Abstract
Collagen-based devices, in various physical conformations, are extensively used for tissue engineering and regenerative medicine applications. Given that the natural cross-linking pathway of collagen does not occur in vitro, chemical, physical, and biological cross-linking methods have been assessed over the years to control mechanical stability, degradation rate, and immunogenicity of the device upon implantation. Although in vitro data demonstrate that mechanical properties and degradation rate can be accurately controlled as a function of the cross-linking method utilized, preclinical and clinical data indicate that cross-linking methods employed may have adverse effects on host response, especially when potent cross-linking methods are employed. Experimental data suggest that more suitable cross-linking methods should be developed to achieve a balance between stability and functional remodeling.
Introduction
Collagen is one of the major structural extracellular matrix (ECM) proteins in mammals, constituting 20% to 30% of the total body proteins. To date, 29 different collagen genes have been identified,1 with all types sharing a unique and common triple helical configuration with a repeated [Gly-X-Y]n sequence, where X is often proline and Y is frequently hydroxyproline.2,3 Among the different collagen types, collagen type I, predominantly localized in the skin, tendon, cornea, and bone, is the most abundant in the body and consequently the most widely studied. Collagen-based materials, in the form of tissue grafts and reconstituted scaffolds, are attractive for biomedical applications, as due to their natural composition and their well-tolerated degradation products are perceived by the host as normal constituents rather than as foreign matter and therefore provide an acceptable host response.4 In addition to their superior mechanical properties, collagen-based devices provide instructive cues to the cells, promoting this way functional tissue repair and regeneration.5,6 It is therefore not surprising that the collagen-based medical device market is estimated to reach 3.7 billion US dollars by 2017.7
The natural cross-linking pathway of lysyl oxidase is responsible for mechanical resilience of tissues and their proteolytic resistance.8 The harsh extraction/purification methods,9 scaffold fabrication technologies,10 and the subsequent sterilization methods11 necessitate the introduction of exogenous cross-links (chemical, physical, or biological in nature) into the molecular structure of collagen implants to control their degradation rate and enhance their mechanical stability.12–14 However, such cross-linking approaches are associated with numerous shortfalls as a function of the cross-linking density/method, including cytotoxicity,15,16 calcification,17–19 and foreign body response.20,21
Herein, we discuss the host/macrophage response, as a function and extent of the cross-linking density/method employed to stabilize collagen-based devices. We recognize that the extent of cross-linking can be assessed by denaturation temperature, quantification of free amine groups, swelling, mechanical properties, and/or resistance to enzymatic degradation. However, denaturation temperature is customarily used to assess cross-linking density due to the simplicity and accuracy of the technique. Thus, herein we define collagen materials that are slightly cross-linked, moderately cross-linked, and heavily cross-linked as those that have exhibited denaturation temperature of <65°C, 65–70°C, and >70°C, respectively.
Collagen Cross-Linking Methods
The fundamental principle of collagen cross-linking is the formation of covalent bonds between collagen molecules using chemical or natural moieties, which bind either to the free amine or carboxyl groups of collagen. The most commonly used chemical cross-linking reagents are aldehydes (e.g., glutaraldehyde [GTA]),22 isocyanates (e.g., hexamethylene diisocyanate [HMDI]),23 and carbodiimides (e.g., 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide [EDC]).24 Photoreactive agents (e.g., rose Bengal,25 riboflavin26), carbohydrates (e.g., ribose,27 glucose28), and plant extracts (e.g., genipin,29,30 oleuropein,31 and Myrica rubra32) have also been used, but to a lesser extent. Recently, the influence of polyethylene glycol (PEG) polymers that can vary in molecular weight, degree of branching, and terminal groups are under intense investigation as means to cross-link and functionalize collagen-based devices.33–36 To avoid cytotoxic effects associated with the chemical cross-linker itself of its residue, physical (e.g., dehydrothermal [DHT]37–42 and UV irradiation41,43–46), and biological (e.g., transglutaminase47–51) methods have also been assessed.
Each cross-linking method has demonstrated a different degree of structural and mechanical stability, largely attributed to the different cross-linking mechanisms, concentration, and exposure time. Cross-linking with GTA involves an heterogeneous cross-linking distribution that occurs only on the surface of the fibrils and fibers, which leads predominantly to intermolecular cross-links connecting distant collagen molecules.52 The ability of GTA to self-polymerize probably accounts for its effectiveness as a cross-linker,53 while the effectiveness of formaldehyde as a cross-linker depends on its abundance rather than on the individual lengths of the cross-links.54 The bifunctional cross-linker HMDI has been used as an alternative to GTA, due to its superior cytocompatibility and proportional mechanical stability.55,56 Carboxyl group cross-linking, through carbodiimide24,57–60 or acyl azide61–69 methods, has been extensively utilized as an alternative to GTA/HMDI; such approaches are more cytocompatible as they do not introduce foreign cross-linking molecules; less resistant to proteolytic attack; and less susceptible to calcification; however, the produced devices are not as strong as such methods can couple proximate collagen molecules.70,71 To increase efficiency and avoid denaturation, the DHT treatment requires high vacuum to reduce the water content before heating at over 100°C for several hours and is usually followed by an EDC step.41,72 Mimicking the in vivo collagen cross-linking, tissue-type (Ca2+ dependent) or microbial (Ca2+ independent) transglutaminase (biological method) has been used to covalently cross-link ECM proteins resulting in a covalent γ-glutamyl-ɛ-lysine isopeptide bond.48 Collagen-based materials that have been cross-linked with mammalian or microbial in origin transglutaminases have demonstrated a moderate increase in denaturation temperature, mechanical resilience, and biological stability, compared to chemical cross-linking approaches.73–76
Physiological Wound Healing
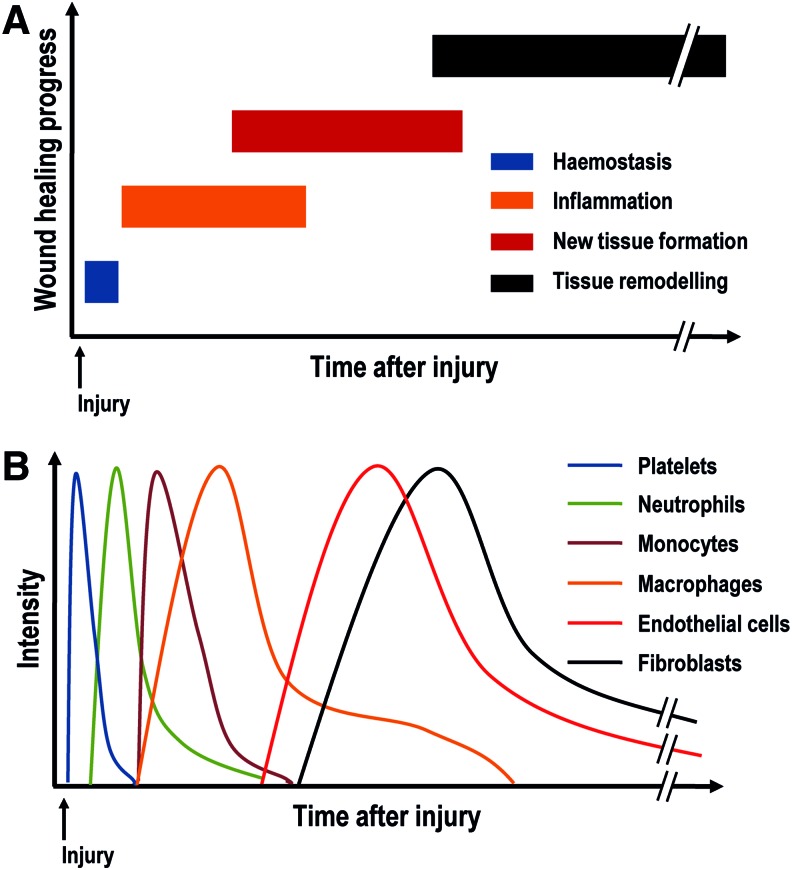
The wound healing process is the innate response of all tissues to any injury or device implantation. It is a complex process that is regulated by several cell types, growth factors, and cytokines that direct the four overlapping phases (Figs. 1 and 2), namely hemostasis, inflammation, new tissue formation, and tissue remodeling.77
FIG. 1.
Temporal distribution of the four overlapping wound healing phases (A) and associated cell type (B). Color images available online at www.liebertpub.com/teb
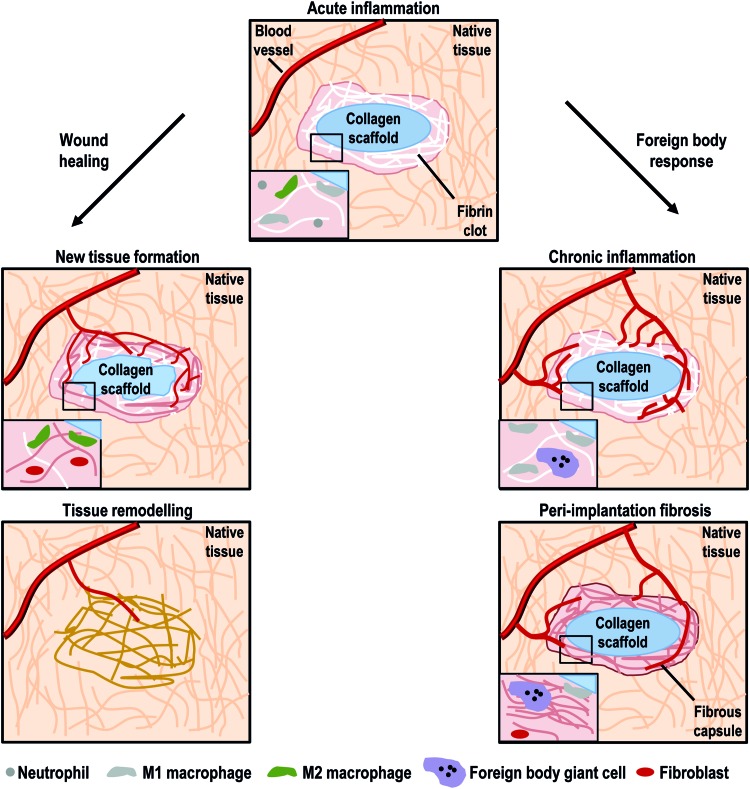
FIG. 2.
Acute inflammation is characterized by the presence of neutrophils, monocytes, and macrophages. Depending on the resolution of the acute inflammation, injury repair could lead to a wound healing process or a classical foreign body response. In wound healing, M2 macrophages attract fibroblast and endothelial cells to secrete a new vascularized tissue. This connective tissue replaces fibrin clot and degraded scaffold. Tissue remodeling is the last healing and could continue for over a year. In foreign body response, acute inflammation persists over time and M1 macrophages aggregate into foreign body giant cells (FBGCs). M1 macrophages and FBGCs fail to degrade the foreign scaffold, resulting in fibroblast recruitment and deposition of a fibrous connective tissue around the scaffold (peri-implantation fibrosis). Color images available online at www.liebertpub.com/teb
Hemostasis occurs immediately after injury or device implantation. Released factors from the tissue induce platelets to secrete clotting factors (e.g., mainly serotonin, thromboxane, platelet-derived growth factor [PDGF], transforming growth factor beta [TGF-β]) to promote coagulation and to develop a fibrin clot.78 This provisional fibrin matrix acts as a scaffold for further cell migration. Simultaneously to the formation of the fibrin clot, a dynamic interaction between blood plasma proteins and the device surface occurs and a provisional matrix around the biomaterial surface is developed; this event is known as the Vroman effect.79 The initial protein adsorption depends on the surface properties of the device, including wettability,80,81 surface charge/chemistry,82,83 topography/roughness,84–86 and stiffness,87 which modulate the cell/inflammatory response and subsequent wound healing.
Following hemostasis, acute inflammation begins between 24 and 48 h after injury. This phase is characterized by the recruitment of neutrophils and mast cells in response to chemokines (cytokines with chemoattractive properties) and other chemoattractants (mainly interleukin [IL]-1, IL-6, IL-8, monocyte chemoattractant protein [MCP]-1, macrophage inflammatory protein [MIP]-1, and tumor necrosis factor alpha [TNF-α]).88,89 Neutrophils and mast cells phagocytize foreign material, bacteria, dead cells, and damaged matrix within the wound. The presence of contaminants/foreign matter at the wound bed increases the neutrophil presence, reactive oxygen species (ROS), and cytokine signaling.88,90 Neutrophils secrete TGF-β, PDGF, platelet factor 4 (PF4), and IL-1 to recruit further mast cells and monocytes. Mast cells secrete histamine and other cytokines that recruit leukocytes into the injury site. After 48–72 h, monocytes migrate and differentiate into macrophages, which secrete TNF-α, IL-6, RANTES (regulated on activation, normal T cell expressed and secreted), MCP-1, and MIP-1, to recruit further macrophages and dominate the cell population at the injury site.91 Initially, macrophages are mainly M1 phenotype, a proinflammatory or classically activated phenotype. M1 macrophages attack potential pathogens or phagocytize at the wound site, as a response to interferon gamma (IFN-γ), TNF-α, or bacterial lipopolysaccharides. Gradually, M1 macrophages change to M2 phenotype, an anti-inflammatory or alternatively activated phenotype. M2 macrophages have been described as displaying different subphenotypes or roles, which are anti-inflammatory (M2a), immunoregulatory or homeostatic (M2b), and prowound healing (M2c).92–94 The macrophage phenotype switch is induced by cytokines, such as IL-4, IL-10, and IL-13, and functional reconstruction depends on the timing of this change. Macrophage activation and polarization is crucial in the coordination of the later inflammation and regeneration phases.95,96 Thus, macrophage polarization and activation is at the forefront of scientific investigation, with various studies aiming to modulate it using biophysical cues (e.g., architectural features,97 topographical patterns85), biochemical signals (e.g., incorporation of glycosaminoglycans98 or drugs99), and biological means (e.g., gene therapy with lipoplexes100 or polyplexes101).
New tissue formation begins 2–10 days after injury and is identified by migration and proliferation of different cell types that produce a new ECM and form the initial wound. In skin, for example, keratinocytes migrate over the dermis and restore the barrier function of the epidermis,88 while fibroblasts, attracted by macrophage cytokines, migrate to the injury site from the wound edge or bone marrow and differentiate into myofibroblasts, contributing to the wound contraction.102 During this stage, fibroblasts and myofibroblasts interact, migrate, proliferate, and secrete ECM proteins to replace the fibrin matrix and form new tissue, predominantly constituted by collagen type III and smaller amounts of fibronectin, elastin, and proteoglycans.88,103 Meanwhile, macrophages and fibroblasts secrete vascular endothelial growth factor (VEGF) and FGF2 that promote endothelial and progenitor cells to produce new blood vessels, respectively.103,104 These new vessels start out from pre-existing vessels adjacent to the wound.88
Tissue remodeling significantly increases 2–3 weeks after injury and could continue for over a year. The remodeling phase is characterized by different cell types undergoing reduction in cell activity and apoptosis and the creation of mature blood vessels. Collagen type III is gradually replaced by collagen type I, an event mainly controlled by matrix metalloproteinases (MMPs), tissue inhibitors of matrix metalloproteinases (TIMPs), and mechanical stress and strain. The properties of the tissue are partially recovered, but the new tissue hardly ever reaches the preinjury state; for example, the dermis reaches up to 70% of its preinjury tensile strength.88
Impaired Wound Healing
Multiple potential factors, local (e.g., injury size, infection, device properties, and degradation products) or systemic (e.g., nutrition, age, health state)105 in nature, can interfere with one or more wound healing stages resulting in improper or impaired wounds. The most common impaired wounds after device implantation are delayed acute wounds, chronic wounds, and peri-implantation fibrosis, the hallmark of which is a patch of inflammatory cells, mainly macrophages and foreign body giant cells (FBGCs), and a disorganized ECM, mostly collagen.88,105 In severe burns, immunosuppression is brought about due to suppression of T-cell proliferation, large macrophage activation, and a high amount of proinflammatory cytokine and free radicals that predispose patients to impaired healing, infection, and systemic organ failure.106,107
Local factors, such as bacterial contamination and foreign material that cannot be cleaned or degraded, respectively, induce the inflammatory cells (monocytes, M1 macrophages, and FBGCs) to remain at the device's interface, prolonging the inflammation phage to over months or years and leading into chronic inflammation and healing failure.88 Systemic factors are associated with the overall health/disease state of the patient. Increased age is often associated with impaired wound healing. For example, in healthy older adults, wound healing suffers a temporal delay associated with dysfunction of macrophage phagocytic capacity108 and polarization,109 delayed angiogenesis,110 and delayed collagen synthesis and re-epithelization.111 Obesity, which nowadays affects over 500 million people worldwide,112 induces hypoxia and a high infection rate due to skin folds and partial suppression of T-cell function113,114; prompts wound dehiscence by increasing tension on wound site115; and alters the adipocyte and macrophage ratio in adipose tissue, inducing increased production of adipocytokines (e.g., TNF-α, IL-6, IL-8, and MCP-1).116 This increase of adipocytokines, in combination with the activation of granulocytes and monocytes that secrete free radicals and proteolytic enzymes,114 compromises the wound healing process. Diabetes, with over 382 million sufferers worldwide in 2013,117 increases ROS production and reduces antioxidant secretion, leading to oxidative stress,118 which when combined with the hypoxic stress of diabetic wounds,119 leads to an increased inflammatory response.105 Furthermore, diabetic patients have several dysregulated cellular functions, including reduction of inflammatory cell recruitment,120 limited bacterial phagocytosis,121 and dysfunction of macrophage polarization (maintaining a strong M1 marker expression and function122 or fibroblast dysfunction123), which result in an unbalanced expression of growth factors and MMPs that inhibit new tissue formation,124 compromising physiological wound healing.
Methods for Assessing In Vitro Inflammatory Response to Collagen-Based Devices
Although numerous cells (e.g., macrophages,22 monocytes,125 neutrophils,126 leukocytes,23 and dendritic cells127) are employed to study the in vitro inflammatory response to biomaterials, macrophages appear to be the preferred cell population for collagen-based devices. This may be due to the determinant role of macrophages in the resolution of inflammation and wound healing and the availability of techniques to characterize macrophage subpopulations and response.128 To date, the most reliable macrophage sources are those isolated from human peripheral blood mononuclear cells (PBMCs)98 and immortalized cell lines, such as the human-derived leukemic monocyte cell line (THP-1)101; the human leukemic lymphoma monocyte cell line (U937)22; and the mouse leukemic monocyte–macrophage cell line (RAW264.7).129 The advantages of immortalized macrophage cell lines include higher accessibility; lower cell phenotype variability; unnecessary addition of inflammatory mediators in media to prevent apoptosis; and cryopreservation without a detrimental effect on cell viability and differentiation.130,131 Nonetheless, immortalized cell lines suffer from certain cell dysfunctions, such as adapted growth in culture, reduced cell–cell interaction, and decreased protein secretion.132
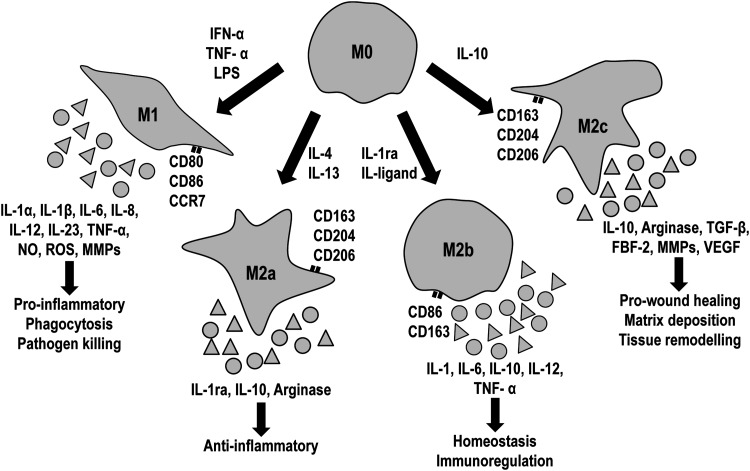
Our understanding of the host response to collagen-based materials is largely attributed to experimental data on macrophage activation and polarization (Fig. 3). Macrophages express different surface markers according to each subpopulation; M1 macrophages are positive for cluster of differentiation 80 (CD80), CD86, and CCR7, while M2 express CD163 and CD206.92,95 Furthermore, the different macrophage subpopulations direct inflammation and tissue repair by secreting cytokines and other reactive species. Specifically, M1 macrophages produce proinflammatory cytokines such as IL-1α, IL-1β, IL-6, IL-8, IL-12, IL-23, and TNF-α, while M2 macrophages secrete IL-1ra, IL-4, IL-10, IL-13, VEGF, TGF-β, and arginase.92–95 M1 macrophages regulate inflammation and collagen-based devices degradation by the secretion of nitrites, ROS, and MMPs.22,133
FIG. 3.
Macrophages polarized from M0 (nonpolarized) to M1 (proinflammatory) or M2 (M2a, anti-inflammatory; M2b, homeostatic; M2c, wound healing) depending on inducing signals. Each macrophage subpopulation expresses different surface markers, cytokines, and reactive species. CD, cluster of differentiation; FBF-2, puf-domain RNA-binding protein; IFN, interferon; IL, interleukin; LPS, lipopolysaccharide; MMP, matrix metalloproteinases; NO, nitric oxide; ROS, reactive oxygen species; TGF-β, transforming growth factor beta; TNF-α, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor.
In Vitro Assessment of Inflammatory Response to Collagen-Based Devices
Cross-linked collagen-based materials have been shown to preferentially alter macrophage response in vitro (Table 1). GTA cross-linked decellularized bovine pericardium (noncommercial material) induced moderate fibroblast cytotoxicity and THP-1 macrophage activation, which secreted a higher amount of TNF-α and IL-6 than the noncross-linked counterpart.134 Additionally, this same material altered U937 macrophage morphology (cell area reduction and disrupted membrane), reduced attachment and viability, increased release of proinflammatory cytokines (TNF-α and IL-6), and changed MMP pattern secretion (upregulated MMP-1 and downregulated MMP-2 and MMP-9), while EDC cross-linked pericardium reduced the release of proinflammatory cytokines, altered MMP pattern, and induced rounded macrophage morphology,22 indicative of M1 macrophages.135 Moreover, PBMCs released a higher amount of TNF-α and IL-6 than IL-10, when cultured on noncommercial GTA cross-linked porcine pulmonary valves.136 With regard to reconstituted collagen materials, noncommercial EDC cross-linked collagen sponges have been shown to increase in vitro resistance to degradation by macrophages; however, these sponges promoted RAW 264.7 macrophage aggregation to form FBGCs that gradually degraded the sponges.137 When incubated with human primary monocytes/macrophages, commercially available slightly cross-linked (HMDI) porcine dermis grafts (Permacol™ Surgical Implant; Covidien, denaturation temperature of 60–61°C,138,139 while its noncross-linked counterpart has denaturation temperature of 56–57°C140) induced low amount of proinflammatory (TNF-α, IL-1β, IL-6, MCP-3, MIP-1α) and anti-inflammatory (IL-1ra, CCL18, MIP-4) cytokines, when compared to other synthetic materials used for soft tissue repair, showing a low M1/M2 protein secretion index.141 Moreover, Permacol materials did not alter in vitro leukocyte viability, activation, and ROS expression. When they were exposed to fresh human peripheral whole blood, they behaved similarly to their noncross-linked counterparts.23 As EDC treatment, noncommercial DHT cross-linked collagen sponges induced FBGC formation, although the treatment increased the enzymatic resistance.137
Table 1.
In Vitro Inflammatory Response Associated with Cross-Linked Collagen-Based Materials
| Cross-linking agent | Summary of in vitro results | References |
|---|---|---|
| GTA (i.e., Peri-Guard® & noncommercial materials) | Alteration of macrophage morphology (cell area reduction and membrane disruption) | 22,134,136 |
| Reduction of macrophage attachment and viability | ||
| Upregulation of proinflammatory cytokines | ||
| Alteration of MMP secretion | ||
| HMDI (i.e., Permacol™ & noncommercial materials) | Increase of enzymatic resistance | 23,141,142 |
| Moderate upregulation of proinflammatory and angiogenic factors/cytokines | ||
| No alteration of leukocyte behavior or release of reactive oxygen species | ||
| EDC (i.e., CollaMend™ & noncommercial materials) | Increase of enzymatic resistance | 22,137,142 |
| Upregulation of proinflammatory and angiogenic factors/cytokines | ||
| Induction of rounded macrophage morphology and macrophage aggregations that form FBGCs to degrade the scaffolds | ||
| DHT (i.e., noncommercial materials) | Increase of enzymatic resistance | 137 |
| Induction of rounded macrophage morphology and macrophage aggregations that form FBGCs to degrade the scaffolds |
DHT, dehydrothermal; EDC, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide; FBGCs, foreign body giant cells; GTA, glutaraldehyde; HMDI, hexamethylene diisocyanate; MMP, matrix metalloproteinase.
Macrophage activation has also been associated with release of chemicals/processing by-products and surface modification. Specifically, released by-products from HMDI and EDC cross-linked porcine dermis grafts (Permacol, and CollaMend™ FM Implant; Bard, denaturation temperature at 66°C138,139) were associated with the increase of proinflammatory (IL-1β, IL-6, IL-8) and vascular (VEGF) cytokine expression of human PBMCs.142 However, a recent publication questioned this theory, as no cross-linking agent traces were detected by nuclear magnetic resonance spectroscopy on conditioned media with a noncommercial GTA cross-linked collagen scaffold, and put forward the notion that the increase of proinflammatory cytokine expression may be induced by collagen surface modification as a function of cross-linking method employed.22 Overall, cross-linking of collagen-based devices has been shown to induce a proinflammatory response: macrophage activation and increase of proinflammatory cytokine release. Despite the significant efforts and the advances in elegant readout systems, the mechanism by which cross-linking alters inflammation has not been elucidated as yet.
In Vivo Models for Assessing Host Response to Collagen-Based Devices
In vivo studies assessing host response can be roughly grouped based on the animal model employed (Table 2). Small animal models are primarily utilized to assess inflammatory response to novel devices, while large animal models are used as close replicates of clinical setting. The most common small animal model for collagen-based devices in vivo characterization is the subcutaneous implantation in mouse or rat for up to a month in duration.21,143 A rat full-thickness skin defect model has also been used to evaluate the wound healing ability of collagen materials combined with plastic dressings in acute and chronic wounds.144,145 The rabbit ear model has also been used to study specific wounds, such as burns146 and hypertrophic scarring.147 Inflammatory response and wound healing are evaluated by routine histological analysis that is sometimes complemented with immunostaining and evaluation of protein and gene expression levels. Collagen-based devices have been extensively assessed in large animal abdominal muscle model repair with significant differences in the size of the defect, time points, and characterization methods (Table 2). However, the rat abdominal model has also been used to evaluate collagen devices for soft tissue repair, despite the lower biomechanical stimulus of small animal models compared to large animals. Obviously, the type of the defect depends on the size of the animal; partial-thickness defect is induced in small animals,148 while full-thickness defect is used in large animals.149 Furthermore, small animal models are primarily used to study early host response; thus, such studies have more early time points (before 30 days). In contrary, large animal models are primarily focused on long-term response, and therefore, early time points (less than 30 days) are hardly ever of interest. Although routine histological analysis is carried out in both small and large animal models, small animal models use more immunohistochemistry, enzyme-linked immunosorbent assay (ELISA), and polymerase chain reaction (PCR) assays to study inflammation cells, surface markers, proteins, and cytokines, while large animal models study functional parameters, such as histomorphometry and mechanical properties of the new tissue. This deviation may be due to the lack of antibodies for large animal models, such as pig, sheep, and bovine species.
Table 2.
Overview of the Animal Models and Experimental Parameters Used to Study Host Response and Wound Healing Associated with Cross-Linked Collagen-Based Materials
| Model size | Species | Material | Model | Time points | Characterization | References |
|---|---|---|---|---|---|---|
| Small animal models | C57BL/6 mouse | Dermal sheep collagen disk (GTA, HMDI) | Subcutaneous implantation | 2, 7, 14, and 21 days | Toluidine blue staining, PCR (IL-4, IL-6, IL-10, IL-13, IFN-γ, TGF-β, MCP-1, MIP-1), IHC (IL-10), in situ zymography | 21,168 |
| Sprague-Dawley rat | Porcine bladder (EDC, GTA), porcine heart valve (GTA), bovine pericardium (GTA) | Subcutaneous implantation | 7, 28, 63, and 180 days | HE and MT staining, quantitative stereological analysis | 143,150 | |
| Sprague-Dawley rat | SIS (EDC, noncross-linked), porcine dermis (EDC, unknown, noncross-linked), | Partial-thickness defect in abdominal muscle | 7, 14, 28, 112 days | MT staining and IHC (CD68, CD80, CD163, CCR7) | 20,148 | |
| Wistar rat | Bovine pericardium (GTA, noncross-linked) | Partial-thickness defect in abdominal muscle | 21 and 90 days | HE and MT staining, IHC (CD80, CD163), lymphocyte transformation test | 159 | |
| Sprague-Dawley rat | Porcine dermis (EDC, HMDI) and human dermis (noncross-linked) | Partial-thickness defect in abdominal muscle | 3, 7, 14, 30, 90, and 180 days | HE, MT, and Verhoeff's staining, IF (COLI, COLIII, FN, elastase, and MMP-9) | 151 | |
| Sprague-Dawley rat | Collagen hydrogel with or without growth factors | Full-thickness skin defect | 3, 7, and 14 days | HE staining, IHC (CD68, COLIV), quantitative stereological analysis, wound contraction | 144,145 | |
| Large animal models | New Zealand white rabbit | Porcine dermis (EDC, HMDI) and SIS (noncross-linked) | Partial-thickness defect in abdominal oblique muscle | 14, 30, 90, and 180 days | MT staining, IF, and qRT-PCR for COLI and COLIII, tensile test | 165,166 |
| New Zealand white rabbit | Collagen sponge with or without growth factors | Second-degree burn defect | 15 days | HE staining, tensile test | 146 | |
| Yucatan minipig | Porcine dermis (EDC, HMDI), bovine pericardium (noncross-linked), and human dermis (noncross-linked) | Full-thickness defect in abdominal muscle | 30, 180, and 360 days | HE staining, stereological analysis, tensile test | 149,158 | |
| Caribbean vervet monkey | Porcine dermis (EDC, HMDI) and SIS (noncross-linked) | Full-thickness defect in abdominal muscle | 180 days | HE staining, IHC (CD3, CD20, CD68), ELISA (immunoglobulin G and M), tensile test | 138,167 |
COL I, collagen type I; ELISA, enzyme-linked immunosorbent assay; HE, hematoxylin and eosin; IF, immunofluorescence; IFN-γ, interferon gamma; IHC, immunohistochemistry; IL, interleukin; MCP-1, monocyte chemoattractant protein-1; MIP-1, macrophage inflammatory protein-1; MT, Masson's trichrome; PCR, polymerase chain reaction; SIS, small intestinal submucosa; TGF-β, transforming growth factor beta.
In Vivo Assessment of Host Response to Collagen-Based Devices
Noncross-linked (Table 3) acellular ECM tissue grafts have shown different host responses depending on their origin. Commercially available porcine small intestinal submucosa (SIS, Surgisis™ Soft Tissue Graft; Cook, denaturation temperature of 61–62°C138,139) and porcine bladder commercially available (MatriStem; Acell)20 or research grade143,150 promoted a dense mononuclear cell infiltration, predominantly neutrophils at week 1 and macrophages (more M2 macrophages than M1) at week 2. At week 4–5, SIS and bladder grafts were completely degraded and were totally replaced by organized collagenous connective tissue and skeletal muscle tissue. Evidence of FBGCs and peri-implantation fibrosis was not observed20,143 or the fibrous tissue surrounding the implants was <52 μm.150 In the same way, commercially available human, porcine, and bovine dermis (Alloderm® Tissue Matrix, LifeCell, denaturation temperature 64°C; Strattice™ Reconstructive Tissue Matrix, LifeCell, denaturation temperature 60°C; SurgiMend™ Collagen Matrix for Soft Tissue Reconstruction, TEI Biosciences, denaturation temperature 57°C139) demonstrated a dense mononuclear cell infiltration, higher M2 macrophage population than M1, and no presence of FBGCs or encapsulation.20,151–153 However, dermis grafts showed a lower cell infiltration, degradation, and new tissue formation than those of SIS and bladder,20,151–153 prolonging graft remodeling over 12 months.152,153 This longer degradation may be due to the higher organization and density of dermis compared to SIS or bladder. With regard to reconstituted collagen materials, noncross-linked materials have been shown to be well-tolerated, to promote tissue regeneration with a minimal inflammatory response.154 Finally, the wound healing capacity (cell infiltration, new tissue deposition, and neovascularization) of noncross-linked collagen materials has also been confirmed in the clinical setting.155
Table 3.
In Vivo Response Associated with Cross-Linked Collagen-Based Materials
| Cross-linking agent | Summary of in vivo results | References |
|---|---|---|
| Noncross-linked | In general, noncross-linked collagen materials | 20,155,158,169,174 |
| Relevant initial cell infiltration | ||
| High-ratio M2/M1 macrophages | ||
| Functional reconstruction, no encapsulation | ||
| Porcine SIS and bladder (i.e., Surgisis™, MatriStem™, or other noncommercial materials) | 143,148,150 | |
| Fast degradation and remodeling ratio | ||
| Human, porcine, and bovine dermis (i.e., Strattice™ or other noncommercial materials) | 20,151,153 | |
| Lower cell infiltration and over-extended remodeling over 12 months | ||
| GTA | Heavily cross-linked collagen-based materials (i.e., Peri-Guard & noncommercial materials) | 21,143,149,150,157–159,162,163 |
| Reduced cell infiltration | ||
| Low-ratio M2/M1 macrophages | ||
| Upregulation of proinflammatory cytokines | ||
| Foreign body response—fibrous encapsulation | ||
| HMDI | Slightly cross-linked collagen-based materials (i.e., Permacol) | 23,151–153,155,164–167,170,171,174 |
| Similar early recruitment of mononuclear cells than its noncross-linked counterpart and less than noncross-linked SIS and bladder. | ||
| Reduced cell infiltration | ||
| Low degradation ratio, over 12–24 months (similar to noncross-linked counterparts) | ||
| Prolonged presence of macrophages around scaffold | ||
| Prolonged remodeling and tissue support | ||
| Heavily cross-linked collagen-based materials (i.e., noncommercial materials) | 21,62,168 | |
| Negligible cell infiltration | ||
| Low-ratio M2/M1 macrophages | ||
| Limited scaffold degradation over 2 years | ||
| Upregulation of IL-10 from FBGCs | ||
| Over-prolonged presence of macrophages and FBGCs around scaffold | ||
| Chronic inflammation and fibrous encapsulation | ||
| EDC | Slightly cross-linked collagen-based materials (i.e., noncommercial materials) | 143,177–180 |
| Relevant initial cell infiltration | ||
| Scaffold degradation and remodeling over 180 days | ||
| New connective tissue replaces degraded scaffold | ||
| Moderately and heavily cross-linked collagen-based materials (i.e., CollaMend & noncommercial materials) | 20,138,148,151,155,165,166,174 | |
| Reduced cell infiltration and low-ratio M2/M1 macrophages | ||
| Limited scaffold degradation over 12 months | ||
| Prolonged presence of macrophages and FBGCs around scaffold | ||
| Chronic inflammation and fibrous encapsulation | ||
| Genipin | Slightly cross-linked collagen-based materials (i.e., noncommercial materials) | 182 |
| Moderate initial cell infiltration | ||
| Scaffold degradation and remodeling up to 12 months | ||
| New connective tissue replaces degraded scaffold | ||
| Heavily cross-linked collagen-based materials (i.e., noncommercial materials) | 181,182 | |
| Reduced cell infiltration | ||
| Limited scaffold degradation at 12 months | ||
| Prolonged presence of macrophages and FBGCs around scaffold | ||
| Chronic inflammation |
The degree of cross-linking regulates scaffold stability and host response.
Chemically cross-linked collagen-based devices demonstrated extended support on the defect area overtime, when compared to the noncross-linked counterparts.156 Commercially available GTA cross-linked tissue grafts (Table 3) (Peri-Guard® Repair Patch, Synovis; denaturation temperature 83°C139) have been shown to elicit chronic inflammation and typical foreign reaction, as evidenced by the early dense accumulation of mononuclear cells and the prolonged presence of macrophages, FBGCs, and fibrous encapsulation surrounding the implant.149,157,158 GTA cross-linked bovine pericardium grafts reduced the M2/M1 macrophage ratio during the inflammation phase, compared to noncross-linked grafts.159 Moreover, noncommercial GTA cross-linked sheep collagen disks induced a massive infiltration of neutrophils that secreted a high amount of MIP-1, MCP-1, and IFN-γ, recruiting and activating macrophages. As a result, macrophages upregulated IL-6 and downregulated IL-10, IL-13, promoting FBGC formation.21 Additionally, commercially available GTA cross-linked collagen sponges for guided bone regeneration and guided tissue regeneration (BioMend® Extend™; Zimmer Dental) promoted ossification in vivo; however, the incidence of mucosa tissue perforation was increased.160 This tissue perforation may be related to the prolonged degradation over 24 weeks, decreased tissue integration and vascularization, and prolonged presence of macrophages and FBGCs around the material.161 Similar to tissue grafts, noncommercial GTA cross-linked collagen hydrogels demonstrated a reduced in vivo degradation (20% degradation after 6 weeks), while their noncross-linked counterparts were largely degraded within a week. However, GTA cross-linked hydrogels reduced cell infiltration and promoted a dense connective tissue layer with inflammatory cells around the hydrogel at an early stage.162 The nonhealing result of GTA cross-linked collagen materials has been attributed to the toxicity of GTA residues163 and the high cross-linking density that prohibit degradation and cell infiltration, even after 2 years of implantation.157
The slightly cross-linked (HMDI) porcine dermis Permacol (Table 3) has displayed high resistance to degradation in vivo and in clinical applications, maintaining the structural integrity for over 2 years.151,164 Permacol has also been shown to induce early recruitment of mononuclear cells around the graft and limited cell infiltration than its noncross-linked counterpart.153 However, this early inflammatory cell population recruitment has been shown to be lower for Permacol than noncross-linked SIS and porcine and human dermis (Surgisis, Strattice, and Alloderm, respectively). However, this response was normalized between all grafts over extended periods.153,165 Regarding cell infiltration, mononuclear cells were detected around Permacol and only infiltrated through material pores; only 20% of implants were colonized at day 14 and the totality of graft was colonized after 1 month.152,165,166 At 90 days, Permacol showed a higher amount of macrophages (RAM-11 positive, specific antibody for rabbit macrophages) and FBGCs than the noncross-linked SIS graft (Surgisis), but a slightly lower macrophage recruitment than other cross-linked porcine dermis grafts (CollaMend).166 After 90 and 180 days of implantation, Permacol implants were surrounded by a new randomly organized connective tissue and fibroblast, supporting tissue integration in its immediate environment. This new tissue adhered to the implants, penetrated them through surface pores, and showed a lower collagen density compared with the typical fibrous tissue.151,165,167 Although Permacol demonstrated a reduction of remodeling ratio, as noncross-linked Strattice and Alloderm, the absence of encapsulation may indicate that these materials are well tolerated and integrated, as they may be assimilated as a normal host matter.
Noncommercial heavily cross-linked (HMDI) dermal grafts (denaturation temperature of 74°C) exhibited extended degradation resistance, induced a limited cell infiltration, and demonstrated a prolonged delay in wound healing.62 These observations may be related to the IL-10 upregulation from FBGCs, which is known to upregulate transcription of TIMP-1, preventing degradation by MMPs. This has also been observed in heavily cross-linked (HMDI) dermal sheep collagen disks21 and heavily cross-linked (HMDI) bovine collagen type I disks.168 The decellularization and delipidation process can also dramatically influence the inflammatory profile of collagen grafts. Specifically, HMDI cross-linked porcine dermis grafts, which were decellularized and delipidated with sodium dodecyl sulfate (SDS) and noncross-linked SIS (Surgisis), have shown increased ROS expression compared to noncross-linked collagen grafts (Strattice and Alloderm™), Permacol, and noncross-linked Permacol.23 This suggests that ROS increase may be processing-dependent. Finally, Permacol has been widely used for human hernia repair with favorable outcomes, compared to synthetic implants.169 Nonetheless, tissue grafts have been associated with a 10% failure rate and 14% chronic inflammation issues for the most complex surgery cases for which no ideal material exists as yet.164,170,171 Commercially available HMDI cross-linked porcine dermis (Zimmer® Collagen Repair Patch; Zimmer) has been used for rotator cuff repair with significant improvement of tendon functionality172; however, chronic inflammation has been reported in few cases.173 It is worth pointing out that these clinical studies were focused on visual observations of the wound and CT scans to evaluate seroma formation, hernia recurrence, and infection. The lack of systematic tissue analysis prohibits precise identification of the cause; is it the graft itself or the comorbidity of the patients?
EDC has also been studied extensively with in vivo degradation and host response depending on the tissue graft characteristics (Table 3). Commercially available EDC cross-linked porcine dermis grafts (CollaMend) showed high resistance to degradation, with no degradation signs and no significant cell infiltration for over 180 days.165 Further, CollaMend induced a disorganized connective tissue with a large amount of macrophages and FBGCs at the implant interface at day 7, reaching the highest cell amount by day 14. By day 30–35, these materials were encapsulated within a dense collagenous tissue and FBGCs20,151; encapsulation and a nonconstructive remodeling layer were evidenced over 180 days, the longest published time point.138,165 Regarding macrophage polarization, CollaMend implants presented the lowest population of M2 macrophages (CD206+) and the highest of M1 macrophages (CCR7+).20 EDC cross-linked porcine dermis has been employed for human abdominal wall reconstruction and clinical data showed similar recurrence to HMDI cross-linked porcine dermis, largely attributed to poor tissue integration and delay in wound healing.155,174,175 However, these clinical studies did not assess the inflammatory response in detail. Another commercially available EDC cross-linked SIS (CuffPatch™, Arthrotek) showed similar results to CollaMend; a higher amount of M1 macrophages (CD80+ and CCR7+) than M2 macrophages (CD163+) and a prolonged presence of macrophages and FBGCs over 16 weeks were reported.148 Interestingly, further investigations demonstrated that the degree of EDC cross-linking of noncommercial decellularized porcine bladder modulated the degradation rate, while it delayed the different stages of reconstructive wound healing.143 Specifically, the low-dose EDC cross-linked tissue grafts (0.0005 mmol per mg of tissue) were completely infiltrated with host cells by day 7 and remained intact, with new collagen being deposited after 28 days. The degradation of these tissue grafts and new collagenous connective tissue deposition was evidenced up to 180 days. The high-dose EDC cross-linked porcine bladder (0.0033 mmol per mg of tissue) displayed the same tendency than the low-dose EDC with some delays in remodeling: low degradation at day 63 and partial degradation with new organized connective tissue by day 180. Furthermore, the observed outstanding cellular infiltration and remodeling features were attributed to the slight cross-linking degree and the fibroporous structure of the materials.143 The introduction of interconnected porosity (30–40 μm pore size) has been demonstrated to promote M2 macrophages and to increase material integration.176 Although scaffold porosity is an important designing parameter, how it modulates macrophage host response is still understudied.
The same host response tendency to EDC cross-linked tissue grafts was observed for EDC cross-linked collagen–elastin sponges (noncommercial materials)177; the low degree of EDC cross-linking (0.3 mM EDC) increased stability of the scaffolds and supported tissue regeneration, although it delayed the wound healing phases. In contrast, the medium degree of EDC cross-linking (0.5 mM EDC) impaired wound healing, induced more macrophages and FBGCs, and scarring was evidenced.177 Furthermore, low-dose EDC cross-linked collagen conduits and sponges (noncommercial materials) have been shown to increase guidance of regenerating axons through distal peripheral nerve sections without obvious macroscopic signs of inflammation or neuroma formation.178–180
As with other cross-linking methods, genipin cross-linking (Table 3) has been shown to increase resistance to degradation for over a year of noncommercial collagen materials, which delayed wound healing.181,182 0.00625%, 0.05%, and 0.625% genipin was used to cross-link bovine pericardium tissue grafts. 0.00625% genipin cross-linked grafts were unable to elicit tissue regeneration due to premature degradation and lack of cell support. 0.05% genipin cross-linked grafts promoted a dense layer of inflammatory cells surrounding the grafts and low cell infiltration at day 3. Cell ingrowth increased with time, reaching maximum by month 3. A gradual graft degradation and new tissue deposition were observed over time; the graft was totally degraded and replaced by connective tissue after 12 months. In contrast, 0.625% genipin cross-linked grafts presented more inflammatory cells, less graft degradation, and less tissue replacement; limited graft surface degradation was observed even after 12 months.182 Ribose has also been used commercially to cross-link collagen sponges (Ossix®; ColBar LifeScience) for guided bone regeneration. This material has demonstrated prolonged degradation, limited cell integration and vascularization, and to induce the presence of macrophages and FBGCs around the material for 24 weeks.161
Overall, in vivo studies demonstrate that host response depends on the cross-linking density and methods employed. Indeed, slightly cross-linked with HMDI, EDC or genipin collagen-based materials support initial cell infiltration and ultimately scaffold replacement by new tissue. Nonetheless, delays in wound healing have also been reported. On the other hand, heavily cross-linked collagen-based materials promote a proinflammatory response (macrophage activation, predominant M1 macrophage population, and increase of proinflammatory cytokine release) that results in impaired wounds or fibrous encapsulation. Despite the extensive investigation into alternative cross-linking methods, no host response studies have been reported as yet.
Conclusions
To date, there is no gold standard method for cross-linking collagen-based materials. With respect to inflammatory response and wound healing, the vast majority of cross-linking methods remain insufficiently studied, with only GTA, HMDI, and EDC to be the most extensively investigated agents. In vitro and in vivo data demonstrate that such chemical cross-linking methods alter the normal wound healing process, even at a low concentration. High cross-linking densities are associated with M1 macrophage response and inhibition of M2 macrophage polarization, reduced cell infiltration, increased proinflammatory cytokine expression, chronic wounds, peri-implantation fibrosis, and delayed wound healing. Detailed information of the processing parameters should be provided to enable better appreciation of the device. Furthermore, preclinical and clinical studies should be accompanied with more detailed analysis (e.g., genes, proteins, immunolabeling, and histology) and information of the general physical state of the subject to enable comparison between different studies. We anticipate that an improved understanding of the mechanisms behind the inflammatory and wound healing response to the cross-linkers, coupled with refined experiments and advances in chemistry, will lead to development of alternative cross-linking processes for collagen-based materials that will display an adequate balance of stability, inflammation, and remodeling. However, with currently available chemical agents, mild collagen crosslinking with better processing to eliminate unreacted site products is recommended in complex clinical cases, where resilience to mechanical loading or resistance to enzymatic degradation are prerequisites.
Acknowledgments
This work is supported by the European Union Seventh Framework Programme (FP7/2007-2013) under agreement number 263289 (Green Nano Mesh) to D.Z.; the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement number 251385 (Tendon Regeneration) to D.Z.; the Science Foundation Ireland, Research Frontiers Programme, under the grant agreement number 09/RFP/ENM2483 to D.Z.; the Health Research Board, Health Research Awards Programme, under the grant agreement number HRA_POR/2011/84 to D.Z.; the Covidien Research Project, under the grant agreement number 81952 to D.Z. and A.P.
Disclosure Statement
L.M.D., A.P., and D.Z. declare no competing financial interests. Y.B. is an employee of Covidien - Sofradim Production.
References
- 1.Zeugolis D.I., and Raghunath M. Collagen: materials analysis and implant uses. In: Ducheyne P., Healy K.E., Hutmacher D.W., Grainger D.W., and Kirkpatrick C.J., eds. Comprehensive Biomaterials. Oxford: Elsevier, 2011, p. 261 [Google Scholar]
- 2.van der Rest M., Garrone R., and Herbage D. Collagen: a family of proteins with many facets. In: Kleinman H.K., ed. Advances in Molecular and Cell Biology. Stamford, CT: JAI Press, Inc., 1993, p. 1 [Google Scholar]
- 3.Kielty C.M., and Grant M.E. The collagen family: structure, assembly and organization in the extracellular matrix. In: Royce P.M., and Steinmann B., eds. Connective Tissue and Its Heritable Disorders: Molecular, Genetic and Medical Aspects, 2nd edition. New York: John Wiley, Inc., 2002, p. 159 [Google Scholar]
- 4.Lee C.H., Singla A., and Lee Y. Biomedical applications of collagen. Int J Pharm 221, 1, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Fratzl P., and Weinkamer R. Nature's hierarchical materials. Prog Mater Sci 52, 1263, 2007 [Google Scholar]
- 6.Paul R.G., and Bailey A.J. Chemical stabilisation of collagen as a biomimetic. ScientificWorldJournal 3, 138, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Global Industry Analysts, Inc. Collagen and HA-Based Biomaterials—Global Strategic Business Report. MCP-1209, 1, 2013
- 8.Smith-Mungo L.I., and Kagan H.M. Lysyl oxidase: properties, regulation and multiple functions in biology. Matrix Biol 16, 387, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Zeugolis D.I., Paul R.G., and Attenburrow G. Factors influencing the properties of reconstituted collagen fibers prior to self-assembly: animal species and collagen extraction method. J Biomed Mater Res A 86, 892, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Zeugolis D.I., Khew S.T., Yew E.S.Y., Ekaputra A.K., Tong Y.W., Yung L.-Y.L., Hutmacher D.W., Sheppard C., and Raghunath M. Electro-spinning of pure collagen nano-fibres—just an expensive way to make gelatin? Biomaterials 29, 2293, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Delgado L., Pandit A., and Zeugolis D.I. Influence of sterilisation methods on collagen-based devices stability and properties. Expert Rev Med Devices 11, 305, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Friess W. Collagen—biomaterial for drug delivery. Eur J Pharm Biopharm 45, 113, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Ramshaw J.A.M., Werkmeister J.A., and Glattauer V. Collagen-based biomaterials. Biotechnol Genet Eng Rev 13, 335, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Zeugolis D.I., Paul G.R., and Attenburrow G. Cross-linking of extruded collagen fibers—a biomimetic three-dimensional scaffold for tissue engineering applications. J Biomed Mater Res A 89, 895, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Gough J.E., Scotchford C.A., and Downes S. Cytotoxicity of glutaraldehyde crosslinked collagen/poly(vinyl alcohol) films is by the mechanism of apoptosis. J Biomed Mater Res 61, 121, 2002 [DOI] [PubMed] [Google Scholar]
- 16.van Wachem P.B., Zeeman R., Dijkstra P.J., Feijen J., Hendriks M., Cahalan P.T., and van Luyn M.J. Characterization and biocompatibility of epoxy-crosslinked dermal sheep collagens. J Biomed Mater Res 47, 270, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Levy R.J., Schoen F.J., Sherman F.S., Nichols J., Hawley M.A., and Lund S.A. Calcification of subcutaneously implanted type I collagen sponges. Effects of formaldehyde and glutaraldehyde pretreatments. Am J Pathol 122, 71, 1986 [PMC free article] [PubMed] [Google Scholar]
- 18.Vasudev S.C., and Chandy T. Effect of alternative crosslinking techniques on the enzymatic degradation of bovine pericardia and their calcification. J Biomed Mater Res 35, 357, 1997 [DOI] [PubMed] [Google Scholar]
- 19.McPherson J., Sawamura S., and Armstrong R. An examination of the biologic response to injectable, glutaraldehyde cross-linked collagen implants. J Biomed Mater Res 20, 93, 1986 [DOI] [PubMed] [Google Scholar]
- 20.Brown B.N., Londono R., Tottey S., Zhang L., Kukla K.A., Wolf M.T., Daly K.A., Reing J.E., and Badylak S.F. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater 8, 978, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye Q., Harmsen M.C., van Luyn M.J., and Bank R.A. The relationship between collagen scaffold cross-linking agents and neutrophils in the foreign body reaction. Biomaterials 31, 9192, 2010 [DOI] [PubMed] [Google Scholar]
- 22.McDade J.K., Brennan-Pierce E.P., Ariganello M.B., Labow R.S., and Michael Lee J. Interactions of U937 macrophage-like cells with decellularized pericardial matrix materials: influence of crosslinking treatment. Acta Biomater 9, 7191, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Bryan N., Ashwin H., Smart N., Bayon Y., Scarborough N., and Hunt J.A. The innate oxygen dependant immune pathway as a sensitive parameter to predict the performance of biological graft materials. Biomaterials 33, 6380, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Olde Damink L.H., Dijkstra P.J., van Luyn M.J., van Wachem P.B., Nieuwenhuis P., and Feijen J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials 17, 765, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Cherfan D., Verter E.E., Melki S., Gisel T.E., Doyle F.J., Jr., Scarcelli G., Yun S.H., Redmond R.W., and Kochevar I.E. Collagen cross-linking using rose bengal and green light to increase corneal stiffness. Invest Ophthalmol Vis Sci 54, 3426, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ibusuki S., Halbesma G.J., Randolph M.A., Redmond R.W., Kochevar I.E., and Gill T.J. Photochemically cross-linked collagen gels as three-dimensional scaffolds for tissue engineering. Tissue Eng 13, 1995, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Roy R., Boskey A., and Bonassar L.J. Processing of type I collagen gels using nonenzymatic glycation. J Biomed Mater Res A 93, 843, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mentink C.J., Hendriks M., Levels A.A., and Wolffenbuttel B.H. Glucose-mediated cross-linking of collagen in rat tendon and skin. Clin Chim Acta 321, 69, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Huang L.L., Sung H.W., Tsai C.C., and Huang D.M. Biocompatibility study of a biological tissue fixed with a naturally occurring crosslinking reagent. J Biomed Mater Res 42, 568, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Sung H.-W., Chang Y., Chiu C.-T., Chen C.-N., and Liang H.-C. Mechanical properties of a porcine aortic valve fixed with a naturally occurring crosslinking agent. Biomaterials 20, 1759, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Antunes A., Attenburrow G., Covington A.D., and Ding J. Utilisation of oleuropein as a crosslinking agent in collagenic films. J Leather Sci 2, 17, 2008 [Google Scholar]
- 32.Zeugolis D.I., Paul R.G., and Attenburrow G. The influence of a natural cross-linking agent (Myrica rubra) on the properties of extruded collagen fibres for tissue engineering applications. Mater Sci Eng C 30, 190, 2010 [Google Scholar]
- 33.Collin E.C., Grad S., Zeugolis D.I., Vinatier C.S., Clouet J.R., Guicheux J.J., Weiss P., Alini M., and Pandit A.S. An injectable vehicle for nucleus pulposus cell-based therapy. Biomaterials 32, 2862, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Cosgriff-Hernandez E., Hahn M.S., Russell B., Wilems T., Munoz-Pinto D., Browning M.B., Rivera J., and Hook M. Bioactive hydrogels based on designer collagens. Acta Biomater 6, 3969, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Ward J., Kelly J., Wang W., Zeugolis D.I., and Pandit A. Amine functionalization of collagen matrices with multifunctional polyethylene glycol systems. Biomacromolecules 11, 3093, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Taguchi T., Xu L., Kobayashi H., Taniguchi A., Kataoka K., and Tanaka J. Encapsulation of chondrocytes in injectable alkali-treated collagen gels prepared using poly(ethylene glycol)-based 4-armed star polymer. Biomaterials 26, 1247, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto K., Nakamura T., Shimizu Y., Ueda H., Sekine T., Yamamoto Y., Kiyotani T., and Takimoto Y. A novel surgical material made from collagen with high mechanical strength: a collagen sandwich membrane. ASAIO J 45, 288, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Cote M.-F., and Doillon C.J. Wettability of cross-linked collagenous biomaterials: in vitro study. Biomaterials 13, 612, 1992 [DOI] [PubMed] [Google Scholar]
- 39.Koide M., Osaki K., Konishi J., Oyamada K., Katakura T., Takahashi A., and Yoshizato K. A new type of biomaterial for artificial skin: dehydrothermally cross-linked composites of fibrillar and denatured collagens. J Biomed Mater Res 27, 79, 1993 [DOI] [PubMed] [Google Scholar]
- 40.Wang M.-C., Pins G.D., and Silver F.H. Collagen fibres with improved strength for the repair of soft tissue injuries. Biomaterials 15, 507, 1994 [DOI] [PubMed] [Google Scholar]
- 41.Weadock K.S., Miller E.J., Bellincampi L.D., Zawadsky J.P., and Dunn M.G. Physical crosslinking of collagen fibers: comparison of ultraviolet irradiation and dehydrothermal treatment. J Biomed Mater Res 29, 1373, 1995 [DOI] [PubMed] [Google Scholar]
- 42.Collins R.L., Christiansen D., Zazanis G.A., and Silver F.H. Use of collagen film as a dural substitute: preliminary animal studies. J Biomed Mater Res 25, 267, 1991 [DOI] [PubMed] [Google Scholar]
- 43.Sionkowska A. Modification of collagen films by ultraviolet irradiation. Polym Degrad Stabil 68, 147, 2000 [Google Scholar]
- 44.Sionkowska A., and Wess T. Mechanical properties of UV irradiated rat tail tendon (RTT) collagen. Int J Biol Macromol 34, 9, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Metreveli N., Namicheishvili L., Jariashvili K., Mrevlishvili G., and Sionkowska A. Mechanisms of the influence of UV irradiation on collagen and collagen-ascorbic acid solutions. Int J Photoenergy 2006, Article ID 76830, 2006 [Google Scholar]
- 46.Vizarova K., Bakos D., Rehakova M., and Macho V. Modification of layered atelocollagen by ultraviolet irradiation and chemical cross-linking: structure stability and mechanical properties. Biomaterials 15, 1082, 1994 [DOI] [PubMed] [Google Scholar]
- 47.Babin H., and Dickinson E. Influence of transglutaminase treatment on the thermoreversible gelation of gelatin. Food Hydrocolloids 15, 271, 2001 [Google Scholar]
- 48.Raghunath M., Hennies H.C., Velten F., Wiebe V., Steinert P.M., Reis A., and Traupe H. A novel in situ method for the detection of deficient transglutaminase activity in the skin. Arch Dermatol Res 290, 621, 1998 [DOI] [PubMed] [Google Scholar]
- 49.Raghunath M., Cankay R., Kubitscheck U., Fauteck J.D., Mayne R., Aeschlimann D., and Schlotzer-Schrehardt U. Transglutaminase activity in the eye: cross-linking in epithelia and connective tissue structures. Invest Ophthalmol Vis Sci 40, 2780, 1999 [PubMed] [Google Scholar]
- 50.Sakamoto H., Kumazawa Y., and Motoki M. Strength of protein gels prepared with microbial transglutaminase as related to reaction conditions. J Food Sci 59, 866, 1994 [Google Scholar]
- 51.Fuchsbauer H.L., Gerber U., Engelmann J., Seeger T., Sinks C., and Hecht T. Influence of gelatin matrices cross-linked with transglutaminase on the properties of an enclosed bioactive material using beta-galactosidase as model system. Biomaterials 17, 1481, 1996 [DOI] [PubMed] [Google Scholar]
- 52.Gorham S.D. Collagen. In: Byrom D., ed. Biomaterials: Novel Materials from Biological Sources. New York: Macmillan Publishers Ltd and ICI Biological Products Business, 1991, p. 55 [Google Scholar]
- 53.Hey K.B., Lachs C.M., Raxworthy M.J., and Wood E.J. Crosslinked fibrous collagen for use as a dermal implant: control of the cytotoxic effects of glutaraldehyde and dimethylsuberimidate. Biotechnol Appl Biochem 12, 85, 1990 [PubMed] [Google Scholar]
- 54.Chapman J.A., Tzaphlidou M., Meek K.M., and Kadler K.E. The collagen fibril—a model system for studying the staining and fixation of a protein. Electron Microsc Rev 3, 143, 1990 [DOI] [PubMed] [Google Scholar]
- 55.Damink L.H.H.O., Dijkstra P.J., van Luyn M.J.A., van Wachem P.B., Nieuwenhuis P., and Feijen J. Crosslinking of dermal sheep collagen using hexamethylene diisocyanate. J Mater Sci Mater Med 6, 429, 1995 [Google Scholar]
- 56.van Luyn M.J.A., van Wachem P.B., Damink L.H.H.O., Dijkstra P.J., Feijen J., and Nieuwenhuis P. Secondary cytotoxicity of cross-linked dermal sheep collagens during repeated exposure to human fibroblasts. Biomaterials 13, 1017, 1992 [DOI] [PubMed] [Google Scholar]
- 57.Nakajima N., and Ikada Y. Mechanism of amide formation by carbodiimide for bioconjugation in aqueous media. Bioconjug Chem 6, 123, 1995 [DOI] [PubMed] [Google Scholar]
- 58.Girardot J.M., and Girardot M.N. Amide cross-linking: an alternative to glutaraldehyde fixation. J Heart Valve Dis 5, 518, 1996 [PubMed] [Google Scholar]
- 59.Pieper J.S., Hafmans T., Veerkamp J.H., and van Kuppevelt T.H. Development of tailor-made collagen-glycosaminoglycan matrices: EDC/NHS crosslinking, and ultrastructural aspects. Biomaterials 21, 581, 2000 [DOI] [PubMed] [Google Scholar]
- 60.Powell H.M., and Boyce S.T. EDC cross-linking improves skin substitute strength and stability. Biomaterials 27, 5821, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Petite H., Frei V., Huc A., and Herbage D. Use of diphenylphosphorylazide for cross-linking collagen-based biomaterials. J Biomed Mater Res A 28, 159, 1994 [DOI] [PubMed] [Google Scholar]
- 62.van Wachem P.B., van Luyn M.J., Olde Damink L.H., Dijkstra P.J., Feijen J., and Nieuwenhuis P. Biocompatibility and tissue regenerating capacity of crosslinked dermal sheep collagen. J Biomed Mater Res 28, 353, 1994 [DOI] [PubMed] [Google Scholar]
- 63.Petite H., Duval J.-L., Frei V., Abdul-Malak N., Sigot-Luizard M.-F., and Herbage D. Cytocompatibility of calf pericardium treated by glutaraldehyde and by the acyl azide methods in an organotypic culture model. Biomaterials 16, 1003, 1995 [DOI] [PubMed] [Google Scholar]
- 64.Zahedi S., Bozon C., and Brunel G. A 2-year clinical evaluation of a diphenylphosphorylazide-cross-linked collagen membrane for the treatment of buccal gingival recession. J Periodontol 69, 975, 1998 [DOI] [PubMed] [Google Scholar]
- 65.Zahedi S., Legrand R., Brunel G., Albert A., Dewe W., Coumans B., and Bernard J.P. Evaluation of a diphenylphosphorylazide-crosslinked collagen membrane for guided bone regeneration in mandibular defects in rats. J Periodontol 69, 1238, 1998 [DOI] [PubMed] [Google Scholar]
- 66.Chevallay B., Abdul_Malak N., and Herbage D. Mouse fibroblasts in long-term culture within collagen three-dimensional scaffolds: influence of crosslinking with diphenylphosphorylazide on matrix reorganization, growth, and biosynthetic and proteolytic activities. J Biomed Mater Res A 49, 448, 2000 [DOI] [PubMed] [Google Scholar]
- 67.Roche S., Ronziere M.-C., Herbage D., and Freyria A.-M. Native and DPPA cross-linked collagen sponges seeded with fetal bovine epiphyseal chondrocytes used for cartilage tissue engineering. Biomaterials 22, 9, 2001 [DOI] [PubMed] [Google Scholar]
- 68.Marinucci L., Lilli C., Guerra M., Belcastro S., Becchetti E., Stabellini G., Calvi E.M., and Locci P. Biocompatibility of collagen membranes crosslinked with glutaraldehyde or diphenylphosphoryl azide: an in vitro study. J Biomed Mater Res A 67, 504, 2003 [DOI] [PubMed] [Google Scholar]
- 69.Jorge-Herrero E., Fernandez P., Turnay J., Olmo N., Calero P., Garcia R., Freile I., and Castillo-Olivares J.L. Influence of different chemical cross-linking treatments on the properties of bovine pericardium and collagen. Biomaterials 20, 539, 1999 [DOI] [PubMed] [Google Scholar]
- 70.Sung H.W., Chang W.H., Ma C.Y., and Lee M.H. Crosslinking of biological tissues using genipin and/or carbodiimide. J Biomed Mater Res A 64, 427, 2003 [DOI] [PubMed] [Google Scholar]
- 71.Barnes C.P., Pemble C.W., Brand D.D., Simpson D.G., and Bowlin G.L. Cross-linking electrospun type II collagen tissue engineering scaffolds with carbodiimide in ethanol. Tissue Eng 13, 1593, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Rault I., Frei V., Herbage D., Abdul-Malak N., and Huc A. Evaluation of different chemical methods for cross-linking collagen gel, films and sponges. J Mater Sci Mater Med 7, 215, 1996 [Google Scholar]
- 73.Zeugolis D.I., Panengad P.P., Yew E.S., Sheppard C., Phan T.T., and Raghunath M. An in situ and in vitro investigation for the transglutaminase potential in tissue engineering. J Biomed Mater Res A 92, 1310, 2010 [DOI] [PubMed] [Google Scholar]
- 74.O Halloran D., Collighan R.J., Griffin M., and Pandit A.S. Characterization of a microbial transglutaminase cross-linked type II collagen scaffold. Tissue Eng 12, 1467, 2006 [DOI] [PubMed] [Google Scholar]
- 75.Lee P.F., Bai Y., Smith R.L., Bayless K.J., and Yeh A.T. Angiogenic responses are enhanced in mechanically and microscopically characterized, microbial transglutaminase crosslinked collagen matrices with increased stiffness. Acta Biomater 9, 7178, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Orban J.M., Wilson L.B., Kofroth J.A., El-Kurdi M.S., Maul T.M., and Vorp D.A. Crosslinking of collagen gels by transglutaminase. J Biomed Mater Res A 68, 756, 2004 [DOI] [PubMed] [Google Scholar]
- 77.Zeng Q., Macri L.K., Prasad A., Clark R.A.F., Zeugolis D.I., Hanley C., Garcia Y., and Pandit A. Skin tissue engineering. In: Ducheyne P., ed. Comprehensive Biomaterials. Oxford: Elsevier, 2011, p. 467 [Google Scholar]
- 78.Martin P. Wound healing—aiming for perfect skin regeneration. Science 276, 75, 1997 [DOI] [PubMed] [Google Scholar]
- 79.Vroman L., Adams A.L., Fischer G.C., and Munoz P.C. Interaction of high molecular weight kininogen, factor XII, and fibrinogen in plasma at interfaces. Blood 55, 156, 1980 [PubMed] [Google Scholar]
- 80.Xu L.C., and Siedlecki C.A. Effects of surface wettability and contact time on protein adhesion to biomaterial surfaces. Biomaterials 28, 3273, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hallab N.J., Bundy K.J., O'Connor K., Moses R.L., and Jacobs J.J. Evaluation of metallic and polymeric biomaterial surface energy and surface roughness characteristics for directed cell adhesion. Tissue Eng 7, 55, 2001 [DOI] [PubMed] [Google Scholar]
- 82.MacDonald D.E., Deo N., Markovic B., Stranick M., and Somasundaran P. Adsorption and dissolution behavior of human plasma fibronectin on thermally and chemically modified titanium dioxide particles. Biomaterials 23, 1269, 2002 [DOI] [PubMed] [Google Scholar]
- 83.Schutte R.J., Parisi-Amon A., and Reichert W.M. Cytokine profiling using monocytes/macrophages cultured on common biomaterials with a range of surface chemistries. J Biomed Mater Res A 88, 128, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deligianni D.D., Katsala N., Ladas S., Sotiropoulou D., Amedee J., and Missirlis Y.F. Effect of surface roughness of the titanium alloy Ti-6Al-4V on human bone marrow cell response and on protein adsorption. Biomaterials 22, 1241, 2001 [DOI] [PubMed] [Google Scholar]
- 85.Chen S., Jones J.A., Xu Y., Low H.-Y., Anderson J.M., and Leong K.W. Characterization of topographical effects on macrophage behavior in a foreign body response model. Biomaterials 31, 3479, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Waterfield J.D., Ali T.A., Nahid F., Kusano K., and Brunette D.M. The effect of surface topography on early NFkappaB signaling in macrophages. J Biomed Mater Res A 95, 837, 2010 [DOI] [PubMed] [Google Scholar]
- 87.Blakney A.K., Swartzlander M.D., and Bryant S.J. The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)-based hydrogels. J Biomed Mater Res A 100, 1375, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li J., Chen J., and Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol 25, 9, 2007 [DOI] [PubMed] [Google Scholar]
- 89.Peterson J.M., and Pizza F.X. Cytokines derived from cultured skeletal muscle cells after mechanical strain promote neutrophil chemotaxis in vitro. J Appl Physiol 106, 130, 2009 [DOI] [PubMed] [Google Scholar]
- 90.Bryan N., Ahswin H., Smart N., Bayon Y., Wohlert S., and Hunt J.A. Reactive oxygen species (ROS)—a family of fate deciding molecules pivotal in constructive inflammation and wound healing. Eur Cell Mater 24, 249, 2012 [DOI] [PubMed] [Google Scholar]
- 91.Anderson J.M., Rodriguez A., and Chang D.T. Foreign body reaction to biomaterials. Semin Immunol 20, 86, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., and Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25, 677, 2004 [DOI] [PubMed] [Google Scholar]
- 93.Gordon S., and Taylor P.R. Monocyte and macrophage heterogeneity. Nat Rev Immunol 5, 953, 2005 [DOI] [PubMed] [Google Scholar]
- 94.Mosser D.M., and Edwards J.P. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8, 958, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brown B.N., and Badylak S.F. Expanded applications, shifting paradigms and an improved understanding of host-biomaterial interactions. Acta Biomater 9, 4948, 2013 [DOI] [PubMed] [Google Scholar]
- 96.Lucas T., Waisman A., Ranjan R., Roes J., Krieg T., Muller W., Roers A., and Eming S.A. Differential roles of macrophages in diverse phases of skin repair. J Immunol 184, 3964, 2010 [DOI] [PubMed] [Google Scholar]
- 97.Garg K., Pullen N.A., Oskeritzian C.A., Ryan J.J., and Bowlin G.L. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials 34, 4439, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Franz S., Allenstein F., Kajahn J., Forstreuter I., Hintze V., Moller S., and Simon J.C. Artificial extracellular matrices composed of collagen I and high-sulfated hyaluronan promote phenotypic and functional modulation of human pro-inflammatory M1 macrophages. Acta Biomater 9, 5621, 2013 [DOI] [PubMed] [Google Scholar]
- 99.Vacanti N.M., Cheng H., Hill P.S., Guerreiro J.D.T., Dang T.T., Ma M., Watson S., Hwang N.S., Langer R., and Anderson D.G. Localized delivery of dexamethasone from electrospun fibers reduces the foreign body response. Biomacromolecules 13, 3031, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bartneck M., Peters F.M., Warzecha K.T., Bienert M., van Bloois L., Trautwein C., Lammers T., and Tacke F. Liposomal encapsulation of dexamethasone modulates cytotoxicity, inflammatory cytokine response, and migratory properties of primary human macrophages. Nanomedicine 2014 [DOI] [PubMed] [Google Scholar]
- 101.Helary C., Browne S., Mathew A., Wang W., and Pandit A. Transfection of macrophages by collagen hollow spheres loaded with polyplexes: a step towards modulating inflammation. Acta Biomater 8, 4208, 2012 [DOI] [PubMed] [Google Scholar]
- 102.Tomasek J.J., Gabbiani G., Hinz B., Chaponnier C., and Brown R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3, 349, 2002 [DOI] [PubMed] [Google Scholar]
- 103.Gurtner G.C., Werner S., Barrandon Y., and Longaker M.T. Wound repair and regeneration. Nature 453, 314, 2008 [DOI] [PubMed] [Google Scholar]
- 104.Spiller K.L., Anfang R.R., Spiller K.J., Ng J., Nakazawa K.R., Daulton J.W., and Vunjak-Novakovic G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 35, 4477, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guo S., and Dipietro L.A. Factors affecting wound healing. J Dent Res 89, 219, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alexander M., Chaudry I.H., and Schwacha M.G. Relationships between burn size, immunosuppression, and macrophage hyperactivity in a murine model of thermal injury. Cell Immunol 220, 63, 2002 [DOI] [PubMed] [Google Scholar]
- 107.Schwacha M.G., Nickel E., and Daniel T. Burn injury-induced alterations in wound inflammation and healing are associated with suppressed hypoxia inducible factor-1alpha expression. Mol Med 14, 628, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Swift M.E., Burns A.L., Gray K.L., and DiPietro L.A. Age-related alterations in the inflammatory response to dermal injury. J Invest Dermatol 117, 1027, 2001 [DOI] [PubMed] [Google Scholar]
- 109.Mahbub S., Deburghgraeve C.R., and Kovacs E.J. Advanced age impairs macrophage polarization. J Interferon Cytokine Res 32, 18, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Swift M.E., Kleinman H.K., and DiPietro L.A. Impaired wound repair and delayed angiogenesis in aged mice. Lab Invest 79, 1479, 1999 [PubMed] [Google Scholar]
- 111.Gosain A., and DiPietro L.A. Aging and wound healing. World J Surg 28, 321, 2004 [DOI] [PubMed] [Google Scholar]
- 112.World Health Organisation. Obesity and overweight. Fact sheet N311. World Health Organisation, 2014. http://www.who.int/mediacentre/factsheets/fs311/en/
- 113.Anaya D.A., and Dellinger E.P. The obese surgical patient: a susceptible host for infection. Surg Infect 7, 473, 2006 [DOI] [PubMed] [Google Scholar]
- 114.Nieman D.C., Henson D.A., Nehlsen-Cannarella S.L., Ekkens M., Utter A.C., Butterworth D.E., and Fagoaga O.R. Influence of obesity on immune function. J Am Diet Assoc 99, 294, 1999 [DOI] [PubMed] [Google Scholar]
- 115.Spiliotis J., Tsiveriotis K., Datsis A.D., Vaxevanidou A., Zacharis G., Giafis K., Kekelos S., and Rogdakis A. Wound dehiscence: is still a problem in the 21th century: a retrospective study. World J Emerg Surg 4, 12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Juge-Aubry C.E., Henrichot E., and Meier C.A. Adipose tissue: a regulator of inflammation. Best Pract Res Clin Endocrinol Metab 19, 547, 2005 [DOI] [PubMed] [Google Scholar]
- 117.International Diabetes Federation. IDF Diabetes Atlas, 6th edition. Brussels, Belgium: International Diabetes Federation, 2013 [Google Scholar]
- 118.Aouacheri O., Saka S., Krim M., Messaadia A., and Maidi I. The investigation of the oxidative stress-related parameters in type 2 diabetes mellitus. Can J Diabetes 2014. [Epub ahead of print]; DOI: 10.1016/j.jcjd.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 119.Martin A., Komada M.R., and Sane D.C. Abnormal angiogenesis in diabetes mellitus. Med Res Rev 23, 117, 2003 [DOI] [PubMed] [Google Scholar]
- 120.Galkowska H., Olszewski W.L., Wojewodzka U., Rosinski G., and Karnafel W. Neurogenic factors in the impaired healing of diabetic foot ulcers. J Surg Res 134, 252, 2006 [DOI] [PubMed] [Google Scholar]
- 121.Lecube A., Pachón G., Petriz J., Hernández C., and Simó R. Phagocytic activity is impaired in type 2 diabetes mellitus and increases after metabolic improvement. PLoS One 6, e23366, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Olefsky J.M., and Glass C.K. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 72, 219, 2010 [DOI] [PubMed] [Google Scholar]
- 123.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 366, 1736, 2005 [DOI] [PubMed] [Google Scholar]
- 124.Wall S.J., Bevan D., Thomas D.W., Harding K.G., Edwards D.R., and Murphy G. Differential expression of matrix metalloproteinases during impaired wound healing of the diabetes mouse. J Invest Dermatol 119, 91, 2002 [DOI] [PubMed] [Google Scholar]
- 125.Kajahn J., Franz S., Rueckert E., Forstreuter I., Hintze V., Moeller S., and Simon J.C. Artificial extracellular matrices composed of collagen I and high sulfated hyaluronan modulate monocyte to macrophage differentiation under conditions of sterile inflammation. Biomatter 2, 226, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Velard F., Laurent-Maquin D., Braux J., Guillaume C., Bouthors S., Jallot E., Nedelec J.M., Belaaouaj A., and Laquerriere P. The effect of zinc on hydroxyapatite-mediated activation of human polymorphonuclear neutrophils and bone implant-associated acute inflammation. Biomaterials 31, 2001, 2010 [DOI] [PubMed] [Google Scholar]
- 127.Sprague L., Muccioli M., Pate M., Meles E., McGinty J., Nandigam H., Venkatesh A.K., Gu M.Y., Mansfield K., Rutowski A., Omosebi O., Courreges M.C., and Benencia F. The interplay between surfaces and soluble factors define the immunologic and angiogenic properties of myeloid dendritic cells. BMC Immunol 12, 35, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brown B.N., Ratner B.D., Goodman S.B., Amar S., and Badylak S.F. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 33, 3792, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ren J.D., Fan L., Tian F.Z., Fan K.H., Yu B.T., Jin W.H., Tan Y.H., and Cheng L. Involvement of a membrane potassium channel in heparan sulphate-induced activation of macrophages. Immunology 141, 345, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Heil T.L., Volkmann K.R., Wataha J.C., and Lockwood P.E. Human peripheral blood monocytes versus THP-1 monocytes for in vitro biocompatibility testing of dental material components. J Oral Rehabil 29, 401, 2002 [DOI] [PubMed] [Google Scholar]
- 131.Qin Z. The use of THP-1 cells as a model for mimicking the function and regulation of monocytes and macrophages in the vasculature. Atherosclerosis 221, 2, 2012 [DOI] [PubMed] [Google Scholar]
- 132.Pan C., Kumar C., Bohl S., Klingmueller U., and Mann M. Comparative proteomic phenotyping of cell lines and primary cells to assess preservation of cell type-specific functions. Mol Cell Proteomics 8, 443, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kwon J., Kim J., Park S., Khang G., Kang P.M., and Lee D. Inflammation-responsive antioxidant nanoparticles based on a polymeric prodrug of vanillin. Biomacromolecules 14, 1618, 2013 [DOI] [PubMed] [Google Scholar]
- 134.Umashankar P.R., Mohanan P.V., and Kumari T.V. Glutaraldehyde treatment elicits toxic response compared to decellularization in bovine pericardium. Toxicol Int 19, 51, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McWhorter F.Y., Wang T., Nguyen P., Chung T., and Liu W.F. Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci U S A 110, 17253, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bayrak A., Tyralla M., Ladhoff J., Schleicher M., Stock U.A., Volk H.D., and Seifert M. Human immune responses to porcine xenogeneic matrices and their extracellular matrix constituents in vitro. Biomaterials 31, 3793, 2010 [DOI] [PubMed] [Google Scholar]
- 137.Yahyouche A., Zhidao X., Czernuszka J.T., and Clover A.J. Macrophage-mediated degradation of crosslinked collagen scaffolds. Acta Biomater 7, 278, 2011 [DOI] [PubMed] [Google Scholar]
- 138.Sandor M., Xu H., Connor J., Lombardi J., Harper J.R., Silverman R.P., and McQuillan D.J. Host response to implanted porcine-derived biologic materials in a primate model of abdominal wall repair. Tissue Eng Part A 14, 2021, 2008 [DOI] [PubMed] [Google Scholar]
- 139.Deeken C.R., Eliason B.J., Pichert M.D., Grant S.A., Frisella M.M., and Matthews B.D. Differentiation of biologic scaffold materials through physicomechanical, thermal, and enzymatic degradation techniques. Ann Surg 255, 595, 2012 [DOI] [PubMed] [Google Scholar]
- 140.Ashwin H., Hunt J., Mausy E., Migliozzi J., Beers D., and Wohlert S. Influence of varying HMDI crosslinking parameters on porcine dermis. In TCES meeting. Eur Cell Mater 23, 64, 2012 [Google Scholar]
- 141.Grotenhuis N., Bayon Y., Lange J.F., Van Osch G.J., and Bastiaansen-Jenniskens Y.M. A culture model to analyze the acute biomaterial-dependent reaction of human primary macrophages. Biochem Biophys Res Commun 433, 115, 2013 [DOI] [PubMed] [Google Scholar]
- 142.Orenstein S.B., Qiao Y., Klueh U., Kreutzer D.L., and Novitsky Y.W. Activation of human mononuclear cells by porcine biologic meshes in vitro. Hernia 14, 401, 2010 [DOI] [PubMed] [Google Scholar]
- 143.Burugapalli K., Chan J.C., Kelly J.L., and Pandit A.S. Efficacy of crosslinking on tailoring in vivo biodegradability of fibro-porous decellularized extracellular matrix and restoration of native tissue structure: a quantitative study using stereology methods. Macromol Biosci 14, 244, 2014 [DOI] [PubMed] [Google Scholar]
- 144.Pandit A., Ashar R., Feldman D., and Thompson A. Investigation of acidic fibroblast growth factor delivered through a collagen scaffold for the treatment of full-thickness skin defects in a rabbit model. Plast Reconstr Surg 101, 766, 1998 [DOI] [PubMed] [Google Scholar]
- 145.Dong Y., Hassan W.U., Kennedy R., Greiser U., Pandit A., Garcia Y., and Wang W. Performance of an in situ formed bioactive hydrogel dressing from a PEG-based hyperbranched multifunctional copolymer. Acta Biomater 10, 2076, 2014 [DOI] [PubMed] [Google Scholar]
- 146.Lee A.R. Enhancing dermal matrix regeneration and biomechanical properties of 2nd degree-burn wounds by EGF-impregnated collagen sponge dressing. Arch Pharm Res 28, 1311, 2005 [DOI] [PubMed] [Google Scholar]
- 147.Hong S.J., Jia S.X., Xie P., Xu W., Leung K.P., Mustoe T.A., and Galiano R.D. Topically delivered adipose derived stem cells show an activated-fibroblast phenotype and enhance granulation tissue formation in skin wounds. PLoS One 8, e55640, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Badylak S.F., Valentin J.E., Ravindra A.K., McCabe G.P., and Stewart-Akers A.M. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A 14, 1835, 2008 [DOI] [PubMed] [Google Scholar]
- 149.Deeken C.R., Melman L., Jenkins E.D., Greco S.C., Frisella M.M., and Matthews B.D. Histologic and biomechanical evaluation of crosslinked and non-crosslinked biologic meshes in a porcine model of ventral incisional hernia repair. J Am Coll Surg 212, 880, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Burugapalli K., and Pandit A. Characterization of tissue response and in vivo degradation of cholecyst-derived extracellular matrix. Biomacromolecules 8, 3439, 2007 [DOI] [PubMed] [Google Scholar]
- 151.Broderick G., McIntyre J., Noury M., Strom H.M., Psoinos C., Christakas A., Billiar K., Hurwitz Z.M., Lalikos J.F., Ignotz R.A., and Dunn R.M. Dermal collagen matrices for ventral hernia repair: comparative analysis in a rat model. Hernia 16, 333, 2012 [DOI] [PubMed] [Google Scholar]
- 152.Bryan N., Ahswin H., Smart N., Bayon Y., Wohlert S., and Hunt J.A. The in vivo evaluation of tissue-based biomaterials in a rat full-thickness abdominal wall defect model. J Biomed Mater Res B Appl Biomater 102, 709, 2014 [DOI] [PubMed] [Google Scholar]
- 153.Bryan N., Ashwin H., Smart N.J., Wohlert S., Bayon Y., and Hunt J.A. Characterisation and comparison of the host response of 6 tissue-based surgical implants in a subcutaneous in vivo rat model. J Appl Biomater Funct Mater 2014. [Epub ahead of print]; DOI: 10.5301/jabfm.5000172 [DOI] [PubMed] [Google Scholar]
- 154.Abou Neel E.A., Bozec L., Knowles J.C., Syed O., Mudera V., Day R., and Hyun J.K. Collagen—emerging collagen based therapies hit the patient. Adv Drug Deliv Rev 65, 429, 2013 [DOI] [PubMed] [Google Scholar]
- 155.Harth K.C., and Rosen M.J. Major complications associated with xenograft biologic mesh implantation in abdominal wall reconstruction. Surg Innov 16, 324, 2009 [DOI] [PubMed] [Google Scholar]
- 156.Gaertner W.B., Bonsack M.E., and Delaney J.P. Experimental evaluation of four biologic prostheses for ventral hernia repair. J Gastrointest Surg 11, 1275, 2007 [DOI] [PubMed] [Google Scholar]
- 157.Badylak S., Kokini K., Tullius B., Simmons-Byrd A., and Morff R. Morphologic study of small intestinal submucosa as a body wall repair device. J Surg Res 103, 190, 2002 [DOI] [PubMed] [Google Scholar]
- 158.Melman L., Jenkins E.D., Hamilton N.A., Bender L.C., Brodt M.D., Deeken C.R., Greco S.C., Frisella M.M., and Matthews B.D. Early biocompatibility of crosslinked and non-crosslinked biologic meshes in a porcine model of ventral hernia repair. Hernia 15, 157, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Umashankar P.R., Arun T., and Kumary T.V. Effect of chronic inflammation and immune response on regeneration induced by decellularized bovine pericardium. J Biomed Mater Res A 101, 2202, 2013 [DOI] [PubMed] [Google Scholar]
- 160.Tal H., Kozlovsky A., Artzi Z., Nemcovsky C.E., and Moses O. Long-term bio-degradation of cross-linked and non-cross-linked collagen barriers in human guided bone regeneration. Clin Oral Implants Res 19, 295, 2008 [DOI] [PubMed] [Google Scholar]
- 161.Rothamel D., Schwarz F., Sager M., Herten M., Sculean A., and Becker J. Biodegradation of differently cross-linked collagen membranes: an experimental study in the rat. Clin Oral Implants Res 16, 369, 2005 [DOI] [PubMed] [Google Scholar]
- 162.Pilipchuk S.P., Vaicik M.K., Larson J.C., Gazyakan E., Cheng M.H., and Brey E.M. Influence of crosslinking on the stiffness and degradation of dermis-derived hydrogels. J Biomed Mater Res A 101, 2883, 2013 [DOI] [PubMed] [Google Scholar]
- 163.Saito H., Murabayashi S., Mitamura Y., and Taguchi T. Characterization of alkali-treated collagen gels prepared by different crosslinkers. J Mater Sci Mater Med 19, 1297, 2008 [DOI] [PubMed] [Google Scholar]
- 164.O'Brien J.A., Ignotz R., Montilla R., Broderick G.B., Christakis A., and Dunn R.M. Long-term histologic and mechanical results of a Permacol abdominal wall explant. Hernia 15, 211, 2011 [DOI] [PubMed] [Google Scholar]
- 165.Pascual G., Rodriguez M., Sotomayor S., Moraleda E., and Bellon J.M. Effects of collagen prosthesis cross-linking on long-term tissue regeneration following the repair of an abdominal wall defect. Wound Repair Regen 20, 402, 2012 [DOI] [PubMed] [Google Scholar]
- 166.Bellon J.M., Rodriguez M., Gomez-Gil V., Sotomayor S., Bujan J., and Pascual G. Postimplant intraperitoneal behavior of collagen-based meshes followed by laparoscopy. Surg Endosc 26, 27, 2012 [DOI] [PubMed] [Google Scholar]
- 167.Connor J., McQuillan D., Sandor M., Wan H., Lombardi J., Bachrach N., Harper J., and Xu H. Retention of structural and biochemical integrity in a biological mesh supports tissue remodeling in a primate abdominal wall model. Regen Med 4, 185, 2009 [DOI] [PubMed] [Google Scholar]
- 168.Ye Q., van Amerongen M.J., Sandham J.A., Bank R.A., van Luyn M.J., and Harmsen M.C. Site-specific tissue inhibitor of metalloproteinase-1 governs the matrix metalloproteinases-dependent degradation of crosslinked collagen scaffolds and is correlated with interleukin-10. J Tissue Eng Regen Med 5, 264, 2011 [DOI] [PubMed] [Google Scholar]
- 169.Smart N.J., Marshall M., and Daniels I.R. Biological meshes: a review of their use in abdominal wall hernia repairs. Surgeon 10, 159, 2012 [DOI] [PubMed] [Google Scholar]
- 170.Hsu P.W., Salgado C.J., Kent K., Finnegan M., Pello M., Simons R., Atabek U., and Kann B. Evaluation of porcine dermal collagen (Permacol) used in abdominal wall reconstruction. J Plast Reconstr Aesthet Surg 62, 1484, 2009 [DOI] [PubMed] [Google Scholar]
- 171.Parker D.M., Armstrong P.J., Frizzi J.D., and North J.H., Jr. Porcine dermal collagen (Permacol) for abdominal wall reconstruction. Curr Surg 63, 255, 2006 [DOI] [PubMed] [Google Scholar]
- 172.Badhe S.P., Lawrence T.M., Smith F.D., and Lunn P.G. An assessment of porcine dermal xenograft as an augmentation graft in the treatment of extensive rotator cuff tears. J Shoulder Elbow Surg 17, 35S, 2008 [DOI] [PubMed] [Google Scholar]
- 173.Soler J.A., Gidwani S., and Curtis M.J. Early complications from the use of porcine dermal collagen implants (Permacol) as bridging constructs in the repair of massive rotator cuff tears. A report of 4 cases. Acta Orthop Belg 73, 432, 2007 [PubMed] [Google Scholar]
- 174.Shah B.C., Tiwari M.M., Goede M.R., Eichler M.J., Hollins R.R., McBride C.L., Thompson J.S., and Oleynikov D. Not all biologics are equal! Hernia 15, 165, 2011 [DOI] [PubMed] [Google Scholar]
- 175.Chavarriaga L.F., Lin E., Losken A., Cook M.W., Jeansonne L.O., White B.C., Sweeney J.F., Galloway J.R., and Davis S.S., Jr. Management of complex abdominal wall defects using acellular porcine dermal collagen. Am Surg 76, 96, 2010 [PubMed] [Google Scholar]
- 176.Madden L.R., Mortisen D.J., Sussman E.M., Dupras S.K., Fugate J.A., Cuy J.L., Hauch K.D., Laflamme M.A., Murry C.E., and Ratner B.D. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc Natl Acad Sci U S A 107, 15211, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Boekema B.K., Vlig M., Olde Damink L., Middelkoop E., Eummelen L., Buhren A.V., and Ulrich M.M. Effect of pore size and cross-linking of a novel collagen-elastin dermal substitute on wound healing. J Mater Sci Mater Med 25, 423, 2014 [DOI] [PubMed] [Google Scholar]
- 178.Daly W.T., Yao L., Abu-rub M.T., O'Connell C., Zeugolis D.I., Windebank A.J., and Pandit A.S. The effect of intraluminal contact mediated guidance signals on axonal mismatch during peripheral nerve repair. Biomaterials 33, 6660, 2012 [DOI] [PubMed] [Google Scholar]
- 179.Yao L., Daly W., Newland B., Yao S., Wang W., Chen B.K., Madigan N., Windebank A., and Pandit A. Improved axonal regeneration of transected spinal cord mediated by multichannel collagen conduits functionalized with neurotrophin-3 gene. Gene Ther 20, 1149, 2013 [DOI] [PubMed] [Google Scholar]
- 180.Bozkurt A., Lassner F., O'Dey D., Deumens R., Bocker A., Schwendt T., Janzen C., Suschek C.V., Tolba R., Kobayashi E., Sellhaus B., Tholl S., Eummelen L., Schugner F., Damink L.O., Weis J., Brook G.A., and Pallua N. The role of microstructured and interconnected pore channels in a collagen-based nerve guide on axonal regeneration in peripheral nerves. Biomaterials 33, 1363, 2012 [DOI] [PubMed] [Google Scholar]
- 181.Chang Y., Tsai C.C., Liang H.C., and Sung H.W. In vivo evaluation of cellular and acellular bovine pericardia fixed with a naturally occurring crosslinking agent (genipin). Biomaterials 23, 2447, 2002 [DOI] [PubMed] [Google Scholar]
- 182.Liang H.C., Chang Y., Hsu C.K., Lee M.H., and Sung H.W. Effects of crosslinking degree of an acellular biological tissue on its tissue regeneration pattern. Biomaterials 25, 3541, 2004 [DOI] [PubMed] [Google Scholar]