. (A) NA activities were compared between rILL346, rILL18, swH1N2, and huH1N1. A 1.0-mg quantity of protein was used to normalize for variation between viral samples. A 1.0-mg quantity of viral protein was mixed with MUNANA for 30 min at 37°C in the absence of light. Viral samples were compared relative to huH1N1 cleavage activity. Data are representative of three independent experiments. (B) Clustal W analysis of the amino acid sequences from the N-terminal and C-terminal regions of the PA from huH1N1 and swH1N2.
DISCUSSION
Swine are considered a mixing vessel for the development of reassortant influenza viruses with pandemic potential. Initial findings suggested that the differential distribution of α2,3- and α2,6-sialic acids along the swine respiratory tract was key to supporting the mixing vessel hypothesis, but more recent studies examining sialic acid receptor distribution profiles in swine and humans indicate that such differences are not significant or observed (48–50). The swine influenza virus TRIG cassette has been hypothesized to have a role in supporting increased glycoprotein exchange in swine, a feature that may increase the reassortment prevalence and increase the overall fitness of influenza virus in swine, perhaps by evasion of herd immunity (20). Calu-3 and LLC-PK1 cells do not have detectable differences in the levels of α2,3- and α2,6-sialic acid expression profiles (data not shown). Additionally, sialic acid binding profiles for the huH1N1 and swH1N2 viruses are not notably different, and both viruses preferentially bind α2,6 terminal sialic acids (72). Thus, it remains unlikely that differences in sialic acid expression and HA specificity are primary causes of differences linked to reassortment potential in these cell types. Thus, the findings in this study support the TRIG cassette as a platform for enabling species-specific reassortment in swine compared to humans. It is shown that coinfection of swine epithelial cells with swine- and human-derived TRIG viruses resulted in a 23% reassortment prevalence with preferential exchange of the NA glycoprotein while no reassortment viruses were identified in human epithelial cells based on mixing of the NA, HA, and PB2 genes.
Influenza reassortant viruses resulting from swine can emerge with pandemic potential as evidenced by the 2009 pH1N1 virus. The pH1N1 virus has established itself as the dominant circulating seasonal H1N1 virus, and based on extensive surveillance studies in swine, reverse zoonosis has been identified worldwide (10–17). pH1N1 harbors the ability to be transmitted very efficiently from human to human based on transmission analyses from the 2009 outbreak and detailed ferret transmission studies (73). With evidence of rapid reverse zoonosis, the potential for continued reassortment in swine is probable. NS, PA, PB2, HA, and NA genes have all been implicated in modulating the pathogenicity of influenza viruses, and this study defines the contribution of swine virus-derived NS, PA, PB2, HA, and NA to enhancing pH1N1 virulence. It also shows that the introduction of swine virus NA and PA directly increases the pathogenicity of the pH1N1 viruses in mice and ferrets, resulting in acute lung injury modulated by increased lymphocyte infiltration and destruction of the endothelial-epithelial barrier. Swine virus NA and PA genes are linked to enhanced expression of MIP-2, a potent neutrophil chemoattractant, which results in elevated levels of neutrophil trafficking to the lungs of infected animals early postinfection. Neutrophils have a notable role in influenza virus pathogenesis, and neutrophilia is consistent with acute respiratory distress syndrome (74). Neutrophils are short-lived, phagocytic granulocytes which may reduce influenza virus infection through phagocytosis of influenza virus-infected epithelial cells or formation of neutrophil extracellular traps (NETs) that reduce infection (75). Neutrophils are directly associated with influenza viral clearance but also, when in abundance in the BAL fluid, correlate with increased disease severity (76). In this study, evidence is provided for this association with both the swine virus NA and PA genes. Neutrophils cause extensive damage to the epithelial-endothelial barrier via production of reactive oxygen species (ROS), secretion of proteases, and robust induction of a proinflammatory cytokine environment (77). Elevated levels of induced IL-6, IL-1β, and TNF-α comprise the elevated proinflammatory environment induced by neutrophils mediated through swine virus NA and PA and are the correlative cause of increased epithelial damage in the lungs. The exact mechanism by which swine virus NA and PA mediate neutrophil-associated acute lung injury needs to be further elucidated.
Neutrophils are essential innate effector cells forming the first line of defense against several pathogens. They have a role in the engulfment of the pathogens and the release of reactive oxygen species (ROS) and proteases that contribute to host defense. Neutrophil recruitment to sites of infection can be mediated by an array of chemoattractants. These chemoattractants synergistically cooperate to mediate recruitment to sites of infection (78). Neutrophils can be recruited to sites of infection via the expression of proinflammatory cytokines, including IL-6, IL-1β, TNF-α, KC, MIP-2, and monocyte chemoattractant protein 1 (MCP-1), which represent inflammatory signals which mediate the trafficking cascade of neutrophils (78, 79). Enhanced IL-6, IL-1β, and TNF-α may also contribute to continued neutrophil recruitment at later times postinfection, an effect that seems linked to swine virus NA and PA genes. In this study, elevated expression of MIP-2 was observed 24 hpi, which appears to correlate with and may be responsible for triggering downstream neutrophil migration that was observed at 48 hpi. The findings of this study suggest that swine virus NA and PA are individually culpable for the elevated MIP-2 expression, and this may be associated with regulation of cellular pathways linked to changes in viral infectivity. NA is responsible for cleavage of sugars in the mucus layer in the lungs and can potentially lead to increased levels of infection (38). The in vivo viral kinetics data do not fully support this hypothesis, but the viral replication kinetics data in human epithelial cells do. The swine virus NA also exhibits increased cleavage activity compared with huH1N1 as evidenced by the increased MUNANA cleavage activity of swH1N2 compared with huH1N1. It is important to note that the PA gene encodes a second protein, PA-X, through a frameshift (70). PA-X can have a direct role in host protein synthesis shutdown, modulating antiviral and apoptotic pathways (36, 70, 71). Clustal W alignments of the PA-X proteins of huH1N1 and swH1N1 show a low degree of similarity. The exact mechanism by which the swine virus NA and PA enhance MIP-2 expression and how MIP-2 is mediating the downstream recruitment of neutrophils to the lung need to be further elucidated.
Collaborative cross talk occurs between neutrophils and NK cells at sites of inflammation mediating recruitment, activation, and maintenance of both neutrophils and NK cells (62). Neutrophil-depleted mice have impaired NK cell maturation exhibiting hyperproliferation and weak survival (80). Neutrophils also express high levels of IL-15, which promotes NK cell activation (63–65). Early recruitment of neutrophils by PA likely mediates the activation and maintenance of NK cells observed at 4 days p.i. NK cells also contribute significantly to influenza virus disease pathogenesis (81–83), but the exact mechanism is not well understood.
The predominant viral strains circulating in swine are typically represented by H1, H2, and H3 strains of IAV, but as preexisting immunity to H1 and H3 subtype viruses is widespread in the human population, it is unlikely that a viral threat would be posed by recombinant IAV representing H1 and H3 subtypes (84). However, H2 viruses harboring an array of neuraminidase proteins circulate in swine and avian populations (85–87). H2 viruses have been absent from the human population for more than 50 years, since the 1957 pandemic (88); therefore, the lack of preexisting immunity to a potential pandemic with H2 viruses elevates the pandemic potential and risk. Not only do the described reassortants here pose a potential risk for elevating the virulence of current H1N1 circulating strains, but also they could elevate the potential risk of H2 viruses emerging with pandemic potential with enhanced virulence compared with the 2009 pandemic H1N1 virus.
In summary, the swine virus TRIG cassette contributes to species-specific reassortment in swine and presents a foundation for establishing swine as a mixing vessel for IAV. As evidenced by extensive global surveillance studies, pH1N1 was rapidly reintroduced into swine, resulting in continued reassortment of pH1N1 with endemic strains of IAV, resulting in variant strains of IAV that result in enhanced virulence to humans as shown by the H3N2v viruses (13, 19, 20). As shown here, swine influenza viruses harbor gene components that can directly modulate the pathogenic outcome of potential reassortant viruses. In particular, swH1N2 NA and PA modulate MIP-2 expression, resulting in pulmonary recruitment of neutrophils which establish a proinflammatory cytokine environment in the lung. The proinflammatory cytokine environment promotes sustained recruitment of neutrophils, leading to their activation in the lung. Modulation of the innate immune response by NA and PA contributes to acute lung injury in both the mouse and the ferret models of infection. These findings necessitate elucidating the mechanisms that promote viral reassortment in swine, and the possibility that the described reassortant viruses described may be actively circulating in swine populations argues for increased surveillance and research on identification of swine influenza virus pathogenicity markers.
ACKNOWLEDGMENTS
We thank Geraldine Saavedra for assistance in sequencing and Karla Stucker for assistance with reverse genetics development.
The studies were fully funded through NIH CEIRS contract HHSN266200700006C and HHSN2722001400004C.
REFERENCES
- 1.Poland GA, Jacobson RM, Targonski PV. 2007. Avian and pandemic influenza: an overview. Vaccine 25:3057–3061. doi: 10.1016/j.vaccine.2007.01.050. [DOI] [PubMed] [Google Scholar]
- 2.Reperant LA, Rimmelzwaan GF, Kuiken T. 2009. Avian influenza viruses in mammals. Rev Sci Tech 28:137–159. [DOI] [PubMed] [Google Scholar]
- 3.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. 2003. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 4.Elderfield R, Barclay W. 2011. Influenza pandemics. Adv Exp Med Biol 719:81–103. doi: 10.1007/978-1-4614-0204-6_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan Y, Vijaykrishna D, Bahl J, Zhu H, Wang J, Smith GJ. 2010. The emergence of pandemic influenza viruses. Protein Cell 1:9–13. doi: 10.1007/s13238-010-0008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E, Deyde V, Okomo-Adhiambo M, Gubareva L, Barnes J, Smith CB, Emery SL, Hillman MJ, Rivailler P, Smagala J, de Graaf M, Burke DF, Fouchier RA, Pappas C, Alpuche-Aranda CM, Lopez-Gatell H, Olivera H, Lopez I, Myers CA, Faix D, Blair PJ, Yu C, Keene KM, Dotson PD Jr, Boxrud D, Sambol AR, Abid SH, St George K, Bannerman T, Moore AL, Stringer DJ, Blevins P, Demmler-Harrison GJ, Ginsberg M, Kriner P, Waterman S, Smole S, Guevara HF, Belongia EA, Clark PA, Beatrice ST, Donis R, Katz J, Finelli L, Bridges CB, Shaw M, Jernigan DB, Uyeki TM, Smith DJ, Klimov AI, Cox NJ. 2009. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 325:197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howden KJ, Brockhoff EJ, Caya FD, McLeod LJ, Lavoie M, Ing JD, Bystrom JM, Alexandersen S, Pasick JM, Berhane Y, Morrison ME, Keenliside JM, Laurendeau S, Rohonczy EB. 2009. An investigation into human pandemic influenza virus (H1N1) 2009 on an Alberta swine farm. Can Vet J 50:1153–1161. [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson MI, Vincent AL, Kitikoon P, Holmes EC, Gramer MR. 2012. Evolution of novel reassortant A/H3N2 influenza viruses in North American swine and humans, 2009–2011. J Virol 86:8872–8878. doi: 10.1128/JVI.00259-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitikoon P, Nelson MI, Killian ML, Anderson TK, Koster L, Culhane MR, Vincent AL. 2013. Genotype patterns of contemporary reassorted H3N2 virus in U.S. swine. J Gen Virol 94:1236–1241. doi: 10.1099/vir.0.51839-0. [DOI] [PubMed] [Google Scholar]
- 10.Charoenvisal N, Keawcharoen J, Sreta D, Chaiyawong S, Nonthabenjawan N, Tantawet S, Jittimanee S, Arunorat J, Amonsin A, Thanawongnuwech R. 2013. Genetic characterization of Thai swine influenza viruses after the introduction of pandemic H1N1 2009. Virus Genes 47:75–85. doi: 10.1007/s11262-013-0927-x. [DOI] [PubMed] [Google Scholar]
- 11.Ali A, Khatri M, Wang L, Saif YM, Lee CW. 2012. Identification of swine H1N2/pandemic H1N1 reassortant influenza virus in pigs, United States. Vet Microbiol 158:60–68. doi: 10.1016/j.vetmic.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Poon LL, Mak PW, Li OT, Chan KH, Cheung CL, Ma ES, Yen HL, Vijaykrishna D, Guan Y, Peiris JS. 2010. Rapid detection of reassortment of pandemic H1N1/2009 influenza virus. Clin Chem 56:1340–1344. doi: 10.1373/clinchem.2010.149179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vijaykrishna D, Poon LL, Zhu HC, Ma SK, Li OT, Cheung CL, Smith GJ, Peiris JS, Guan Y. 2010. Reassortment of pandemic H1N1/2009 influenza A virus in swine. Science 328:1529. doi: 10.1126/science.1189132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harder TC, Grosse Beilage E, Lange E, Meiners C, Dohring S, Pesch S, Noe T, Grund C, Beer M, Starick E. 2013. Expanded cocirculation of stable subtypes, emerging lineages, and new sporadic reassortants of porcine influenza viruses in swine populations in northwest Germany. J Virol 87:10460–10476. doi: 10.1128/JVI.00381-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vincent A, Awada L, Brown I, Chen H, Claes F, Dauphin G, Donis R, Culhane M, Hamilton K, Lewis N, Mumford E, Nguyen T, Parchariyanon S, Pasick J, Pavade G, Pereda A, Peiris M, Saito T, Swenson S, Van Reeth K, Webby R, Wong F, Ciacci-Zanella J. 2014. Review of influenza A virus in swine worldwide: a call for increased surveillance and research. Zoonoses Public Health 61:4–17. doi: 10.1111/zph.12049. [DOI] [PubMed] [Google Scholar]
- 16.Zhou NN, Senne DA, Landgraf JS, Swenson SL, Erickson G, Rossow K, Liu L, Yoon K, Krauss S, Webster RG. 1999. Genetic reassortment of avian, swine, and human influenza A viruses in American pigs. J Virol 73:8851–8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma W, Lager KM, Lekcharoensuk P, Ulery ES, Janke BH, Solorzano A, Webby RJ, Garcia-Sastre A, Richt JA. 2010. Viral reassortment and transmission after co-infection of pigs with classical H1N1 and triple-reassortant H3N2 swine influenza viruses. J Gen Virol 91:2314–2321. doi: 10.1099/vir.0.021402-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vijaykrishna D, Smith GJ, Pybus OG, Zhu H, Bhatt S, Poon LL, Riley S, Bahl J, Ma SK, Cheung CL, Perera RA, Chen H, Shortridge KF, Webby RJ, Webster RG, Guan Y, Peiris JS. 2011. Long-term evolution and transmission dynamics of swine influenza A virus. Nature 473:519–522. doi: 10.1038/nature10004. [DOI] [PubMed] [Google Scholar]
- 19.Jhung MA, Epperson S, Biggerstaff M, Allen D, Balish A, Barnes N, Beaudoin A, Berman L, Bidol S, Blanton L, Blythe D, Brammer L, D'Mello T, Danila R, Davis W, de Fijter S, Diorio M, Durand LO, Emery S, Fowler B, Garten R, Grant Y, Greenbaum A, Gubareva L, Havers F, Haupt T, House J, Ibrahim S, Jiang V, Jain S, Jernigan D, Kazmierczak J, Klimov A, Lindstrom S, Longenberger A, Lucas P, Lynfield R, McMorrow M, Moll M, Morin C, Ostroff S, Page SL, Park SY, Peters S, Quinn C, Reed C, Richards S, Scheftel J, Simwale O, Shu B, Soyemi K, Stauffer J, Steffens C, Su S, Torso L, Uyeki TM, Vetter S, Villanueva J, Wong KK, Shaw M, Bresee JS, Cox N, Finelli L. 2013. Outbreak of variant influenza A(H3N2) virus in the United States. Clin Infect Dis 57:1703–1712. doi: 10.1093/cid/cit649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biggerstaff M, Reed C, Epperson S, Jhung MA, Gambhir M, Bresee JS, Jernigan DB, Swerdlow DL, Finelli L. 2013. Estimates of the number of human infections with influenza A(H3N2) variant virus, United States, August 2011-April 2012. Clin Infect Dis 57(Suppl 1):S12–S15. doi: 10.1093/cid/cit273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell PJ, Danzy S, Kyriakis CS, Deymier MJ, Lowen AC, Steel J. 2014. The M segment of the 2009 pandemic influenza virus confers increased neuraminidase activity, filamentous morphology, and efficient contact transmissibility to A/Puerto Rico/8/1934-based reassortant viruses. J Virol 88:3802–3814. doi: 10.1128/JVI.03607-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. 2014. CDC key points: first U.S. human infection with H3N2v virus in 2014. Centers for Disease Control and Prevention, Atlanta, GA: https://linksweb.oph.dhh.louisiana.gov/linksweb/docs/CDC%20Influenza%20Key%20Points%20Aug%2022%202014.doc. [Google Scholar]
- 23.Marc D. 2014. Influenza virus non-structural protein NS1: interferon antagonism and beyond. J Gen Virol 95:2594–2611. doi: 10.1099/vir.0.069542-0. [DOI] [PubMed] [Google Scholar]
- 24.Gabriel G, Fodor E. 2014. Molecular determinants of pathogenicity in the polymerase complex. Curr Top Microbiol Immunol 385:35–60. doi: 10.1007/82_2014_386. [DOI] [PubMed] [Google Scholar]
- 25.Gabriel G, Abram M, Keiner B, Wagner R, Klenk HD, Stech J. 2007. Differential polymerase activity in avian and mammalian cells determines host range of influenza virus. J Virol 81:9601–9604. doi: 10.1128/JVI.00666-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan S, Hatta M, Kim JH, Le MQ, Neumann G, Kawaoka Y. 2014. Amino acid changes in the influenza A virus PA protein that attenuate avian H5N1 viruses in mammals. J Virol 88:13737–13746. doi: 10.1128/JVI.01081-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheung PP, Watson SJ, Choy KT, Fun Sia S, Wong DD, Poon LL, Kellam P, Guan Y, Malik Peiris JS, Yen HL. 2014. Generation and characterization of influenza A viruses with altered polymerase fidelity. Nat Commun 5:4794. doi: 10.1038/ncomms5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei K, Sun H, Sun Z, Sun Y, Kong W, Pu J, Ma G, Yin Y, Yang H, Guo X, Chang KC, Liu J. 2014. Influenza A virus acquires enhanced pathogenicity and transmissibility after serial passages in swine. J Virol 88:11981–11994. doi: 10.1128/JVI.01679-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ducatez MF, Ilyushina NA, Fabrizio TP, Rehg JE, Bovin NV, Webster RG, Webby RJ. 2012. Both influenza hemagglutinin and polymerase acidic genes are important for delayed pandemic 2009 H1N1 virus clearance in the ferret model. Virology 432:389–393. doi: 10.1016/j.virol.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subbarao EK, London W, Murphy BR. 1993. A single amino acid in the PB2 gene of influenza A virus is a determinant of host range. J Virol 67:1761–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehle A, Dugan VG, Taubenberger JK, Doudna JA. 2012. Reassortment and mutation of the avian influenza virus polymerase PA subunit overcome species barriers. J Virol 86:1750–1757. doi: 10.1128/JVI.06203-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kashiwagi T, Leung BW, Deng T, Chen H, Brownlee GG. 2009. The N-terminal region of the PA subunit of the RNA polymerase of influenza A/HongKong/156/97 (H5N1) influences promoter binding. PLoS One 4:e5473. doi: 10.1371/journal.pone.0005473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakabe S, Takano R, Nagamura-Inoue T, Yamashita N, Nidom CA, Quynh Le M, Iwatsuki-Horimoto K, Kawaoka Y. 2013. Differences in cytokine production in human macrophages and in virulence in mice are attributable to the acidic polymerase protein of highly pathogenic influenza A virus subtype H5N1. J Infect Dis 207:262–271. doi: 10.1093/infdis/jis523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q, Zhang S, Jiang H, Wang J, Weng L, Mao Y, Sekiguchi S, Yasui F, Kohara M, Buchy P, Deubel V, Xu K, Sun B, Toyoda T. 2012. PA from an H5N1 highly pathogenic avian influenza virus activates viral transcription and replication and induces apoptosis and interferon expression at an early stage of infection. Virol J 9:106. doi: 10.1186/1743-422X-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fodor E, Mingay LJ, Crow M, Deng T, Brownlee GG. 2003. A single amino acid mutation in the PA subunit of the influenza virus RNA polymerase promotes the generation of defective interfering RNAs. J Virol 77:5017–5020. doi: 10.1128/JVI.77.8.5017-5020.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jagger BW, Wise HM, Kash JC, Walters KA, Wills NM, Xiao YL, Dunfee RL, Schwartzman LM, Ozinsky A, Bell GL, Dalton RM, Lo A, Efstathiou S, Atkins JF, Firth AE, Taubenberger JK, Digard P. 2012. An overlapping protein-coding region in influenza A virus segment 3 modulates the host response. Science 337:199–204. doi: 10.1126/science.1222213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Els MC, Laver WG, Air GM. 1989. Sialic acid is cleaved from glycoconjugates at the cell surface when influenza virus neuraminidases are expressed from recombinant vaccinia viruses. Virology 170:346–351. doi: 10.1016/0042-6822(89)90394-2. [DOI] [PubMed] [Google Scholar]
- 38.Cohen M, Zhang XQ, Senaati HP, Chen HW, Varki NM, Schooley RT, Gagneux P. 2013. Influenza A penetrates host mucus by cleaving sialic acids with neuraminidase. Virol J 10:321. doi: 10.1186/1743-422X-10-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang X, Steukers L, Forier K, Xiong R, Braeckmans K, Van Reeth K, Nauwynck H. 2014. A beneficiary role for neuraminidase in influenza virus penetration through the respiratory mucus. PLoS One 9:e110026. doi: 10.1371/journal.pone.0110026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mantovani A, Cassatella MA, Costantini C, Jaillon S. 2011. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 41.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 42.Matrosovich M, Matrosovich T, Garten W, Klenk HD. 2006. New low-viscosity overlay medium for viral plaque assays. Virol J 3:63. doi: 10.1186/1743-422X-3-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walker SE, Lorsch J. 2013. RNA purification-precipitation methods. Methods Enzymol 530:337–343. doi: 10.1016/B978-0-12-420037-1.00019-1. [DOI] [PubMed] [Google Scholar]
- 44.Dlugolenski D, Jones L, Tompkins SM, Crameri G, Wang LF, Tripp RA. 2013. Bat cells from Pteropus alecto are susceptible to influenza A virus infection and reassortment. Influenza Other Respir Viruses 7:900–903. doi: 10.1111/irv.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LaBarre DD, Lowy RJ. 2001. Improvements in methods for calculating virus titer estimates from TCID50 and plaque assays. J Virol Methods 96:107–126. doi: 10.1016/S0166-0934(01)00316-0. [DOI] [PubMed] [Google Scholar]
- 46.Henle W, Liu OC. 1951. Studies on host-virus interactions in the chick embryo-influenza virus system. VI. Evidence for multiplicity reactivation of inactivated virus. J Exp Med 94:305–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Powell JD, Dlugolenski D, Nagy T, Gabbard J, Lee C, Tompkins SM, Tripp RA. 2014. Polymerase discordance in novel swine influenza H3N2v constellations is tolerated in swine but not human respiratory epithelial cells. PLoS One 9:e110264. doi: 10.1371/journal.pone.0110264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Evans GO. 1994. Removal of blood from laboratory mammals and birds. Lab Anim 28:178–179. doi: 10.1258/002367794780745209. [DOI] [PubMed] [Google Scholar]
- 49.Frank AL, Puck J, Hughes BJ, Cate TR. 1980. Microneutralization test for influenza A and B and parainfluenza 1 and 2 viruses that uses continuous cell lines and fresh serum enhancement. J Clin Microbiol 12:426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou B, Donnelly ME, Scholes DT, St George K, Hatta M, Kawaoka Y, Wentworth DE. 2009. Single-reaction genomic amplification accelerates sequencing and vaccine production for classical and swine origin human influenza a viruses. J Virol 83:10309–10313. doi: 10.1128/JVI.01109-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou B, Li Y, Belser JA, Pearce MB, Schmolke M, Subba AX, Shi Z, Zaki SR, Blau DM, Garcia-Sastre A, Tumpey TM, Wentworth DE. 2010. NS-based live attenuated H1N1 pandemic vaccines protect mice and ferrets. Vaccine 28:8015–8025. doi: 10.1016/j.vaccine.2010.08.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ito T, Couceiro JN, Kelm S, Baum LG, Krauss S, Castrucci MR, Donatelli I, Kida H, Paulson JC, Webster RG, Kawaoka Y. 1998. Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J Virol 72:7367–7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lipatov AS, Kwon YK, Sarmento LV, Lager KM, Spackman E, Suarez DL, Swayne DE. 2008. Domestic pigs have low susceptibility to H5N1 highly pathogenic avian influenza viruses. PLoS Pathog 4:e1000102. doi: 10.1371/journal.ppat.1000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou P, Hong M, Merrill MM, He H, Sun L, Zhang G. 2014. Serological report of influenza A (H7N9) infections among pigs in Southern China. BMC Vet Res 10:203. doi: 10.1186/s12917-014-0203-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nelli RK, Kuchipudi SV, White GA, Perez BB, Dunham SP, Chang KC. 2010. Comparative distribution of human and avian type sialic acid influenza receptors in the pig. BMC Vet Res 6:4. doi: 10.1186/1746-6148-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Poucke SG, Nicholls JM, Nauwynck HJ, Van Reeth K. 2010. Replication of avian, human and swine influenza viruses in porcine respiratory explants and association with sialic acid distribution. Virol J 7:38. doi: 10.1186/1743-422X-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scholtissek C. 1995. Molecular evolution of influenza viruses. Virus Genes 11:209–215. doi: 10.1007/BF01728660. [DOI] [PubMed] [Google Scholar]
- 58.Oxford JS, Callow KA, Corcoran T, Beare AS. 1982. Plaquing characteristics of influenza A virus recombinants of defined genetic composition. Arch Virol 74:227–232. doi: 10.1007/BF01314716. [DOI] [PubMed] [Google Scholar]
- 59.Spensieri F, Borgogni E, Zedda L, Bardelli M, Buricchi F, Volpini G, Fragapane E, Tavarini S, Finco O, Rappuoli R, Del Giudice G, Galli G, Castellino F. 2013. Human circulating influenza-CD4+ ICOS1+IL-21+ T cells expand after vaccination, exert helper function, and predict antibody responses. Proc Natl Acad Sci U S A 110:14330–14335. doi: 10.1073/pnas.1311998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sladkova T, Kostolansky F. 2006. The role of cytokines in the immune response to influenza A virus infection. Acta Virol 50:151–162. [PubMed] [Google Scholar]
- 61.Sorrell EM, Schrauwen EJ, Linster M, De Graaf M, Herfst S, Fouchier RA. 2011. Predicting ‘airborne’ influenza viruses: (trans-) mission impossible? Curr Opin Virol 1:635–642. doi: 10.1016/j.coviro.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Costantini C, Cassatella MA. 2011. The defensive alliance between neutrophils and NK cells as a novel arm of innate immunity. J Leukoc Biol 89:221–233. doi: 10.1189/jlb.0510250. [DOI] [PubMed] [Google Scholar]
- 63.Miyazaki S, Ishikawa F, Shimizu K, Ubagai T, Edelstein PH, Yamaguchi K. 2007. Gr-1high polymorphonuclear leukocytes and NK cells act via IL-15 to clear intracellular Haemophilus influenzae in experimental murine peritonitis and pneumonia. J Immunol 179:5407–5414. doi: 10.4049/jimmunol.179.8.5407. [DOI] [PubMed] [Google Scholar]
- 64.Verbist KC, Klonowski KD. 2012. Functions of IL-15 in anti-viral immunity: multiplicity and variety. Cytokine 59:467–478. doi: 10.1016/j.cyto.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verbist KC, Rose DL, Cole CJ, Field MB, Klonowski KD. 2012. IL-15 participates in the respiratory innate immune response to influenza virus infection. PLoS One 7:e37539. doi: 10.1371/journal.pone.0037539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, Davis DM, Strominger JL, Yewdell JW, Porgador A. 2001. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature 409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 67.Kreijtz JH, Fouchier RA, Rimmelzwaan GF. 2011. Immune responses to influenza virus infection. Virus Res 162:19–30. doi: 10.1016/j.virusres.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 68.Ge MQ, Ho AW, Tang Y, Wong KH, Chua BY, Gasser S, Kemeny DM. 2012. NK cells regulate CD8+ T cell priming and dendritic cell migration during influenza A infection by IFN-gamma and perforin-dependent mechanisms. J Immunol 189:2099–2109. doi: 10.4049/jimmunol.1103474. [DOI] [PubMed] [Google Scholar]
- 69.Wang JP, Bowen GN, Padden C, Cerny A, Finberg RW, Newburger PE, Kurt-Jones EA. 2008. Toll-like receptor-mediated activation of neutrophils by influenza A virus. Blood 112:2028–2034. doi: 10.1182/blood-2008-01-132860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yewdell JW, Ince WL. 2012. Virology frameshifting to PA-X influenza. Science 337:164–165. doi: 10.1126/science.1225539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shi M, Jagger BW, Wise HM, Digard P, Holmes EC, Taubenberger JK. 2012. Evolutionary conservation of the PA-X open reading frame in segment 3 of influenza A virus. J Virol 86:12411–12413. doi: 10.1128/JVI.01677-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bradley KC, Jones CA, Tompkins SM, Tripp RA, Russell RJ, Gramer MR, Heimburg-Molinaro J, Smith DF, Cummings RD, Steinhauer DA. 2011. Comparison of the receptor binding properties of contemporary swine isolates and early human pandemic H1N1 isolates (novel 2009 H1N1). Virology 413:169–182. doi: 10.1016/j.virol.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 73.Munster VJ, de Wit E, van den Brand JM, Herfst S, Schrauwen EJ, Bestebroer TM, van de Vijver D, Boucher CA, Koopmans M, Rimmelzwaan GF, Kuiken T, Osterhaus AD, Fouchier RA. 2009. Pathogenesis and transmission of swine-origin 2009 A(H1N1) influenza virus in ferrets. Science 325:481–483. doi: 10.1126/science.1177127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weiland JE, Davis WB, Holter JF, Mohammed JR, Dorinsky PM, Gadek JE. 1986. Lung neutrophils in the adult respiratory distress syndrome. Clinical and pathophysiologic significance. Am Rev Respir Dis 133:218–225. [DOI] [PubMed] [Google Scholar]
- 75.Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA, Phoon MC, van Rooijen N, Chow VT. 2011. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol 179:199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tate MD, Ioannidis LJ, Croker B, Brown LE, Brooks AG, Reading PC. 2011. The role of neutrophils during mild and severe influenza virus infections of mice. PLoS One 6:e17618. doi: 10.1371/journal.pone.0017618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Short KR, Kroeze EJ, Fouchier RA, Kuiken T. 2014. Pathogenesis of influenza-induced acute respiratory distress syndrome. Lancet Infect Dis 14:57–69. doi: 10.1016/S1473-3099(13)70286-X. [DOI] [PubMed] [Google Scholar]
- 78.Chou RC, Kim ND, Sadik CD, Seung E, Lan Y, Byrne MH, Haribabu B, Iwakura Y, Luster AD. 2010. Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a murine model of inflammatory arthritis. Immunity 33:266–278. doi: 10.1016/j.immuni.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. 2010. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 80.Jaeger BN, Donadieu J, Cognet C, Bernat C, Ordonez-Rueda D, Barlogis V, Mahlaoui N, Fenis A, Narni-Mancinelli E, Beaupain B, Bellanne-Chantelot C, Bajenoff M, Malissen B, Malissen M, Vivier E, Ugolini S. 2012. Neutrophil depletion impairs natural killer cell maturation, function, and homeostasis. J Exp Med 209:565–580. doi: 10.1084/jem.20111908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schultz-Cherry S. 2015. Role of NK cells in influenza infection. Curr Top Microbiol Immunol 386:109–120. doi: 10.1007/82_2014_403. [DOI] [PubMed] [Google Scholar]
- 82.Zhou G, Juang SW, Kane KP. 2013. NK cells exacerbate the pathology of influenza virus infection in mice. Eur J Immunol 43:929–938. doi: 10.1002/eji.201242620. [DOI] [PubMed] [Google Scholar]
- 83.Abdul-Careem MF, Mian MF, Yue G, Gillgrass A, Chenoweth MJ, Barra NG, Chew MV, Chan T, Al-Garawi AA, Jordana M, Ashkar AA. 2012. Critical role of natural killer cells in lung immunopathology during influenza infection in mice. J Infect Dis 206:167–177. doi: 10.1093/infdis/jis340. [DOI] [PubMed] [Google Scholar]
- 84.Nabel GJ, Wei CJ, Ledgerwood JE. 2011. Vaccinate for the next H2N2 pandemic now. Nature 471:157–158. doi: 10.1038/471157a. [DOI] [PubMed] [Google Scholar]
- 85.Jones JC, Baranovich T, Marathe BM, Danner AF, Seiler JP, Franks J, Govorkova EA, Krauss S, Webster RG. 2014. Risk assessment of H2N2 influenza viruses from the avian reservoir. J Virol 88:1175–1188. doi: 10.1128/JVI.02526-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pappas C, Yang H, Carney PJ, Pearce MB, Katz JM, Stevens J, Tumpey TM. 2015. Assessment of transmission, pathogenesis and adaptation of H2 subtype influenza viruses in ferrets. Virology 477C:61–71. doi: 10.1016/j.virol.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Richt JA, Rockx B, Ma W, Feldmann F, Safronetz D, Marzi A, Kobasa D, Strong JE, Kercher L, Long D, Gardner D, Brining D, Feldmann H. 2012. Recently emerged swine influenza A virus (H2N3) causes severe pneumonia in Cynomolgus macaques. PLoS One 7:e39990. doi: 10.1371/journal.pone.0039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Scholtissek C, Rohde W, Von Hoyningen V, Rott R. 1978. On the origin of the human influenza virus subtypes H2N2 and H3N2. Virology 87:13–20. doi: 10.1016/0042-6822(78)90153-8. [DOI] [PubMed] [Google Scholar]