ABSTRACT
Hydrogen sulfide (H2S) is an endogenous gaseous mediator that has gained increasing recognition as an important player in modulating acute and chronic inflammatory diseases. However, its role in virus-induced lung inflammation is currently unknown. Respiratory syncytial virus (RSV) is a major cause of upper and lower respiratory tract infections in children for which no vaccine or effective treatment is available. Using the slow-releasing H2S donor GYY4137 and propargylglycin (PAG), an inhibitor of cystathionine-γ-lyase (CSE), a key enzyme that produces intracellular H2S, we found that RSV infection led to a reduced ability to generate and maintain intracellular H2S levels in airway epithelial cells (AECs). Inhibition of CSE with PAG resulted in increased viral replication and chemokine secretion. On the other hand, treatment of AECs with the H2S donor GYY4137 reduced proinflammatory mediator production and significantly reduced viral replication, even when administered several hours after viral absorption. GYY4137 also significantly reduced replication and inflammatory chemokine production induced by human metapneumovirus (hMPV) and Nipah virus (NiV), suggesting a broad inhibitory effect of H2S on paramyxovirus infections. GYY4137 treatment had no effect on RSV genome replication or viral mRNA and protein synthesis, but it inhibited syncytium formation and virus assembly/release. GYY4137 inhibition of proinflammatory gene expression occurred by modulation of the activation of the key transcription factors nuclear factor κB (NF-κB) and interferon regulatory factor 3 (IRF-3) at a step subsequent to their nuclear translocation. H2S antiviral and immunoregulatory properties could represent a novel treatment strategy for paramyxovirus infections.
IMPORTANCE RSV is a global health concern, causing significant morbidity and economic losses as well as mortality in developing countries. After decades of intensive research, no vaccine or effective treatment, with the exception of immunoprophylaxis, is available for this infection as well as for other important respiratory mucosal viruses. This study identifies hydrogen sulfide as a novel cellular mediator that can modulate viral replication and proinflammatory gene expression, both important determinants of lung injury in respiratory viral infections, with potential for rapid translation of such findings into novel therapeutic approaches for viral bronchiolitis and pneumonia.
INTRODUCTION
Hydrogen sulfide (H2S) is an endogenous gaseous transmitter that participates in the regulation of the respiratory system's physiological functions and pathophysiological alterations, including chronic obstructive pulmonary disease (COPD), asthma, pulmonary fibrosis, and hypoxia-induced pulmonary hypertension, as it regulates lung functions such as airway constriction, pulmonary circulation, cell proliferation/apoptosis, fibrosis, oxidative stress, and inflammation (reviewed in reference 1). H2S is produced endogenously in mammals, including humans, by three enzymes: cystathionine-γ-lyase (CSE), cystathionine-β-synthase (CBS), and 3-mercaptopyruvate sulfurtransferase (MST) (2–4). Sulfide salts such as sodium hydrosulfide (NaHS) and sodium sulfide (Na2S) have been widely used to study the biological effects of hydrogen sulfide in many cells, tissues, and animals. These salts generate a large burst of H2S over a short time period, when used in cell culture. GYY4137 is a novel water-soluble H2S donor that releases H2S slowly over a period of hours (5). H2S donors have been used to demonstrate how therapeutic H2S administration exerts significant effects on various animal models of inflammation, reperfusion injury, and circulatory shock (6). There are no studies investigating the role of H2S generation in pathophysiology of viral infections or the use of H2S donors as a pharmacological intervention for virus-induced diseases.
Respiratory tract infections are a leading cause of morbidity and mortality worldwide. Paramyxoviruses, which include respiratory syncytial virus (RSV) and human metapneumovirus (hMPV), represent a major cause of pediatric upper and lower respiratory tract infections (7, 8). These viruses are associated with bronchiolitis, pneumonia, flu-like syndromes, as well as asthma exacerbations and represent a substantial public health problem for the community. Nipah virus (NiV) is a zoonotic emerging pathogen that also belongs to the Paramyxoviridae family and can cause severe and often fatal respiratory disease and/or encephalitis in humans (9). No vaccine or effective treatment is available for RSV, hMPV, or NiV, with the exception of immunoprophylaxis for RSV. Our previous studies have shown that these viruses induce the expression of a variety of proinflammatory genes, including cytokines and chemokines, in airway epithelial cells (AECs), the main target of infection (10–12), which are likely to play a major role in disease pathogenesis. Cytokine and chemokine gene expression in virus-infected cells is orchestrated by the activation of two key transcription factors, nuclear factor κB (NF-κB) and interferon regulatory factor 3 (IRF-3). A number of virus-inducible inflammatory and immunoregulatory genes require NF-κB for their transcription and/or are dependent on an intact NF-κB signaling pathway (13, 14), and IRF-3 is necessary for viral induction of RANTES transcription and gene expression (15, 16).
To address the role of H2S generation/administration in viral infections, we used an in vitro model of RSV infection of AECs. We found that RSV infection led to decreased expression of CSE, a reduced ability to generate cellular H2S, as well as increased H2S degradation. Inhibition of H2S generation by using PAG was associated with increased generation of virus infectious particles as well as increased proinflammatory mediator secretion, suggesting an important role of endogenous H2S in controlling viral replication and proinflammatory gene expression. GYY4137 treatment of both A549 (a lung carcinoma cell line retaining features of type II alveolar epithelial cells) and primary small alveolar epithelial (SAE) cells significantly reduced virus-induced proinflammatory mediator release, and it significantly inhibited viral replication at a step subsequent to viral adsorption. GYY4137 administration blocked RSV replication without significantly reducing viral mRNA synthesis, viral genome replication, and viral protein synthesis, indicating that it affects steps involved in viral assembly and/or release. It also resulted in significant inhibition of syncytium formation, indicating a modulatory effect on virus-induced cellular fusion.
GYY4137 treatment of AECs infected with RSV did not affect the initial step of virus-induced activation of IRF-3 and NF-κB, as shown by the lack of changes in their nuclear translocation; however, it significantly reduced IRF-3 and NF-κB binding to the endogenous promoter of proinflammatory genes, resulting in an inhibition of chemokine gene transcription, indicating an important effect of H2S on cellular signaling, independent of its antiviral activity.
MATERIALS AND METHODS
Materials.
GYY4137 [morpholin-4-ium-4-methoxyphenyl(morpholino)phosphinodithioate], a novel water-soluble, slow-releasing H2S compound, and dl-propargylglycin (PAG), an inhibitor of the H2S-generating enzyme cystathionine-γ-lyase (CSE), were purchased from Sigma-Aldrich (St. Louis, MO, USA). Solutions were prepared freshly in culture medium and filtered through a 0.2-μm filter before treatment. Sulfidefluor-7-acetoxymethyl ester (SF7-AM), a fluorescent probe that allows direct, real-time visualization of endogenous H2S produced in live human cells (17), was generously provided by Christopher J. Chang (Department of Chemistry, University of California, Berkeley). An SF7-AM stock solution was prepared in dimethyl sulfoxide (DMSO) and diluted in serum-free medium at least a thousandfold.
Virus preparation.
The RSV Long strain was grown in HEp-2 cells and purified by centrifugation on discontinuous sucrose gradients, as described previously (18, 19), and titers of viral pools in PFU/ml were determined by using a methylcellulose plaque assay, as described previously (20). No contaminating cytokines or lipopolysaccharide (LPS), tested by the Limulus hemocyanin agglutination assay, was found in these virus preparations. Virus pools were aliquoted, quick-frozen on dry ice-alcohol, and stored at −80°C until use.
hMPV strain CAN97-83 was obtained from the Centers for Disease Control and Prevention (CDC), Atlanta, GA, with permission from Guy Boivin at the Research Center in Infectious Diseases, Regional Virology Laboratory, Laval University, Quebec City, Canada; propagated on LLC-MK2 cells; and purified on sucrose cushions, as previously described (21). Titers of virus pools in PFU/ml were determined by immunostaining, as previously described (21).
The Nipah virus Bangladesh strain (NiV-B) was obtained from the Special Pathogens Branch of the Centers for Disease Control and Prevention (Atlanta, GA). The virus was propagated on Vero cells, as previously described (10). Titers of virus pools were determined by a 50% tissue culture infective dose (TCID50) assay, as previously described (10). All infectious work with NiV was performed in a class II biological safety cabinet in a biosafety level 4 (BSL4) laboratory at the Galveston National Laboratory.
Cell culture and viral infection.
A549 cells, a human alveolar type II-like epithelial cell line (American Type Culture Collection, Manassas, VA), and small alveolar epithelial (SAE) cells (Clonetics, San Diego, CA), derived from terminal bronchioli of cadaveric donors, were grown in F12K medium and SAE cell growth medium, respectively, containing 10% (vol/vol) fetal bovine serum (FBS), 10 mM glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin for F12K medium and 7.5 mg/ml bovine pituitary extract (BPE), 0.5 mg/ml hydrocortisone, 0.5 μg/ml human epidermal growth factor (hEGF), 0.5 mg/ml epinephrine, 10 mg/ml transferrin, 5 mg/ml insulin, 0.1 μg/ml retinoic acid, 0.5 μg/ml triiodothyronine, 50 mg/ml gentamicin, and 50 mg/ml bovine serum albumin (BSA) for SAE cell medium. When SAE cells were used for RSV infection, they were changed to basal medium, not supplemented with growth factors, 6 h prior to and throughout the experiment. Confluent cell monolayers were infected with RSV or hMPV at multiplicity of infection (MOI) of 1, as previously described (22), unless otherwise stated. NiV infection was performed at an MOI of 0.01 (10). For PAG experiments, cells were seeded into 6-well or 24-well plates, infected with RSV for 1 h at 37°C in 5% CO2, and then treated with PAG after the viral inoculum was removed. For GYY4137 experiments, cells were seeded into 6-well or 24-well plates and treated either prior to infection, but not throughout the duration of infection, or at different times postinfection (p.i.), after the viral inoculum was removed. There was no effect of either compound on uninfected-cell viability, as assessed by trypan blue exclusion, or on basal cellular mediator secretion.
Methylene blue assay.
H2S production was measured by use of a colorimetric methylene blue assay, as previously described (23). Briefly, cells were homogenized, incubated at 37°C for 5 min, and then cooled on ice for 10 min. l-Cysteine (1 and 3 mmol/liter) and pyridoxal 5-phosphate (2 mmol/liter) were added and incubated for 1 h at 37°C. Zinc acetate (1%) and 10% trichloroacetic acid solutions were used to terminate the reaction. After the addition of N,N-dimethylphenylendiamine sulfate and FeCl3 for 15 min, the optical absorbance of the solutions was measured at 650 nm.
SF7-AM fluorescence assay.
A549 cells were grown in eight-well Lab-Tek II glass chamber slides (Thermo Scientific, Pittsburgh, PA, USA) and incubated with 5 μM SF7-AM probe at 37°C for 30 min. After washing with culture medium, A549 cells were infected with RSV and treated with GYY4137, as described above. Confocal fluorescence imaging studies were performed with a Zeiss 710 laser scanning microscope with a 20× water objective lens, with Zen 2009 software (Carl Zeiss). SF7-AM was excited by using a 488-nm argon laser, and emission was collected by using a Meta detector at wavelengths of between 500 and 650 nm. Cells were imaged at 37°C with 5% CO2 throughout the experiment. Image analysis was performed by using Metamorph software (Carl Zeiss), and fluorescence was quantified by using the mean pixel intensity after setting a common threshold for all images.
Luciferase assay.
A549 cells were transiently transfected by using a NF-κB- or interferon-stimulated responsive element (ISRE)-driven luciferase reporter plasmid containing five repeats of the NF-κB site of the IgG promoter or three repeats of the RANTES ISRE promoter, respectively, linked to the luciferase reporter gene, using Fugene 6 (Roche Diagnostic Corp., Indianapolis, IN), as previously described (16, 24). A total of 0.5 μg of the reporter gene plasmid and 0.05 μg of β-galactosidase expression plasmid/well were premixed with Fugene 6 and added to the cells in regular medium. The next day, cells were infected with RSV for 1 h, followed by treatment with GYY4137, and harvested at either 15 or 24 h p.i. to independently measure luciferase and β-galactosidase reporter activities, as previously described (24). Luciferase activity was normalized to the activity of the internal control β-galactosidase. Results are expressed in arbitrary units.
Determination of lactate dehydrogenase activity.
Lactate dehydrogenase (LDH) activity in the medium, an index of cellular damage, was measured by a colorimetric assay using a commercially available kit (Cayman Chemical, MI, USA) according to the manufacturer's instructions.
Quantitative real-time PCR.
Total RNA was extracted by using a ToTALLY RNA kit (catalog number AM1910; Ambion, Austin, TX). RNA samples were quantified by using a NanoDrop spectrophotometer (Thermo Fisher Scientific Inc., Wilmington, DE), and quality was analyzed on an RNA Nano or Pico chip by using the Agilent 2100 bioanalyzer (Agilent Technologies). Synthesis of cDNA was performed with 1 μg of total RNA in a 20-μl reaction mixture by using the TaqMan Reverse Transcription Reagents kit from ABI (catalog number N8080234; Applied Biosystems). The reaction conditions were as follows: 25°C for 10 min, 48°C for 30 min, and 95°C for 5 min. Quantitative real-time PCR amplification (performed in triplicate) was done with 1 μl of cDNA in a total volume of 25 μl by using Faststart Universal SYBR green master mix (catalog number 04913850001; Roche Applied Science). The final concentration of the primers was 300 nM. 18S RNA was used as a housekeeping gene for normalization. PCR assays were run with the ABI Prism 7500 sequence detection system with the following conditions: 50°C for 2 min, 95°C for 10 min, and then 95°C for 15 s and 60°C for 1 min for 40 cycles. The RSV N-specific reverse transcriptase (RT) primer contained a tag sequence from the bacterial chloramphenicol resistance (Cmr) gene to generate the cDNA, because of self-priming exhibited by RSV RNA. Duplicate cycle threshold (CT) values were analyzed in Microsoft Excel by the comparative CT (ΔΔCT) method according to the manufacturer's instructions (Applied Biosystems). The amount of target (2−ΔΔCT) was obtained by normalization to the endogenous reference (18S) sample. To detect RSV N transcripts, we used RT primer 5′-CTGCGATGAGTGGCAGGCTTTTTTTTTTTTAACTCAAAGCTC-3′; the tag is underlined. For PCR assays, we used RSV tag reverse primer CTGCGATGAGTGGCAGGC and forward primer ACTACAGTGTATTAGACTTRACAGCAGAAG. To detect the genome minus strand, we used RSV N RT primer 5′-CTGCGATGAGTGGCAGGCACTACAGTGTATTAGACTTRACAGCAGAAG-3′. For PCR assays, we used RSV tag primer CTGCGATGAGTGGCAGGC and primer RSV P GCATCTTCTCCATGRAATTCAGG.
Western blotting.
Nuclear extracts of uninfected and infected cells were prepared by using hypotonic/nonionic detergent lysis, according to a protocol described previously by Schreiber et al. (25). To prevent contamination with cytoplasmic proteins, isolated nuclei were purified by centrifugation through 1.7 M sucrose buffer for 30 min at 12,000 rpm, before nuclear protein extraction, as previously described (26). Total cell lysates were prepared from uninfected and infected A549 cells by the addition of ice-cold lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EGTA, 0.25% sodium deoxycholate, 1 mM Na3VO4, 1 mM NaF, 1% Triton X-100, and 1 μg/ml of aprotinin, leupeptin, and pepstatin). After incubation on ice for 10 min, the lysates were collected, and detergent-insoluble materials were removed by centrifugation at 4°C at 14,000 × g. Proteins (10 to 20 μg per sample) were then boiled in 2× Laemmli buffer and resolved on SDS-PAGE gels. Proteins were transferred onto a Hybond polyvinylidene difluoride membrane (Amersham, Piscataway, NJ), and nonspecific binding sites were blocked by immersing the membrane in Tris-buffered saline–Tween (TBST) containing 5% skim milk powder or 5% bovine serum albumin for 30 min. After a short wash in TBST, membranes were incubated with the primary antibody for 1 h at room temperature or overnight at 4°C, depending on the antibody used, followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibody (Sigma, St. Louis, MO), diluted 1:10,000 in TBST, for 30 min at room temperature. After washing, proteins were detected by using an enhanced chemiluminescence system (RPN 2016; Amersham, GE Healthcare, United Kingdom) and visualized by autoradiography. Antibodies used for Western blot assays were goat anti-RSV polyclonal antibody from Ab D SeroTec; rabbit anti-p65, anti-Ser536, or anti-Ser276 p65 from Cell Signaling Technology Inc., Danvers, MA; and rabbit anti-IRF-3 from Santa Cruz Biotechnology, Santa Cruz, CA.
Bio-Plex assay.
Cell-free supernatants were tested for multiple cytokines and chemokines by using the Bio-Plex Cytokine Human multiplex panel (Bio-Rad Laboratories, Hercules, CA), according to the manufacturer's instructions. Interleukin-8 (IL-8) and RANTES were also quantified by an enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's protocol (DuoSet; R&D Systems, Minneapolis, MN). Prior to analysis, NiV samples were inactivated on dry ice by gamma radiation (5 megarads).
Chromatin immunoprecipitation and quantitative genomic PCR.
For chromatin immunoprecipitation (ChIP) assays, we used a ChIP-It express kit from Active Motif (Carlsbad, CA) according to the manufacturer's instructions, with some modifications. Briefly, A549 cells in a 10-cm plate were washed three times with phosphate-buffered saline (PBS) and fixed with freshly prepared 2 mM disuccinimidyl glutarate (DSG) for 45 min at room temperature. After three washes with PBS, cells were fixed with freshly prepared formaldehyde for 10 min and neutralized with glycine for 5 min at room temperature. Cells were harvested and disrupted by using a Dounce homogenizer to isolate nuclei. Nuclei were sheared by sonication to obtain DNA fragments of 200 to 1,500 bp. Twenty micrograms of sheared chromatin was immunoprecipitated with 5 μg of ChIP-grade anti-NF-κB (catalog number sc-722X) or anti-IRF-3 (catalog number sc-369X) antibodies from Santa Cruz Biotechnology and magnetic beads conjugated with protein G at 4°C overnight. Immunoprecipitation with IgG antibody was used as a negative control. Chromatin was reverse cross-linked, eluted from magnetic beads, and purified by using a Qiagen PCR purification kit (Qiagen, USA). Quantitative genomic PCR (Q-gPCR) was done by SYBR green-based real-time PCR using primers spanning the IL-8 gene NF-κB promoter site (forward primer AGGTTTGCCCTGAGGGGATG and reverse primer GGAGTGCTCCGGTGGCTTTT) or primers spanning the RANTES gene ISRE promoter site (forward primer AGCGGCTTCCTGCTCTCTGA and reverse primer CAGCTCAGGCTGGCCCTTTA). Total input chromatin DNA for immunoprecipitation was included as a positive control for PCR amplification.
Statistical analysis.
Statistical analyses were performed with the InStat 3.05 Biostatistics package from GraphPad, San Diego, CA. To ascertain differences between two groups, Student's t test was used, and if more than two groups were compared, one-way analysis of variance was performed, followed by Tukey's post hoc test. P values of <0.05 were considered statistically significant. When indicated, values of measurements are expressed as means ± standard errors of the means (SEM) in the figures.
RESULTS
RSV infection affects H2S generation in airway epithelial cells.
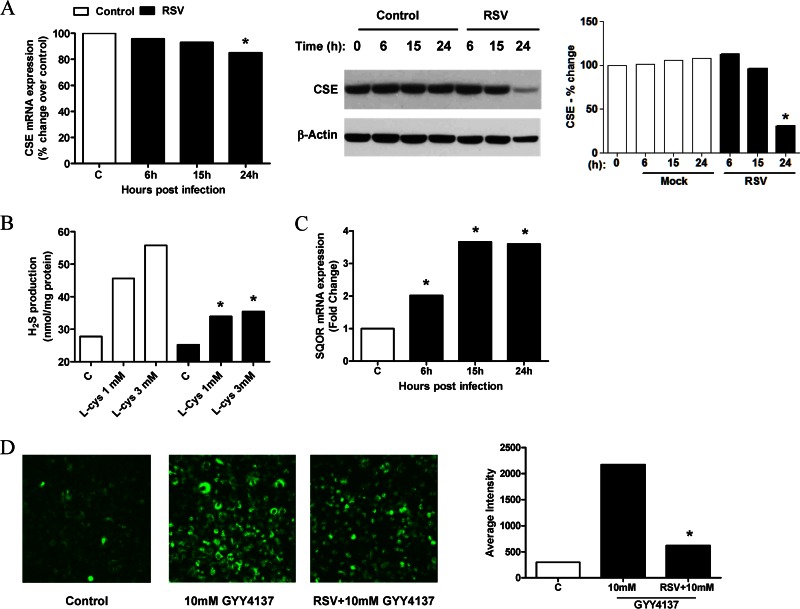
Of the three H2S-generating enzymes CSE, CBS, and MST, CSE represents the major source of H2S in lung tissue, and it uses cysteine as the main substrate. Sulfide:quinone oxidoreductase (SQOR) is a membrane-bound enzyme that catalyzes the first step in the mitochondrial metabolism of H2S (27). To determine whether RSV induced changes in H2S-generating and -metabolizing enzymes in AECs, A549 cells were infected for 6, 15, and 24 h and harvested for extraction of total RNA and measurement of CSE, CBS, and SQOR mRNA levels by real-time PCR. We found that CSE mRNA and protein expression levels were decreased by RSV infection only at later time points (Fig. 1A), while there was no significant change in the CBS mRNA level (data not shown). On the other hand, there was a significant time-dependent increase in the SQOR mRNA expression level in RSV-infected cells compared to uninfected cells (Fig. 1C). To investigate whether RSV modulated the capacity of airway epithelial cells to generate H2S, A549 cells were infected for 15 h and harvested to prepare total cell lysates. H2S production was then measured by a methylene blue assay. There was a significant reduction in H2S generation in RSV-infected cells, compared to uninfected cells, when cysteine was supplied at 1 and 3 mM concentrations as the CSE substrate (Fig. 1B). When A549 cells were treated with the slow-releasing H2S donor GYY4137, there was a significant increase in the intracellular level of H2S detected by the fluorescent probe SF7-AM, which was significantly lower in infected cells, suggesting an increase in H2S degradation following RSV infection (Fig. 1D).
FIG 1.
Effect of RSV infection on H2S production in airway epithelial cells. A549 cells were infected with RSV for 6, 15, and 24 h and harvested to prepare total RNA or total cell lysates. (A and C) CSE (A, left) and SQOR (C) mRNA levels in uninfected and RSV-infected cells were measured by reverse transcriptase quantitative PCR (qRT-PCR). CSE cellular levels were also measured by Western blotting of total cell lysates. The membrane was stripped and reprobed for β-actin to determine equal loading of the samples (A, middle). Densitometric analysis of CSE band intensity, normalized to β-actin, was performed by using Alpha Ease software version 2200 (2.2d) (Alpha Innotech Co., San Leandro, CA) (A, right). Results are representative of data from three independent experiments. *, P < 0.05 compared to uninfected cells. (B) A549 cells were infected with RSV for 15 h and harvested to prepare total cell lysates. H2S production in uninfected and RSV-infected cells was determined by a methylene blue colorimetric assay. Results are representative of data from three independent experiments. *, P < 0.05 compared to uninfected cells. (D) A549 cells were incubated with 5 μM the fluorescent probe SF7-AM and infected with RSV for 1 h. Medium or 10 mM GYY4137 was added to uninfected or infected cells and incubated for 15 h. (Left) Images of uninfected and untreated cells (control) and uninfected or infected cells treated with 10 mM GYY4137. (Right) Average fluorescence intensity quantified by confocal microscopy using Zeiss Metamorph software. Results are representative of data from three independent experiments. *, P < 0.05 compared to uninfected, treated cells.
CSE inhibition enhances RSV-induced chemokine production and viral replication.
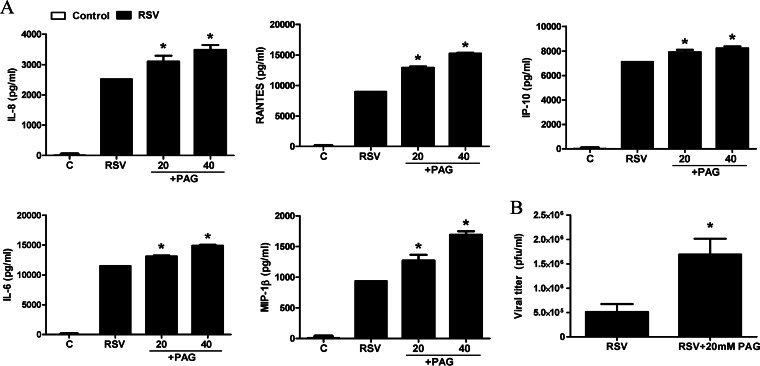
To examine the effect of CSE inhibition on virus-induced cellular responses, A549 cells were infected with RSV for 1 h and then treated with different concentrations of dl-propargylglycin (PAG). Cell supernatants were harvested at 24 h p.i. to measure virus-induced chemokine secretion. PAG administration significantly increased the levels of production of several cytokines and chemokines in response to RSV infection in a dose-dependent manner (Fig. 2A). PAG treatment of A549 cells also resulted in a significant increase in viral infectious-particle formation (3- to 4-fold increase), assessed by a plaque assay (Fig. 2B), indicating a role of endogenous H2S production in viral replication and proinflammatory cellular responses.
FIG 2.
Effect of CSE inhibition on RSV-induced cytokine and chemokine production and viral replication. A549 cells were infected with RSV for 1 h and then incubated in the presence or absence of 20 or 40 mM PAG. (A) Cell supernatants from uninfected and RSV-infected cells, treated or untreated, were assayed at 24 h p.i. for cytokine and chemokine secretion by a Bio-Plex assay. Results are expressed as means ± standard errors. Results are representative of data from three independent experiments run in triplicate. (B) Cells were treated as described above for panel A and harvested at 24 h p.i. to determine viral titers by a plaque assay. *, P < 0.05 compared to untreated RSV-infected cells.
Effect of H2S treatment on RSV-induced proinflammatory mediator production.
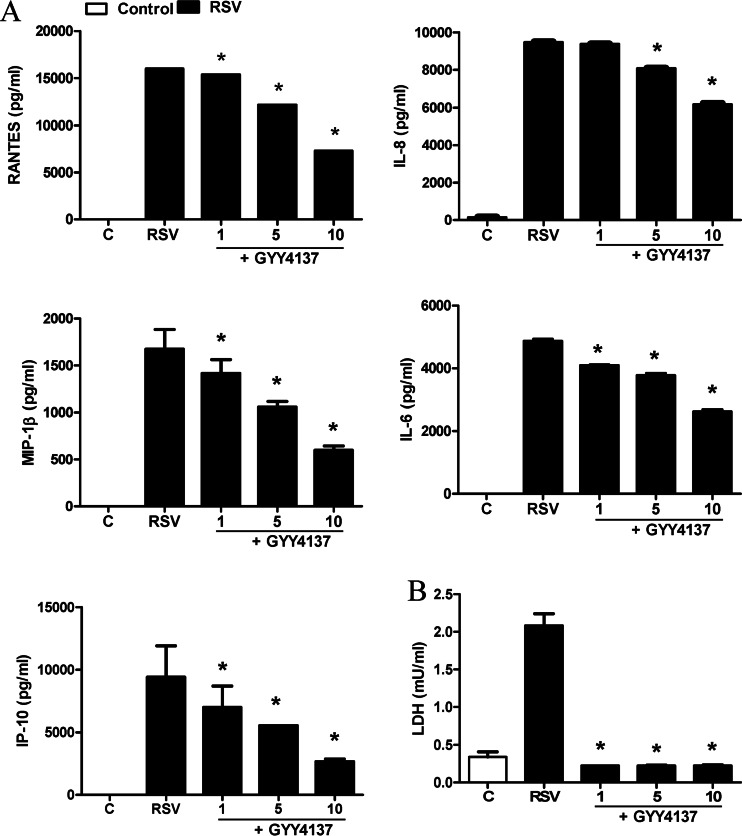
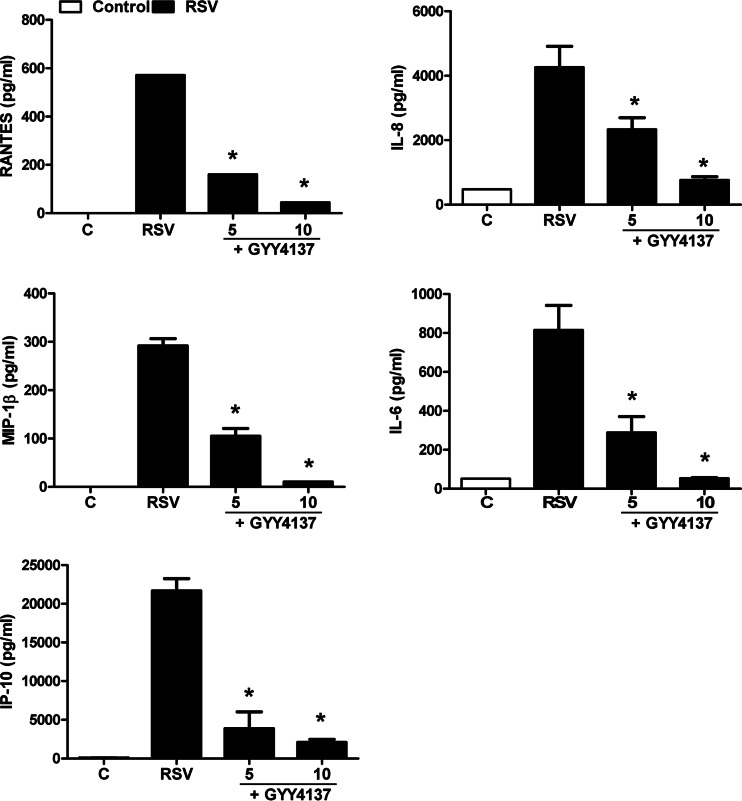
To investigate the effect of increasing intracellular H2S levels on viral responses, we determined levels of cytokine and chemokine secretion in A549 cells infected with RSV in the presence or absence of GYY4137, a slow-releasing H2S donor. A549 cells were infected with RSV for 1 h, followed by incubation with different concentrations of GYY4137, and harvested to collect the cell supernatant at 24 h p.i. to measure proinflammatory mediator release by ELISAs and Bio-Plex assays. RSV-induced secretion of several cytokines and chemokines, such as IL-6, IL-8, RANTES, macrophage inflammatory protein 1β (MIP-1β), and interferon-induced protein 10, was decreased by GYY4137 treatment in a dose-dependent manner (Fig. 3A). To investigate possible GYY4137 cytotoxicity, supernatants of uninfected or infected and treated or untreated A549 cells were harvested and tested for LDH release. There was no enhanced cellular damage; on the contrary, we observed a protective effect against virus-induced cytotoxicity in response to GYY4137 treatment (Fig. 3B). Inhibition of proinflammatory secretion, following RSV infection, by GYY4137 administration was also confirmed in SAE cells, normal human AECs, which we have shown to behave very similarly to A549 cells in terms of chemokine/cytokine gene expression and transcription factor and signaling pathway activation in response to RSV infection (12, 19, 22, 28–31) (Fig. 4).
FIG 3.
Effect of H2S donor treatment on RSV-induced cytokine and chemokine production in A549 cells. Cells were infected with RSV for 1 h and then incubated in the presence or absence of GYY4137 at 1, 5, and 10 mM for 24 h. Cell supernatants were assayed for cytokine and chemokine secretion by an ELISA or a Bio-Plex assay (A) and for cytotoxicity by an LDH release assay (B). Results are expressed as means ± standard errors and are representative of data from at least three independent experiments run in triplicate for the experiments described above for panel A. *, P < 0.05 compared to untreated RSV-infected cells.
FIG 4.
Effect of H2S donor treatment on RSV-induced cytokine and chemokine production in SAE cells. Cells were infected with RSV for 1 h and then incubated in the presence or absence of GYY4137 at 5 and 10 mM for 24 h. Cell supernatants were assayed for cytokine and chemokine secretion by an ELISA or a Bio-Plex assay. Results are expressed as means ± standard errors and are representative of data from three independent experiments run in triplicate. *, P < 0.05 compared to untreated RSV-infected cells.
Effects of H2S treatment on RSV replication.
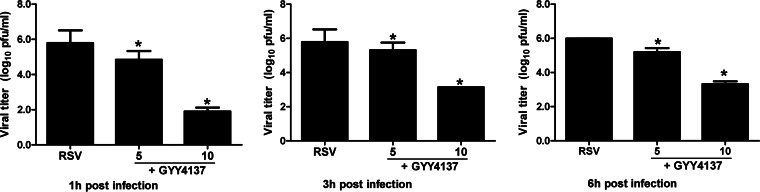
To determine whether increasing intracellular H2S levels affect viral replication, A549 cells were treated with different concentrations of GYY4137 either 1 h prior to RSV adsorption, until adsorption but not during infection, or 1 h after RSV adsorption and throughout infection and harvested at 24 p.i. to measure viral titers by a plaque assay. There was no change in viral titers when GYY4137 was given before infection (data not shown), while there was a significant decrease in RSV replication when GYY4137 was added after adsorption, in particular with the highest dose of the H2S donor, in the order of a several-log reduction (Fig. 5, left), indicating significant antiviral activity of H2S administration. To investigate whether this effect was reproducible if GYY4137 was administered several hours after infection, A549 cells were treated at 3 and 6 h p.i. and harvested to measure viral titers. We observed a significant decrease in RSV replication with both treatments, although the decrease was somewhat less striking than that with administration at 1 h p.i. (Fig. 5, middle and right), indicating that GYY4137 can affect viral replication when infection is already established.
FIG 5.
Effect of H2S donor treatment on RSV replication. A549 cells were infected with RSV for 1, 3, or 6 h and then incubated in the presence or absence of GYY4137 at 5 and 10 mM for 24 h. Cells were harvested to determine viral titers by a plaque assay. Results are expressed as means ± standard errors and are representative of data from five independent experiments run in triplicate. *, P < 0.05 compared to untreated RSV-infected cells.
H2S treatment affects virus particle release and syncytium formation.
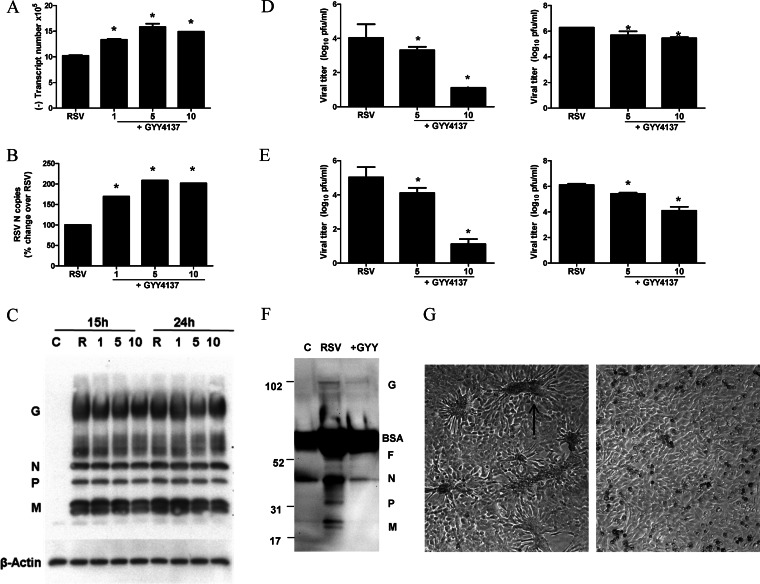
To further investigate how H2S treatment affected viral replication, we used several approaches, including quantification of viral gene transcription, genome replication, viral antigen detection, and viral particle release. GYY4137 administration did not decrease the number of RSV genome copies and N gene copies; on the contrary, they were somewhat increased at all concentrations tested (Fig. 6A and B). Viral protein expression, assessed by a Western blot assay of total cell lysates, was not significantly affected by GYY4137 treatment at any of the doses tested (Fig. 6C). When viral titers were assessed separately on cell supernatants and cell pellets, we found that GYY4137 administration dramatically reduced the number of infectious virus particles present in the cell supernatant, with a much less robust effect on those associated with the cell pellet (Fig. 6D, left versus right), suggesting that H2S treatment affects viral replication in part at the level of virus assembly but mostly at the level of virus release. When viral replication was assessed in a multicycle replication system, this resulted in a significant inhibition of the cell-associated virus content in addition to the almost complete absence of virus in the cell supernatant of infected cells treated with the higher dose of GYY4173 (Fig. 6E). To determine whether the reduction in viral titers in cell supernatants was due to fewer virus particles released or to a loss of infectivity, we performed a Western blot analysis of viral proteins in supernatants from cells infected in the absence or presence of GYY4173. We found clear decreases in the levels of most of the viral proteins, with the exception of the G protein, which represents in good part a secreted protein (Fig. 6F). Moreover, we observed a striking reduction in cellular syncytium formation, suggesting that GYY4137 treatment can significantly affect virus-induced cellular fusion (Fig. 6G).
FIG 6.
Effect of H2S donor treatment on different steps of viral replication. A549 cells were infected with RSV for 1 h and then incubated in the presence or absence of GYY4137 for 24 h. (A to C) Cells were harvested to prepare either total RNA to measure viral genome copy numbers (A) or RSV N gene copy numbers (B) by qRT-PCR or total cell lysates to measure viral protein expression by Western blotting (C). The membrane was stripped and reprobed with β-actin as a control for equal loading of the samples. Data are representative of data from three independent experiments with similar results. (D) A549 cells were infected with RSV for 1 h and then incubated in the presence or absence of GYY4137 at 5 and 10 mM for 24 h. Cell supernatants (left) and cell pellets (right) were harvested separately to determine viral titers by a plaque assay. Results are expressed as means ± standard errors and are representative of data from three independent experiments run in triplicate. *, P < 0.05 compared to untreated RSV-infected cells. (E) HEp-2 cells were infected with RSV at an MOI of 0.01 in the presence or absence of GYY4137 at 5 and 10 mM for 48 h. Cell supernatants (left) and cell pellets (right) were harvested separately to determine viral titers by a plaque assay. Results are expressed as means ± standard errors and are representative of two independent experiments run in triplicate. *, P < 0.05 compared to untreated RSV-infected cells. (F) A549 cells were infected with RSV for 1 h and then incubated in the presence or absence of GYY4137 for 24 h. Cell supernatants were harvested to measure viral protein expression by Western blotting. Data are representative of data from two independent experiments with similar results. (G) Light microscopy photograph (magnification, ×20) of HEp-2 cells infected with RSV at an MOI of 0.01 for 48 h in the presence (right) or absence (left) of GYY4137 at 10 mM. The arrow indicates one of the many syncytia present in the cell monolayer as a result of viral infection.
Effect of GYY4137 on RSV-induced cellular signaling.
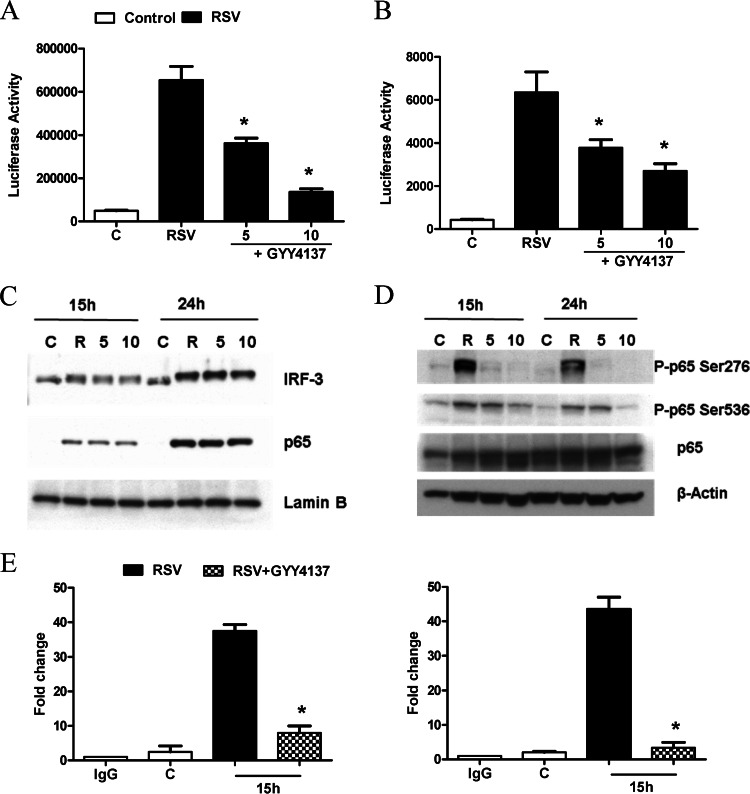
Cytokine and chemokine gene expression in A549 cells infected by RSV is orchestrated by the activation of the two key transcription factors NF-κB and IRF-3. To determine whether changes in RSV-induced cytokine and chemokine production observed with GYY4137 treatment affected NF-κB- and IRF-3-dependent gene transcription, we performed reporter gene assays. Cells were transiently transfected with either a NF-κB- or IRF-driven luciferase reporter plasmid and then treated with GYY4137 after 1 h of viral adsorption and harvested at 24 h p.i. to measure luciferase activity. RSV infection significantly enhanced both IRF-3- and NF-κB-dependent gene transcription, which was significantly inhibited by GYY4137 treatment in a dose-dependent manner (Fig. 7A and B), consistent with the observed reduction in IL-8 and RANTES secretion.
FIG 7.
Effect of H2S donor treatment on virus-induced signaling. (A and B) A549 cells were transiently transfected with an ISRE-driven (A) or NF-κB-driven (B) reporter gene plasmid, infected with RSV for 1 h, and then treated with 5 and 10 mM GYY4137. Cells were harvested at 15 or 24 h p.i. to measure luciferase and β-galactosidase reporter activities. Luciferase activity was normalized to the activity of the internal control β-galactosidase. Results are representative of data from three independent experiments run in triplicate. Data are expressed as means ± standard errors for normalized luciferase activity. *, P < 0.05 relative to untreated, RSV-infected cells. (C) A549 cells were infected with RSV for 1 h, followed by GYY4137 treatment at different concentrations, and harvested at 15 and 24 h p.i. to prepare either total cell lysates or nuclear extracts. IRF-3 and p65 nuclear translocation was assessed by Western blotting of nuclear extracts. Membranes were stripped and reprobed with lamin B to determine equal loading of the samples. (D) Total Ser276 and Ser536 p65 phosphorylation levels were determined by Western blotting of total cell lysates. The membrane was stripped and reprobed for total p65 and β-actin to determine equal loading of the samples. Data are representative of data from three independent experiments with similar results. (E) Chromatin DNA from uninfected and RSV-infected A549 cells in the presence or absence of GYY4137 for 15 h was immunoprecipitated by using an anti-NF-κB antibody (left), an anti-IRF-3 antibody (right), or IgG as a negative control. Q-gPCR was performed by using primers spanning either the NF-κB-binding site of the IL-8 promoter or the ISRE-binding site of the RANTES promoter. Total input chromatin DNA for immunoprecipitation was included as positive control for Q-gPCR amplification. The fold change was calculated compared to the IgG control. Results are representative of data from two independent experiments. *, P < 0.05 relative to untreated, RSV-infected cells.
To determine whether GYY4137 treatment was able to modulate virus-induced NF-κB and IRF-3 activation, A549 cells were infected with RSV for 1 h, incubated with or without GYY4137, and harvested at 15 and 24 h p.i. to prepare either total cell lysates or nuclear extracts. NF-κB and IRF-3 nuclear levels or cellular levels of phosphorylated serine in p65, the major NF-κB subunit activated in response to RSV infection (22), were assessed by Western blotting. Nuclear translocation of both transcription factors was not changed by GYY4137 treatment compared to RSV infection alone (Fig. 7C); however, there was a significant decrease in RSV-induced p65 Ser276 and Ser536 phosphorylation (Fig. 7D), two important posttranslational modifications that affect NF-κB transcriptional activity (32). In addition, GYY4137 treatment significantly reduced p65 and IRF occupancy of their cognate binding site on the IL-8 and RANTES endogenous promoters, assessed by a two-step chromatin immunoprecipitation (XChIP) and genomic PCR (Q-gPCR) assay (Fig. 7E). Taken together, these results indicate that increasing cellular H2S levels by using a slow-releasing donor can effectively modulate the strong proinflammatory cellular response induced by RSV infection through blocking IRF- and NF-κB-dependent gene transcription.
Effects of H2S treatment on chemokine production and viral replication induced by other paramyxoviruses.
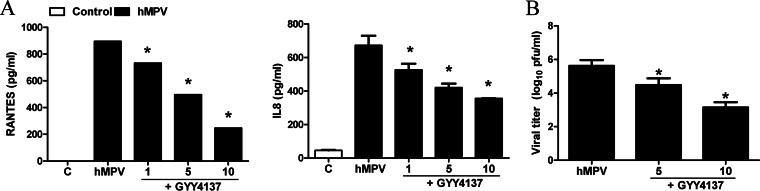
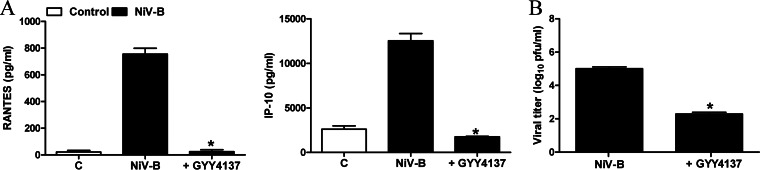
To investigate whether GYY4137 had similar antiviral and anti-inflammatory effects on other paramyxoviruses, we measured chemokine secretion and viral replication in A549 cells in response to hMPV infection. A549 cells were infected with hMPV for 1 h and incubated in the presence or absence of GYY4137 for a total of 24 h. Cell supernatants were collected to measure levels of IL-8 and RANTES induction by an ELISA, while viral titers were determined by immunostaining. hMPV-induced IL-8 and RANTES secretion was significantly decreased by GYY4137 treatment in a dose-dependent manner (Fig. 8A). Similarly, viral replication was also significantly reduced by GYY4137 treatment (Fig. 8B). A similar experiment was conducted by using a model of SAE cells infected with NiV-B. Similarly to RSV and hMPV, GYY4137 treatment led to a significant reduction of virus-induced cytokine and chemokine secretion (Fig. 9A) and inhibition of viral replication (Fig. 9B). In addition, GYY4137 treatment inhibited syncytium formation in response to both hMPV and NiV infection (data not shown), suggesting that GYY4137 has a broad antiviral effect on paramyxoviruses.
FIG 8.
Effect of H2S donor treatment on hMPV-induced chemokine production and viral replication. A549 cells were infected with hMPV for 1 h, followed by treatment with different millimolar concentrations of GYY4137. (A) Cell supernatants from uninfected and hMPV-infected cells, treated or untreated, were assayed at 24 h p.i. for cytokine and chemokine secretion by a Bio-Plex assay. Results are expressed as means ± standard errors. Results are representative of data from two independent experiments run in triplicate. *, P < 0.05 compared to untreated hMPV-infected cells. (B) Viral replication was determined at 24 h postinfection by titration of viral infectious particles released into the cell supernatants by a plaque assay. Results are representative of data from two independent experiments run in triplicate. *, P < 0.05 compared to untreated hMPV-infected cells.
FIG 9.
Effect of H2S donor treatment on NiV-induced chemokine production and viral replication. SAE cells were infected with NiV at an MOI of 0.1 for 1 h, followed by treatment with a 5 mM concentration of GYY4137. (A) Cell supernatants from uninfected and NiV-infected cells, treated or untreated, were assayed at 24 h p.i. for cytokine and chemokine secretion by a Bio-Plex assay. Results are expressed as means ± standard errors. Results are representative of data from two independent experiments run in triplicate. *, P < 0.05 compared to untreated NiV-infected cells. (B) Viral replication was determined at 24 h postinfection by titration of viral infectious particles released into cell supernatants by a plaque assay. Results are representative of data from two independent experiments run in triplicate. *, P < 0.05 compared to untreated NiV-infected cells.
DISCUSSION
In this study, we investigated the role of H2S in airway epithelial cell responses to viral infection. Paramyxoviruses, in particular RSV and hMPV, are a primary cause of severe lower respiratory tract infections in children as well as in other populations, leading to increased morbidity and mortality, for which there is no vaccine or treatment besides supportive measures. The virus-induced lung inflammatory response, triggered by the secretion of cytokines and chemokines from virus-infected airway-resident cells such as AECs and alveolar macrophages, plays an important role in disease pathogenesis. We and others have shown that modulation of the inflammatory response is associated with an amelioration of clinical illness in animal models of RSV infection (33–36), making it an important target for the development of effective treatment strategies.
H2S is an important endogenous gaseous mediator that has recently been the focus of intense investigation, leading to supportive evidence that it plays an important role in vasoactive, cytoprotective, anti-inflammatory, and antioxidant cellular responses (reviewed in reference 37). Our study shows for the first time that H2S has a protective role in RSV infection by modulating both inflammatory gene expression and viral replication. AECs infected with RSV displayed a decreased ability to generate H2S and enhanced degradation of H2S released by the donor GYY4137, indicating that viral infection leads to changes in H2S cellular homeostasis. Endogenous H2S production appears to play an important role in modulating virus-induced chemokine secretion and viral replication, as both were significantly enhanced by treatment of AECs with the CSE inhibitor PAG, while increased H2S cellular levels, as a result of the administration of GYY4137, were associated with a significant reduction of proinflammatory mediator production and, most importantly, a striking reduction of late-stage viral replication.
GYY4137 administration resulted in a strong inhibition of viral replication at a step subsequent to viral adsorption. It dramatically reduced the amount of infectious virus present in the cell supernatant, with a much less robust effect on cell-associated virus, without having a significant effect on viral gene transcription, protein synthesis, or genome replication. These findings suggest that H2S treatment inhibits viral replication in part at the level of virus assembly but mostly at the level of virus release, which in part explains the increases in cellular viral mRNA and genomic RNA levels observed with H2S treatment. To produce progeny virions, ribonuclear protein complexes, which form cytoplasmic inclusions and contain newly synthesized genomic RNA together with several viral proteins translated in the cytoplasm, have to be assembled with the surface glycoproteins that have trafficked to the cell surface through the secretory pathway and are then released to form mature infectious virus (reviewed in reference 38). The apical recycling endosome (ARE) has been implicated in RSV protein trafficking and membrane scission, and downregulation of specific proteins such as myosin Vb or Rab11 disrupts virion formation and results in diminished numbers of viral progeny. To date, it is not known whether H2S, or any other endogenous gaseous transmitters, modulates ARE functions. The final step in viral assembly and budding involves a membrane scission event to separate the assembled viral particle from the host cell membrane, which often involves multivesicular body formation and the endosomal sorting complex required for transport (ESCRT) protein system, with membrane scission being performed by the ATPase Vps4 (38). In the case of RSV, budding is unaffected by the inhibition of Vps4, suggesting that RSV uses a novel mechanism for this final step of replication. Some evidence suggests that surface glycoproteins, both F and G, could actively contribute to the budding process leading to RSV egress from infected cells (38). Although we did not detect significant changes in the levels of expression of most viral proteins, we have not investigated whether H2S treatment could affect their routing to the cell membrane. Changes in the cellular localization of the F protein, for example, could explain the changes observed in syncytium formation and assembly/release following H2S treatment.
Endogenous H2S production and exogenous H2S administration have been associated with both proinflammatory and anti-inflammatory effects in various models of disease (reviewed in reference 39). In the context of acute pancreatitis and in burn injury, for example, H2S seems to play a proinflammatory role, while in other pathologies, such as asthma, COPD, LPS-induced inflammation, and ischemia reperfusion, it displays anti-inflammatory properties. In models of lung injury, administration of H2S donors has often been associated with an anti-inflammatory effect. For example, in a mouse model of hyperoxia, treatment with NaHS was associated with reduced lung permeability and inflammation, due to decreased levels production of proinflammatory mediators such as IL-1β, monocyte chemoattractant protein 1 (MCP-1), and MIP-2 and increased levels of anti-inflammatory cytokine expression (40). Similar results were obtained in other models of acute lung injury, such as the one associated with hemorrhagic shock or with bleomycin treatment (41, 42).
Recent studies have established that H2S is indeed a biologically relevant signaling molecule, similar to the other gaseous mediators nitric oxide and carbon monoxide (reviewed in reference 43). In several models of inflammatory diseases, the inhibition of proinflammatory mediator expression was paralleled by the inhibition of NF-κB activation. NaHS administration inhibited NF-κB activation in a mouse model of hemorrhagic shock as well as in a rat model of acute lung injury. Similarly, H2S donor treatment in a rat model of bleomycin-induced pulmonary inflammation and fibrosis led to the inhibition of activation of the NF-κB subunit p65 (44). In vitro, NaHS and GYY4137 have been shown to inhibit LPS-induced NF-κB activation in cultured macrophages (5). Garlic compounds such as diallyl sulfide, a possible H2S donor, can also downregulate NF-κB activation (41). GYY4137 treatment of AECs infected with RSV did not change primary virus-induced activation of IRF-3 and NF-κB, as shown by the lack of changes in their nuclear translocation, in agreement with the absence of differences due to GYY4137 treatment in viral RNA generation, the major trigger of cellular signaling in RSV-infected cells through the activation of the virus-sensing cytosolic receptor RIG-I (45). GYY4137 treatment, however, significantly reduced IRF-3 and NF-κB binding to RANTES and IL-8 endogenous promoters, indicating a direct effect of H2S on cellular signaling. An important mechanism by which H2S can modulate cellular signaling is through its direct and indirect antioxidant activity (reviewed in reference 43). Administration of H2S has been shown to increase cellular glutathione levels, and it has also been associated with increased activation of Nrf2, a transcription factor that regulates oxidative stress by affecting the gene expression of several key antioxidant enzymes (43). Inducible phosphorylation on distinct serine residues, including Ser276 and Ser536, has been shown to regulate NF-κB transcriptional activity without modification of nuclear translocation or DNA-binding affinity (32). We have recently shown that the inhibition of RSV-induced reactive oxygen species (ROS) formation by treatment of AECs with antioxidants significantly reduces RSV-dependent NF-κB serine phosphorylation, resulting in the inhibition of RSV-induced expression of several NF-κB-dependent genes without affecting nuclear translocation (46). Our finding that H2S treatment significantly reduced p65 Ser276 and Ser536 phosphorylation suggests that modulation of ROS cellular levels could be a major mechanism by which H2S affects virus-induced cellular signaling.
In conclusion, we have shown that modulation of cellular H2S significantly impacts cellular responses and viral replication in an in vitro model of RSV infection. Our finding that H2S donor treatment also affects replication and proinflammatory mediator production in a model of hMPV and NiV infection suggests that H2S possesses broad antiviral activity against paramyxoviruses. We are currently investigating the effect of H2S donor treatment in the context of other nonparamyxovirus infections to determine the potential antiviral spectrum of these compounds. We are also determining the role of endogenous H2S production in RSV infection in vivo, taking advantage of knockout mice available for the enzymes CSE and CBS. Indeed, preliminary studies indicate that CSE is an important modulator of disease and airway hyperresponsiveness as well as lung inflammation triggered by RSV infection (A. Casola, unpublished data), similar to what was recently reported for allergic airway inflammation triggered by ovalbumin sensitization in mice (47). Of interest is the observation that premature infants, who are at high risk of developing severe bronchiolitis following RSV and other respiratory viral infections, have very low tissue CSE activity compared to full-term infants (48). We are also evaluating the efficacy of H2S donors in a mouse model of RSV infection to determine whether their administration has therapeutic potential for the prevention and treatment of virus-induced lung disease.
ACKNOWLEDGMENTS
This project was supported by grants R01 AI062885, R21 AI111042, R21 AI103565, P01 AI07924602, GM107846, P30 ES006676, and W81XWH1010146-DoD; the UTMB John Sealy Memorial Endowment Fund (A.C.); and a UTMB Center for Tropical Diseases postdoctoral fellowship (O.E.).
We thank Kimberly Palkowetz and Tianshuang Liu for technical assistance and Cynthia Tribble for manuscript submission.
REFERENCES
- 1.Chen Y, Wang R. 2012. The message in the air: hydrogen sulfide metabolism in chronic respiratory diseases. Respir Physiol Neurobiol 184:130–138. doi: 10.1016/j.resp.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Wang P, Zhang G, Wondimu T, Ross B, Wang R. 2011. Hydrogen sulfide and asthma. Exp Physiol 96:847–852. doi: 10.1113/expphysiol.2011.057448. [DOI] [PubMed] [Google Scholar]
- 3.Wang R. 2012. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev 92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 4.Wang R. 2011. Signaling pathways for the vascular effects of hydrogen sulfide. Curr Opin Nephrol Hypertens 20:107–112. doi: 10.1097/MNH.0b013e3283430651. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, Moore PK. 2008. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation 117:2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 6.Szabo C. 2007. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov 6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 7.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, Grijalva CG, Zhu Y, Szilagyi P. 2009. The burden of respiratory syncytial virus infection in young children. N Engl J Med 360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams JV, Harris PA, Tollefson SJ, Halburnt-Rush LL, Pingsterhaus JM, Edwards KM, Wright PF, Crowe JE Jr. 2004. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med 350:443–450. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escaffre O, Borisevich V, Rockx B. 2013. Pathogenesis of Hendra and Nipah virus infection in humans. J Infect Dev Ctries 7:308–311. doi: 10.3855/jidc.3648. [DOI] [PubMed] [Google Scholar]
- 10.Escaffre O, Borisevich V, Carmical JR, Prusak D, Prescott J, Feldmann H, Rockx B. 2013. Henipavirus pathogenesis in human respiratory epithelial cells. J Virol 87:3284–3294. doi: 10.1128/JVI.02576-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garofalo RP, Haeberle H. 2000. Epithelial regulation of innate immunity to respiratory syncytial virus. Am J Respir Cell Mol Biol 23:581–585. doi: 10.1165/ajrcmb.23.5.f204. [DOI] [PubMed] [Google Scholar]
- 12.Bao X, Liu T, Spetch L, Kolli D, Garofalo RP, Casola A. 2007. Airway epithelial cell response to human metapneumovirus infection. Virology 368:91–101. doi: 10.1016/j.virol.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bitko V, Velazquez A, Yank L, Yang Y-C, Barik S. 1997. Transcriptional induction of multiple cytokines by human respiratory syncytial virus requires activation of NF-kB and is inhibited by sodium salicylate and aspirin. Virology 232:369–378. doi: 10.1006/viro.1997.8582. [DOI] [PubMed] [Google Scholar]
- 14.Tian B, Zhang Y, Luxon B, Garofalo RP, Casola A, Sinha M, Brasier AR. 2002. Identification of NF-kB dependent gene networks in respiratory syncytial virus-infected cells. J Virol 76:6800–6814. doi: 10.1128/JVI.76.13.6800-6814.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin R, Heylbroeck C, Genin P, Pitha PM, Hiscott J. 1999. Essential role of interferon regulatory factor 3 in direct activation of RANTES chemokine transcription. Mol Cell Biol 19:959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casola A, Garofalo RP, Haeberle H, Elliott TF, Lin A, Jamaluddin M, Brasier AR. 2001. Multiple cis regulatory elements control RANTES promoter activity in alveolar epithelial cells infected with respiratory syncytial virus. J Virol 75:6428–6439. doi: 10.1128/JVI.75.14.6428-6439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin VS, Lippert AR, Chang CJ. 2013. Cell-trappable fluorescent probes for endogenous hydrogen sulfide signaling and imaging H2O2-dependent H2S production. Proc Natl Acad Sci U S A 110:7131–7135. doi: 10.1073/pnas.1302193110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ueba O. 1978. Respiratory syncytial virus. I. concentration and purification of the infectious virus. Acta Med Okayama 32:265–272. [PubMed] [Google Scholar]
- 19.Olszewska-Pazdrak B, Casola A, Saito T, Alam R, Crowe SE, Mei F, Ogra PL, Garofalo RP. 1998. Cell-specific expression of RANTES, MCP-1, and MIP-1alpha by lower airway epithelial cells and eosinophils infected with respiratory syncytial virus. J Virol 72:4756–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kisch AL, Johnson KM. 1963. A plaque assay for respiratory syncytial virus. Proc Soc Exp Biol Med 112:583–589. [DOI] [PubMed] [Google Scholar]
- 21.Kolli D, Bao X, Liu T, Hong C, Wang T, Garofalo RP, Casola A. 2011. Human metapneumovirus glycoprotein G inhibits TLR4-dependent signaling in monocyte-derived dendritic cells. J Immunol 187:47–54. doi: 10.4049/jimmunol.1002589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garofalo RP, Sabry M, Jamaluddin M, Yu RK, Casola A, Ogra PL, Brasier AR. 1996. Transcriptional activation of the interleukin-8 gene by respiratory syncytial virus infection in alveolar epithelial cells: nuclear translocation of the RelA transcription factor as a mechanism producing airway mucosal inflammation. J Virol 70:8773–8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asimakopoulou A, Panopoulos P, Chasapis CT, Coletta C, Zhou Z, Cirino G, Giannis A, Szabo C, Spyroulias GA, Papapetropoulos A. 2013. Selectivity of commonly used pharmacological inhibitors for cystathionine beta synthase (CBS) and cystathionine gamma lyase (CSE). Br J Pharmacol 169:922–932. doi: 10.1111/bph.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casola A, Garofalo RP, Jamaluddin M, Vlahopoulos S, Brasier AR. 2000. Requirement of a novel upstream response element in RSV induction of interleukin-8 gene expression: stimulus-specific differences with cytokine activation. J Immunol 164:5944–5951. doi: 10.4049/jimmunol.164.11.5944. [DOI] [PubMed] [Google Scholar]
- 25.Schreiber E, Matthias P, Muller MM, Schaffner W. 1989. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res 17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brasier AR, Spratt H, Wu Z, Boldogh I, Zhang Y, Garofalo RP, Casola A, Pashmi J, Haag A, Luxon B, Kurosky A. 2004. Nuclear heat shock response and novel nuclear domain 10 reorganization in respiratory syncytial virus-infected A549 cells identified by high-resolution two-dimensional gel electrophoresis. J Virol 78:11461–11476. doi: 10.1128/JVI.78.21.11461-11476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson MR, Melideo SL, Jorns MS. 2012. Human sulfide:quinone oxidoreductase catalyzes the first step in hydrogen sulfide metabolism and produces a sulfane sulfur metabolite. Biochemistry 51:6804–6815. doi: 10.1021/bi300778t. [DOI] [PubMed] [Google Scholar]
- 28.Casola A, Burger N, Liu T, Jamaluddin M, Brasier AR, Garofalo RP. 2001. Oxidant tone regulates RANTES gene transcription in airway epithelial cells infected with respiratory syncytial virus: role in viral-induced interferon regulatory factor activation. J Biol Chem 276:19715–19722. doi: 10.1074/jbc.M101526200. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Luxon B, Casola A, Garofalo RP, Jamaluddin M, Brasier AR. 2001. Expression of respiratory syncytial virus-induced chemokine gene networks in lower airway epithelial cells revealed by cDNA microarrays. J Virol 75:9044–9058. doi: 10.1128/JVI.75.19.9044-9058.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pazdrak K, Olszewska-Pazdrak B, Liu B, Takizawa R, Brasier AR, Garofalo RP, Casola A. 2002. MAPK activation is involved in posttranscriptional regulation of RSV-induced RANTES gene expression. Am J Physiol Lung Cell Mol Physiol 283:L364–L372. doi: 10.1152/ajplung.00331.2001. [DOI] [PubMed] [Google Scholar]
- 31.Hosakote YM, Jantzi PD, Esham DL, Spratt H, Kurosky A, Casola A, Garofalo RP. 2011. Viral-mediated inhibition of antioxidant enzymes contributes to the pathogenesis of severe respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med 183:1550–1560. doi: 10.1164/rccm.201010-1755OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong H, Voll RE, Ghosh S. 1998. Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell 1:661–671. doi: 10.1016/S1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]
- 33.Haeberle HA, Kuziel WA, Dieterich HJ, Casola A, Gatalica Z, Garofalo RP. 2001. Inducible expression of inflammatory chemokines in respiratory syncytial virus-infected mice: role of MIP-1alpha in lung pathology. J Virol 75:878–890. doi: 10.1128/JVI.75.2.878-890.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haeberle HA, Casola A, Gatalica Z, Petronella S, Dieterich HJ, Ernst PB, Brasier AR, Garofalo RP. 2004. IkappaB kinase is a critical regulator of chemokine expression and lung inflammation in respiratory syncytial virus infection. J Virol 78:2232–2241. doi: 10.1128/JVI.78.5.2232-2241.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bennett BL, Garofalo RP, Cron SG, Hosakote YM, Atmar RL, Macias CG, Piedra PA. 2007. Immunopathogenesis of respiratory syncytial virus bronchiolitis. J Infect Dis 195:1532–1540. doi: 10.1086/515575. [DOI] [PubMed] [Google Scholar]
- 36.Castro SM, Guerrero-Plata A, Suarez-Real G, Adegboyega PA, Colasurdo GN, Khan AM, Garofalo RP, Casola A. 2006. Antioxidant treatment ameliorates respiratory syncytial virus-induced disease and lung inflammation. Am J Respir Crit Care Med 174:1361–1369. doi: 10.1164/rccm.200603-319OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kimura H. 2014. Production and physiological effects of hydrogen sulfide. Antioxid Redox Signal 20:783–793. doi: 10.1089/ars.2013.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El Najjar F, Schmitt AP, Dutch RE. 2014. Paramyxovirus glycoprotein incorporation, assembly and budding: a three way dance for infectious particle production. Viruses 6:3019–3054. doi: 10.3390/v6083019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whiteman M, Winyard PG. 2011. Hydrogen sulfide and inflammation: the good, the bad, the ugly and the promising. Expert Rev Clin Pharmacol 4:13–32. doi: 10.1586/ecp.10.134. [DOI] [PubMed] [Google Scholar]
- 40.Li HD, Zhang ZR, Zhang QX, Qin ZC, He DM, Chen JS. 2013. Treatment with exogenous hydrogen sulfide attenuates hyperoxia-induced acute lung injury in mice. Eur J Appl Physiol 113:1555–1563. doi: 10.1007/s00421-012-2584-5. [DOI] [PubMed] [Google Scholar]
- 41.Kalayarasan S, Sriram N, Sudhandiran G. 2008. Diallyl sulfide attenuates bleomycin-induced pulmonary fibrosis: critical role of iNOS, NF-kappaB, TNF-alpha and IL-1beta. Life Sci 82:1142–1153. doi: 10.1016/j.lfs.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 42.Xu DQ, Gao C, Niu W, Li Y, Wang YX, Gao CJ, Ding Q, Yao LN, Chai W, Li ZC. 2013. Sodium hydrosulfide alleviates lung inflammation and cell apoptosis following resuscitated hemorrhagic shock in rats. Acta Pharmacol Sin 34:1515–1525. doi: 10.1038/aps.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li L, Rose P, Moore PK. 2011. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol 51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 44.Cao H, Zhou X, Zhang J, Huang X, Zhai Y, Zhang X, Chu L. 2014. Hydrogen sulfide protects against bleomycin-induced pulmonary fibrosis in rats by inhibiting NF-kappaB expression and regulating Th1/Th2 balance. Toxicol Lett 224:387–394. doi: 10.1016/j.toxlet.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Liu P, Jamaluddin M, Li K, Garofalo RP, Casola A, Brasier AR. 2007. Retinoic acid-inducible gene I mediates early antiviral response and Toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J Virol 81:1401–1411. doi: 10.1128/JVI.01740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jamaluddin M, Tian B, Boldogh I, Garofalo RP, Brasier AR. 2009. Respiratory syncytial virus infection induces a reactive oxygen species-MSK1-phospho-Ser-276 RelA pathway required for cytokine expression. J Virol 83:10605–10615. doi: 10.1128/JVI.01090-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benetti LR, Campos D, Gurgueira SA, Vercesi AE, Guedes CE, Santos KL, Wallace JL, Teixeira SA, Florenzano J, Costa SK, Muscara MN, Ferreira HH. 2013. Hydrogen sulfide inhibits oxidative stress in lungs from allergic mice in vivo. Eur J Pharmacol 698:463–469. doi: 10.1016/j.ejphar.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 48.Vina J, Vento M, Garcia-Sala F, Puertes IR, Gasco E, Sastre J, Asensi M, Pallardo FV. 1995. L-cysteine and glutathione metabolism are impaired in premature infants due to cystathionase deficiency. Am J Clin Nutr 61:1067–1069. [DOI] [PubMed] [Google Scholar]