Abstract
Autologous bone grafting (ABG) remains entrenched as the gold standard of treatment in bone regenerative surgery. Consequently, many marginally successful bone tissue engineering strategies have focused on mimicking portions of ABG's “ideal” osteoconductive, osteoinductive, and osteogenic composition resembling the late reparative stage extracellular matrix (ECM) in bone fracture repair, also known as the “hard” or “bony” callus. An alternative, less common approach that has emerged in the last decade harnesses endochondral (EC) ossification through developmental engineering principles, which acknowledges that the molecular and cellular mechanisms involved in developmental skeletogenesis, specifically EC ossification, are closely paralleled during native bone healing. EC ossification naturally occurs during the majority of bone fractures and, thus, can potentially be utilized to enhance bone regeneration for nearly any orthopedic indication, especially in avascular critical-sized defects where hypoxic conditions favor initial chondrogenesis instead of direct intramembranous ossification. The body's native EC ossification response, however, is not capable of regenerating critical-sized defects without intervention. We propose that an underexplored potential exists to regenerate bone through the native EC ossification response by utilizing strategies which mimic the initial inflammatory or fibrocartilaginous ECM (i.e., “pro-” or “soft” callus) observed in the early reparative stage of bone fracture repair. To date, the majority of strategies utilizing this approach rely on clinically burdensome in vitro cell expansion protocols. This review will focus on the confluence of two evolving areas, (1) native ECM biomaterials and (2) developmental engineering, which will attempt to overcome the technical, business, and regulatory challenges that persist in the area of bone regeneration. Significant attention will be given to native “raw” materials and ECM-based designs that provide necessary osteo- and chondro-conductive and inductive features for enhancing EC ossification. In addition, critical perspectives on existing stem cell-based therapeutic strategies will be discussed with a focus on their use as an extension of the acellular ECM-based designs for specific clinical indications. Within this framework, a novel realm of unexplored design strategies for bone tissue engineering will be introduced into the collective consciousness of the regenerative medicine field.
Introduction
The human body has an extensive capacity to regenerate bone tissue after trauma. Disruption of the surrounding vasculature and bone marrow resulting from a bone fracture initially facilitates a cascade of coagulation and inflammatory events within the fracture space (Fig. 1).1–8 The subsequent bone-healing process overlaps with this inflammatory phase spatiotemporally via both bone developmental pathways: intramembranous (IM) and endochondral (EC) ossification.1–8 Large defects above a “critical-size,” however, cannot be restored without intervention and often lead to nonunion.9,10 Although current surgical intervention strategies, most notably autologous bone grafting (ABG), have yielded favorable bone healing outcomes in these situations, it is clear from the past decade's surge in clinically available tissue-engineered bone implants, some coupled with stem cell-based therapeutics (SCBTs), that alternative bone regenerative strategies have gained a significant market share.11
FIG. 1.
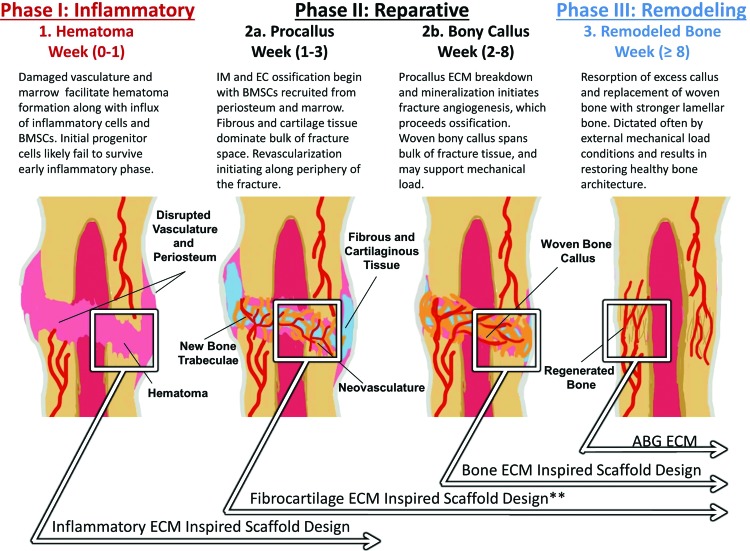
Both IM and EC ossification occurs during the bone-healing process in three overlapping regeneration phases. Critical-sized bone defects favor EC ossification over direct IM ossification, primarily due to the large, avascular nature of these defects. Four ECM-based biomaterial strategies (boxes) are highlighted with regard to bone regeneration. The intermediate fibrocartilaginous ECM remains underexplored (**) as a strategy to potentially enhance quality and extent of EC ossification and overall bone regeneration. ABG, autologous bone grafting; EC, endochondral; ECM, extracellular matrix; IM, intramembranous. Color images available online at www.liebertpub.com/teb
Clinically, however, ABG remains the gold standard of treatment due to its inherent osteoconductivity, osteoinductivity, and thus osteogenic capabilities.6,12–14 Despite these advantages, ABG has key clinical limitations, including donor site morbidity and pain, increased risk of infection, limited handling capacity, graft resorption problems, and restricted tissue availability.6,12–14 Nearly three decades of bone tissue engineering research have been aimed at overcoming these limitations by finding performance competitive alternatives to ABG. As a result, many bone regeneration strategies have focused on mimicking portions of the complex composition and bioactive signals present in ABG, such as demineralized bone matrix (DBM), which resembles the late reparative stage extracellular matrix (ECM) in bone fracture repair (i.e., “hard” or “bony” callus).1–8 These efforts have yielded dozens of clinical products, including synthetic scaffolds, allografts, and xenografts15,16; however, the majority of orthopedic procedures continue to utilize ABG.6,12–14
To date, acellular biomaterial-based products have dominated the alternative bone implant market at the commercial level.11,15,16 A major characteristic of these products is the ability to mimic the biological ECM environment that is specific for bone regeneration. These materials possess both conductive scaffold moieties and inductive signaling molecules that have been difficult to match with synthetic designs.17–21 Within the realm of bone biomimetics, native ECM-based biomaterials (e.g., DBM) have been a key protagonist.15 However, there may be underexplored potential to enhance the bone regenerative response with native ECM biomaterials that instead mimic the initial inflammatory or fibrocartilaginous ECM (i.e., “pro-” or “soft” callus) observed in the early reparative stage of the native bone fracture healing process.
Addressing this avenue of research is the emerging paradigm of “developmental engineering” first introduced in 2009 in two reviews by Lenas et al.,22,23 which offer an innovative approach to enhance the regenerative capacity of tissue-engineered implants, including bone tissue applications, by focusing on path-dependent precursor tissue formation. This new strategy involves engineering developmental “processes” in vitro in addition to “tissues,” and it recognizes that the molecular and cellular mechanisms involved in developmental skeletogenesis, specifically EC ossification, are closely paralleled during native bone healing.1–8 Primarily, these strategies have involved in vitro chondrogenic priming of various adult and embryonic stem cells (ESCs) for subsequent implantation and EC ossification in vivo. To date, however, no review has critically analyzed these in vitro priming approaches across various stem cell sources as a collective endeavor for the purpose of advancing bone regenerative medicine. While these priming strategies have successfully produced EC ossification in several animal models, their clinical translation remains tethered to burdensome donor cell expansion protocols.
The incorporation of donor cells via SCBT strategies may provide advantageous osteo- and chondrogenic capabilities to acellular bone implants, which rely solely on recruited cells from the peripheral host tissue. These benefits are likely the reason behind the increasing saturation of product pipelines (i.e., preclinical and clinical level) with SCBTs across the areas of tissue engineering and regenerative medicine.11 As a result, the bone regeneration market is likely to see an influx of SCBT products, either as exclusive therapies or combined with biomaterial implants, within the next decade. The clinical acceptance of these products will primarily depend on achieving (1) improved patient outcomes while simultaneously reducing procedural (2) complications and (3) expenses compared with current standards of treatment.11,24,25 Key to the success of SCBTs will be the reduction in time and cost-consuming manufacturing practices (multiple days to weeks) associated with traditional in vitro cell expansion. Critical-sized bone defects often require surgical intervention within hours as opposed to weeks, because delayed bone healing leads to a higher risk of nonunion.9 Therefore, traditional cell expansion procedures represent a nonfeasible clinical option in most critical-sized defect indications. Advances in intraoperative SCBT strategies offer innovative solutions to overcome this challenge by combining cell harvest, isolation, and implantation in one surgical setting without compromising progenitor cell and signal efficacy, thus eliminating the prolonged cell expansion phase.25 Although SCBT strategies could revolutionize the orthopedic market, basic biomaterial designs incorporating the appropriate native ECM components to accompany these SCBTs will be crucial in the advancement of bone regenerative medicine.
An alternative strategy to reduce or remove the cell expansion step may reside in extending developmental engineering principles to in vivo designs (i.e., within the graft or implant), thus removing in vitro chondrogenic priming requirements altogether. Inspiration for in vivo developmental designs already exists with native ECM biomaterials, specifically acellular DBM grafts, which have already been shown to elicit some EC ossification in addition to the IM pathway.26–32 However, as previously mentioned, DBM composition reflects the physicochemical properties of the late bone reparative stage ECM (Fig. 1)6,9,33,34 and suggests that designs aimed at mimicking the composition of bone ECM may insufficiently elicit an EC ossification response inside healing fractures.
This review will highlight essential design criteria involved in the underexplored regenerative area of developmentally engineering bone in vivo (Fig. 2). Traditional strategies have approached tissue-engineered construction through a combination of factors described in the tissue engineering triad: cells, signals, and scaffolds. However, the use of “raw” ECM biomaterials may serve to bridge the gap between the latter two components of this triad by recognizing their integrated contribution to the local ECM,20 potentially obviating the need for additional incorporation of cells and signaling molecules. Crucial to developmentally engineering the process of EC ossification inside an implant will not only be the selection of native biomaterials that mimic the relevant tissue's ECM composition but also materials which serve to modulate the developmental process. The primary focus will be on acellular ECM strategies, where the physicochemical cues that native ECM biomaterials possess may sufficiently elicit a bone regenerative response. Within this framework, the advantages of incorporating SCBT strategies will be discussed along with design criteria related to specific bone defect indications.
FIG. 2.
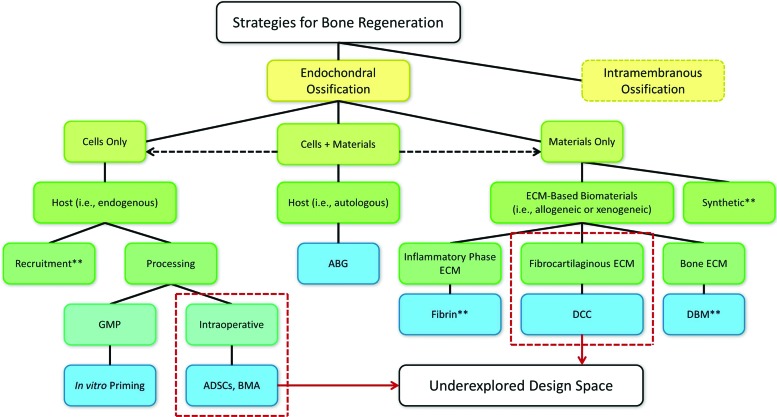
Current landscape of EC ossification strategies (blue boxes) along with underexplored design space (dashed outlines) highlighted in this review. Some combined cell and material strategies are not shown explicitly (dashed arrows). This includes several in vitro priming studies that utilized cell and material approaches (**) and are further explored elsewhere (Tables 2–4). All strategies will be critically evaluated with regard to technical, regulatory, and commercial challenges. ABG, autologous bone grafting; ADSCs, adipose-derived stem cells; BMA, bone marrow aspirate; DBM, demineralized bone matrix; DCC, decellularized cartilage; GMP, good manufacturing practice. Color images available online at www.liebertpub.com/teb
Overview of the Natural Bone-Healing Process
As mentioned earlier, the human body has an extensive capacity to regenerate bone tissue after trauma and fracture. The cellular and molecular processes involved in developmental skeletogenesis are closely paralleled during native bone healing.1–9 Characterization of the complex mechanisms involved in bone healing has been the focus of several extensive reviews,1,2,9,33 and readers interested in further details surrounding this cascade of events should direct their attention to these recommended citations.
In general, the fracture-healing process is most often described in three overlapping phases: Inflammatory, Reparative, and Remodeling (Fig. 1), where each phase represents a complex spatiotemporal distribution of cells, ECM, and bioactive signals.1–9 Initially, disrupted vasculature and bone marrow during the inflammatory phase facilitates a coagulation cascade along with an influx of progenitor cells, including bone mesenchymal stem cells (BMSCs), into the fracture space forming a hematoma. Without permanent vasculature, however, the fracture space becomes hypoxic, and it remains unclear whether enough of these stem cells survive the initial inflammatory phase to play an active role in subsequent tissue regeneration.1–9 Established bioactive signaling from the inflammatory phase ECM subsequently recruits progenitor cells from both the exposed periosteum and bone marrow that migrate into the fracture space, initiating both bone developmental pathways: IM and EC ossification.1–8 Primary differences in these pathways reside in precursor requirements. While IM requires condensation and proliferation of progenitor BMSCs for ossification, EC requires cartilage and fibrous intermediate tissue formation before ossification.1–9 EC ossification, therefore, can further be divided into three generalized steps: (1) chondrogenesis, (2) cartilage hypertrophy, and (3) ossification.1–9 Regardless of the pathway, however, neo-vascularization and angiogenesis are necessary before ossification can proceed.5,6,35–38
In contrast, chondrogenesis predominately occurs in avascular environments where oxygen tension is low.39–43 The main consequence of this difference is that IM and EC ossification is manifested in physiologically distinct regions of the fracture space, where the former primarily occurs adjacent to the fracture ends and the latter occurs primarily in the avascular bulk of the fracture space.2,5,6 Collectively, this early reparative stage climaxes in the formation of a spanning fibrocartilaginous callus (i.e., “pro-” or “soft” callus) in the majority of the fracture space developing from EC ossification. The procallus serves both a chemical and mechanical function and acts as a primed template for subsequent osteoblast infiltration, woven bone ossification (i.e., “hard” or “bony” callus), and, finally, bone remodeling to restore healthy lamellar bone architecture.1–9
While the process mentioned earlier describes successful bone regeneration within the body, it is well understood that large defects above a “critical-size” are incapable of completely restoring native bone structure without intervention and often lead to nonunion.9,10 It is important to note that critical-sized defects are inherently large, avascular spaces that present favorable conditions for EC ossification as opposed to IM. This review will outline several studies (Tables 1–3) that have leveraged EC ossification and developmental engineering strategies within critical-sized defects in various animal models. Collectively, this evidence suggests that enhancing EC ossification may be a viable, underexplored strategy for improving bone regeneration.
Table 1.
Priming In Vitro Chondrogenesis with Bone Mesenchymal Stem Cells for In Vivo Endochondral Ossification
| Reference(s) | Year | Cell source | Additional materials | In vitro priming method | Animal model | Highlighted results |
|---|---|---|---|---|---|---|
| Yamada et al.104 | 2003 | BMSCs | Fibrin and beta-TCP | Isolated rat BMSCs were chondrogenically primed on beta-TCP blocks and implanted with fibrin glue. | Implanted subcutaneously in nude mice. | EC ossification was only seen in primed rBMSC constructs. Noncellular and unprimed rBMSC scaffolds did not produce EC ossification. |
| Simmons et al.103 | 2004 | BMSCs | Alginate Matrix (RGD conjugation) | Isolated rat BMSCs were chondrogenically primed and implanted with dual growth factor encapsulation (BMP-2 and TGF-β). | Implanted subcutaneously in nude mice. | Significantly higher amount of EC ossification was seen in scaffolds with dual GF compared with single GF and nonloaded controls. |
| Pelttari et al.72 | 2006 | BMSCs | Fibrin Matrix | Isolated human BMSCs were primed in chondrogenic medium for 4 to 7 weeks. Human ACs were isolated for implantation, and a portion of hACs were expanded in chondrogenic medium. | Implanted subcutaneously in nude mice. | hBMSCs exhibited extensive EC ossification, while hACs remained stable cartilage. Chondrogenically expanded hACs did not undergo EC ossification, suggesting that they are locked into a stable cartilage phase. |
| Farrell et al.64 | 2009 | BMSCs | Collagen and GAG | Isolated human BMSCs were cultured for 21 days in chondrogenic medium. Three groups were cultured for 14 extra days in (a) the same medium, (b) mineralizing chondrogenic medium, or (c) osteogenic medium. Unprimed control medium was also used. | Implanted subcutaneously in nude mice. | Chondrogenic priming led to collagen II and X production along with vessel ingrowth in vivo while osteogenic priming led to mineralized matrix, no vascularization, and poor cell viability. Unprimed control conditions yielded no bone. Mineralized chondrogenic priming severely diminished production of inductive growth factors. |
| Janicki et al.66 | 2010 | BMSCs | beta-TCP or Hydroxyapatite (HA/TCP) | Isolated human BMSCs were primed in chondrogenic medium (6 weeks), in osteogenic medium, or unprimed in control medium. | Implanted subcutaneously in nude mice. | Osteogenic priming led to poor bone formation. Unprimed hBMSCs on beta-TCP exhibited IM ossification. With chondrogenic priming, hBMSCs formed bone via EC ossification, while hematopoietic marrow formed from murine origin. This was hypothesized to be the result of direct osteogenesis of cartilage-resident hBMSCs or transdifferentiation from chondrocytes to osteoblasts. |
| Scotti et al.74 | 2010 | BMSCs | None, direct pellet implantation | Isolated human BMSCs were primed to prechondrogenic, early hypertrophic, and late hypertrophic phase tissues | Implanted subcutaneously in nude mice. | Hypertrophic cartilage templates were required to form bone trabeculae. Prehypertrophic hBMSCs resorbed quickly and did not form bone. Results indicate that no external scaffolding was required to undergo EC ossification. |
| Farrell et al.63 | 2011 | BMSCs | Collagen and GAG | Isolated human BMSCs and rat BMSCs were cultured in osteogenic, chondrogenic, or control medium (28 days). In some cases, induced mineralization in chondrogenic medium lasted for 7 days. | Implanted subcutaneously in nude co-isogenic hPLAP-transgenic F344 rats | Bone and bone marrow was observed in chondrogenic primed constructs but not in unprimed control and osteogenic primed constructs. Late switching (last 7 days) to osteogenic medium prevented bone formation, but beta-glycophosphate-induced mineralization did not prevent bone formation in chondrogenic primed constructs. Osteoblasts in formed bone were almost entirely of host origin, but osteocytes were of both host and donor origin. |
| Scotti et al.73 | 2013 | BMSCs | Collagen Type I Meshes | Isolated human BMSCs were primed to hypertrophic stage and seeded onto collagen type I meshes. | Implanted subcutaneously in nude mice. | Functional bone was formed heterotopically with cortical-like bone originating from host murine cells, and inner trabecular-like bone from EC ossification and originating from hBMSC primed hypertrophic cartilage constructs. |
| Sheehy et al.60 | 2013 | BMSCs | Agarose | Isolated porcine chondrocytes and BMSCs were cultured and chondrogenically primed before seeding on bilayer osteochondral scaffolds. | Implanted subcutaneously in nude mice. | EC ossification was restricted to the BMSC primed layer of the scaffold in vivo, while primed chondrocytes maintained chondrogenic phenotype. This led to a bilayer osteochondral construct. |
| Sheehy et al.107 | 2014 | BMSCs | Agarose and “Channeled” Agarose | Isolated porcine BMSCs were chondrogenically primed for 5 weeks followed by hypertrophic priming for an additional week. | Implanted subcutaneously in nude mice. | EC ossification was observed in hypertrophic primed pBMSCs encapsulated in agarose gel. In addition, channeled agarose gels with pBMSCs showed increased vascularization and mineralization compared with solid agarose counterparts, which indicated that EC ossification may be enhanced with improved vascular support. |
| Bahney et al.110 | 2014 | BMSCs | None, direct pellet implantation | Isolated human BMSCs were chondrogenically primed for three weeks. In addition, cartilage grafts were isolated from healing callus at 7 days after fracture and re-implanted in vivo. Implants were compared with living isografts, devitalized allografts, and sham groups. | Implanted into mid-tibia diaphysis osteotomy in nude mice. | Cartilage grafts and primed hBMSC pellets produce well vascularized and integrated bone regenerate via EC ossification; however, hBMSC pellets did not integrate with each other in vivo. Cartilage graft was equally likely to integrate with adjacent bone as bone isografts and outperformed devitalized allografts. Osteocytes in regenerated bone were primarily of donor origin rather than host recruited cells. |
| van der Stok et al.106 | 2014 | BMSCs | None, direct pellet implantation | Isolated human BMSCs were chondrogenically primed for three weeks and compared with hBMSCs cultured in chondrogenic media for only 3 days. | Implanted in 6 mm mid-femur diaphysis defects in Rowett nude rats. | Significantly higher EC ossification was observed in 21 day chondrogenically primed hBMSC pellets compared with 3 day undifferentiated hBMSC controls. Primed hBMSC can result in complete bridging of the 6 mm defect; however, the effect was donor dependent. |
| Harada et al.105 | 2014 | BMSCs | PLGA | Isolated rat BMSCS were chondrogenically primed for 21 days and compared with rBMSCS that were cultured in standard medium. Implants were also compared with cell-free scaffolds and contralateral femur controls. | Implanted into 5 and 15 mm mid-femur diaphysis defects in Fischer 344 rats. | Significantly higher EC ossification was observed in chondrogenically primed rBMSCs seeded onto PLGA scaffolds compared with undifferentiated rBMSC controls. Histological, micro-CT, and mechanical analysis of primed cell scaffolds revealed functionalized bone regeneration as early as 2 weeks after implantation in both 5 and 15 mm defect models. By 8 weeks, mechanical strength of primed cell scaffolds was statistically equivalent to contralateral femur controls in 5 mm defects, while scaffolds implanted in 15 mm defects exhibited 75% of the mean strength of native bone. |
ACs, articular chondrocytes; BMSC, bone mesenchymal stem cell; beta-TCP, beta tricalcium phosphate; EC, endochondral; GAG, glycosaminoglycan; hAC, human AC; hBMSC, human BMSC; IM, intramembranous; pBMSC, porcine BMSC; rBMSC, rat BMSC; PLGA, poly(lactic-co-glycolic) acid.
Table 2.
Priming In Vitro Chondrogenesis with Embryonic Stem Cells, Synovium-Derived Stem Cells, Articular Chondrocytes, and Induced Pluripotent Stem Cells for In Vivo Endochondral Ossification
| Reference(s) | Year | Cell source | Additional materials | In vitro priming method | Animal model | Highlighted results |
|---|---|---|---|---|---|---|
| Montufar-Solis et al.27 | 2004 | ESCs | None, direct pellet implantation | Isolated murine ESC constructs were chondrogenically primed for 3 weeks. | Implanted adjacent to and within murine skull defects of same lineage. | Implants placed both adjacently and directly into skull defects exhibited EC ossification, while control groups without primed implants did not. |
| Doan et al.62 | 2010 | ESCs | None, direct pellet implantation | Isolated murine ESC constructs were chondrogenically primed for 3 weeks. | Implanted into murine skull defects of same lineage. | EC Ossification was only seen in rBMSC primed cartilage implants. |
| Shoji et al.108 | 2010 | ADSCs | Collagen | Isolated human ADSCs expanded from SVF | Implanted in nude rat femurs | hADSCs exhibited greater EC ossification than PBS and hFB (fibroblast) implant controls in collagen matrix. |
| Case et al.61 | 2003 | ACs | PGA | Isolated rabbit chondrocytes from patellar groove and femoral condyles were chondrogenically primed on PGA scaffolds for 4 weeks. | Implanted into empty bone chambers in rabbit femoral metaphyses. | Cell-loaded constructs exhibited EC ossification and led to increased bone formation compared with devitalized constructs. Thus, cells had a significant impact on outcome. |
| Oliveira et al.70,71 | 2009 | ACs | Chitosan Sponge | Isolated chondrocytes from chicken embryo sterna expanded on chitosan sponge and hypertrophically primed for 3 weeks. Control chondrocytes were taken from caudal region of sternum, while transient chondrocytes were taken from cephalic region of sternum. | Implanted subcutaneously in nude mice. | Maturation and hypertrophy seen in treated chondrocyte-chitosan scaffolds. EC ossification was only seen in transient cartilage templates compared with permanent cartilage scaffold (control). |
| Tam et al.109 | 2014 | iChons | CopiOs (Zimmer) Bone Void Filler | Induced chondrocytes (iChons) were directly reprogrammed from murine dermal fibroblasts by two methods: iChon (Con) and iChon (Ind). After induction, cells were chondrogenically primed for 7 days, and hypertrophically primed for 14 days. | Implanted subcutaneously in nude mice. | Both induced cell sources underwent chondrogenic differentiation in vitro, but hypertrophic differentiation was only seen in iChon (Ind) cells. In vivo, iChon (Con) cells formed stable cartilage, while iChon (Ind) cells exhibited EC ossification markers. Osteoblasts in formed bone were of host origin, while chondrocytes were of both donor and host origin. |
ADSC, adipose-derived stem cell; ESC, embryonic stem cell; hADSC, human ADSC; hFB, human fibroblast; PBS, phosphate-buffered saline; PGA, polyglycolic acid; SVF, stromal vascular fraction.
Table 3.
Comparing In Vitro Chondrogenically Primed Cell Sources for In Vivo Endochondral Ossification
| Reference(s) | Year | Cell source | Additional materials | In vitro priming method | Animal model | Highlighted results |
|---|---|---|---|---|---|---|
| Dickhut et al.77 | 2009 | BMSCs, ADSCs, SDSCs and ACs | None, direct pellet implantation | Isolated human BMSCs, ADSCs, SDSCs, and ACs were cultured in chondrogenic medium (5 weeks). | Implanted subcutaneously in nude mice. | Chondrogenic priming led to similar collagen type II vs. type I levels and were positive for type X in all cells. SDSCs and ACs had significantly lower levels of ALP activity after priming, which correlated with poor EC ossification after implantation. While primed BMSCs and ADSCs exhibited EC ossification in vivo, SDSCs and ACs exhibited fibrous dedifferentiation and degradation. |
| Jukes et al.67 | 2008 | ESCs, BMSCs, and ACs | Ceramic particles | Isolated goat and human BMSCs were osteogenically primed for 7 or 21 days. In addition, these cells along with murine ESCs and goat ACs were chondrogenically primed for approximately 21 days. | Implanted into nude mouse skull defects. | Osteogenic priming led only to direct IM ossification. Chondrogenic priming was necessary for EC ossification, but not sufficient as only mESCs underwent EC ossification. Goat and human BMSCs underwent only marginal EC ossification and goat ACs did not exhibit any EC ossification when cultured in a similar fashion. |
| Tortelli et al.111 | 2009 | BMSCs and OBs | Hydroxyapatite | Isolated rate BMSCs and OBs were cultured in standard medium. | Implanted subcutaneously in nude mice. | The capacity to recruit host cells and the ossification pathway were dependent on donor cell commitment. rBMSC implants led to formation of bone from host cells through the EC ossification pathway, and donor rOBs exhibited direct IM ossification. No studies were conducted with chondrogenically primed cells. |
| Both et al.76 | 2011 | BMSCs and ESCs | BCP | Isolated human BMSCs, rat ESCs, and human ESCs were cultured in a series of differentiation mediums, including chondrogenic priming followed by osteogenic priming. Cells were seeded on BCP scaffolds. | Implanted subcutaneously in nude mice. | ESCs were indistinguishable from BMSCs after in vitro osteogenic priming. Osteogenic priming of BMSCs led to direct (IM) osteogenesis and extensive bone formation. Both rESCs and hESCs did not form substantial bone but exhibited signs of limited EC ossification. |
| Vinardell et al.75 | 2012 | BMSCs, FPSCs, SDSCs and ACs | Agarose | Isolated porcine BMSCs, SDSCs, FPSCs, and ACs were chondrogenically primed (3 weeks). An additional group was primed in hypertrophic medium for 4 weeks. | Implanted subcutaneously in nude mice. | Only BMSCs exhibited EC ossification. SDSCs and FPSCs underwent fibrous dedifferentiation and resorption, and ACs appeared to maintain chondrogenic stability. |
ALP, alkaline phosphatase; BCP, biphasic calcium phosphate; FPSCs, fat-pad derived stem cells; OB, osteoblast; rOB, human OB; SDSC, synovium-derived stem cell.
Strategies to Enhance EC Ossification
Harnessing the potential of developmental engineering
Developmental engineering, a term first introduced into the tissue engineering community with two reviews in 2009 by Lenas et al.,22,23 involves the engineering of developmental “processes” and “tissues” in vitro, and recognizes that the embryonic and morphological paradigms involved in developmental skeletogenesis are closely paralleled during native bone healing.1–8 This approach attempts to recapitulate aspects bone regeneration by leveraging several concepts in developmental biology, including22,23
(1) Path dependence: successive developmental tissue relies on previous tissue formation
(2) Robustness: tissue developmental process resistant to unintended external perturbation
(3) Semi-autonomy: partially self-governed tissue development
EC ossification is a feasible route to utilize these guiding principles of developmental engineering, and the current review will cover advances made over the past decade in engineering EC ossification for bone regeneration (Tables 1–3).
While the Lenas reviews only covered replicating in vivo developmental processes in an in vitro environment,22,23 the current review aims to expand their previous concept further to include reproduction of developmental processes in an in vivo environment (i.e., within implanted grafts/scaffolds). If evidenced to produce similar regenerative outcomes compared with their in vitro counterparts, in vivo developmental designs would obviate the need for cost- and time-consuming cell expansion protocols, which remain a critical challenge in the translation of SCBTs.11,24,25 However, there remains a deficiency in both fundamental research and translatable products within the expansive framework of in vivo developmental designs.
Coupling in vivo developmental engineering with native ECM biomaterials
Characterizing the influence of DBM on ossification pathway
Inspiration for in vivo developmental designs already exists in the form of native ECM biomaterials. There is significant evidence that EC ossification occurs in acellular DBM grafts,26–29 which inherently resemble the composition of late reparative stage bone-healing ECM.1–8,15 In a critical-sized rat femoral defect model, Oakes et al.26 observed histological evidence of increased EC ossification foci in human DBM implants suspended in a hyaluronic acid carrier fluid compared with similar DBM implants suspended in a glycerol solution. However, radiographic scoring at 16 weeks postimplantation revealed no significant difference in their mineral content.26 Although the authors did not further pursue developmental differences between groups, this evidence suggests that both EC and IM pathways were utilized within DBM implants. This could have been due to differences in carrier fluid. Hyaluronic acid is known to be a major component of cartilage ECM4 and, therefore, could play a regulatory role in both chondrogenesis and EC ossification.8
Regardless of this, the DBM composition containing conductive and inductive biological agents likely influences developmental pathways during bone healing.26–29 DBM composition, however, is not uniform throughout the body. There exist compositional differences in bone ECM originating from IM sources during fetal development (e.g., cranium) and EC sources (e.g., femur).44 Furthermore, DBM originating from IM and EC bone have been shown in a series of studies by Rabie et al.28,30–32 to elicit different healing pathways during regeneration of parietal bone defects in New Zealand White Rabbits. The parietal bone is formed by IM ossification during fetal development,44 suggesting that only implants directed toward IM ossification will possess regenerative potential. However, evidence from Rabie et al.28,30,31 showed that IM and EC ossification pathways can be utilized, exclusively or in combination, to regenerate parietal defects based entirely on the source of DBM. In addition, the amount of bone regeneration was shown to be source dependent, where grafts favoring IM ossification displayed a significantly higher degree of newly formed bone observed by serial histological sectioning.32 While these studies collectively support the “same for same” surgical practice in bone regeneration,32 where DBM from IM and EC sources is matched on a developmental basis to the respective bone defect site, the major limitation of these studies was the short timeframe of healing (14 days) observed after implantation. Since Oakes et al.26 observed no evidence of a difference in bone regeneration at 16 weeks postimplantation, similar longitudinal studies are necessary to determine whether DBM implants from EC or IM sources display significant regenerative capacities.
The differences in regenerative potential and developmental pathways seen in the Rabie studies were attributed to varying inductive and conductive ECM factors within DBM.28,30–32 Inductive factors such as bone morphogenic proteins (BMPs) have been implicated in bone morphogenesis dating back to their initial isolation from DBM, reviewed in chronological detail by Gruskin et al.15 Consequently, BMP-2 content, release, and bioactivity has become an important guideline in determining efficacy and quality control of commercially available DBM products.15 BMPs, however, represent only a fraction of the growth factors in the spatiotemporal cascade of inductive molecules involved during bone healing, extensively reviewed by Mehta et al.9 Inductive signaling alone could have been responsible for the regenerative effect seen in DBM as well as account for differences seen between DBM from IM and EC sources. However, the conductive proteins present in DBM may also provide key regulatory control, as it has become increasingly evident that structural components of native ECM along with mechanical forces acting on the tissue have a significant influence on cellular and molecular bioactivity.21,45
Collectively, there is significant evidence to suggest that EC ossification can occur within native ECM biomaterials in the form of acellular DBM implants. While there has been extensive research into the bone regenerative capacity of DBM, an underexplored avenue of research is the focus on the developmental pathway's effect on regenerative capacity of these implants. The characterization of DBM's influence, and perhaps limitation, in stimulating EC ossification should be elucidated in the future to advance the understanding of the bone-healing process and potentially enhance bone regenerative designs.
Exploring EC ossification potential in ECM biomaterials
In general, native ECM refers to both soluble and insoluble biomolecules that may be utilized as cell scaffolding and bioactive signaling. In addition, the effectiveness of autologous, allogeneic, and xenogeneic ECM biomaterials can be assessed in terms of physical, chemical, and mechanical properties of the tissue. While it remains debatable whether native ECM represents nature's ideal biological scaffold, particularly because resident cells exhibit physiologically relevant synthesis and maintenance within it, these materials have received significant attention for their potential efficacy in the regenerative tissue market.17,18,20,21
Traditional bone ECM strategies: ABG intrinsically involves the grafting of native bone ECM tissue with associated autologous cells into bone defects, and it remains the gold standard of treatment in bone regeneration due to its inherent osteoconductivity, osteoinductivity, and osteogenic capacity.6,12–14 It intuitively followed that designs mimicking the native bone ECM (Fig. 1) held potential for bone regeneration, and, as a result, many alternative bone regeneration strategies focused on mimicking portions of the complex composition and bioactive signals present in ABG, which resembles the late reparative stage ECM in bone fracture repair (i.e., “hard” or “bony” callus).1–8 Some of these strategies were discussed in the previous DBM characterization section where allogeneic tissue was used to elicit bone regeneration. Subsequently, efforts in the bone ECM biomimetic area have yielded dozens of commercially available products.15,16 Two reviews by Gruskin et al.15 and Bohner16 compare an extensive number of these products that can be divided into two broad categories: (1) organic scaffolds (e.g., allografts and xenografts) and (2) inorganic scaffolds (e.g., calcium phosphate cements); however, the majority of orthopedic procedures continue to utilize ABG.6,12–14 This is likely due to the fact that bone ECM biomimetic strategies fail to sufficiently mimic the complex three-dimensional (3D) physical, chemical, and cellular composition of healthy autologous bone.6,12–14
It is important to note, however, that the functional and structural properties of ECM during the bone-healing process are spatiotemporally dynamic and do not resemble healthy native bone until well into the reparative and remodeling stages of healing (Fig. 1).6,9,33,34 This delay in resemblance suggests that designs aimed at mimicking the composition of bone ECM may insufficiently elicit a regenerative response inside healing fractures and could be a worthwhile focus of future investigation in bone regeneration.
Alternative ECM biomaterial strategies for EC ossification: By instead leveraging the concepts of both developmental engineering and the spatiotemporal dynamics of ECM in bone healing, there may be potential to enhance the extent and quality of EC ossification. Specifically, native ECM biomaterials that mimic the initial inflammatory stage ECM or fibrocartilaginous ECM (i.e., “pro-” or “soft” callus) in the early reparative stage of bone fracture healing may be selected instead of materials which mimic the composition of bone ECM. These scaffolds or grafts based on inflammatory or fibrocartilaginous ECM may harness the path-dependent, robust, and semi-autonomous nature of EC ossification, potentially leading to extensive bone regeneration.
Characterization of the complex cascade of signaling events and ECM changes involved in bone healing and EC ossification has been the focus of several recent reviews,6,9,33,34 and readers interested in further multifaceted details surrounding relevant biomolecules should direct their attention to these recommended citations. While the characterization of the spatiotemporal distribution of these molecules may be of value for developmental engineering, a few efforts have been made to mimic the appropriate array of bioactive scaffolding and signaling molecules with native ECM biomaterials that correspond to the early and intermediate stages of native bone healing.
Native ECM biomaterials possess both conductive and inductive potential that are difficult to match with synthetic designs (e.g., non-native polymers).17–20 In addition, it is increasingly evident that the structural components of native ECM also provide a regulatory role for key processes in cellular development and tissue regeneration.21,45 These processes include cell adhesion, proliferation, differentiation, migration, and survival along with modulating signaling activity of soluble bioactive molecules.21,45 Furthermore, the native ECM's integrated approach to combine the traditional signaling and scaffolding components of the tissue engineering triad suggests that acellular designs may obviate the need for burdensome cell expansion protocols and additional bioactive signal incorporation which introduce major challenges that threaten commercial success in bone regeneration.24,25
To date, a few studies have attempted to coordinate developmental bone engineering strategies with acellular native ECM biomaterials (Figs. 1 and 2). Subsequent sections will address key features of existing technology and more importantly focus on worthwhile areas of future investigation for the purpose of enhancing EC ossification and bone regeneration.
Inflammatory stage ECM strategies for EC ossification: Since most tissues primarily rely on broken vasculature to supply damaged areas with inflammatory signals and cells, modulating the body's initial inflammatory response after any tissue trauma has been well studied.46,47 As a result, many regenerative design strategies have focused on isolating and concentrating portions of human blood such as platelets and plasma (e.g., platelet-rich plasma [PRP]) or pro-coagulation molecules (e.g., fibrin sealants) to enhance endogenous healing responses.48–54 It remains to be seen whether a scaffold with only inflammation-related chemical and mechanical cues can elicit enough of an EC ossification response to heal critical-sized bone defects. Pure fibrin scaffolds and PRP alone do not appear to exhibit sufficient cartilage48–50,52 and bone51,53,54 regeneration in critical-sized defects, which suggests that additional regenerative stimuli such as bioactive molecules and progenitor stem cells may be required.
One strategy to enhance the regenerative response in inflammatory stage ECM strategies has been to supply the fracture space with stem cells from bone marrow aspirate, as fractured bone is supplied with bone marrow intrusion when the inner marrow space is disrupted.1–9 This intrusion of marrow allows supplementary signaling molecules and progenitor cells, including BMSCs, to infiltrate the fracture space. However, hypoxic conditions within the mechanically unstable fracture lead to significant cell mortality, and it remains unclear whether enough of these stem cells survive the initial inflammatory phase to actively participate in subsequent tissue regeneration.1–9 Jakob et al.25 reviewed some bone engineering strategies which included isolated and concentrated bone marrow aspirate to enhance regeneration, but concluded that the fraction of BMSCs present in bone marrow (1 in 10,000 nucleated cells) limited the regenerative capacity of these designs. Extensive work has also been conducted to additionally isolate, differentiate, and expand the BMSCs within bone marrow to enhance the regenerative capacity of engineered bone implants, discussed in subsequent sections on SCBT strategies (Tables 2–4). However, the expansion of BMSCs requires financially burdensome and time-consuming protocols that limit the commercial application of these strategies.24,25 Therefore, cell expansion should likely be considered a contingent reserve to cell recruitment strategies.
Table 4.
Modulators of Chondrocyte Hypertrophy
| Differentiation pathway | Chemical | Physical | Mechanical |
|---|---|---|---|
| Chondrogenesis | TGF-β, BMPs, Dexamethasone | Low oxygen tension | Lower stiffness and adhesion, intermittent and cyclic compression load conditions |
| Hypertrophy and osteogenesis | Vitamin D3, Retinoic acid, Leptin, Insulin, Thyroxine, β-glycerophosphate, BMPs, MMPs, IL-1β, Calcium ions | Normal oxygen tension | Higher stiffness and adhesion, lower magnitude, and higher fluid shear load conditions |
BMPs, bone morphogenic proteins; MMP, matrix metalloproteinase; TGF, transforming growth factor; IL-1β, interleukin-1β.
Coupling the evidence of low progenitor count with poor cell survival rate observed in hypoxic bone defects suggests the importance of spatiotemporal cellular recruitment strategies for bone regeneration. As the native bone healing process continues into the reparative stages of healing, the exposed periosteum and bone marrow continue to serve as reservoirs of progenitor cells that subsequently migrate into the fracture space.6,9,33,34 Several important reviews have focused on physical (e.g., osmotic gradients and hydrodynamic forces) and chemical (e.g., chemokines) cellular recruitment strategies for tissue regeneration.55,56 Within the realm of developmental engineering and native ECM biomaterials, the subsequent differentiation pathway should also be emphasized with regard to overall cellular recruitment strategy. Since critical-sized defects are primarily avascular and hypoxic.2,5,6 initial chondrogenesis involved in EC ossification may be a more favorable condition to exploit in this lower oxygen tension environment compared with direct osteogenesis in IM ossification.39–43
Collectively, the evidence surrounding the use of inflammatory stage ECM scaffolds, signals, and recruited cells indicates that current designs remain insufficient in regenerating bone in critical-sized defects. To date, no studies have focused on controlling the recruitment and differentiation of BMSCs down the EC ossification pathway within the realm of inflammatory stage ECM strategies. This underexplored area may be an advantageous condition to exploit in future bone regeneration strategies.
Procallus stage ECM strategies for EC ossification: An intuitive source for enhancing stimulation of EC ossification is the procallus ECM, as natural bone healing progresses to this stage early in the regenerative process.1–9 Insights into the conductive and inductive biomolecules that control EC ossification have been essential to tissue engineering strategies focusing on cartilage formation,57,58 osteochondral defects,59,60 and, more recently, bone regeneration (Tables 2–4).27,43,57,58,61–76 While cartilage tissue engineering strategies have focused on inducing and maintaining chondrogenic phenotypes, the induction and modulation of cartilage hypertrophy is critical to the progression of EC ossification in bone regeneration designs.9,33,34,37–43,72,77–85 The key transcription factor involved in initial chondrogenesis is SOX9, whereas the primary hypertrophy associated factors are runt-related transcription factor 2 (Runx2) and myocyte enhancer factor 2C (MEF2C).33,34,41,78 In this critical maturation stage of EC ossification, chondrocytes experience a large increase in volume (∼5- to 10-fold), and downstream proteins activated by Runx2 and MEF2C transcription factors begin to remodel the surrounding ECM.33,34,41,78 These targets include matrix metalloproteinases that degrade cartilaginous ECM,1–8 Indian Hedgehog (Ihh) that induces proliferation of nonhypertrophic chondrocytes,33,34,41,78 angiogenic factors such as vascular endothelial growth factor (VEGF) to promote neo-vascularization34,35,72,77,78 along with collagen type X, alkaline phosphatase (ALP), and matrix vesicles for ECM mineralization.86–88 While several in vitro studies have shown that external chemical,9,33,38,41,73,80–85,89,90 physical,39–42 and mechanical cues41,45 stimulated BMSCs to stay locked in a stable cartilage state or progress into a hypertrophic phenotype (Table 4), it remains to be seen whether acellular procallus ECM designs have the potential to elicit similar responses from incorporated or recruited stem cells.
In summary, the success of in vivo developmental engineering designs for EC ossification will likely depend on the incorporation of physiologically relevant bioactive chemical mediators along with physical and mechanical cues drawn from these important in vitro studies. Coupling these results with the previously discussed evidence regarding DBM's influence on EC ossification26–32 provides a potent array of strategies for future bone regeneration designs. Furthermore, it is evident that the selection of appropriate native ECM components is essential for exploiting the advantages of EC ossification. However, strategies to efficiently and feasibly incorporate these biomolecules into bone regenerative implant designs require consideration and, thus, will be further discussed next.
Isolating, identifying, and delivering native ECM for EC ossification
Utilizing step-wise ECM strategies
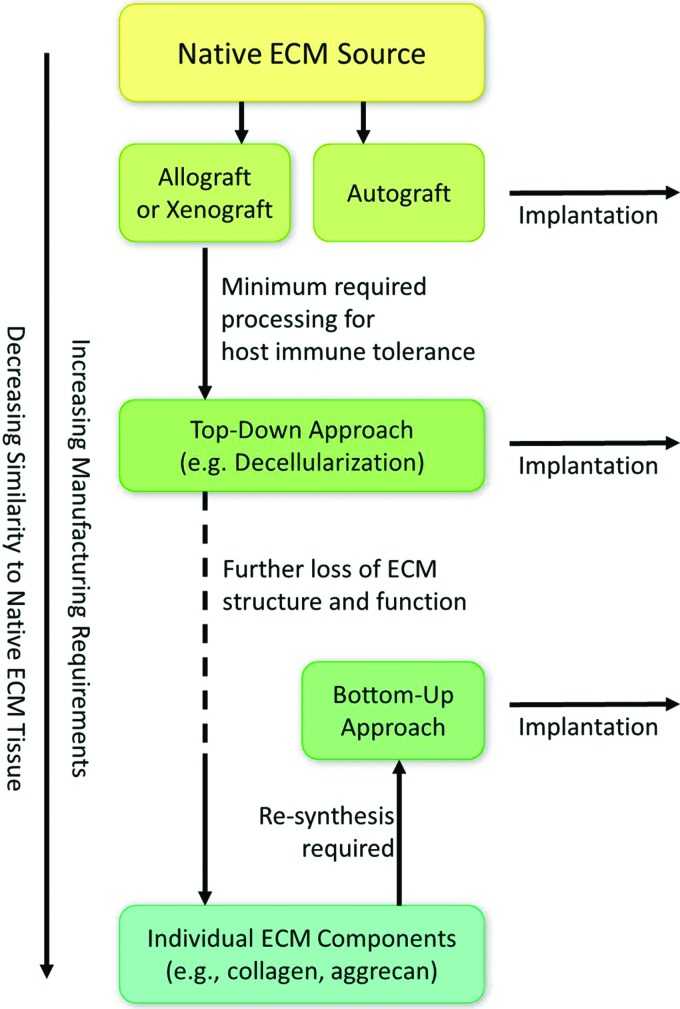
Strategies to design scaffolds with incorporated native ECM components can be divided into two main categories: (1) Bottom-up approach or (2) Top-down approach (i.e., step-wise designs) (Fig. 3). While the former aims at mimicking native ECM with complex biomaterial re-synthesis strategies (e.g., collagen polymerization), the latter step-wise approach attempts to process native ECM from autologous, allogeneic, or xenogeneic sources without completely compromising tissue structure and function (e.g., decellularization and demineralization). Two extensive reviews by Badylak17 and Renth et al.20 provided detailed experimental outcomes and current clinical products utilizing “raw” native ECM biomaterials for tissue regeneration, which include both bottom-up and step-wise strategies. In general, nearly all step-wise design approaches described in these reviews, with the exception of autologous tissue harvesting, require some form of processing to avoid immunogenicity with host (i.e., recipient) tissue, as allogeneic and xenogeneic cellular antigens are recognized as foreign.17–19,91 To address this immunogenicity, a variety of physical, chemical, and enzymatic processing methods have demonstrated the possibility of sufficiently devitalizing or decellularizing these tissues to suppress host rejection without compromising the conductive, inductive, and mechanical components of the ECM.17–19,91
FIG. 3.
Top-down approaches to mimic native ECM tissue require less processing before implantation and retain more of the ECM's physicochemical composition, structure, and function compared with bottom-up re-synthesis approaches. Color images available online at www.liebertpub.com/teb
In a comprehensive review of current tissue and whole organ decellularization methods by Crapo et al.,91 it was recognized that all current techniques cause some degree of ECM disruption. As manufacturing requirements increase, the complex physical and chemical components of the native ECM tissue decrease along with corresponding mechanical structures and functions.17–21,91 At the extreme end of this spectrum, where individual “raw” ECM components are isolated (e.g., collagen, glycosaminoglycans, and soluble growth factors),20 designs necessarily require some form of bottom-up re-synthesis to regain a portion of the physiologically relevant ECM structure and functionality; however, it poorly resembles the initial native ECM tissue (Fig. 3).17 The main advantage of using “raw” biomaterial re-synthesis, however, is that designs can be modular, which means that separate elements of the design can be tested independently. As a result, each component can be characterized for its contribution to the process as a whole in a full-factorial experimental design (e.g., scaffolding protein vs. soluble factor vs. scaffolding protein+soluble factor). Nevertheless, the problem with modular re-synthesis-based designs is that mimicking complex environments, such as the complex cascade of signaling events involved in bone healing,6,9,33,34 is nearly impossible in addition to being economically infeasible.92 This is compounded by the fact that the characterization of the complex 3D structures and functions of native ECM remains incomplete.17,91 A modular design coupling both conductive and inductive biomolecules present in native bone ECM yields nearly infinite permutations; however, this has remained entrenched as the primary philosophy of bone tissue engineering over the past three decades.92 Even with current sophisticated manufacturing technology, including 3D printing, complex polymerization, and chemical conjugation techniques, artificial re-synthesis of this biological material remains physiologically dissimilar compared with its native tissue counterpart in terms of relevant physicochemical composition.17–21 Although future technological advances may someday improve bottom-up re-synthesis designs, the main consequence is that step-wise design strategies may currently represent the more favorable option when attempting to mimic native ECM.91
Step-wise designs from both allogeneic and xenogeneic tissue sources have been utilized clinically for cartilage and bone tissue engineering along with dermal, vascular, nerve, and urogenital tissue.15,17,18,20,93 Furthermore, previous evidence was presented as linking step-wise-derived DBM to extensive bone regeneration via IM and EC ossification,26–32 an attribute that likely contributes to its role as a key protagonist in the bone graft substitute market.15 If alternative ECM biomaterials are to be used to enhance bone regeneration as proposed in the current review, then key technical, regulatory, and commercial features surrounding the use and approval of DBM should be utilized as a guiding template for potential success.
Decellularized cartilage for enhancing EC ossification
Intuitively, it follows that designs mimicking the native bone or cartilage ECM hold vast potential for eliciting ossification and chondrogenesis, respectively. Therefore, it is understandable that for many years, bone and cartilage tissue engineering strategies have focused primarily on treating these two tissues as separate regenerative endeavors. Although a pivotal review by Singh et al.55 established chemical and mechanical-based spatial gradients as an advantageous design approach in osteochondral interfacial tissue regeneration, these strategies still involve a binary differentiation pathway, where the transition zone is only a spatial gradient formed by a gradual change in either chondrogenic or osteogenic cues. EC ossification, however, is a spatiotemporal transition process where cartilage precursor gradually transitions to bone over extended periods of time.1,2,9,33 Thus, the chondrogenic signaling cues initially present in a scaffold may be required to transition over time to influence hypertrophy and osteogenesis.
Previously mentioned in this review was emerging evidence which has shown the potential of DBM to prompt EC ossification in vivo,26–32 and subsequent sections will discuss evidence that in vitro primed cartilage constructs may also prompt EC ossification in vivo (Tables 2–4). Coupling this evidence with motifs in developmental engineering, including path dependence, robustness, and semi-autonomy, suggests that decellularized cartilage (DCC), which mimics portions of the native procallus ECM, may provide extensive potential in prompting EC ossification within an implant and could be a worthwhile focus of future investigation in bone regeneration.
DCC, from both allogeneic and xenogeneic sources, is a poorly explored native ECM biomaterial. Recently, it has emerged as a source that is rich in both chondroinductive and chondroconductive potential.18,20,21,93–95 Current commercially available products utilizing articular cartilage and/or DCC include Biocartilage® (Arthrex), De Novo NT® and ET® Live Graft (Zimmer), and Chondrofix® (Zimmer). However, all of these products use cartilage ECM for the purpose of regenerating only cartilage tissue. In the framework of developmental engineering, DCC provides a source for modulating and enhancing EC ossification; however, there remains an extreme deficiency in both fundamental research and translatable products incorporating this material. Hyaline cartilage is avascular in nature, which may be advantageous to pursue for critical-sized bone defects, as chondrocytes are better suited for hypoxic environments compared with osteoblasts. Moreover, an avascular tissue may minimize potential developmental disturbance from unintended external perturbations.1–9,22,23 An additional consequence of this isolation is that the progression of cartilage to bone during EC ossification is primarily dictated within the tissue by intercellular signaling, incorporating semi-autonomous control into the process.1–9,22,23 Furthermore, many forms of cartilage exist in the body, including hyaline cartilage (e.g., femoral condyles and tibial plateau), fibrocartilage (e.g., temporomandibular joint disc and knee meniscus), elastic cartilage (e.g., nose and ear), and even hypertrophic cartilage (e.g., fetal bones and growth plates).4 Fibrocartilage may provide an even better procallus mimetic tissue than hyaline cartilage, as the procallus is fibrocartilaginous in nature. In addition, fetal bones undergoing EC ossification during skeletogenesis may provide primed hypertrophic cartilage that could facilitate rapid ossification in a bone defect. Thus, there exists a range of DCC-derived tissues that may be explored in relation to EC ossification.
Delivery strategies for decellularized tissues
Crucial to the future success of these ECM materials will be the strategies used to incorporate and deliver them within engineered implants. If maximum retention of native inductive, conductive, and mechanical potential of ECM is desired, then minimal required processing is preferred to keep the microarchitecture of the native tissue intact.17–21 However, this severely limits native ECM's potential to be incorporated within an engineered implant or delivered surgically to a defect site. Large sections of native ECM tissue have been broken down by various morselization and solubilization strategies, which can be used to enhance decellularization efficiency.15,18–20 However, the effect of particle size on regenerative capacity of these materials remains unclear. A recent review of DBM clinical products and procurement by Gruskin et al.15 indicated that larger particles of DBM (420–840 μm) might be more osteoinductive than smaller particles (<250 μm) but warned readers that the majority of preclinical data regarding particle-size effects are inconsistent due to varying animal models and subjective outcome measures. Furthermore, the authors of this article are not aware of any studies that analyze chondrogenesis as a function of DCC particle size or EC ossification as a function of ECM particle size.
While morselization strategies most often include some form of mechanical breakdown (e.g., pulverization or cyrogrinding), solubilization uses chemical and enzymatic methods (e.g., demineralization and collagenase) to break down ECM.18,19 The primary advantage of breaking down native ECM into micron- or sub-micron-sized particles is an increased relative surface area per volume of material. This can be exploited to improve decellularization efficiency, but it may also be essential in exposing cellular adhesion proteins, modulating growth factor release, and uncovering potential cross-linking moieties to facilitate synthetic reconstruction strategies (e.g., polymerization).
In summary, the extent of native ECM processing required to enhance the regenerative capacity of a bone implant will be application specific. These specifications will include issues such as load-bearing requirements, defect geometry (e.g., irregular vs. machined defects), bone architecture (e.g., cortical vs. cancellous), and vulnerable surrounding tissues (e.g., the spinal cord in vertebral fusion or brain tissue in calvarial repair). Existing design paradigms already used to support DBM particles, such as solid scaffolding or chemically cross-linked hydrogels15 along with emerging design paradigms such as shear-responsive and self-assembling colloidal gels,96–100 will likely yield an extensive array of application-specific bone regenerative scaffolds with EC ossification potential.
Incorporating SCBTs into the design
Priming chondrogenesis in vitro for EC ossification
While acellular ECM design strategies possess great conductive and inductive potential for eliciting in vivo EC ossification, SCBTs may provide advantageous osteo- and chondrogenic capacity within bone implants. Without the incorporation of donor cells, acellular implants such as allografts, xenografts, or synthetic scaffolds should rely solely on recruited cells from the peripheral host tissue. Recruitment strategies often involve biochemical agents (e.g., chemokines) or induced physical migration (e.g., osmotic gradients or hydrodynamic forces), which may be insufficient for some indications with limited access to progenitor cell reservoirs in the bone marrow and periosteum. BMSC recruitment also involves successful cellular migration into the scaffold, which may be limited by physical constraints within the implant (e.g., small pore size, limited pore connectivity).101 By incorporating a donor stem cell population within an implant instead, these cellular recruitment challenges may be overcome.
The bone regeneration community has attempted to harness the advantages of SCBT strategies, either as exclusive therapies or combined and encapsulated within biomaterial implants, to overcome cell recruitment limitations and to obviate ABG as the standard of treatment for bone fractures.102 Gamie et al.102 provided a comprehensive review of various sources of stem cells in combination with bone graft substitutes for bone tissue engineering through osteogenic differentiation. Sources of these cells range from ESCs to an array of cells from adult mesenchymal origin, including umbilical cord stem cells, bone marrow and periosteum-derived stem cells, adipose-derived stem cells (ADSCs), synovium-derived stem cells (SDSCs), and, more recently, induced pluripotent stem cells (iPSCs) were shown to lead to osteogenesis both in vitro and in vivo.41,102 Cells from these sources possess some degree of pluripotency, where differentiation can lead to a number of distinct cell types, including osteoblasts and chondrocytes.
Within the realm of SCBTs for bone regeneration, a majority of strategies have focused on stimulating cell populations to undergo direct osteogenesis rather than chondrogenesis. However, as previously mentioned, the EC ossification pathway holds key advantages over IM ossification. This is especially true in critical-sized defects that are inherently large avascular spaces and favor intermediate chondrogenesis of tissue rather than direct ossification.1–9 An emerging group of successful research efforts have focused on exploiting this condition over the past decade. These recent publications (Tables 2–4) have demonstrated the capacity of cartilage tissue constructs to promote EC ossification in vivo after in vitro priming with seeded BMSCs,60,63,64,66,67,72–76,103–107 ESCs,27,62,67,76 ADSCs,108 iPSCs,109 and articular chondrocytes (ACs).61,67,70,71,75 Most of these studies utilized a heterotopic bone formation model in immunocompromised animals to provide evidence of osteo- and chondro-conductivity and inductivity. However, more recent studies by Bahney et al.,110 van der Stok et al.,106 Harada et al.,105 and Shoji et al.108 (Table 2) showed critical-size defect regeneration in rat femurs and mice tibias via EC ossification. Likewise, studies by Montufar-Solis et al.27 and Doan et al.62 (Table 3) showed critical-sized defect regeneration in murine calvaria. Collectively, these studies indicated that primed cartilage constructs can regenerate bone through EC ossification regardless of fetal developmental origin.
While the majority of the tissue priming research aimed at establishing feasibility from a particular cell population (Tables 2 and 3), several studies compared various cell sources to elucidate differences in their capacity to stimulate EC ossification (Table 4).67,75,76 Most of these comparative studies concluded that only certain cell sources, most often ESCs or BMSCs, were capable of inducing EC ossification in animal models67,75–77,111; however, other studies clearly showed significant EC ossification with ADSCs,108 iPSCs,109 and even ACs in vivo.61,70,71 The only stem cell population that did not exhibit EC ossification were SDSCs, which instead went through fibrous degradation and resorption in vivo.75,77 This contradicting evidence highlights the fact that the spatiotemporal control of cell culture priming conditions is essential in determining implant fate in vivo. A review by Gawlitta et al.41 focused on the modulation of these conditions to optimize the in vitro priming of BMSCs for EC ossification. However, a comprehensive overview of modulating EC priming conditions between comparative sources of stem cells has not been addressed and could be a worthwhile focus of future investigation in enhancing EC ossification and bone regeneration designs.
Regardless of the cell source used for priming EC ossification, several generalized conclusions from the reviewed primed in vitro stem cell studies (Tables 2–4) can be summarized as follows:
(1) Chondrogenic cell priming in vitro was required to elicit EC ossification in vivo.
(2) Cartilage templates were necessary, but not sufficient to elicit EC ossification in vivo.
(a) Stable or permanent AC templates progressed through EC ossification.
(3) Osteogenic priming alternatively favored IM over EC ossification.
(4) Hypertrophic chondrocyte priming elicited the most extensive EC ossification.
While the collective evidence from these studies suggested that EC ossification remains necessarily tethered to the burden of in vitro cell priming and expansion, previous evidence was already presented as linking EC ossification to acellular DBM grafts.26–32 This evidence directly contradicts the notion that priming is necessary to elicit an EC response (Tables 2–4) and instead only represents a sufficient condition which can also be addressed by acellular native ECM biomaterials.
Consequently, this contradictory evidence suggests that an intermediate and underexplored strategy for bone regeneration may exist which incorporates advantages exhibited by both DBM biomaterials and primed cartilage constructs, such as the enhancement of EC ossification, and possibly minimizes their limitations, for example the extensive costs, risks, and time-consuming manufacturing practices associated with in vitro cell expansion. Extensive supporting evidence has been previously presented in this review to suggest that native, acellular ECM biomaterials (e.g., DCC) mimicking the procallus healing microenvironment may be a worthwhile focus for future investigation in this underexplored design space for bone regeneration.
Intraoperative SCBT strategies with native ECM biomaterials
An emerging strategy to streamline SCBTs is to consolidate necessary cell protocols, including the harvesting, isolation, stimulation, and implantation of autologous cells, into one surgical (i.e., intraoperative) setting. Intraoperative SCBTs usually involve the use of multiple surgical sites (e.g., a bone defect site and a bone marrow aspiration site) but obviates the need for cell expansion protocols. By definition, ABG falls under the description of an intraoperative SCBT, as autologous stem cells are harvested and implanted within one surgical setting. Recent intraoperative strategies aim at minimizing the limitations associated with traditional stem cell expansion protocols and ABG while also maximizing stem cell efficacy.
To date, however, no intraoperative SCBT approaches using either BMSCs or ADSCs derived from the stromal vascular fraction (SVF) have been able to regenerate bone in critical-sized defects without the inclusion of additional stimulatory factors.25 In a study by Helder et al.,112 isolated SVF cells failed to generate bone when implanted in a goat intervertebral model. Likewise, Follmar et al.113 saw limited angiogenesis and no evidence of ossification in ADSCs-loaded fibrin glues implanted subcutaneously in rabbits. Muller et al.114 observed some evidence of combined angiogenesis and osteoid structures, identified with histology and immunostaining for bone sialoprotein and osteocalcin, in human ADSC-loaded fibrin glues combined with beta-tricalcium phosphate, hydroxyapatite, and acellular bone xenografts implanted subcutaneously in nude mice. However, the authors found no evidence of murine derived bone structures in the implants, indicating a lack of osteoinductivity.114 More recent data from the same group indicated that SVF combined with 250 ng of BMP-2 and encapsulated in a fibrin and porous calcium phosphate composite gel could form heterotropic bone when injected subcutaneously in nude mice.115 Overall, this combined evidence suggests that additional inductive stimulation of stem cells within the brief surgical time frame may be crucial in improving efficacy of intraoperative designs.
Emerging evidence in this area suggests that quick stimulation of isolated stem cells (e.g., minutes to hours) may improve differentiation and gene expression outcomes compared with untreated cells.25,46,47,116 Some strategies for stimulating isolated stem cells include the use of inductive and conductive signaling molecules, either in purified form (e.g., BMP-2)114,115 or from extracted endogenous tissue and fluids (e.g., PRP).117 Kitamura et al.117 showed histological and mechanical evidence that BMSCs loaded with PRP regenerated bone in a dog mandible defect model comparable to autologous bone controls.
Meanwhile, it should be reiterated and emphasized that native ECM biomaterials from allogeneic and xenogeneic sources possess both conductive and inductive biomolecules.17,18,20,21 These biomaterials alone may have the potential to stimulate stem cells in an intraoperative setting. As previously discussed, native ECM representing both the early and late stages of bone repair have the ability to induce both chondro- and osteogenesis by seeded stem cells.26–29,93 A critical area of future investigation, therefore, should be devoted to examining the capacity of these biomaterials to stimulate stem cell differentiation and gene expression within the time constrained intraoperative setting for the purpose of enhancing bone regeneration.
In summary, establishing the regenerative potential of acellular ECM biomaterial designs will help elucidate the additional benefits of incorporating either SCBTs or other purified inductive and conductive biomolecules into intraoperative manufacturing strategies for bone tissue engineering. Regardless of these future outcomes, it is critical for these intraoperative designs to maximize implant efficacy while simultaneously reducing cost, time, and complexity of the surgical procedure compared with current standards of treatment.
Discussion: Converging Framework of EC Ossification in the Future of Bone Regeneration
EC ossification, a process that naturally occurs in almost all bone-healing events,1–9 can be utilized to enhance bone regeneration for nearly any orthopedic indication, especially in avascular critical-sized defects where hypoxic conditions favor chondrogenesis instead of direct IM ossification. The native EC ossification response, however, remains insufficient in regenerating critical-sized defects without intervention but can potentially be enhanced by combining in vivo developmental engineering strategies with biomimetic ECM biomaterials. Evidence to support this claim of utility resides in two evolving arenas of bone tissue engineering: (1) in vitro primed chondrogenic constructs (Tables 2–4) and (2) native DBM allografts and xenografts.26–32 Both strategies have been shown to elicit EC ossification in heterotopic animal models and, more importantly, regenerate critical-sized defects in bone that originated during fetal development from EC ossification (e.g., femur) and IM ossification (e.g., cranium). This latter evidence gives credence to the potential versatility of strategies enhancing EC ossification for a vast array of clinical orthopedic indications.
Currently, however, both in vitro priming and DBM strategies face formidable technical, business, and regulatory challenges that limit their feasibility as commercially competitive alternatives to the standard of treatment, ABG. While the former approach remains tethered to burdensome, costly, and commercially inhibitive in vitro cell expansion protocols, the latter may elicit an insufficient EC ossification response due to its primarily osteoinductive and osteoconductive ECM cues, which instead favor IM ossification.
In addition, regulatory challenges exist for both strategies.118 The in vitro expansion of autologous stem cells inherently involves a high level of manufacturing risk that should meet stringent quality assurance and control for approval by the U.S. Food and Drug Administration (FDA),118 potentially resulting in an expensive premarket approval (PMA) process for each orthopedic indication. Furthermore, off-site facilities may be required to expand cells, which add to the associated risks involved in maintaining viability and preventing contamination of cells. Meanwhile, native ECM biomaterials also face regulatory approval challenges outlined by the FDA and American Association of Tissue Banks (AATB).15 Allogeneic tissue (e.g., DBM or DCC) by itself is not considered a medical device by the FDA unless it is combined with a carrier material, such as hyaluronic acid (e.g., DBX®; MTF/Synthes), in which case it should go through 510(k) approval. Instead, these materials (e.g., Puros® DBM; Zimmer) are categorized under the heading of human cells, tissues, and cellular and tissue-based products (HCT/P). However, the majority of orthopedic DBM products have undergone 510(k) approval by the FDA.15 Based on this knowledge of the regulatory landscape surrounding these strategies, it is evident that acellular ECM biomaterials face fewer challenges than SBCT strategies for approval and, therefore, may represent the more favorable route to clinical translation.
An alternative, and underexplored, developmental engineering strategy integrates the advantages of these two EC ossification approaches with a new class of ECM biomaterials that instead resemble the early fibrocartilaginous bone-healing microenvironment and may enhance the EC ossification response from damaged host tissue. The primary native ECM candidates proposed to potentially elicit such a response are decellularized biomaterials originating from articular, fibrocartilaginous, or hypertrophic cartilage sources. These tissues inherently possess relevant chondroconductive and chondroinductive biomolecules that may potentially modulate EC ossification within an implant. In addition, decellularized ECM constructs may reduce or remove the need for time- and cost-consuming in vitro cell expansion protocols, expanding the developmental engineering paradigm22,23 to include in vivo design strategies. Likewise, the FDA regulatory approval associated with this new class of ECM biomaterials may possibly avoid the more costly and time-consuming PMA process and instead follow a less stringent 510(k) approval process due to its similarity to existing commercially available DBM products.
Although significant focus was given to acellular ECM strategies, critical perspectives on SCBTs were also discussed. While the reviewed chondrogenic priming studies collectively concluded that in vitro stimulation was a necessary step to elicit EC ossification, contradicting evidence with acellular DBM implants suggests that the inclusion and priming of stem cells is not a requirement and may be obviated with the proper inclusion of native ECM components.26–32 Regardless of this contradiction, SCBT strategies provided evidence that modulating hypertrophic signals in bone regenerative implants may lead to a significant enhancement of EC ossification (Tables 2–4). Furthermore, SCBTs provide additional chondrogenic and osteogenic potential within implants compared with acellular ECM biomaterials that alternatively rely on stem cell recruitment from surrounding host tissue. It is not clear whether current orthopedic products in the preclinical and clinical development phase utilize in vitro expansion strategies, but according to Jaklenec et al.,11 a majority of SCBTs across the entire area of tissue engineering and regenerative medicine favor autologous cells (59%) compared with allogeneic (39%) and xenogeneic (2%) cell sources. This indicates the industry's growing interest in developing SCBTs, but it should be emphasized that regulatory approval is not a guarantee of commercial success. Intraoperative SCBT strategies may address these concerns by reducing or removing the burden of in vitro cell expansion without compromising the chondrogenic and osteogenic potential of incorporated stem cells, thus furthering the bone regenerative potential of ECM implants along with overall commercial feasibility.
In summary, native ECM biomaterials inherently possessing ideal conductive, inductive, and mechanical properties have yet to be considered with regard to EC ossification and developmental engineering principles in general. A variety of ECM biomaterial criteria have been reviewed here, which are promising for regenerative investigations spanning bone, cartilage, and osteochondral defects. In the future, in vivo comparisons will be necessary to provide substantial evidence that native ECM biomaterials mimicking aspects of the reparative procallus microenvironment will provide superior performance compared with traditional tissue engineering strategies and current standards of treatment.
Acknowledgments
The authors are grateful for the support from the NIH (R01 DE022472 and R01 AR056347) and the NIGMS Predoctoral Biotechnology Training Grant Program (T32 GM-08359).
Disclosure Statement
No competing financial interests exist.
References
- 1.Einhorn T.A. The science of fracture healing. J Orthop Trauma 19, S4, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Gerstenfeld L.C., Cullinane D.M., Barnes G.L., Graves D.T., and Einhorn T.A. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem 88, 873, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Kalfas I.H. Principles of bone healing. Neurosurg Focus 10, E1, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Meyer U., and Weismann H.P. Bone and cartilage. In: Schroeder G., ed. Bone and Cartilage Engineering. New York, NY: Springer, 2006, pp. 7–46 [Google Scholar]
- 5.Miclau T., Schneider R.A., Eames B.F., and Helms J.A. Common molecular mechanisms regulating fetal bone formation and adult fracture repair. In: Lieberman J.R., and Friedlaender G.E., eds. Bone Regeneration and Repair: Biology and Clinical Application. Totowa, NJ: Humana Press, Inc., 2005, pp. 45–55 [Google Scholar]
- 6.Sfeir C., Ho L., Doll B.A., Azari K., and Hollinger J.O. Fracture repair. In: Lieberman J.R., and Friedlaender G.E., eds. Bone Regeneration and Repair: Biology and Clinical Applications. Totowa, NJ: Humana Press, Inc., 2005, pp. 21–44 [Google Scholar]
- 7.Schindeler A., McDonald M.M., Bokko P., and Little D.G. Bone remodeling during fracture repair: the cellular picture. Semin Cell Dev Biol 19, 459, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Mackie E.J., Ahmed Y.A., Tatarczuch L., Chen K.S., and Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol 40, 46, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Mehta M., Schmidt-Bleek K., Duda G.N., and Mooney D.J. Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Adv Drug Deliv Rev 64, 1257, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu C., Miclau T., Hu D., Hansen E., Tsui K., Puttlitz C., et al. . Cellular basis for age-related changes in fracture repair. J Orthop Res 23, 1300, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaklenec A., Stamp A., Deweerd E., Sherwin A., and Langer R. Progress in the tissue engineering and stem cell industry “are we there yet?” Tissue Eng Part B Rev 18, 155, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Burg K.J., Porter S., and Kellam J.F. Biomaterial developments for bone tissue engineering. Biomaterials 21, 2347, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Frohlich M., Grayson W.L., Wan L.Q., Marolt D., Drobnic M., and Vunjak-Novakovic G. Tissue engineered bone grafts: biological requirements, tissue culture and clinical relevance. Curr Stem Cell Res Ther 3, 254, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mistry A.S., and Mikos A.G. Tissue engineering strategies for bone regeneration. Adv Biochem Eng Biotechnol 94, 1, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Gruskin E., Doll B.A., Futrell F.W., Schmitz J.P., and Hollinger J.O. Demineralized bone matrix in bone repair: history and use. Adv Drug Deliv Rev 64, 1063, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bohner M. Design of ceramic-based cements and putties for bone graft substitution. Eur Cells Mater 20, 1, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Badylak S.F. The extracellular matrix as a biologic scaffold material. Biomaterials 28, 3587, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Benders K.E., van Weeren P.R., Badylak S.F., Saris D.B., Dhert W.J., and Malda J. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol 31, 169, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Gilbert T.W., Sellaro T.L., and Badylak S.F. Decellularization of tissues and organs. Biomaterials 27, 3675, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Renth A.N., and Detamore M.S. Leveraging “raw materials” as building blocks and bioactive signals in regenerative medicine. Tissue Eng Part B Rev 18, 341, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsang K.Y., Cheung M.C., Chan D., and Cheah K.S. The developmental roles of the extracellular matrix: beyond structure to regulation. Cell Tissue Res 339, 93, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Lenas P., Moos M., and Luyten F.P. Developmental engineering: a new paradigm for the design and manufacturing of cell-based products. Part II: from genes to networks: tissue engineering from the viewpoint of systems biology and network science. Tissue Eng Part B Rev 15, 395, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Lenas P., Moos M., and Luyten F.P. Developmental engineering: a new paradigm for the design and manufacturing of cell-based products. Part I: from three-dimensional cell growth to biomimetics of in vivo development. Tissue Eng Part B Rev 15, 381, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Hollister S.J., and Murphy W.L. Scaffold translation: barriers between concept and clinic. Tissue Eng Part B Rev 17, 459, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jakob M., Saxer F., Scotti C., Schreiner S., Studer P., Scherberich A., et al. . Perspective on the evolution of cell-based bone tissue engineering strategies. Eur Surg Res 49, 1, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Oakes D.A., Lee C.C., and Lieberman J.R. An evaluation of human demineralized bone matrices in a rat femoral defect model. Clin Orthop Relat Res 281, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Montufar-Solis D., Nguyen H.C., Nguyen H.D., Horn W.N., Cody D.D., and Duke P.J. Using cartilage to repair bone: an alternative approach in tissue engineering. Ann Biomed Eng 32, 504, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Rabie A.B., Chay S.H., and Wong A.M. Healing of autogenous intramembranous bone in the presence and absence of homologous demineralized intramembranous bone. Am J Orthod Dentofacial Orthop 117, 288, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Wang J., Glimcher M.J., Mah J., Zhou H.Y., and Salih E. Expression of bone microsomal casein kinase II, bone sialoprotein, and osteopontin during the repair of calvarial defects. Bone 22, 621, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Rabie A.B., Dan Z., and Samman N. Ultrastructural identification of cells involved in the healing of intramembranous and endochondral bones. Int J Oral Maxillofac Surg 25, 383, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Rabie A.B., and Lie Ken Jie R.K. Integration of endochondral bone grafts in the presence of demineralized bone matrix. Int J Oral Maxillofac Surg 25, 311, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Rabie A.B., Wong R.W., and Hagg U. Composite autogenous bone and demineralized bone matrices used to repair defects in the parietal bone of rabbits. Br J Oral Maxillofac Surg 38, 565, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Dimitriou R., Tsiridis E., and Giannoudis P.V. Current concepts of molecular aspects of bone healing. Injury 36, 1392, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Studer D., Millan C., Ozturk E., Maniura-Weber K., and Zenobi-Wong M. Molecular and biophysical mechanisms regulating hypertrophic differentiation in chondrocytes and mesenchymal stem cells. Eur Cells Mater 24, 118, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Carmeliet P., and Jain R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centola M., Abbruzzese F., Scotti C., Barbero A., Vadala G., Denaro V., et al. . Scaffold-based delivery of a clinically relevant anti-angiogenic drug promotes the formation of in vivo stable cartilage. Tissue Eng Part A 19, 1960, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.des Rieux A., Ucakar B., Mupendwa B.P., Colau D., Feron O., Carmeliet P., et al. . 3D systems delivering VEGF to promote angiogenesis for tissue engineering. J Controlled Release 150, 272, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Zhou Z., Apte S.S., Soininen R., Cao R., Baaklini G.Y., Rauser R.W., et al. . Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci U S A 97, 4052, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malda J., Klein T.J., and Upton Z. The roles of hypoxia in the in vitro engineering of tissues. Tissue Eng 13, 2153, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Sheehy E.J., Buckley C.T., and Kelly D.J. Oxygen tension regulates the osteogenic, chondrogenic and endochondral phenotype of bone marrow derived mesenchymal stem cells. Biochem Biophys Res Commun 417, 305, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Gawlitta D., Farrell E., Malda J., Creemers L.B., Alblas J., and Dhert W.J. Modulating endochondral ossification of multipotent stromal cells for bone regeneration. Tissue Eng Part B Rev 16, 385, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Lewis M.C., Macarthur B.D., Malda J., Pettet G., and Please C.P. Heterogeneous proliferation within engineered cartilaginous tissue: the role of oxygen tension. Biotechnol Bioeng 91, 607, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Scotti C., Osmokrovic A., Wolf F., Miot S., Peretti G.M., Barbero A., et al. . Response of human engineered cartilage based on articular or nasal chondrocytes to interleukin-1beta and low oxygen. Tissue Eng Part A 18, 362, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scott C.K., and Hightower J.A. The matrix of endochondral bone differs from the matrix of intramembranous bone. Calcif Tissue Int 49, 349, 1991 [DOI] [PubMed] [Google Scholar]
- 45.Discher D.E., Mooney D.J., and Zandstra P.W. Growth factors, matrices, and forces combine and control stem cells. Science 324, 1673, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anitua E., Sanchez M., and Orive G. Potential of endogenous regenerative technology for in situ regenerative medicine. Adv Drug Deliv Rev 62, 741, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Chen F.M., Zhang J., Zhang M., An Y., Chen F., and Wu Z.F. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials 31, 7892, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Dare E.V., Griffith M., Poitras P., Wang T., Dervin G.F., Giulivi A., et al. . Fibrin sealants from fresh or fresh/frozen plasma as scaffolds for in vitro articular cartilage regeneration. Tissue Eng Part A 15, 2285, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Eyrich D., Brandl F., Appel B., Wiese H., Maier G., Wenzel M., et al. . Long-term stable fibrin gels for cartilage engineering. Biomaterials 28, 55, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Eyrich D., Gopferich A., and Blunk T. Fibrin in tissue engineering. Adv Exp Med Biol 585, 379, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Grageda E. Platelet-rich plasma and bone graft materials: a review and a standardized research protocol. Implant Dent 13, 301, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Kaufman M.R., Westreich R., Ammar S.M., Amirali A., Iskander A., and Lawson W. Autologous cartilage grafts enhanced by a novel transplant medium using fibrin sealant and fibroblast growth factor. Arch Facial Plast Surg 6, 94, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Mazor Z., Peleg M., Garg A.K., and Luboshitz J. Platelet-rich plasma for bone graft enhancement in sinus floor augmentation with simultaneous implant placement: patient series study. Implant Dent 13, 65, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Santos S.G., Lamghari M., Almeida C.R., Oliveira M.I., Neves N., Ribeiro A.C., et al. . Adsorbed fibrinogen leads to improved bone regeneration and correlates with differences in the systemic immune response. Acta Biomater 9, 7209, 2013 [DOI] [PubMed] [Google Scholar]
- 55.Singh M., Berkland C., and Detamore M.S. Strategies and applications for incorporating physical and chemical signal gradients in tissue engineering. Tissue Eng Part B Rev 14, 341, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee S.H., and Shin H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv Drug Deliv Rev 59, 339, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Pelttari K., Steck E., and Richter W. The use of mesenchymal stem cells for chondrogenesis. Injury 39 Suppl 1, S58, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Jukes J.M., Moroni L., van Blitterswijk C.A., and de Boer J. Critical Steps toward a tissue-engineered cartilage implant using embryonic stem cells. Tissue Eng Part A 14, 135, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Sundelacruz S., and Kaplan D.L. Stem cell- and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Semin Cell Dev Biol 20, 646, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheehy E.J., Vinardell T., Buckley C.T., and Kelly D.J. Engineering osteochondral constructs through spatial regulation of endochondral ossification. Acta Biomater 9, 5484, 2013 [DOI] [PubMed] [Google Scholar]
- 61.Case N.D., Duty A.O., Ratcliffe A., Muller R., and Guldberg R.E. Bone formation on tissue-engineered cartilage constructs in vivo: effects of chondrocyte viability and mechanical loading. Tissue Eng 9, 587, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Doan L., Kelley C., Luong H., English J., Gomez H., Johnson E., et al. . Engineered cartilage heals skull defects. Am J Orthod Dentofacial Orthop 137, 162.e1; discussion 162, 2010 [DOI] [PubMed] [Google Scholar]
- 63.Farrell E., Both S.K., Odorfer K.I., Koevoet W., Kops N., O'Brien F.J., et al. . In-vivo generation of bone via endochondral ossification by in-vitro chondrogenic priming of adult human and rat mesenchymal stem cells. BMC Musculoskelet Disord 12, 31, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Farrell E., van der Jagt O.P., Koevoet W., Kops N., van Manen C.J., Hellingman C.A., et al. . Chondrogenic priming of human bone marrow stromal cells: a better route to bone repair? Tissue Eng Part C Methods 15, 285, 2009 [DOI] [PubMed] [Google Scholar]
- 65.Janicki P., Boeuf S., Steck E., Egermann M., Kasten P., and Richter W. Prediction of in vivo bone forming potency of bone marrow-derived human mesenchymal stem cells. Eur Cells Mater 21, 488, 2011 [DOI] [PubMed] [Google Scholar]
- 66.Janicki P., Kasten P., Kleinschmidt K., Luginbuehl R., and Richter W. Chondrogenic pre-induction of human mesenchymal stem cells on beta-TCP: enhanced bone quality by endochondral heterotopic bone formation. Acta Biomater 6, 3292, 2010 [DOI] [PubMed] [Google Scholar]
- 67.Jukes J.M., Both S.K., Leusink A., Sterk L.M., van Blitterswijk C.A., and de Boer J. Endochondral bone tissue engineering using embryonic stem cells. Proc Natl Acad Sci U S A 105, 6840, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jukes J.M., van Blitterswijk C.A., and de Boer J. Skeletal tissue engineering using embryonic stem cells. J Tissue Eng Regen Med 4, 165, 2010 [DOI] [PubMed] [Google Scholar]
- 69.Li J., and Pei M. Optimization of an in vitro three-dimensional microenvironment to reprogram synovium-derived stem cells for cartilage tissue engineering. Tissue Eng Part A 17, 703, 2011 [DOI] [PubMed] [Google Scholar]
- 70.Oliveira S.M., Amaral I.F., Barbosa M.A., and Teixeira C.C. Engineering endochondral bone: in vitro studies. Tissue Eng Part A 15, 625, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oliveira S.M., Mijares D.Q., Turner G., Amaral I.F., Barbosa M.A., and Teixeira C.C. Engineering endochondral bone: in vivo studies. Tissue Eng Part A 15, 635, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pelttari K., Winter A., Steck E., Goetzke K., Hennig T., Ochs B.G., et al. . Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum 54, 3254, 2006 [DOI] [PubMed] [Google Scholar]
- 73.Scotti C., Piccinini E., Takizawa H., Todorov A., Bourgine P., Papadimitropoulos A., et al. . Engineering of a functional bone organ through endochondral ossification. Proc Natl Acad Sci U S A 110, 3997, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scotti C., Tonnarelli B., Papadimitropoulos A., Scherberich A., Schaeren S., Schauerte A., et al. . Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci U S A 107, 7251, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vinardell T., Sheehy E.J., Buckley C.T., and Kelly D.J. A comparison of the functionality and in vivo phenotypic stability of cartilaginous tissues engineered from different stem cell sources. Tissue Eng Part A 18, 1161, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Both S.K., van Apeldoorn A.A., Jukes J.M., Englund M.C., Hyllner J., van Blitterswijk C.A., et al. . Differential bone-forming capacity of osteogenic cells from either embryonic stem cells or bone marrow-derived mesenchymal stem cells. J Tissue Eng Regen Med 5, 180, 2011 [DOI] [PubMed] [Google Scholar]
- 77.Dickhut A., Pelttari K., Janicki P., Wagner W., Eckstein V., Egermann M., et al. . Calcification or dedifferentiation: requirement to lock mesenchymal stem cells in a desired differentiation stage. J Cell Physiol 219, 219, 2009 [DOI] [PubMed] [Google Scholar]
- 78.Gauci S.J., Golub S.B., Tutolo L., Little C.B., Sims N.A., Lee E.R., et al. . Modulating chondrocyte hypertrophy in growth plate and osteoarthritic cartilage. J Musculoskelet Neuronal Interact 8, 308, 2008 [PubMed] [Google Scholar]
- 79.Hattori T., Muller C., Gebhard S., Bauer E., Pausch F., Schlund B., et al. . SOX9 is a major negative regulator of cartilage vascularization, bone marrow formation and endochondral ossification. Development 137, 901, 2010 [DOI] [PubMed] [Google Scholar]
- 80.Inada M., Wang Y., Byrne M.H., Rahman M.U., Miyaura C., Lopez-Otin C., et al. . Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc Natl Acad Sci U S A 101, 17192, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mumme M., Scotti C., Papadimitropoulos A., Todorov A., Hoffmann W., Bocelli-Tyndall C., et al. . Interleukin-1beta modulates endochondral ossification by human adult bone marrow stromal cells. Eur Cells Mater 24, 224, 2012 [DOI] [PubMed] [Google Scholar]
- 82.Ortega N., Behonick D.J., and Werb Z. Matrix remodeling during endochondral ossification. Trends Cell Biol 14, 86, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ortega N., Wang K., Ferrara N., Werb Z., and Vu T.H. Complementary interplay between matrix metalloproteinase-9, vascular endothelial growth factor and osteoclast function drives endochondral bone formation. Dis Models Mech 3, 224, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Page-McCaw A., Ewald A.J., and Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 8, 221, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stickens D., Behonick D.J., Ortega N., Heyer B., Hartenstein B., Yu Y., et al. . Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development 131, 5883, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boskey A.L., and Roy R. Cell culture systems for studies of bone and tooth mineralization. Chem Rev 108, 4716, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hessle L., Johnson K.A., Anderson H.C., Narisawa S., Sali A., Goding J.W., et al. . Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci U S A 99, 9445, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johnson K., Moffa A., Chen Y., Pritzker K., Goding J., and Terkeltaub R. Matrix vesicle plasma cell membrane glycoprotein-1 regulates mineralization by murine osteoblastic MC3T3 cells. J Bone Miner Res 14, 883, 1999 [DOI] [PubMed] [Google Scholar]
- 89.Mahmoudifar N., and Doran P.M. Chondrogenesis and cartilage tissue engineering: the longer road to technology development. Trends Biotechnol 30, 166, 2012 [DOI] [PubMed] [Google Scholar]
- 90.Puetzer J.L., Petitte J.N., and Loboa E.G. Comparative review of growth factors for induction of three-dimensional in vitro chondrogenesis in human mesenchymal stem cells isolated from bone marrow and adipose tissue. Tissue Eng Part B Rev 16, 435, 2010 [DOI] [PubMed] [Google Scholar]
- 91.Crapo P.M., Gilbert T.W., and Badylak S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 32, 3233, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Evans C.H. Barriers to the clinical translation of orthopedic tissue engineering. Tissue Eng Part B Rev 17, 437, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ye K., Felimban R., Moulton S.E., Wallace G.G., Di Bella C., Traianedes K., et al. . Bioengineering of articular cartilage: past, present and future. Regen Med 8, 333, 2013 [DOI] [PubMed] [Google Scholar]
- 94.Schwarz S., Koerber L., Elsaesser A.F., Goldberg-Bockhorn E., Seitz A.M., Durselen L., et al. . Decellularized cartilage matrix as a novel biomatrix for cartilage tissue-engineering applications. Tissue Eng Part A 18, 2195, 2012 [DOI] [PubMed] [Google Scholar]
- 95.Sutherland A.J., Converse G.L., Hopkins R.A., and Detamore M.S. The bioactivity of cartilage extracellular matrix in articular cartilage regeneration. Adv Healthc Mater 2014. [Epub ahead of print]; DOI: 10.1002/adhm.201400165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guvendiren M., Lu H.D., and Burdick J.A. Shear-thinning hydrogels for biomedical applications. Soft Matter 8, 260, 2012 [Google Scholar]
- 97.Wang Q., Gu Z., Jamal S., Detamore M.S., and Berkland C. Hybrid hydroxyapatite nanoparticle colloidal gels are injectable fillers for bone tissue engineering. Tissue Eng Part A 19, 2586, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Q., Jamal S., Detamore M.S., and Berkland C. PLGA-chitosan/PLGA-alginate nanoparticle blends as biodegradable colloidal gels for seeding human umbilical cord mesenchymal stem cells. J Biomed Mater Res Part A 96, 520, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang Q., Wang J., Lu Q., Detamore M.S., and Berkland C. Injectable PLGA based colloidal gels for zero-order dexamethasone release in cranial defects. Biomaterials 31, 4980, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lin Y., Boker A., He J., Sill K., Xiang H., Abetz C., et al. . Self-directed self-assembly of nanoparticle/copolymer mixtures. Nature 434, 55, 2005 [DOI] [PubMed] [Google Scholar]
- 101.Loh Q.L., and Choong C. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng Part B Rev 19, 485, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gamie Z., Tran G.T., Vyzas G., Korres N., Heliotis M., Mantalaris A., et al. . Stem cells combined with bone graft substitutes in skeletal tissue engineering. Expert Opin Biol Ther 12, 713, 2012 [DOI] [PubMed] [Google Scholar]
- 103.Simmons C.A., Alsberg E., Hsiong S., Kim W.J., and Mooney D.J. Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone 35, 562, 2004 [DOI] [PubMed] [Google Scholar]
- 104.Yamada Y., Boo J.S., Ozawa R., Nagasaka T., Okazaki Y., Hata K., et al. . Bone regeneration following injection of mesenchymal stem cells and fibrin glue with a biodegradable scaffold. J Craniomaxillofac Surg 31, 27, 2003 [DOI] [PubMed] [Google Scholar]
- 105.Harada N., Watanabe Y., Sato K., Abe S., Yamanaka K., Sakai Y., et al. . Bone regeneration in a massive rat femur defect through endochondral ossification achieved with chondrogenically differentiated MSCs in a degradable scaffold. Biomaterials 35, 7800, 2014 [DOI] [PubMed] [Google Scholar]
- 106.van der Stok J., Koolen M.K., Jahr H., Kops N., Waarsing J.H., Weinans H., et al. . Chondrogenically differentiated mesenchymal stromal cell pellets stimulate endochondral bone regeneration in critical-sized bone defects. Eur Cells Mater 27, 137; discussion 48, 2014 [DOI] [PubMed] [Google Scholar]
- 107.Sheehy E.J., Vinardell T., Toner M.E., Buckley C.T., and Kelly D.J. Altering the architecture of tissue engineered hypertrophic cartilaginous grafts facilitates vascularisation and accelerates mineralisation. PLoS One 9, e90716, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shoji T., Ii M., Mifune Y., Matsumoto T., Kawamoto A., Kwon S.M., et al. . Local transplantation of human multipotent adipose-derived stem cells accelerates fracture healing via enhanced osteogenesis and angiogenesis. Lab Invest 90, 637, 2010 [DOI] [PubMed] [Google Scholar]
- 109.Tam W.L., O D.F., Hiramatsu K., Tsumaki N., Luyten F.P., and Roberts S.J. Sox9 reprogrammed dermal fibroblasts undergo hypertrophic differentiation in vitro and trigger endochondral ossification in vivo. Cell Reprogram 16, 29, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bahney C.S., Hu D.P., Taylor A.J., Ferro F., Britz H.M., Hallgrimsson B., et al. . Stem cell-derived endochondral cartilage stimulates bone healing by tissue transformation. J Bone Miner Res 29, 1269, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tortelli F., and Cancedda R. Three-dimensional cultures of osteogenic and chondrogenic cells: a tissue engineering approach to mimic bone and cartilage in vitro. Eur Cells Mater 17, 1, 2009 [DOI] [PubMed] [Google Scholar]
- 112.Helder M.N., Knippenberg M., Klein-Nulend J., and Wuisman P.I.J.M. Stem cells from adipose tissue allow challenging new concepts for regenerative medicine. Tissue Eng 13, 1799, 2007 [DOI] [PubMed] [Google Scholar]
- 113.Follmar K.E., Prichard H.L., DeCroos F.C., Wang H.T., Levin L.S., Klitzman B., et al. . Combined bone allograft and adipose-derived stem cell autograft in a rabbit model. Ann Plast Surg 58, 561, 2007 [DOI] [PubMed] [Google Scholar]
- 114.Muller A.M., Mehrkens A., Schafer D.J., Jaquiery C., Guven S., Lehmicke M., et al. . Towards an intraoperative engineering of osteogenic and vasculogenic grafts from the stromal vascular fraction of human adipose tissue. Eur Cells Mater 19, 127, 2010 [DOI] [PubMed] [Google Scholar]
- 115.Mehrkens A., Saxer F., Guven S., Hoffmann W., Muller A.M., Jakob M., et al. . Intraoperative engineering of osteogenic grafts combining freshly harvested, human adipose-derived cells and physiological doses of bone morphogenetic protein-2. Eur Cells Mater 24, 308, 2012 [DOI] [PubMed] [Google Scholar]
- 116.Evans C.H., Palmer G.D., Pascher A., Porter R., Kwong F.N., Gouze E., et al. . Facilitated endogenous repair: making tissue engineering simple, practical, and economical. Tissue Eng 13, 1987, 2007 [DOI] [PubMed] [Google Scholar]
- 117.Kitamura S., Ohgushi H., Hirose M., Funaoka H., Takakura Y., and Ito H. Osteogenic differentiation of human bone marrow-derived mesenchymal cells cultured on alumina ceramics. Artif Organs 28, 72, 2004 [DOI] [PubMed] [Google Scholar]
- 118.Lee M.H., Arcidiacono J.A., Bilek A.M., Wille J.J., Hamill C.A., Wonnacott K.M., et al. . Considerations for tissue-engineered and regenerative medicine product development prior to clinical trials in the United States. Tissue Eng Part B Rev 16, 41, 2010 [DOI] [PubMed] [Google Scholar]