Abstract
Background: This study reports an open trial of family-based cognitive-behavioral therapy (CBT) in children and adolescents with obsessive-compulsive disorder (OCD) exhibiting an onset pattern consistent with pediatric acute-onset neuropsychiatric syndrome (PANS).
Methods: Eleven primarily Caucasian youth with PANS-related OCD (range=4–14 years; 6 boys) who were incomplete responders to antibiotic treatment, received family-based CBT delivered either face-to-face or via web camera.
Results: All participants completing treatment (8 of 8) were considered improved at posttreatment, and average obsessive-compulsive symptom severity was reduced by 49%. Significant reductions in obsessive-compulsive symptom severity and in clinician- and parent-rated OCD-related impairment were noted. Reductions in parent- and child-rated anxiety, child-rated OCD-related impairment, and comorbid neuropsychiatric symptoms were not statistically significant.
Conclusions: Gains were maintained at follow-up, with 100% (6 of 6) of those assessed remaining improved. Implications for treatment and further research are discussed.
Introduction
Pediatric acute-onset neuropsychiatric syndrome (PANS) (Swedo et al. 2012), an umbrella term also encompassing pediatric autoimmune neuropsychiatric disorder associated with streptococcus (PANDAS) (Swedo et al. 1998; Murphy et al. 2010), is a phenotype of obsessive-compulsive disorder (OCD) wherein symptoms present with an abrupt and sudden onset, episodic course, concomitant neuropsychiatric sequelae (e.g., rigid or repetitive behaviors, tics, worsening fine motor skills, frequent urination), and occurrence of obsessive-compulsive symptoms (Murphy and Pichichero 2002; Murphy et al. 2014). The nature of onset and clinical presentation for PANS differs from the typical pattern of gradual onset found in traditional pediatric OCD (Swedo et al. 2012), and is associated with significant functional impairment across multiple domains (Murphy et al. 2012; Swedo et al. 2012; Bernstein et al. 2013; Murphy et al. 2014a).
The majority of treatments researched for PANS-type OCD have been pharmacological (e.g., intravenous immunoglobulin [IVIG], plasmapheresis, prednisone) (Allen et al. 1995; Perlmutter et al. 1999; Murphy et al. 2014a). For example, Perlmutter et al. (1999) supported the efficacy of IVIG and plasma exchange via reductions of 45% and 58%, respectively, on the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) (Goodman et al. 1989). However, the side effect profile, and common experience of incomplete response or symptom relapse for many youth utilizing such treatments (Murphy and Pichichero 2002) warrant further investigation into alternative treatment approaches.
The use of prophylactic antibiotic therapy has also been examined in youth with PANDAS/PANS. Snider et al. (2005) compared penicillin and azithromycin in a 12 month parallel baseline design among 23 youth with PANDAS. Although no significant difference was found between the penicillin and azithromycin groups, youth in both conditions showed significant decreases in the average instances of exacerbated neuropsychiatric symptoms and streptococcal infection as compared with the baseline period. Other data have supported prophylactic antibiotic therapy (Garvey et al. 1999), and, although controlled data are limited, numerous clinical accounts suggest the benefit of acute antibiotic therapy among youth with PANS (Murphy et al. 2014b). However, some youth remain symptomatic after an adequate course of antibiotic treatment, with some children experiencing symptom recurrence after terminating antibiotic regimen, subsequent exposure to infection, or for undetermined reasons.
Although the etiology of PANS remains unknown (Murphy et al. 2014), there is an attenuation of behavioral impulse control functioning, as evidenced by commonly comorbid difficulties with emotion regulation, oppositionality, and developmental regression, thereby increasing the difficulty of suppressing a behavioral response to anxiety and habituating to subjective distress (Murphy and Pichichero 2002). Behavioral theory holds relevance in understanding why some children who receive benefit (as well as those who do not) from immune modulating therapies remain symptomatic. Obsessive-compulsive symptoms are maintained through a combination of classical and operant conditioning, wherein a specific event, setting, or situation becomes paired with anxiety, resulting in a classically conditioned relationship between the previously neutral stimulus and the conditioned response (CR), namely anxiety. Once this relationship has been established, it is strengthened or maintained through operant contingencies. First, the act of avoiding the conditioned stimulus (CS) or engaging in rituals when exposed results in distress relief, thereby negatively reinforcing the conditioned relationship between CS and CR. Second, to reduce child distress/impairment, family members accommodate OCD symptoms, which similarly reinforces the obsessive-compulsive cycle vis-à-vis distress reduction. Although immunotherapies may be successful in addressing the etiological determinants that facilitated the initial establishment of a relationship between CS and CR, the learning of the fear relationship may be maintained, thus perpetuating symptom expression.
Given the efficacy of cognitive-behavioral therapy (CBT) for pediatric OCD (Abramowitz et al. 2005; Pediatric OCD Treatment Study Team 2004; Piacentini et al. 2011; Storch et al. 2013) and the aforementioned behavioral theory, CBT may present an efficacious approach to treating this population, especially in the instance of partial response. Building on a prior case report (Storch et al. 2004a), Storch et al. (2006) conducted an open trial of intensive outpatient CBT among 7 youth 9–13 years of age with PANDAS. Eighty-six percent of youth met criteria for treatment response after acute treatment, with 71% of cases meeting criteria for OCD remission. The waxing and waning nature of symptoms among youth with PANDAS was evident at follow-up assessment, as three of the six responders experienced partial or full relapse; both participants experiencing full relapse presented with comorbid oppositional behavior, which was not targeted by the treatment protocol.
The present study reports an open trial examining the preliminary efficacy of CBT for PANS-type OCD, delivered in person or via web camera, among youth who were partial responders or nonresponders to sequentially provided antibiotic treatment. We predicted that CBT would be associated with a reduction in obsessive-compulsive symptom severity, functional impairment, and anxiety symptoms.
Methods
Participants
Participants were 11 children (6 boys) 4–14 years of age (mean=9.4±2.7 years), 91% (10/11) Caucasian, identified as meeting criteria for the PANS subtype (Table 1 provides principal symptoms). Anxiety disorder diagnoses were determined via administration of Anxiety Disorder Interview Schedule for DSM-IV-Child Interview Schedule – Parent version (ADIS-IV-P) (Silverman and Albano 1996), and additional psychiatric diagnoses confirmed through use of best estimate procedures (Leckman et al. 1982) in which consensus was sought between two licensed child and adolescent clinical psychologists, regarding the diagnosis of OCD and the presence of elements required for inclusion. In this procedure, the two clinicians discussed clinical information provided by the child and that child's family, in addition to reviewing the participant's completed measures and past clinical records to provide an accurate diagnostic profile. Exclusion criteria included absence of 100% agreement for primary diagnosis. No youth were excluded based upon diagnostic disagreement.
Table 1.
Individual Presentation Data
| Subject | Age | Gender | Primary obsessions | Primary compulsions |
|---|---|---|---|---|
| 1 | 9 | Female | Contamination, Eating | Repeating, Eating, Food Aversion |
| 2 | 10 | Female | Harming others, Morality, Need to know | Checking, Repeating, Reassurance |
| 3 | 4 | Male | Contamination, Harming self/others, Forbidden images, Losing things | Washing, Hoarding, Confessing |
| 4 | 9 | Female | Need to know, Need for “just right” | Checking, Repeating, Symmetry, Touch/Tap |
| 5 | 11 | Female | Contamination, Violent images, Thoughts of numbers/sounds | Washing, Repeating, Walking, Confessing |
| 6 | 6 | Female | Contamination, Need to know, Dysmorphophobia, Religiosity | Washing, Checking, Repeating, Symmetry, Walking, Confessing |
| 7 | 9 | Male | Harming self, Religiosity | Washing, Checking, Repeating, Symmetry, Arranging |
| 8 | 8 | Male | Contamination, Harming self/others, Forbidden thoughts, Losing things, Religiosity | Washing, Checking, Hoarding, Mental rituals |
| 9 | 11 | Male | Forbidden images, Religiosity | Avoiding, Mental rituals |
| 10 | 12 | Male | Contamination, Religiosity | Washing, Repeating |
| 11 | 14 | Male | Harming self, Religiosity, Magical thinking | Repeating, Symmetry, Hoarding, Walking |
PANS criteria were confirmed by an experienced board-certified child and adolescent psychiatrist. Many participants (6 of 11; 54%) had an exacerbation or symptom onset within several weeks of group A streptococcal (GAS) exposure, infection, or sore throat and fever. All participants shared a history of dramatic onset. Participants were considered to have been incomplete responders if they received a minimum of 4 weeks of antibiotic treatment deemed by the treating physician (T.K.M.) to be of reasonable clinical dosage and duration based on the child's age, body mass index, and tolerability, with no symptom remission after 4 weeks. Although there are no formal guidelines delineating the specific antibiotic dosage for use in PANS, the 4 week duration was based upon the physician's clinical experience suggesting that most of the potential clinical response would be achieved in this window.
Inclusion criteria included: 1) Primary diagnosis of OCD at pretreatment derived from the ADIS-IV-P (Silverman and Albano 1996), with a clinical severity rating of ≥4; 2) symptom onset recent (≤6 months) and of dramatic fashion; 3) completion of a 4 week regimen of antibiotic treatment; 4) Children's Yale-Brown Obsessive-Compulsive Scale (CY-BOCS) total score ≥16, or CY-BOCS Compulsion Severity Score of ≥8 in the absence of articulated obsessions; and 5) being between the ages of 4 and 14 years. Children were excluded if they met any of the following criteria: 1) Autism spectrum disorder diagnosis, organic brain syndrome, or intellectual deficiency; 2) current suicidality measured by the ADIS-IV-P and/or all available clinical information; 3) recent change in any antidepressant medication in the preceding 8 weeks; or 4) concurrent psychotherapy. One participant was taking a selective serotonin reuptake inhibitor (SSRI) on presentation, and all participants maintained antibiotic regimen during course of CBT.
Procedures
Study procedures were approved by the local institutional review board. Participants were recruited from the patient flow at a university-based specialty clinic. The first or second author (J.M.N. or C.J.) obtained written parental consent, and child assent for youth≥6 years of age. Thereafter, a trained clinician administered the CY-BOCS to the child and parent in a private office, according to assessment recommendations for youth (e.g., Lewin and Piacentini 2010). Following administration of clinician-rated instruments, a research assistant provided instructions for completing child- and parent-report measures. A member of the research staff remained available to answer any questions and/or to assist the child with completion of the assessment packet. In all, five total assessments were conducted by an independent evaluator not involved in treatment: Pretreatment, Session 4, Session 8, posttreatment, and 1–4 month follow-up. The assessments immediately following CBT sessions 4 and 8 only involved administration of measures examining parent- and clinician-rated OCD symptom severity, whereas other visits included administration of all measures. Although follow-up assessment was originally planned for 3 months following treatment, difficulty in gathering responses from families resulted in actual follow-up assessment times ranging from 1 month to 4 months (mean=2 months, 1 week) after treatment completion.
Treatment
The treatment protocol was a modified version of the Pediatric OCD Treatment Study Manual (2004). It was designed to provide a maximum of 14 CBT sessions (∼ 50 minutes each) in person or via web camera on a twice-weekly schedule. Twice weekly sessions were implemented because of the possibility of the impracticality of maintaining any existing antibiotic regimen for the duration of a typical course of CBT (e.g., 12–16 weeks). Provision of CBT via web camera was offered to all families to better address factors that restricted the use of CBT (e.g., travel and schedule limitations, cost to family because of missing work and/or school), as well as to maximize exposure authenticity (conducting exposure tasks in natural settings). The majority of participating families (9 of 11) requested therapy via web camera, although two families (living less than 5 minutes from our clinic) participated in in-person sessions. Among youth with OCD, CBT delivered via web camera has demonstrated similar treatment effects to those of in-person treatment (Storch et al. 2011).
The modified protocol included modules providing: Psychoeducation related to OCD, PANS, and CBT (sessions 1–2); hierarchy development (sessions 2–3); cognitive restructuring introduction and practice when developmentally appropriate (sessions 3–4); and exposure activities (sessions 5–14). Although the protocol followed a general structure consistent with the Pediatric OCD Treatment Study (2004), various modifications were incorporated to address individual needs associated with age (e.g., developmental scaling of materials, techniques to increase parent involvement) and maladaptive interaction dynamics between parent and child (e.g., accommodation).
Measures
CY-BOCS
The CY-BOCS (Scahill et al. 1997) is a semistructured psychometrically sound (Storch et al. 2004b; Lewin et al. 2013) clinician-administered assessment of obsessive-compulsive symptom presence and severity. The CY-BOCS consists of two five-item subscales: Obsessions severity and compulsions severity, the sum of which provides a total score. Internal consistency rating was 0.76 for the current sample.
Clinical Global Impressions–Severity (CGI-Severity)
The CGI-Severity (National Institute of Mental Health 1985) is a single-item rating of illness severity allowing the clinician to rate the global severity of obsessive-compulsive symptoms, with scores ranging from 0 (no illness) to 6 (serious illness). The CGI-Severity was rated at pretreatment, posttreatment, and follow-up.
CGI-Improvement
The CGI-Improvement (Guy 1976) is a single-item rating of clinical improvement that allows the clinician to rate improvement in obsessive-compulsive symptoms, with scores ranging from “very much worse” to “very much improved.” The CGI-Improvement was rated at posttreatment and follow-up. Participants rated as “much improved” and “very much improved” were defined as treatment responders.
Child-Global Assessment Scale (C-GAS)
The C-GAS (Shaffer et al. 1983) was used to assess the child's overall level of functioning. Based on clinical information gathered via observation and interview of the parent and child, the clinician provided a rating of overall psychological functioning on a scale of 0–100.
Screen for Childhood Anxiety Related Emotional Disorders (SCARED)
The parent and child version of the SCARED (Birmaher et al. 1997) measure symptoms of anxiety, including the most common symptoms of panic/somatized anxiety, generalized anxiety, separation anxiety, social phobia, and school phobia. The SCARED has demonstrated good parent–child agreement, internal consistency, test–retest reliability, and discriminant validity, and is sensitive to treatment (RUPP Anxiety Study Group 2001). Internal consistency ratings were 0.97 and 0.98 for the child and parent versions, respectively.
Child Obsessive Compulsive Impact Scale-Child/Parent Versions (COIS-C/P)
The COIS-C/P (Piacentini and Jaffer 1999) assess the extent to which pediatric OCD causes impairment in specific areas of child psychosocial functioning. Items specify potential difficulties in school (16 items), social (19 items), and home/family (17 items) activities. The four remaining questions assess global impairment related to school, social activities, going places, and home/family activities. Internal consistency ratings were 0.95 and 0.96 for the child and parent versions, respectively.
Child Behavior Checklist (CBCL)
The CBCL (Achenbach and Rescorla 2001) is a commonly used, psychometrically sound 118 item self-report scale assessing specific child behaviors from the parent's perspective. The CBCL provides a total behavior problem score, several subscale scores, and scores on two dimensions of behavioral dysfunction: Internalizing (e.g., anxiety, depression) and Externalizing (e.g., aggression, impulsivity).
Data analysis
Using procedures provided by Schlomer et al. (2010), missing data were determined to be at random. Given that listwise deletion occurred with variables of interest in <5% of response opportunities (Arbuckle 1996), multiple imputation was conducted using SOLAS 4.0. Examination of all data at pretreatment, session 4, session 8, posttreatment, and follow-up assessment points demonstrated normal distributions. Results were analyzed using repeated measures t tests comparing pre- and posttreatment. In addition, CY-BOCS data were compared across all time points using repeated measures ANOVA. Correction for family-wise error rate was not conducted, given the preliminary nature of the study. Diagnostic outcomes were reviewed using descriptive and graphical methods. To determine the degree to which gains were maintained after treatment, follow-up was compared with posttreatment. The clinical relevance of findings was calculated via Cohen's d (Cohen 1988).
Results
Of the 11 consenting participants, 2 were lost to attrition prior to the session 4 assessments; in both cases, participants' symptom intensity was such that more intensive treatment options were sought, and study-based treatment terminated accordingly. An additional participant completed all sessions, but dropped out prior to completing posttreatment assessment for unknown reasons. The remaining eight participants completed an average of 12.75 sessions (range 4–14). Two of the eight treatment-completing participants were lost to attrition prior to completing the follow-up assessment. All instruments administered at follow-up were completed by the six remaining participants.
Primary outcome measures
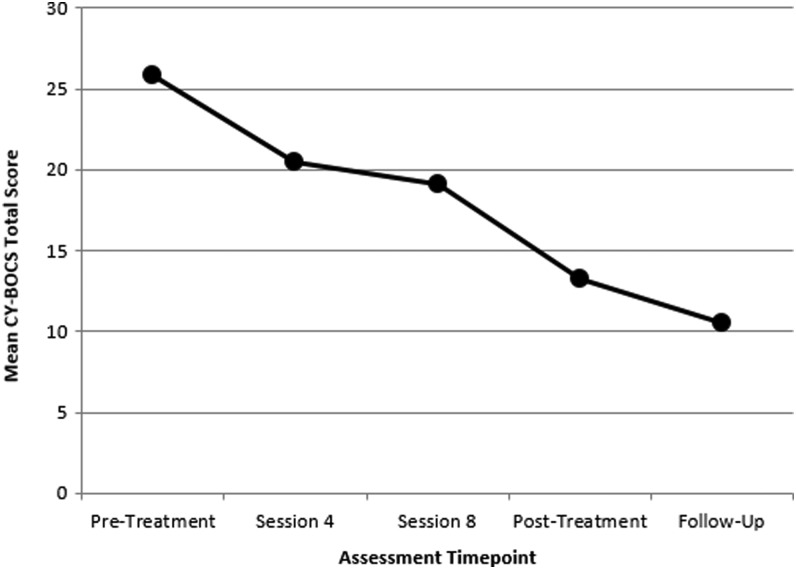
Table 2 lists mean values for all primary and secondary outcome measures across pretreatment, posttreatment, and follow-up assessment points. Repeated measures t tests indicated that the youth received significantly lower CY-BOCS scores at posttreatment relative to pretreatment (T[7]=3.72, ρ=0.01, d=1.88). This difference was maintained at follow-up assessment (T[5]=0.86, ρ=0.43). As a whole, the group demonstrated positive treatment response on the CGI-Severity, indicating significant improvement from pre- to posttreatment (T[7]=2.99, ρ=0.022). Correspondingly, 75% (6 of 8) of treatment-completing youth demonstrated at least one or more grade of improvement on this scale at posttreatment assessment. Specifically, posttreatment illness severity was classified as “no illness” for one child (subject 9), “slight” for one (subject 3), “mild” for two (subjects 1 and 8), and “moderate” for four (subjects 4, 5, 6, and 10). Improvement was sustained at follow-up assessment (T[5]=1.00, ρ=0.36), with illness severity classified as “slight” for one child (subject 8), “mild” for four (subjects 1, 3, 4, and 6), and “moderate” for one (subject 10). At posttreatment, all youth completing posttreatment assessment (8 of 8) were considered to be treatment responders on the CGI-Improvement. All participants completing the follow-up assessment (6 of 6) retained their treatment responder status. An analysis of variance (ANOVA) on CY-BOCS scores yielded significant decrease among assessment points (F[4,30]=6.96, ρ<0.001). A post-hoc Tukey test indicated that posttreatment and follow-up assessment points differed significantly at ρ<0.01; session 4 and session 8 assessment points were not significantly different from the other assessment points. Figure 1 provides a plot of mean CY-BOCS total scores across assessment administration time points.
Table 2.
Primary and Secondary Outcome Measures Data
| Scale | Pre mean (SD) (n,8) | Post mean (SD) (n,8) | Pre-post effect size | Follow-up mean (SD) (n,6) | Post-follow-up effect size |
|---|---|---|---|---|---|
| CY-BOCS | 25.8 (4.6) | 13.2* (8.3) | 1.88 | 12.8 (4.7) | 0.06 |
| CGI-Severity | 3.8 (1.0) | 2.1* (1.1) | 1.62 | 2.0 (0.6) | 0.11 |
| SCARED-P | 29.8 (21.6) | 16.1 (13.6) | 0.76 | 14.0 (9.4) | 0.18 |
| SCARED-C | 26.4 (19.4) | 22.2 (12.7) | 0.26 | 13.5 (14.8) | 0.63 |
| COIS-P | |||||
| Home | 9.3 (4.8) | 4.3* (3.7) | 1.17 | 4.5 (1.9) | −0.07 |
| School | 7.9 (7.1) | 2.4* (2.6) | 1.03 | 5.2 (4.5) | −0.76 |
| Social | 7.1 (6.4) | 0.6* (1.1) | 1.42 | 2.5 (3.0) | −0.84 |
| COIS-C | |||||
| Home | 8.5 (4.8) | 5.3 (3.7) | 0.75 | 2.0 (1.4) | 1.18 |
| School | 7.8 (7.7) | 3.2 (2.3) | 0.81 | 2.5 (0.7) | 0.41 |
| Social | 4.8 (5.8) | 2.5 (1.4) | 0.54 | 0.5 (0.7) | 1.81 |
| CBCL-Int | 16.9 (11.7) | 7.8* (7.8) | 0.92 | 10.0 (9.9) | −0.25 |
| CBCL-Ext | 11.8 (9.3) | 10.6 (6.0) | 0.15 | 9.0 (7.5) | 0.24 |
Effect size (Cohen's d). Post-follow-up effect size compared follow-up administration results with those obtained at posttreatment.
ρ<0.05.
CY-BOCS, Children's Yale-Brown Obsessive-Compulsive Inventory; CGI-Severity, Clinical Global Impressions-Severity; SCARED-P, Screen for Childhood Anxiety Related Emotional Disorders –Parent Version; SCARED-C, Screen for Childhood Anxiety Related Emotional Disorders –Child Version; COIS-P, Child Obsessive Compulsive Impact Scale –Parent Version; COIS-C, Child Obsessive Compulsive Impact Scale –Child Version; CBC-Int, Child Behavior Checklist Internalizing Subscale; CBCL-Ext, Child Behavior Checklist Externalizing Subscale.
FIG. 1.
Plot of mean Yale-Brown Obsessive-Compulsive Scale (CY-BOCS) total scores across assessment administration time points.
Secondary outcome measures
Significantly lower parent ratings on internalizing problems from pre- to posttreatment (T[7]=2.84, ρ=0.04) were observed. This difference was maintained at follow-up assessment (T[5]=0.98, ρ=0.43). Parent ratings on externalizing problems did not demonstrate significant improvement (T[7]=1.90 ρ=0.25) at posttreatment assessment. Comparison of SCARED total ratings failed to demonstrate significant improvement from pretreatment at the posttreatment for parent (T[7]=0.88, ρ=0.42) or child (T[7]=0.05, ρ=0.97) ratings. Parent-rated impairment demonstrated significant improvement at posttreatment assessment across all domains: Home (T[7]=2.64, ρ=0.04); School (T[7]=2.55, ρ=0.04); and Social (T[7]=2.52, ρ=0.05). Additionally, these improvements were maintained at follow-up assessment across all domains: Home (T[5]=1.44, ρ=0.24); School (T[5]=3.00, ρ=0.06); and Social (T[5]=1.70, ρ=0.19). Child-rated impairment failed to indicate significant improvement from pre- to posttreatment for any domain: Home (T[7]=1.13, ρ=0.31); School (T[7]=0.00, ρ=1.0); and Social (T[7]=0.54, ρ=0.61).
Discussion
Presentation of PANS-type OCD is characterized by sudden and abrupt onset or exacerbation of symptoms. A variety of pharmacological treatments have been used to address symptoms; however, many times obsessive-compulsive symptoms show limited or partial response. Limited evidence to date (Storch et al. 2004a, 2006) suggests that CBT may hold promise as a treatment for PANS-related OCD.
Youth who completed treatment showed significantly improved ratings at posttreatment and follow-up on the CY-BOCS, with a large effect size consistent with the Pediatric OCD Treatment Study Team (2004) outcomes and with previous work in this area (Storch et al. 2006). Relative to pretreatment, CY-BOCS reductions of 49% and 50% were observed at posttreatment and follow-up, respectively. Similarly, significant reductions in parent-rated internalizing symptoms and parent-rated impairment across all domains were observed at posttreatment. All participants who completed treatment were much or very much improved at posttreatment and follow-up. No evidence of partial or full relapse with respect to OCD symptoms – as evidenced by improved ratings on instruments – was observed among those participants completing follow-up assessment.
Reductions in child- and parent-reported anxiety were not found, potentially reflecting the nature of treatment (i.e., focus limited to OCD symptoms). Reductions in child-rated impairment and parent-rated externalizing problems were nonsignificant. These findings may be the result of increased within-subject error resulting from poor and/or inconsistent reporting among youth. Alternately, the treatment did not target externalizing behaviors (which often were subclinical prior to treatment); therefore, reductions were not expected.
Given the episodic nature of PANS presentation, the short-term maintenance of gains may reflect the prophylactic utility of combining CBT with continued antibiotic treatment against cyclical exacerbations commonly associated with the PANS group. Utilization of antibiotic treatment to reduce acute symptom loading in conjunction with sequential implementation of CBT may improve the level of competency among youth and their parents in utilizing learned adaptive behaviors (e.g., exposure activities) and altering accommodation-based routines, even if reexposure to infectious trigger(s) should occur.
Limitations
The findings of the current study must be examined against the backdrop of limitations. First, the study did not utilize a control condition. However, it should be noted that use of a waitlist or no-treatment control is problematic, given the impairing and episodic symptom course characteristic of PANS-type OCD. Second, the study presents data from a small sample size of eight treatment-completing participants, resulting in findings that may not generalize to the population of youth with PANS-type OCD. Third, it is unclear to what extent, if any, continuation of antibiotic treatment accounted for OCD symptom reductions; however, it appears clear in all cases that the child and family members benefited from skills learned during CBT. Unfortunately, the waxing and waning nature of symptoms likely contributed to participant attrition. Finally, given a single follow-up assessment point, the episodic nature of PANS-type OCD symptoms was not adequately captured. Future research should seek to make use of longer follow-up duration to assess the maintenance of CBT-related gains in this population.
Conclusions
This study focused on the utility of CBT to address symptoms of PANS-type OCD resistant to antibiotic prophylaxis treatment. Findings of the current study are consistent with those found in studies of traditional (i.e., non-PANS-type) presentations of OCD; specifically, significant reductions in OCD symptoms were endorsed by all participants who completed a full course of CBT. In addition to lending additional evidence to support the efficacy of CBT among youth with PANS-type OCD, these findings suggest its appropriateness in conjunction with antibiotic treatment, as a potential means of altering the symptom trajectory commonly associated with PANS presentation.
Clinical Significance
The use of CBT for PANS-type OCD symptoms demonstrated promising treatment effects as an augmentation strategy to antibiotic therapy, for active or residual symptoms. Although PANS-type OCD has a biological etiology, it is evident that such symptoms are maintained by a variety of environmental and behavioral characteristics (avoidance, family accommodation, negative reinforcement). Therefore, CBT, particularly subsequent to the initial phase of symptom onset, may attenuate the episodic pattern of symptom exacerbation that is a hallmark of PANS, and interrupt the behavioral cycle of ritual engagement and family accommodation that serves to maintain some level of functional impairment.
Disclosures
Drs. Nadeau and Jordan, Mr. Selles, Ms. Wu, Ms. King, Ms. Patel, Ms. Hanks, and Ms. Arnold have no research or other financial support to disclose. Dr. Lewin receives grant funding from the Agency for Healthcare Research and Quality, Centers for Disease Control, International OCD Foundation, Joseph Drown Foundation, National Alliance for Research on Schizophrenia and Affective Disorders, National Institutes of Health, and the University of South Florida Research Council. He is a consultant for Prophase, Inc. and has received speaker's honorariums from the Tourette Syndrome Association. He received travel reimbursement from Roche Pharmaceuticals. Dr. Murphy has received research support in the past 3 years from All Children's Hospital Research Foundation, AstraZeneca Neuroscience iMED, Centers for Disease Control, International OCD Foundation, National Institutes of Health, Ortho McNeil Scientific Affairs, Otsuka, Pfizer Pharmaceuticals, Roche Pharmaceuticals, Shire, Sunovion Pharmaceuticals Inc., and Tourette Syndrome Association, Transcept Pharmaceuticals, Inc. Dr. Murphy is on the Medical Advisory Board for the Tourette Syndrome Association and on the Scientific Advisory Board for the International OCD Foundation. She receives a textbook honorarium from Lawrence Erlbaum. Dr. Storch has received grant funding in the last 3 years from the Agency for Healthcare Research and Quality, All Children's Hospital Research Foundation, Centers for Disease Control, International OCD Foundation, National Institutes of Health, and Ortho McNeil Scientific Affairs. He receives textbook honorariums from American Psychological Association, Lawrence Erlbaum, Springer Publishers, and Wiley. Dr. Storch has been an educational consultant for Rogers Memorial Hospital. He is a consultant for CroNos, Inc., and Prophase, Inc. and is on the Speaker's Bureau and Scientific Advisory Board for the International OCD Foundation.
References
- Abramowitz JS, Whiteside SP, Deacon BJ: The effectiveness of treatment for pediatric obsessive-compulsive disorder: A meta-analysis. Behav Ther 36:55–63, 2005 [Google Scholar]
- Achenbach TM, Rescorla L: Manual for the Achenbach System of Empirically Based Assessment (ASEBA) School-Age Forms and Profiles. Burlington, VT: ASEBA; 2001 [Google Scholar]
- Allen AJ, Leonard HL, Swedo SE: Case study: A new infection-triggered, autoimmune subtype of pediatric OCD and Tourette's syndrome. J Am Acad Child Adolesc Psychiatry 34:307–311, 1995 [DOI] [PubMed] [Google Scholar]
- Arbuckle JL: Full Information Estimation in the Presence of Incomplete Data. Mahwah, NJ: Erlbaum; 1996 [Google Scholar]
- Bernstein GA, Victor AM, Nelson PM, Lee SS: Pediatric obsessive-compulsive disorder: Symptom patterns and confirmatory factor analysis. J Obsessive Compuls Relat Disord 2:299–305, 2013 [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, Neer SM: The screen for child anxiety related emotional disorders: scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry 36:545–553, 1997 [DOI] [PubMed] [Google Scholar]
- Cohen J: Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Hillsdale, NJ: Erlbaum; 1988 [Google Scholar]
- Garvey MA, Perlmutter SJ, Allen AJ, Hamburger S, Lougee L, Leonard HL, Witowski ME, Dubbert B, Swedo SE: A pilot study of penicillin prophylaxis for neuropsychiatric exacerbations triggered by streptococcal infections. Biol Psychiatry 45:1564–1571, 1999 [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Henninger GR, Charney DS: The Yale-Brown Obsessive Compulsive Scale: I. Development, use, and reliability. Arch Gen Psychiatry 46:1006–1011, 1989 [DOI] [PubMed] [Google Scholar]
- Guy W: ECDEU Assessment Manual for Psychopharmacology–Revised. Volume Department of Health, Education, and Welfare Publ. No. ADM 76 338. Rockville, MD: U.S. Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs, pp 218–222, 1976 [Google Scholar]
- Leckman JF, Sholomaskas D, Thompson WE, Belanger A, Weissman MM: Best estimate of lifetime psychiatric diagnosis: A methodological study. Arch Gen Psychiatry 39:879–883, 1982 [DOI] [PubMed] [Google Scholar]
- Lewin AB, Piacentini J: Evidence-based assessment of child obsessive compulsive disorder: Recommendations for clinical practice and treatment research. Child & Youth Care Forum. 39:73–89, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin AB, Piacentini J, DN A.S., Jones AM, Peris T, Geffken GR, Geller DA, Nadeau JM, Murphy TK, Storch EA: Defining clinical severity in pediatric obsessive-compulsive disorder. Psychol Assess 26:679–684, 2013 [DOI] [PubMed] [Google Scholar]
- Murphy TK, Gerardi DM, Leckman JF: Pediatric acute-onset neuropsychiatric syndrome. Psychiatr Clin North Am 37:353–374, 2014 [DOI] [PubMed] [Google Scholar]
- Murphy TK, Kurlan R, Leckman J: The immunobiology of Tourette's disorder, pediatric autoimmune neuropsychiatric disorders associated with Streptococcus, and related disorders: A way forward. J Child Adolesc Psychopharmacol 20:317–331, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TK, Parker–Athill EC, Lewin AB, Storch EA, Mutch PJ: Cefdinir for recent onset pediatric neuropsychiatric disorders: A pilot randomized trial. J Child Adolesc Psychopharmacol 25:57–64, 2015a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TK, Patel PD, McGuire JF, Kennel A, Mutch PJ, Athill EP, Hanks CE, Lewin AB, Storch EA, Toufexis MD, Dadlani GH, Rodriguez CA: Characterization of the pediatric acute-onset neuropsychiatric syndrome phenotype. J Child Adolesc Psychopharmacol 25:14–25, 2015b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy ML, Pichichero ME: Prospective identification and treatment of children with pediatric autoimmune neuropsychiatric disorder associated with group A streptococcal infection (PANDAS). Arch Pediatr Adolesc Med 156:356–361, 2002 [DOI] [PubMed] [Google Scholar]
- Murphy TK, Storch EA, Lewin AB, Edge PJ, Goodman WK: Clinical factors associated with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. J Pediatr 160:314–319, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Mental Health: Clinical Global Impressions Scale (CGI) Psychopharmacol Bull 21:747–748, 1985 [Google Scholar]
- Pediatric OCD Treatment Study Team: Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: The Pediatric OCD Treatment Study (POTS) Randomized Controlled Trial. J Am Med Assoc 292:1969–1976, 2004 [DOI] [PubMed] [Google Scholar]
- Perlmutter SJ, Leitman SF, Garvey MA, Hamburger S, Feldman E, Leonard HL, Swedo SE: Therapeutic plasma exchange and intravenous immunoglobulin for obsessive-compulsive disorder and tic disorders in childhood. Lancet 354:1153–1158, 1999 [DOI] [PubMed] [Google Scholar]
- Piacentini J, Bergman RL, Chang S, Langley A, Peris T, Wood JJ, McCracken J: Controlled comparison of family cognitive behavioral therapy and psychoeducation/relaxation training for child obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry 50:1149–1161, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentini JC, Jaffer MP: Measuring Functional Impairment in Youngsters with OCD: Manual for the Child OCD Impact Scale (COIS). Los Angeles: UCLA Department of Psychiatry; 1999 [Google Scholar]
- RUPP Anxiety Study Group: Fluvoxamine for the treatment of anxiety disorders in children and adolescents. N Engl J Med 344:1279–1285, 2001 [DOI] [PubMed] [Google Scholar]
- Scahill L, Riddle MA, McSwiggin–Hardin M, Ort SI, King RA, Goodman WK, Cicchetti D, Leckman JF: Children's Yale–Brown Obsessive Compulsive Scale: Reliability and validity. J Am Acad Child Adolesc Psychiatry 36:844–852, 1997 [DOI] [PubMed] [Google Scholar]
- Schlomer GL, Bauman S, Card NA: Best practices for missing data management in counseling psychology. J Couns Psychol 57:1–10, 2010 [DOI] [PubMed] [Google Scholar]
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, Aluwahlia S: A Children's Global Assessment Scale (CGAS). Arch Gen Psychiatry 40:1228–1231, 1983 [DOI] [PubMed] [Google Scholar]
- Silverman WK, Albano AM: Anxiety Disorders Interview Schedule for DSM-IV: Parent Interview Schedule, Vol 1 New York: Oxford University Press; 1996 [Google Scholar]
- Snider LA, Lougee L, Slattery M, Grant P, Swedo SE: Antibiotic prophylaxis with azithromycin or penicillin for childhood-onset neuropsychiatric disorders. Biol Psychiatry 57:788–792, 2005 [DOI] [PubMed] [Google Scholar]
- Storch EA, Bussing R, Small BJ, Geffken GR, McNamara JP, Rahman O, Lewin AB, Garvan CS, Goodman WK, Murphy TK: Randomized, placebo-controlled trial of cognitive-behavioral therapy alone or combined with sertraline in the treatment of pediatric obsessive–compulsive disorder. Behav Res Ther 51:823–829, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch EA, Caporino NE, Morgan JR, Lewin AB, Rojas A, Brauer L, Larson MJ, Murphy TK: Preliminary investigation of web-camera delivered cognitive-behavioral therapy for youth with obsessive-compulsive disorder. Psychiatry Res 189:407–412, 2011 [DOI] [PubMed] [Google Scholar]
- Storch EA, Gerdes A, Adkins J, Geffken GR, Star J, Murphy TK: Behavioral treatment of child with pediatric autoimmune neuropsychiatric disorder associated with group A streptococcal infection. J Am Acad Child Adolesc Psychiatry 43:510–511, 2004a [DOI] [PubMed] [Google Scholar]
- Storch EA, Murphy TK, Geffken GR, Mann G, Adkins J, Merlo LJ, Duke D, Munson M, Swaine Z, Goodman WK: Cognitive-behavioral therapy for PANDAS-related obsessive-compulsive disorder: Findings from a preliminary waitlist controlled open trial. J Am Acad Child Adolesc Psychiatry 45:1171–1178, 2006 [DOI] [PubMed] [Google Scholar]
- Storch EA, Murphy TK, Geffken GR, Soto O, Sajid M, Allen P, Roberti JW, Killiany EM, Goodman WK: Psychometric evaluation of the Children's Yale–Brown Obsessive–Compulsive Scale. Psychiatry Res 129:91–98, 2004b [DOI] [PubMed] [Google Scholar]
- Swedo SE, Leckman JF, Rose NR: From research subgroup to clinical syndrome: modifying the PANDAS criteria to describe PANS (pediatric acute-onset neuropsychiatric syndrome).Pediatr Ther 2:2,2012 [Google Scholar]
- Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S, Dow S, Zamkoff J, Dubbert BK, Lougee L. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry 155:264–271, 1998 [DOI] [PubMed] [Google Scholar]