Abstract
Background: Cystic fibrosis (CF) is a common life-shortening genetic disease in which women have been described to have worse outcomes than males, particularly in response to respiratory infections with Pseudomonas aeruginosa. However, as advancements in therapies have improved life expectancy, this gender disparity has been challenged. The objective of this study is to examine whether a gender-based survival difference still exists in this population and determine the impact of common CF respiratory infections on outcomes in males versus females with CF.
Methods: We conducted a retrospective cohort analysis of 32,766 patients from the United States Cystic Fibrosis Foundation Patient Registry over a 13-year period. Kaplan-Meier and Cox proportional hazards models were used to compare overall mortality and pathogen based survival rates in males and females.
Results: Females demonstrated a decreased median life expectancy (36.0 years; 95% confidence interval [CI] 35.0–37.3) compared with men (38.7 years; 95% CI 37.8–39.6; p<0.001). Female gender proved to be a significant risk factor for death (hazard ratio 2.22, 95% CI 1.79–2.77), despite accounting for variables known to influence CF mortality. Women were also found to become colonized earlier with several bacteria and to have worse outcomes with common CF pathogens.
Conclusions: CF women continue to have a shortened life expectancy relative to men despite accounting for key CF-related comorbidities. Women also become colonized with certain common CF pathogens earlier than men and show a decreased life expectancy in the setting of respiratory infections. Explanations for this gender disparity are only beginning to be unraveled and further investigation into mechanisms is needed to help develop therapies that may narrow this gender gap.
Introduction
Cystic fibrosis (CF) is one of the most common genetic, fatal diseases in the United States. It is a multisystem disorder that is characterized by nutritional deficiency and recurrent respiratory infections due to thick mucus, caused by lack of chloride transport across surface epithelium. Advancements in therapies have improved the median life expectancy from less than 5 years of age in the 1950s to nearly 40 years of age currently.1 These advancements began with the advent of pancreatic enzyme supplementation in the 1940s to help with malnutrition and progressed to therapies aimed at thinning airway mucus and fighting lung infections such as dornase alpha and nebulized Tobramycin respectively in the 1990s.2,3 Most recently ivacaftor, a therapy that works to correct the underlying chloride channel protein defect, was approved for patients with certain genetic mutations.4 However, despite these improvements in treatment, females with CF have worse outcomes than males for reasons that are only beginning to be understood.5 Chronic respiratory infections remain the largest contributor to morbidity and mortality in CF patients, and we therefore hypothesized that females have more severe responses to respiratory infections then males, contributing to their poorer outcomes.
A variety of respiratory diseases show a gender disparity in prevalence and/or outcomes. For example, lymphangioleiomyomatosis is a rare cystic lung disease of unknown etiology well known to disproportionately affect young women of childbearing age.6 In fact, very few men have ever been reported to have this disease. This disease was hypothesized to be hormone mediated, but hormone based therapies have not proven to be clearly beneficial as of yet.6 Observational data suggest other diseases such as idiopathic pulmonary hypertension are more prevalent in women, whereas idiopathic pulmonary fibrosis, disproportionately affects men.7–9 Further, in relationship to malignancies in the lung, malignant mesothelioma disproportionately affects men, whereas women are more likely to have adenocarcinoma, yet men have worse outcomes when affected by adenocarcinoma.10–12 Multiple epidemiologic studies have established that women have worse outcomes than men in several airway diseases beyond CF, such as asthma and chronic obstructive pulmonary disease (COPD).5,13–16 Post-puberty, girls with asthma have more frequent and severe exacerbations than boys.17 In COPD, women develop evidence of airway obstruction after fewer numbers of cigarettes per lifetime than men, and women with severe COPD have a 50% increased risk of death compared to men.16 In non-CF bronchiectasis, females have been shown to have a higher prevalence of the disease than males, particularly in conjunction with nontuberculous mycobacterial infections, despite no known difference in immunologic susceptibility.13
Reports that women with CF have lower survival rates than men first appeared in the 1990s.18–20 Rosenfeld and colleagues published a large cohort analysis using data from the United States Cystic Fibrosis Foundation Patient Registry (CFFPR) between 1988 through 1992, showing that women had shorter life expectancies than men.5 This difference was significant even after adjusting for confounding morphometric and nutritional factors. Another group demonstrated that CF females acquired chronic Pseudomonas aeruginosa infection, one of the most prevalent and virulent bacteria in this population, at an earlier age (median of 9.5 years in females and 11.2 years in males) and had a more rapid decline in lung function after P. aeruginosa colonization.21 Since that time, several reports have refuted the gender gap observation in CF, arguing that improved airway clearance therapies have narrowed the gender difference.22–25
Despite the controversy, multiple reports have evaluated mechanisms behind the gender disparity in CF and focused on the role of female sex hormones. In particular, estrogen has been implicated as a hormone that decreases air surface liquid on bronchial epithelial cells by modulating ion channels on airway epithelium and therefore renders CF females at a greater disadvantage than males by enhancing mucus viscosity. In addition, estrogen has been shown to promote conversion of P. aeruginosa from a nonmucoid to mucoid form in CF, which is the more drug resistant and pathogenic form.22–25 Increasing evidence is demonstrating that pathogens beyond P. aeruginosa are important variables in survival of patients with CF, including methicillin resistant Staphylococcus aureus (MRSA).26
We, therefore, sought to utilize the United States CFFPR to determine if a survival difference remains in men and women with CF and evaluate the effect of respiratory infections on outcomes between males and females with CF.
Methods
Participants
The CF Foundation Patient Registry contains demographic and clinical data collected at United States centers accredited by the CF Foundation and captures 92% to 97% of CF deaths reported in the U.S.20 The United States CFFPR has evolved to allow the examination of more and more CF pathogens to be feasible. Reported microbiology data in the CFFPR changed in 1994 to include a much more detailed profile than in the past. From 1994 to 1997, positive cultures were reported annually.26 Cultures were reported quarterly from 1998 through 2002, and at each encounter since 2003. Due to these changes in collection, we utilized only data from the annual data set to stay consistent over the time course of analysis. Positive culture data were defined as greater than or equal to two positive cultures across annual data sets for all bacteria included other than Burkholderia cepacia, which was defined as present if ever positive. Subjects for this study were CF patients with data entered from CFF-accredited care centers between January 1, 1995 and December 31, 2007.
Design and statistical analysis
Differences in demographic variables were determined using chi-squared tests for categorical variables and t-tests for continuous variables. We performed a survival analysis using Kaplan-Meier methodology. Age at entry into the cohort was defined as age of diagnosis. The primary outcome was the time from age at entry into the cohort until age at death from any cause, as reported in previous CF literature.26 The CFF registry captures 90 percent of patient deaths. Therefore, at the end of the study period, subjects were either categorized as dead or assumed to be alive, and censored at their age on December 31, 2007. Patients who underwent lung transplantation were excluded. A log-rank analysis was used to determine statistical differences in survival times between men and women.
Cox proportional hazards models were used to analyze the effects of multiple variables on mortality. Time independent variables included were gender, race, CF genotype, date of birth (cohorts were grouped by increments of 10 years), cause of death, highest recorded sweat chloride value, pancreatic functional status (defined as whether or not the subject was using enzyme replacements), and presence or absence CF-related diabetes (CFRD). Diabetes was defined as whether or not the subject was listed as having CFRD or having a hemoglobin A1c greater than 6.1.27 Positive culture data were defined as greater than or equal to two positive cultures across annual data sets for all bacteria included other than B. cepacia, which was defined as present if ever positive. Burkholderia species information in the CFFPR does not differentiate mucoid from nonmucoid strains.
Time-dependent variables analyzed in the Cox proportional hazard model were height and body mass index (BMI) (z-scores), percent of forced expiratory volume in 1 second (%FEV1), and rate of pulmonary exacerbations. Pulmonary exacerbations were defined as use of intravenous antibiotics for the purpose of treating a CF exacerbation. The effect of continuous variables on mortality rates was further analyzed using a lag-two method in order to account for possible differences that may be due to imbalances in severity of illness or aggressive treatment in the last years of life. In other words, for time-dependent covariates, the covariate values at age t-2 were used as predictors. Similar results were noted using both methods of analysis and no interactions were found between variables in the model. Individual pathogen influence on gender-based survival was analyzed using a multivariate Cox proportional hazard analysis with gender as a time independent variable and pathogen as a time-dependent variable.
Age of pathogen acquisition as a mean with standard deviations and life expectancy after infection as a median with confidence intervals were evaluated using independent two-tailed t-tests. All data was analyzed using SAS software, version 9.3 (SAS Institute). This study was approved by the University of Texas Southwestern Medical Center Institutional Review Board and was waived of informed consent given subjects had previously agreed to participation in the CFF registry.
Results
Cohort characteristics
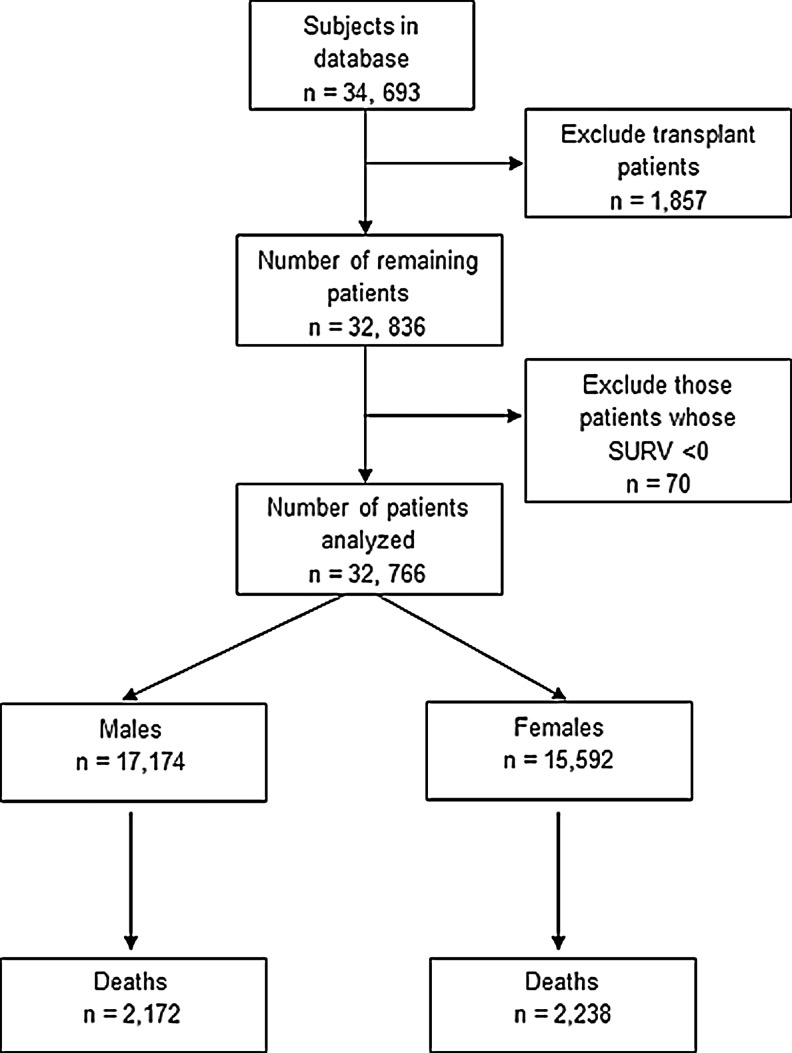
This study is a retrospective cohort analysis of 34,693 patients in the CFFPR (Fig. 1). Patients who received an organ transplant (1,857) were excluded given that their survival would be altered by organ transplantation, leaving 32,836 subjects. A group of 70 subjects were found to have a negative value for survival, likely due to data entry error and were also excluded. Therefore, a total of 32,766 subjects were included in the final analysis with 17,174 males and 15,592 females, of which 12.6% of males died and 14.3% of females died (Fig. 1).
FIG. 1.
Diagram of the study population of cystic fibrosis patients from the Cystic Fibrosis Foundation Patient Registry analyzed by gender. A total of 34,693 subjects were evaluated for this analysis. Subjects who received a lung transplant were excluded, resulting in 32,836 subjects. There were 70 patients whose survival lengths (SURV) were found to be less than zero and were therefore not included. There remained 32,766 subjects, of whom 17,174 were men and 15,592 were women.
Comparisons of important CF demographic and clinical characteristics between men and women in this cohort are shown in Table 1. Distribution of race and genotypes in males and females were similar though females had lower sweat tests. Date of birth cohort and lung function as measured by %FEV1 were statistically different between groups but without extreme imbalances in subgroups. The percentage of men with pancreatic insufficiency was higher, while the highest risk for an abnormal BMI was in females. CF-related diabetes was more prevalent in females. Presence of mucoid and nonmucoid P. aeruginosa, MRSA, and Achromobacter xylosoxidans was higher in females, while presence of B. cepacia was higher in males. Finally, more females died of respiratory and cardio-respiratory causes than males and females had on average an increased number of acute exacerbations per year than males.
Table 1.
Demographic Characteristics of Male and Female Cystic Fibrosis Patients Seen at Cystic Fibrosis Foundation-Accredited U.S. Care Centers
| Male n=17,174 (52.4%) | Female n=15,592 (47.6%) | p value | |
|---|---|---|---|
| Racea | 0.373 | ||
| White | 14,974 (87.2%) | 13,653 (87.6%) | |
| Hispanic | 1,316 (7.6%) | 1,145 (7.3%) | |
| African American | 734 (4.3%) | 639 (4.1%) | |
| Sweat chloride, mean±SD | 99.1±20.8 | 98.3±21.0 | <0.001 |
| n=14,701 | n=13,192 | ||
| CFTR genotype,an (%) | 0.116 | ||
| F508del/F508del | 6,853 (39.9%) | 6,311 (40.5%) | |
| F508del/Other | 5,302 (30.9%) | 5,043 (32.3%) | |
| Other/Other | 2,066 (12.0%) | 1,821 (11.7%) | |
| Date of birth cohort,an (%) | 0.0001 | ||
| 1958–1967 | 3,734 (21.7%) | 3,687 (23.6%) | |
| 1968–1977 | 5,089 (29.6%) | 4,643 (29.8%) | |
| 1978–1987 | 4,557 (26.5%) | 4,040 (25.9%) | |
| 1988–1997 | 2,509 (14.6%) | 2,098 (13.5%) | |
| 1998–2007 | 1,285 (7.5%) | 1,116 (7.2%) | |
| BMI risk,a,bn (%) | <0.001 | ||
| Low (≥22) | 2,265 (13.2%) | 1,514 (9.7%) | |
| Mild (20–21.9) | 3,076 (17.9%) | 2,919 (18.7%) | |
| Moderate (18–19.9) | 2,867 (16.7) | 2,728 (17.5%) | |
| High (<18) | 8,966 (52.2%) | 8,431 (54.1%) | |
| Height (percent),b mean±SD | 33.6±27.3% | 34.9±27.3% | <0.001 |
| n=15,077 | n=13,711 | ||
| FEV1,a,b % predicted, n (%) | <0.001 | ||
| >80 | 5,645 (32.9%) | 4,746 (30.4%) | |
| 60–79 | 2,690 (15.7%) | 2,475 (15.9%) | |
| 40–59 | 2,114 (12.3%) | 2,255 (14.5%) | |
| <40 | 6,725 (39.1%) | 6,116 (39.2%) | |
| Number of pulmonary exacerbations per year, mean±SD | 1.7±3.1 | 2.2±3.4 | <0.001 |
| n=16,175 | n=14,667 | ||
| Pancreatic insufficiency, n (%) | 14,960 (87.1%) | 13,093 (84.0) | <0.0001 |
| CF-related diabetes, n (%) | 2,214 (12.9%) | 2,499 (16.0%) | <0.001 |
| Pseudomonas aeruginosa, nonmucoid, n (%) | 10,762 (62.7%) | 10,222 (65.6%) | <0.0001 |
| P. aeruginosa, mucoid, n (%) | 8,442 (49.2%) | 7,933 (50.9%) | 0.002 |
| MSSA, n (%) | 14,521 (84.6%) | 13,137 (84.3%) | 0.456 |
| MRSA, n (%) | 4,277 (24.9%) | 4,087 (26.2%) | 0.007 |
| Haemophilus influenzae, n (%) | 9,902 (57.7%) | 8,892 (57.0%) | 0.251 |
| Klebsiella pneumonia, n (%) | 2,057(12.0%) | 1,965 (12.6%) | 0.085 |
| Escherichia coli, n (%) | 2,361 (13.8%) | 2,329 (15.0%) | 0.002 |
| Achromobacter xylosoxidans, n (%) | 5,070 (29.5%) | 5,152 (33.0%) | <0.0001 |
| Other gram negatives, n (%) | 7,815 (45.5%) | 7,198 (46.2%) | 0.231 |
| Burkholderia cepacia, n (%) | 1,494 (8.7%) | 1,221 (7.8%) | 0.004 |
| Aspergillus species, n (%) | 5,783 (33.7%) | 5,345 (34.3%) | 0.246 |
| Nontuberculous mycobacterium, n (%) | 921 (5.4%) | 893 (5.4%) | 0.942 |
| Cause of death,an (%) | <0.001 | ||
| Cardiopulmonary | 1,823 (83.9%) | 1,975 (88.2%) | |
| Liver disease | 63 (2.9%) | 40 (1.8%) | |
| Trauma | 60 (2.8%) | 18 (0.8%) | |
| Suicide | 12 (0.6%) | 1 (0.1%) | |
| Other | 135 (6.2%) | 127 (5.7%) | |
| Unknown | 79 (3.6%) | 77 (3.4%) |
Using a Bonferroni adjustment for multiple tests, a p value of <0.002 should be considered statistically significant. This adjustment controls the overall table error rate for the table at 0.05.
Variables analyzed as lag 2 (2 years prior to death) for demographics.
BMI, body mass index; CFTR, cystic fibrosis transmembrane conductance regulator; F508del, deltaF508 gene mutation; FEV1, forced expiratory volume in 1 second; MRSA, methicillin resistant staphylococcus aureus; MSSA, methicillin sensitive staphylococcus aureus; SD, standard deviation
Survival analysis
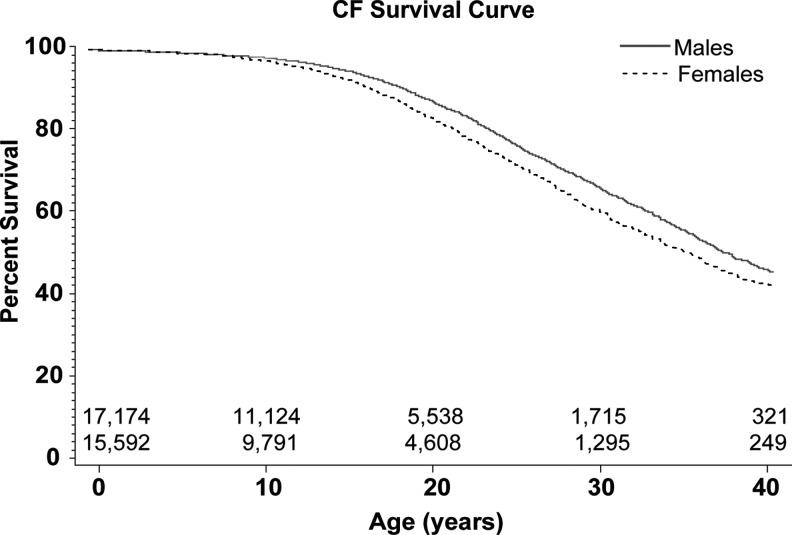
Kaplan-Meier analysis demonstrated a significant difference in survival of CF males versus females (p<0.001) with a median life expectancy of 38.7 years (95% confidence interval [95% CI] 37.8–39.6) in males and 36.0 years (95% CI 35.0 37.3) in females (Fig. 2). Risk of death was analyzed using Cox proportional analysis with all variables included in this study (Table 2). Importantly, females were more likely to die than males (hazard ratio [HR] 1.252, 95% CI 1.180–1.328, p<0.001). Variables that have been previously described to impact survival in CF were similarly found to be significant risk factors for death in our study, including BMI, pancreatic insufficiency, %FEV1, CF-related diabetes, and colonization with P. aeruginosa, MRSA, and B. cepacia.18,19,28,29 Other pathogens found to be associated with increased risk of death included Klebsiella pneumoniae, Escherichia coli, A. xylosoxidans, other gram negative organisms, and Aspergillus species, but not methicillin-sensitive Staphylococcus aureus (MSSA) or Haemophilus infuenzae. Mortality also increased with increasing number of pulmonary exacerbations as described previously (1.7 in males and 2.2 in females, p<0.001).30
FIG. 2.
Kaplan Meier estimate of survival comparing cystic fibrosis (CF) women with men seen at accredited U.S. CF care centers from 1995 to 2007. Females had poorer survival, with a median life expectancy of 36.0 years (95% CI 35.0–37.3) versus 38.7 years (95% CI 37.8–39.6) in men (p<0.001).
Table 2.
Univariate Cox Regression for Survival
| Variables | Hazard ratio (95% CI) | p value |
|---|---|---|
| Female | 1.252 (1.180–1.328) | <0.0001 |
| Racea | ||
| White | 0.771 (0.661–0.887) | 0.0003 |
| Hispanic | 1.937 (1.720–2.181) | <0.0001 |
| African American | 1.191 (1.018–1.394) | 0.0295 |
| Sweat Chloride | 1.001 (0.999–1.003) | 0.2732 |
| CFTR genotypea | ||
| F508del/F508del | 1.068 (0.992–1.150) | 0.0956 |
| F508del/other | 0.895 (0.828–0.967) | 0.0049 |
| Other/other | 1.107 (0.982–1.248) | 0.0824 |
| Date of birth cohorta | ||
| 1958–1967 | 0.512 (0.468–0.560) | <0.0001 |
| 1968–1977 | 1.014 (0.950–1.083) | 0.6695 |
| 1978–1987 | 1.414 (1.321–1.512) | <0.0001 |
| 1988–1997 | 1.193 (1.072–1.328) | 0.0013 |
| 1998–2007 | 1.668 (1.309–2.124) | <0.0001 |
| BMI (z-score)b | 0.245 (0.231–.0261) | <0.0001 |
| Height (z-score)b | 0.687 (0.676–0.697) | <0.0001 |
| FEV1, % predicted | 0.947 (0.944–0.951) | <0.0001 |
| Number of pulmonary exacerbations per year, mean | 1.141 (1.137–1.146) | <0.001 |
| Pancreatic insufficiency | 1.403 (1.062–1.854) | 0.0173 |
| CF-related diabetes | 2.509 (2.035–3.092) | <0.0001 |
| Pseudomonas aeruginosa, nonmucoid | 1.360 (1.169–1.583) | <0.0001 |
| P. aeruginosa, mucoid | 2.183 (1.835–2.598) | <0.0001 |
| MSSA | 0.714 (0.627–0.813) | <0.0001 |
| MRSA | 2.371 (1.999–2.811) | <0.0001 |
| Haemophilus influenzae | 0.562 (0.446–0.708) | <0.0001 |
| Klebsiella pneumonia | 2.197 (1.498–3.221) | 0.0003 |
| Escherichia coli | 2.150 (1.514–3.054) | 0.0001 |
| Alcaligenes xylosoxidans | 2.765 (2.246–3.404) | <0.0001 |
| Achromobacter xylosoxidans | 1.780 (1.473–2.152) | <0.0001 |
| Other gram negatives | 1.641 (1.372–1.963) | <0.0001 |
| Burkholderia cepacia | 2.615 (2.104–3.251) | <0.0001 |
| Aspergillus species | 1.665 (1.407–1.971) | <0.0001 |
| Nontuberculous mycobacteria | 1.283 (0.769–2.139) | 0.3390 |
Using a Bonferroni adjustment for multiple testing, a p value of<0.0016 should be considered statistically significant. This adjustment controls the overall table error rate for the table at 0.05.
BMI and height calculated as z-scores using infant National Center for Health Statistics (NHS) and Centers for Disease Control and Prevention (CDC) tables.22
95% CI, 95% confidence interval; CFTR, cystic fibrosis transmembrane conductance regulator.
In order to account for the influence of other variables on gender-related outcomes, all variables from the univariate analysis were included in a multivariate model (Table 3). There remained an increased risk of death associated with female gender (adjusted HR 2.227, 95% CI 1.790–2.771, p<0.0001). Other variables in the model associated with increased risk of death when accounting for all other variables included number of pulmonary exacerbations, pancreatic insufficiency, CF-related diabetes, mucoid and nonmucoid P. aeruginosa, MRSA, and B. cepacia, as previously reported.18,19,29 Date of birth cohorts were included in the multivariate model, but did not reveal statistical significance and are therefore not displayed on Table 3.
Table 3.
Multivariate Cox Regression for Survival
| Variables adjusted for | Relative hazard ratio (95% CI) | p value |
|---|---|---|
| Female | 2.227 (1.790–2.771) | <0.0001 |
| White race | 0.457 (0.256–0.815) | 0.008 |
| Hispanic race | 1.723 (1.284–2.313) | 0.0003 |
| African American race | 0.443 (0.215–0.912) | 0.027 |
| F508del/F508del | 0.749 (0.548–1.023) | 0.069 |
| F508del/other | 0.913 (0.667–1.250) | 0.571 |
| BMIa (z-score) | 0.348 (0.264–0.459) | <0.0001 |
| Heighta (z-score) | 0.609 (0.564–0.657) | <0.0001 |
| FEV1, % predicted | 0.955 (0.949–0.960) | <0.0001 |
| Number of pulmonary exacerbations per year | 1.088 (1.069–1.107) | <0.0001 |
| Pancreatic insufficiency | 7.914 (2.871–21.814) | <0.0001 |
| CF-related diabetes | 1.533 (1.164–2.018) | 0.002 |
| Pseudomonas aeruginosa, nonmucoid | 1.190 (0.971–1.458) | 0.094 |
| P. aeruginosa, mucoid | 1.413 (1.175–1.775) | 0.003 |
| MSSA | 0.896 (0.732–1.097) | 0.288 |
| MRSA | 1.544 (1.222–1.952) | 0.0003 |
| Haemophilus influenzae | 0.636 (0.412–0.981) | 0.041 |
| Klebsiella pneumoniae | 2.104 (1.113–3.975) | 0.022 |
| Escherichia coli | 1.227 (0.648–2.325) | 0.530 |
| Achromobacter xylosoxidans | 1.181 (0.881–1.584) | 0.265 |
| Other gram negatives | 1.264 (0.957–1.670) | 0.099 |
| Burkholderia cepacia | 1.638 (1.120–2.397) | 0.011 |
| Aspergillus species | 1.250 (0.999–1.563) | 0.051 |
| Nontuberculous mycobacteria | 1.230 (0.671–2.254) | 0.504 |
BMI and height calculated as z-scores using infant NHS and CDC tables.30 [30]
Gender differences in age of infection and outcomes
We further evaluated gender-based differences in microbial infection. We assessed mean age of first pathogen acquisition and impact of infection on survival outcomes in males versus females (Table 4). Females acquired nonmucoid and mucoid P. aeruginosa, MSSA, MRSA, H. influenza, A. xylosoxidans, other gram negative organisms, Aspergillus species, and nontuberculous mycobacterium at significantly earlier ages than males, with the largest age difference being in those with atypical mycobacterium. Moreover, we evaluated gender specific mortality in males versus females infected with these pathogens and found females to have a decreased life expectancy with all pathogens except K. pneumonia. Importantly, bacteria previously reported to impact survival in CF such as P. aeruginosa, MRSA, and B. cepacia were associated with poorer survival outcomes in females.18,19,26 In addition, pathogens in a variety of categories including nontuberculous mycobacterium, fungi and bacteria showed disparities.
Table 4.
Pathogen Acquisition and Life Expectancy in CF Males Versus Females
| Variable | Sex | Number of subjects | Mean age of first culture (years)±SD | p value (time to first culture) | Median age of death (years) (95%CI) | p value (survival) |
|---|---|---|---|---|---|---|
| Pseudomonas aeruginosa, nonmucoid | M | 10,762 | 10.87±9.44 | <0.0001 | 41.7 (40.5–43.6) | <0.0001 |
| F | 10,222 | 10.03±9.12 | 39.5 (38.2–40.9) | |||
| P. aeruginosa, mucoid | M | 8,442 | 13.32±8.99 | <0.0001 | 41.5 (39.6–43.5) | <0.0001 |
| F | 7,933 | 12.46±8.80 | 38.9 (38.0–40.7) | |||
| MSSA | M | 14,521 | 5.68±7.65 | 0.0008 | 39.8 (38.8–41.7) | <0.0001 |
| F | 13,137 | 5.37±7.38 | 37.6 (36.1–38.6) | |||
| MRSA | M | 4,277 | 12.40±9.33 | 0.0007 | 41.5 (38.8–45.9) | 0.0003 |
| F | 4,087 | 11.72±9.12 | 40.7 (38.6–44.6) | |||
| Haemophilus influenzae | M | 9,902 | 6.16±7.52 | 0.0208 | 39.9 (38.8–42.2) | <0.0001 |
| F | 8,892 | 5.91±7.44 | 39.0 (37.8–40.9) | |||
| Klebsiella pneumoniae | M | 2,057 | 4.42±8.36 | 0.2930 | 40.0 (36.0–45.1) | 0.0215 |
| F | 1,965 | 4.70±8.50 | 40.3 (35.0–45.2) | |||
| Escherichia coli | M | 2,070 | 4.96±8.87 | 0.5230 | 41.1 (36.8–45.9) | 0.0013 |
| F | 1,965 | 4.78±8.52 | 38.9 (33.7–43.6) | |||
| Achromobacter xylosoxidans | M | 5,070 | 10.47±9.02 | 0.0104 | 40.3 (38.8–43.7) | <0.0001 |
| F | 5,152 | 10.01±8.83 | 38.5 (36.5–40.1) | |||
| Gram negatives | M | 7,815 | 8.71±9.43 | 0.0130 | 39.8 (38.5–41.0) | <0.0001 |
| F | 7,198 | 8.33±9.20 | 38.5 (36.6–39.7) | |||
| Burkholderia cepacia | M | 1,494 | 13.71±8.41 | 0.0930 | 32.3 (31.3–33.7) | 0.0008 |
| F | 1,221 | 13.16±8.36 | 29.1 (28.0–30.4) | |||
| Aspergillus species | M | 5,783 | 13.13±8.69 | 0.0003 | 41.5 (39.4–43.0) | <0.0001 |
| F | 5,345 | 12.54±8.48 | 37.2 (35.4–38.6) | |||
| Nontuberculous mycobacteria | M | 921 | 16.27±9.56 | 0.0006 | 46.4 (44.2–49.7) | 0.0039 |
| F | 839 | 14.73±9.24 | 44.6 (39.0–49.1) |
Finally, we examined the individual effects of pathogens on female gender-based survival using multivariate analysis (Table 5). All pathogens increased female-based risk of death from the original hazard ratio of 1.252. Interestingly, MRSA had a similar impact on female risk of death as mucoid and nonmucoid P. aeruginosa, implying that these gender-based mechanisms may not be unique to gram negative bacteria or to P. aeruginosa.
Table 5.
Multivariate Cox Regression for Survival
| Variables | Hazard ratio (95% CI) | p value |
|---|---|---|
| Female | 1.598 (1.384–1.844) | <0.0001 |
| Pseudomonas aeruginosa, nonmucoid | 1.346 (1.156–1.566) | 0.0001 |
| Female | 1.606 (1.392–1.854) | <0.0001 |
| P. aeruginosa, mucoid | 2.179 (1.832–2.592) | <0.0001 |
| Female | 1.508 (1.330–1.709) | <0.0001 |
| MSSA | 0.723 (0.635–0.823) | <0.0001 |
| Female | 1.593 (1.399–1.813) | <0.0001 |
| MRSA | 2.348 (1.980–2.784) | <0.0001 |
| Female | 1.518 (1.339–1.720) | <0.0001 |
| Haemophilus influenzae | 0.562 (0.448–0.711) | <0.0001 |
| Female | 1.520 (1.340–1.723) | <0.0001 |
| Klebsiella pneumonia | 2.183 (1.489–3.199) | <0.0001 |
| Female | 1.523 (1.344–1.727) | <0.0001 |
| Escherichia coli | 2.171 (1.528–3.083) | <0.0001 |
| Female | 1.511 (1.333–1.713) | <0.0001 |
| Achromobacter xylosoxidans | 1.756 (1.453–2.123) | <0.0001 |
| Female | 1.519 (1.340–1.722) | <0.0001 |
| Other gram negatives | 1.637 (1.369–1.958) | <0.0001 |
| Female | 1.533 (1.352–1.738) | <0.0001 |
| Burkholderia cepacia | 2.656 (2.137–3.302) | <0.0001 |
| Female | 1.516 (1.337–1.718) | <0.0001 |
| Aspergillus species | 1.655 (1.398–1.959) | <0.0001 |
| Female | 1.521 (1.342–1.724) | <0.0001 |
| Nontuberculous mycobacteria | 1.279 (0.767–2.133) | 0.3452 |
Discussion
This large registry analysis demonstrated that women with CF have worse outcomes than men, are predisposed to earlier respiratory infections and have decrease life expectancies once colonized with respiratory pathogens. We examined 32,766 CF patients in the United States, and show for the first time that there are gender-based differences in microbial infection in CF airways beyond P. aeruginosa.
Previous groups have demonstrated worse outcomes in CF women relative to men, particularly in the 1990s.5,18,19 Since that time, a host of literature has supported or refuted this gender disparity. The potential explanations for a gender-based difference in CF patients are only beginning to be revealed. Some of the hypotheses for the worse outcomes in women with CF have included descriptions of an immunologic difference resulting in earlier infections and poorer responses to organisms such as P. aeruginosa.21,31,32 Chotirmall and colleagues demonstrated that estrogen induces mucoid conversion of P. aeruginosa, a more antibiotic resistant form of the bacteria, and showed an association between increased exacerbations and mucoid conversion in women with CF during high estrogen states.31 We found that not only do women acquire P. aeruginosa at an earlier age, but they also acquire MSSA, MRSA, H. influenzae, A. xylosoxidans, B. cepacia, Aspergillus species, and nontuberculous mycobacterium at earlier ages, often even prior to puberty. This earlier acquisition was combined with increased mortality in women versus men in those with mucoid and nonmucoid P. aeruginosa, MRSA, A. xylosoxidans, B. cepacia, Aspergillus species, and nontuberculous mycobacterium among others. Therefore the sensitivity of women to infections in CF is not unique to P. aeruginosa and spans other gram negative organisms, gram positive organisms, nontuberculous mycobacterium, and fungal infections. Descriptions of a gender disparity in nontuberculous mycobacterial infections outside of CF have been well described where women are at increased risk for infection, but primarily later in life, particularly after menopause.33–35 A genetic basis may underlie this as at least one study found that 36% of patients with mycobacterial infection who did not have CF did carry one cystic fibrosis transmembrane conductance regulator (CFTR) disease-causing gene, while others have hypothesized morphometric or nutritional explanations.33–35 Though the cause of the gender disparity in infectious susceptibility is unclear, preventing early acquisition of infections serves as a potential avenue of future studies and treatments.
Several groups have also reported that sex hormones alter components of the mucociliary apparatus, comprised of mucus, cilia, and the airway epithelial air surface liquid layer. The mucociliary apparatus serves as a unique mechanism by which the airways protect the lungs from infection by trapping pathogens and propelling them out of the lungs. Reports demonstrated that sex hormones impact airway epithelial cell apical sodium and chloride transport,36,37 alter airway surface liquid (ASL) volume,38 influence inflammatory mediators that can predispose to infection and colonization,31 and change cilia beat frequency in the airways.39 It is still unknown if these effects directly translate to poorer female outcomes; however, estrogen was shown to decrease chloride secretion via calcium mediated chloride channels in women with CF as measured by changes in nasal potential difference, and resulted in decreased airway surface liquid volume in CF airway epithelium.38 This altered mucociliary clearance apparatus may explain in part the disadvantage females have to an array of pathogens in CF. Respiratory infections typically trigger CF exacerbations in these patients and our group also demonstrated that post-puberty CF women have a distinct increase in rate of pulmonary exacerbations relative to men.40 However, sex hormones are likely not the only explanation for this gender disparity given that some reports suggest the gender difference in CF may start before pubertal hormone surges and that we demonstrated earlier bacterial infection in females in this study prior to puberty in several cases.5
Other evidence hypothesizes possible differences in innate immune response. One report showed that estrogen inhibited interleukin-8 production in CF bronchial epithelial cells in vitro and hypothesized that this would hinder neutrophil recruitment and inflammatory responses in women.41–43 Others have shown that estrogen inhibits the respiratory burst in neutrophils, potentially hindering their killing capacity. Others believe that the gender disparity has very little to do with host immunity and more to do with gender-based differences in morphometrics, such as airway diameter or airway branch angles, which has not yet been evaluated. This type of data was not available in this study given the lack of radiology imaging in association with this database, but would be interesting to explore at a more local CF center level.
Given that our study was retrospective in design, there are several other inherent limitations to this study. For example, variables not available in the registry that could impact results include patient exercise habits and adherence to medications. We also did not incorporate medications prescribed in the analysis. Though we lacked these data, previous studies showed no differences in adherence to therapies between males and females with CF.44 Further, we were unable to determine if earlier radiographic changes were seen in females versus males, as is often described to occur even prior to changes in lung function or if bronchiectasis severity scores were worse in women relative to men. These types of analysis would be interesting to perform at single centers that can perform radiographic correlates. In addition, details regarding pulmonary exacerbations such as which patients received antibiotics for a true exacerbation versus a “tune-up” or “clean-out” was also unavailable. The microbiology data in this study suggests that women have a higher prevalence and worse outcome once infected with several bacteria beyond P. aeruginosa, however, we submit that we excluded transplant patients from this analysis, which could have impacted the results. Finally, we did not have data on socioeconomic status or environmental factors that could impact male versus female outcomes and acknowledge that these kinds of variables could affect the results. Despite these limitations, this is the largest analysis to suggest that women with CF have worse outcomes in the setting of infections beyond P. aeruginosa. Further work into mechanisms behind this including the impact of hormones on innate immunity is warranted.
Finally, the gender disparity in CF is not unique to this airway disease. A host of literature supports worse outcomes in females with asthma and COPD. Interestingly, this disparity is opposite to that seen in the global United States population where the life expectancy for females is 80.9 versus 76.3 in males as of 2010, a 4.6 year difference in favor of women as opposed to the 2.7 year difference in favor of men found in our analysis, demonstrating a theoretical 7.3 year disadvantage in women with CF relative to the general population. Therefore, further research into the mechanisms behind this disparity is warranted and may shed light into potential therapeutic options such as hormone modulating therapies that may be delivered directly to the airways.
Conclusions
In conclusion, in evaluating the impact of gender on outcomes in a large CF cohort, females were found to have an increased mortality, more commonly acquire infections at an earlier age than males, and have shorter life expectancies in the setting of respiratory infections. These findings were seen in association with a number of pathogens including gram positive, gram negative, fungal, and nontubercuous mycobacterial organisms. This study corroborates a host of literature describing potential disadvantages of women in relation to airway diseases and supports the need for further work to understand mechanisms behind this gender disparity.
Acknowledgments
We would like to thank the Cystic Fibrosis Foundation for use of the CF Patient Registry data and funding support, as well as Children's Clinical Research Advisory Committee for funding, and the University of Texas Southwestern Clinical and Translational Research Center for statistical support.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Marshall BC. Cystic Fibrosis Foundation Patient Registry: 2013. Annual Data Report. Available at http://cff.org Accessed on December5, 2014
- 2.Fuchs HJ, Borowitz DS, Christiansen DH, et al. . Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med 1994;331:637–642 [DOI] [PubMed] [Google Scholar]
- 3.Ramsey BW, Pepe MS, Quan JM, et al. . Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med 1999;340:23–30 [DOI] [PubMed] [Google Scholar]
- 4.Ramsey BW, Davies J, McElvaney NG, et al. . A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011;365:1663–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenfeld M, Davis R, FitzSimmons S, Pepe M. Ramsey B. Gender gap in cystic fibrosis mortality. Am J Epidemiol 1997;145:794–803 [DOI] [PubMed] [Google Scholar]
- 6.Taveira-DaSilva AM. Moss J. Optimizing treatments for lymphangioleiomyomatosis. Expert Rev Respir Med 2012;6:267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thenappan T, Shah SJ, Rich S. Gomberg-Maitland M. A USA-based registry for pulmonary arterial hypertension: 1982–2006. Eur Respir J 2007;30:1103–1110 [DOI] [PubMed] [Google Scholar]
- 8.Badesch DB, Raskob GE, Elliott CG, et al. . Pulmonary arterial hypertension: Baseline characteristics from the REVEAL Registry. Chest 2010;137:376–387 [DOI] [PubMed] [Google Scholar]
- 9.Raghu G, Chen SY, Yeh WS, et al. . Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: Incidence, prevalence, and survival, 2001–11. Lancet Respir Med 2014;2:566–572 [DOI] [PubMed] [Google Scholar]
- 10.Hyland RA, Ware S, Johnson AR. Yates DH. Incidence trends and gender differences in malignant mesothelioma in New South Wales, Australia. Scand J Work Environ Health 2007;33:286–292 [DOI] [PubMed] [Google Scholar]
- 11.Wisnivesky JP. Halm EA. Sex differences in lung cancer survival: Do tumors behave differently in elderly women? J Clin Oncol 2007;25:1705–1712 [DOI] [PubMed] [Google Scholar]
- 12.Cerfolio RJ, Bryant AS, Scott E, et al. . Women with pathologic stage I, II, and III non-small cell lung cancer have better survival than men. Chest 2006;130:1796–1802 [DOI] [PubMed] [Google Scholar]
- 13.Morrissey BM. Harper RW. Bronchiectasis: Sex and gender considerations. Clin Chest Med 2004;25:361–372 [DOI] [PubMed] [Google Scholar]
- 14.Farha S, Asosingh K, Laskowski D, et al. . Effects of the menstrual cycle on lung function variables in women with asthma. Am J Respir Crit Care Med 2009;180:304–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Celli B, Vestbo J, Jenkins CR, et al. . Sex differences in mortality and clinical expressions of patients with chronic obstructive pulmonary disease. The TORCH experience. Am J Respir Crit Care Med 2011;183:317–322 [DOI] [PubMed] [Google Scholar]
- 16.Han MK, Postma D, Mannino DM, et al. . Gender and chronic obstructive pulmonary disease: Why it matters. Am J Respir Crit Care Med 2007;176:1179–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. European Network for Understanding Mechanisms of Severe Asthma. Eur Respir J 2003;22:470–477 [DOI] [PubMed] [Google Scholar]
- 18.Corey M. Farewell V. Determinants of mortality from cystic fibrosis in Canada, 1970–1989. Am J Epidemiol 1996;143:1007–1017 [DOI] [PubMed] [Google Scholar]
- 19.Liou TG, Adler FR, Fitzsimmons SC, et al. . Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol 2001;153:345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.FitzSimmons SC. The changing epidemiology of cystic fibrosis. J Pediatr 1993;122:1–9 [DOI] [PubMed] [Google Scholar]
- 21.Demko CA, Byard PJ, Davis PB. Gender differences in cystic fibrosis: Pseudomonas aeruginosa infection. J Clin Epidemiol 1995;48:1041–1049 [DOI] [PubMed] [Google Scholar]
- 22.Nick JA, Chacon CS, Brayshaw SJ, et al. . Effects of gender and age at diagnosis on disease progression in long-term survivors of cystic fibrosis. Am J Respir Crit Care Med 2010;182:614–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Assael BM, Castellani C, Ocampo MB, et al. . Epidemiology and survival analysis of cystic fibrosis in an area of intense neonatal screening over 30 years. Am J Epidemiol 2002;156:397–401 [DOI] [PubMed] [Google Scholar]
- 24.Viviani L, Bossi A. Assael BM. Absence of a gender gap in survival. An analysis of the Italian registry for cystic fibrosis in the paediatric age. J Cyst Fibros 2011;10:313–317 [DOI] [PubMed] [Google Scholar]
- 25.Verma N, Bush A. Buchdahl R. Is there still a gender gap in cystic fibrosis? Chest 2005;128:2824–2834 [DOI] [PubMed] [Google Scholar]
- 26.Dasenbrook EC, Checkley W, Merlo CA, et al. . Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA 2010;303:2386–2392 [DOI] [PubMed] [Google Scholar]
- 27.Moran A, Brunzell C, Cohen RC, et al. . Clinical care guidelines for cystic fibrosis-related diabetes: A position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care 2010;33:2697–2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fogarty AW, Britton J, Clayton A. Smyth AR. Are measures of body habitus associated with mortality in cystic fibrosis? Chest 2012;142:712–717 [DOI] [PubMed] [Google Scholar]
- 29.Chamnan P, Shine BS, Haworth CS, Bilton D. Adler AI. Diabetes as a determinant of mortality in cystic fibrosis. Diabetes Care 2010;33:311–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Boer K, Vandemheen KL, Tullis E, et al. . Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax 2011;66:680–685 [DOI] [PubMed] [Google Scholar]
- 31.Chotirmall SH, Smith SG, Gunaratnam C, et al. . Effect of estrogen on pseudomonas mucoidy and exacerbations in cystic fibrosis. N Engl J Med 2012;366:1978–1986 [DOI] [PubMed] [Google Scholar]
- 32.Maselli JH, Sontag MK, Norris JM, et al. . Risk factors for initial acquisition of Pseudomonas aeruginosa in children with cystic fibrosis identified by newborn screening. Pediatr Pulmonol 2003;35:257–262 [DOI] [PubMed] [Google Scholar]
- 33.Mirsaeidi M, Hadid W, Ericsoussi B, Rodgers D. Sadikot RT. Non-tuberculous mycobacterial disease is common in patients with non-cystic fibrosis bronchiectasis. Int J Infect Dis 2013;17:e1000–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsuyuguchi K, Suzuki K, Matsumoto H, et al. . Effect of oestrogen on Mycobacterium avium complex pulmonary infection in mice. Clin Exp Immunol 2001;123:428–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim RD, Greenberg DE, Ehrmantraut ME, et al. . Pulmonary nontuberculous mycobacterial disease: Prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med 2008;178:1066–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sweezey NB, Ghibu F. Gagnon S. Sex hormones regulate CFTR in developing fetal rat lung epithelial cells. Am J Physiol 1997;272:L844–851 [DOI] [PubMed] [Google Scholar]
- 37.Singh AK, Schultz BD, Katzenellenbogen JA, et al. . Estrogen inhibition of cystic fibrosis transmembrane conductance regulator-mediated chloride secretion. J Pharmacol Exp Ther 2000;295:195–204 [PubMed] [Google Scholar]
- 38.Coakley RD, Sun H, Clunes LA, et al. . 17beta-Estradiol inhibits Ca2+-dependent homeostasis of airway surface liquid volume in human cystic fibrosis airway epithelia. J Clin Invest 2008;118:4025–4035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jain R, Ray JM, Pan JH. Brody SL. Sex hormone-dependent regulation of cilia beat frequency in airway epithelium. Am J Respir Cell Mol Biol 2012;46:446–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sutton S, Rosenbluth D, Raghavan D, Zheng J. Jain R. Effects of puberty on cystic fibrosis related pulmonary exacerbations in women versus men. Pediatr Pulmonol 2014;49:28–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chotirmall SH, Greene CM, Oglesby IK, et al. . 17Beta-estradiol inhibits IL-8 in cystic fibrosis by up-regulating secretory leucoprotease inhibitor. Am J Respir Crit Care Med 2010;182:62–72 [DOI] [PubMed] [Google Scholar]
- 42.Chotirmall SH, Greene CM.McElvaney NG. Immune, inflammatory and infectious consequences of estrogen in women with cystic fibrosis. Expert Rev Respir Med 2012;6:573–575 [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Cela E, Gagnon S. Sweezey NB. Estrogen aggravates inflammation in Pseudomonas aeruginosa pneumonia in cystic fibrosis mice. Respir Res 2010;11:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masterson TL, Wildman BG, Newberry BH. Omlor GJ. Impact of age and gender on adherence to infection control guidelines and medical regimens in cystic fibrosis. Pediatr Pulmonol 2011;46:295–301 [DOI] [PubMed] [Google Scholar]